Abstract
Keratin 8 (K8) variants predispose to human liver injury via poorly understood mechanisms. We generated transgenic mice that overexpress the human disease-associated K8 Gly61-to-Cys (G61C) variant and showed that G61C predisposes to liver injury and apoptosis and dramatically inhibits K8 phosphorylation at serine 73 (S73) via stress-activated kinases. This led us to generate mice that overexpress K8 S73-to-Ala (S73A), which mimicked the susceptibility of K8 G61C mice to injury, thereby providing a molecular link between K8 phosphorylation and disease-associated mutation. Upon apoptotic stimulation, G61C and S73A hepatocytes have persistent and increased nonkeratin proapoptotic substrate phosphorylation by stress-activated kinases, compared with wild-type hepatocytes, in association with an inability to phosphorylate K8 S73. Our findings provide the first direct link between patient-related human keratin variants and liver disease predisposition. The highly abundant cytoskeletal protein K8, and possibly other keratins with the conserved S73-containing phosphoepitope, can protect tissue from injury by serving as a phosphate “sponge” for stress-activated kinases and thereby provide a novel nonmechanical function for intermediate filament proteins.
Introduction
Keratins are intermediate filament (IF) proteins that are preferentially expressed in epithelial cells and epidermal appendages (Fuchs and Weber, 1994; Coulombe and Omary, 2002). In epithelial cells, the prominent IFs include keratins 1–20 (K1–K20), which are further classified into type I (K9–K20) and II (K1–K8) keratins, which form obligate, noncovalent type I/II keratin heteropolymers (Moll et al., 1982; Herrmann and Aebi, 2004). Unique keratin complements serve as cell-specific markers that distinguish different epithelial cell types. For example, basal epidermal keratinocytes preferentially express K5/K14, and suprabasal keratinocytes in the upper layer of the skin express K1/K10, whereas K8/K18 are the IF proteins of adult hepatocytes (Moll et al., 1982; Coulombe and Omary, 2002). K8/K18 is also the prototype keratin pair of simple-type epithelia and, as such, is broadly expressed in epithelial components of glandular tissues, including the pancreas and intestine, with variable levels of K19, K20, and K7, depending on the cell type (Ku et al., 1999). All IF proteins, including keratins, contain a central and conserved coiled coil–forming α-helical “rod” domain that is flanked by relatively nonconserved non–α-helical NH2-terminal “head” and COOH-terminal “tail” domains (Fuchs and Weber, 1994; Herrmann and Aebi, 2004). The flanking head and tail domains are the more exposed portions of IF proteins, which explains why all IF phosphorylation sites reside in these domains (Omary et al., 1998). Several in vivo K8/K18 phosphorylation sites have been identified that include K8 Ser23/Ser73/Ser431 and K18 Ser33/Ser52 (Omary et al., 1998). K8/K18 hyperphosphorylation correlates with disease progression in patients with chronic liver disease (Toivola et al., 2004; Zatloukal et al., 2004) and plays an essential role in regulating keratin filament organization, association with binding partners such as 14-3-3 proteins, and turnover (Coulombe and Omary, 2002).
Keratin mutations are associated with several skin, oral, esophageal, ocular, hair, and liver diseases that reflect the tissue-specific expression of the particular keratin (Fuchs and Cleveland, 1998; Omary et al., 2004). The resulting disease-related tissue defects are manifestations of the clearly defined function of keratins that allows cells to cope with mechanical stresses. This keratin-related cytoprotective effect is most evident in the keratinocyte fragility phenotype of human epidermolysis bullosa simplex (EBS), which is caused by K5/K14 mutations, and is evident in the phenotypes of several animal models that lack or express mutant keratins (Fuchs and Cleveland, 1998; Magin et al., 2004; Omary et al., 2004). Emerging evidence also indicates that keratins protect cells from nonmechanical injury via mechanisms that include keratin regulation of cell signaling cascades, regulation of susceptibility to apoptosis, and modulation of protein targeting to subcellular compartments (Coulombe and Omary, 2002; Toivola et al., 2005). For example, livers of K8- or K18-null mice or mice that express K18 Arg89-to-Cys (an EBS-like mutation) manifest a remarkable predisposition to injury and apoptosis (Ku et al., 1996, 2003; Loranger et al., 1997; Caulin et al., 2000; Gilbert et al., 2001). K18 R89C and K14 R125 residues and their surrounding amino acids are highly conserved, and K14 R125 mutations cause the severest form of EBS and are the most common in keratin-related skin diseases (Fuchs and Cleveland, 1998; Porter and Lane, 2003).
Most human keratin-associated diseases are caused by autosomal-dominant keratin missense mutations with near complete penetrance, and most of these mutations are located at highly conserved regions at the ends of the rod domain (Porter and Lane, 2003; Omary et al., 2004). Exceptions include mutations in K8/K18, which pose a risk for the subsequent development of cirrhosis and liver disease progression (Ku et al., 1997, 2001, 2005; Strnad et al., 2006a,b), and may also be associated with inflammatory bowel disease (Owens et al., 2004). All known human K8/K18 mutations do not involve the highly conserved ends of the rod domain. For example, the EBS-like K18 Arg89-to-Cys mutation, which causes hepatocyte fragility and predisposes to hepatocyte injury and apoptosis in mice (Ku et al., 1995, 1996, 2003), has not been found in humans, and it is hypothesized that such mutations are embryolethal (Omary et al., 2002; Porter and Lane, 2003). The prevalence odds ratio for the association of K8/K18 mutations with human cirrhosis is 3.8 (95% confidence interval of 2.1–7.1), and the association with liver disease is highly significant when comparing a large American cohort of liver disease patients who underwent liver transplantation with a control group (P < 0.0001; Ku et al., 2005). In addition, a study using a large German patient cohort with chronic hepatitis C showed a significant association of exonic K8 variants with increased fibrosis (Strnad et al., 2006a). However, direct evidence for the predisposition to liver injury via any natural human K8/K18 mutation has not been described.
To address the in vivo significance of human liver disease–associated keratin mutations, we generated transgenic mice that overexpress wild-type (WT) or Gly61-to-Cys (G61C) human K8 (hK8) and compared their susceptibility to stress-induced liver injury. We targeted K8 G61 for the following reasons: (a) K8 G61 is highly conserved among type II keratins (Fig. 1 A), (b) K8 G61C is the second most prevalent among K8/K18 variants that are associated with cirrhosis and fibrosis progression (Ku et al., 2005; Strnad et al., 2006a), and (c) in transfected cells, G61C interferes with keratin filament reorganization and cross-links hK8 under oxidative conditions (Ku et al., 2001, 2005). The G61C mice unmasked an important relationship between K8 G61C mutation and K8 S73 phosphorylation by stress-activated protein kinases (SAPKs). This relationship was further explored by generating transgenic mice that overexpress K8 S73A.
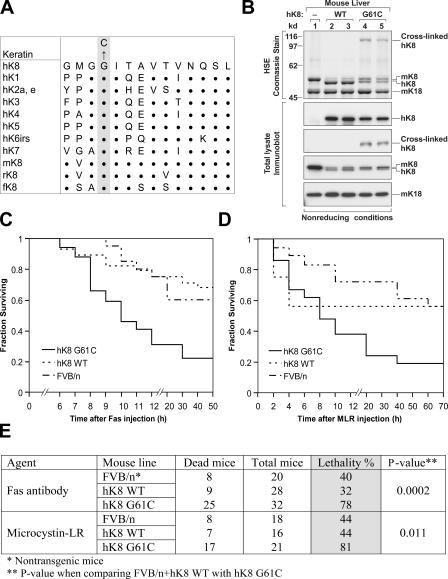
Figure 1.
hK8 G61C mice have increased susceptibility to liver injury. (A) Alignment of the hK8 sequence that is proximal to G61 (shaded residue) with other type II keratins. m, mouse; r, rat; and f, frog. Single letter abbreviations are used for amino acids, and bold dots indicate amino acids that are identical to hK8. (B) Livers were isolated from nontransgenic (lane 1) or transgenic mice that overexpress hK8 WT (lanes 2 and 3) or G61C (lanes 4 and 5). Two independent transgenic lines per genotype were used (i.e., each lane from 2 to 5 represents one mouse per transgenic line). Livers were homogenized, followed by HSE to purify the total keratin pool, or were solubilized in 2% SDS-containing sample buffer to obtain total lysates (Ku et al., 2004). Isolates were separated by SDS-PAGE, and then visualized by Coomassie staining (HSE preparations) or by immunoblotting (total liver homogenates) with Abs to m/hK8, hK8, and mK18. Cross-linked K8 is detected only in livers from K8 G61C mice, and disappears if samples are analyzed under reducing conditions (not depicted). (C and D) The following three mouse genotypes were used: nontransgenic FVB/n, transgenic hK8 WT, or G61C mutant mice (16–32 age- and sex-matched mice per genotype). Each analyzed genotype consisted of nearly equal portions of the two generated independent lines (WT1 and WT2; G61C1 and G61C2). After Fas (C) or MLR (D) injection, mice were assessed hourly for 12 h, and then every 10 h for 3 d. The analyses show survival curves for each administered agent. (E) Summary of the mouse lethality data shown in C and D. No significant difference in mortality was observed when comparing FVB/n with hK8 WT mice, whereas mortality was markedly increased in hK8 G61C mice as compared with the combined FVB/n + hK8 WT mice.
Results
K8 G61C predisposes to stress-induced liver injury in transgenic mice
Transgene expression of the WT and G61C K8 mice was confirmed by blotting total liver homogenates with antibodies (Abs) specific to hK8, and by Coomassie staining of a cytoskeletal high salt extract (HSE) that shows the total liver keratin composition and distinguishes endogenous from exogenous keratins (Fig. 1 B). Importantly, protein levels of the endogenous mouse WT K8 and human G61C transgene product are similar, which mimics what is seen in human liver disease patients with heterozygous K8 G61C expression. Cross-linked hK8 is found only in G61C, but not WT, livers (Fig. 1 B) because of the absence of Cys in WT hK8. Both WT and G61C mice were viable, fertile, and had no obvious phenotype under basal conditions. Previously described transgenic mice that overexpress high levels of WT K8, using the same WT hK8 we used, also do not have liver abnormalities under basal conditions, but develop pancreatic insufficiency that is possibly related to the WT hK8 expression level (Casanova et al., 1999). The K8 WT and G61C mice described herein do not have abnormal histology in their pancreata under basal conditions (unpublished data).
We tested the consequence of G61C on susceptibility to liver injury using the Fas (which causes hepatocyte apoptosis; Ku et al., 2003) or microcystin-LR (MLR; which causes hemorrhagic hepatitis; Ku et al., 1998) injury models. Fas or MLR administration causes significant lethality (Fig. 1, C and D), and the G61C mice were markedly more susceptible to lethal liver injury as compared with nontransgenic and hK8 WT mice (∼80% G61C vs. ∼40% control; Fig. 1 E). The increased lethality of G61C, as compared with WT mice, is caused by severe liver hemorrhage and apoptosis (Fig. 2 A). Although K8 G61C forms normal-appearing keratin filaments under basal conditions, Fas administration causes hepatocyte drop-off and a more prominent keratin filament collapse in G61C, as compared with WT mice (Fig. 2 B). Fas administration also modulates K8/K18 phosphorylation (Ku et al., 2003), including an increase in K8 S73/S431 and a decrease in K18 S33 phosphorylation in all transgenic lines (Fig. 2 C). However, K8 S73 hyperphosphorylation (Fig. 2 C) was significantly less in G61C, as compared with WT livers (65% less, as determined using quantitative blotting with anti-K8 pS73 Ab; not depicted). The increased apoptosis in K8 G61C-expressing hepatocytes was also confirmed by enhanced formation of the caspase-generated K18 fragment (Fig. 2 C). Therefore, the natural K8 G61C mutation causes a dramatic increase in transgenic mouse susceptibility to stress-induced liver injury.
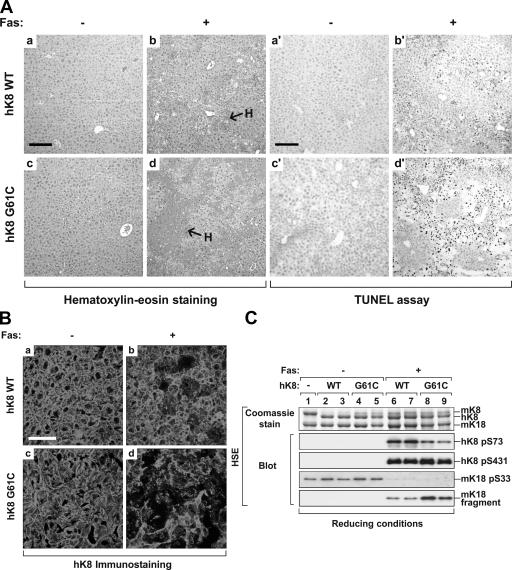
Figure 2.
K8 G61C accentuates Fas-induced liver injury. Livers were obtained from the indicated transgenic mice 4 h after PBS or Fas Ab administration. (A) Histological analysis (a–d) and TUNEL assay (a′–d′) of transgenic mouse livers that were isolated after PBS (−) or Fas (+) intraperitoneal injection. Note the severe hemorrhage (H) and pyknotic nuclei in hK8 G61C (d and d′) as compared with hK8 WT liver (b and b′) after Fas administration. (B) Immunofluorescence staining of livers similar to those shown in A using an Ab that is specific to hK8. Note the similar staining pattern of hK8 WT and G61C livers under basal conditions (a and c), but the marked hepatocyte drop-off and the collapse of keratin filaments in G61C livers compared with WT mice after Fas injection (b and d). (C) Mouse livers were homogenized, followed by isolation of the total keratin pool by HSE. The extracts were separated by SDS-PAGE, and then visualized by Coomassie staining or blotting with Abs to the indicated epitopes. Each lane represents the analysis of one independent mouse liver. Bars: (A) 160 μm; (B) 40 μm.
Comparison of the effect of K8 and K18 mutation, or absence, in transfected cells and mouse livers
We compared the effect of K8 G61C (i.e., the patient-related variant) and K18 R89C mutation, or K8/K18 absence in transfected cells and mouse livers. K18 R89C is not a natural mutation, but K18 R89 is highly conserved among all IF proteins and the homologous residue is a mutation “hotspot” in epidermal keratin-related diseases such as EBS (Porter and Lane, 2003). Overexpression of K18 R89C in transgenic mice causes hepatocyte keratin filament disruption, hepatocyte fragility, and enhanced susceptibility to liver injury and apoptosis (Ku et al., 1995, 2003) and was the major clue that led us to search for, and then associate, K8/K18 variants with human liver disease (Ku et al., 1997). When tested in transfected cells, K8 G61C behaves similarly to K18 R89C (a control for Cys mutation); both are highly insoluble and generate keratin cross-links upon oxidative challenge when compared with WT K8 and another natural K8 (R340H) variant (Fig. 3 A). The cross-linked K8 species are clearly detected in total lysates, but not cytosol (Fig. 3 A, lane 11 vs. 15), which indicates that most of these species are in the filament fraction. K8 G61C insolubility (Fig. 3 B) and cross-linking after Fas or paraquat administration (Fig. 3 C) were similarly noted in transgenic mouse livers.
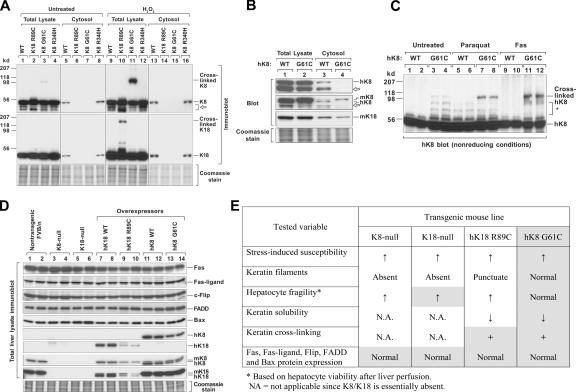
Figure 3.
K8 G61C decreases keratin solubility and promotes keratin cross-linking without altering hepatocyte fragility. (A) BHK cells were transiently cotransfected with the indicated mutant and/or WT keratins (K8 WT + K18 WT; K8 WT + K18 R89C; K18 WT + K8 G61C or R340H). After 3 d, cells were cultured in the presence or absence of 20 mM H2O2 for 1 h, and then divided into two portions. One portion was used to isolate a detergent-free cytosolic soluble fraction and the second to generate a total cell lysate. The isolates were subjected to SDS-PAGE under nonreducing conditions (to visualize cross-linked K8) and blotted with Abs specific to hK8 or hK18. Coomassie staining is also shown to demonstrate equal gel loading. Arrow indicates degraded K8. (B) Liver perfusion was used to isolate hepatocytes from hK8 WT and G61C mice. Total lysates and detergent-free cytosolic fractions were prepared from the isolated hepatocytes, followed by blotting with Abs specific to hK8, m/hK18, and mK18. Open arrows indicate degraded K8. (C) Oxidative stress or apoptosis was induced in mice by intraperitoneal administration of paraquat or Fas Ab, respectively. Total liver lysates were isolated and blotted with anti-hK8 Ab. Asterisk indicates nonspecific bands. Each lane represents the analysis of one independent mouse liver. (D) Total homogenates were prepared from livers of the indicated mouse genotypes (2 mice/genotype), followed by SDS-PAGE and blotting with Abs to the indicated proteins. A duplicate gel was stained with Coomassie blue to verify equal protein loading. Note that the K8 and K18 proteins are absent in both K8- and K18-null livers (lanes 3–6) because of degradation of the partner protein (Baribault et al., 1994; Magin et al., 1998). (E) Liver perfusion of K18-null, K18 R89C, K8 WT, and K8 G61C was performed to assess hepatocyte fragility. K18 R89C was used as a control and hepatocytes from these mice are already known to be fragile (Ku et al., 1995). Hepatocytes from K18-null and hK18 R89C livers had 16–24% viability (n = 3–6 livers/genotype), whereas hK8 WT and G61C hepatocytes had 82–89% viability (n = 4–6 livers/genotype). A summary of the phenotypes of transgenic mice that lack keratin expression (K8- and K18-null) or that express mutant K8 or K18 is shown. Shaded parameters are those examined in this study, whereas unshaded parameters represent summaries of previous studies (Marceau et al., 2001; Omary et al., 2002; Zatloukal et al., 2004).
We then assessed hepatocyte fragility and the expression of several apoptosis-related proteins in K8 G61C mice as compared with K18 R89C, K8-, and K18-null mice. The K8- or K18-null mice, and K18 R89C mice, have a dramatically increased susceptibility to liver injury and apoptosis (Marceau et al., 2001; Omary et al., 2002; Zatloukal et al., 2004). We examined the expression of several apoptosis-associated proteins because c-Flip protein, but not mRNA, was reported to be absent in K8-null mouse liver, and c-Flip absence may account for the increased susceptibility of K8-null livers to apoptosis (Gilbert et al., 2004). We did not observe any difference in c-Flip (using two independent anti–c-Flip Abs) or several other apoptosis-associated protein levels between these four mouse groups and nontransgenic mouse groups (Fig. 3 D), which indicates that the previously reported results with c-Flip (Gilbert et al., 2004) were likely caused by differences in Ab specificity.
We then examined hepatocyte fragility in K18-null, K8 WT, and K8 G61C mice upon liver perfusion because K8-null and the EBS-like K18 R89C livers have remarkably fragile hepatocytes (Ku et al., 1995; Loranger et al., 1997). Nontransgenic and K18 R89C mice were used as controls. Hepatocytes isolated from nontransgenic, K8 WT, and K8 G61C livers had 82–89% (n = 4–6 livers/genotype) viability as compared with hepatocytes from K18-null livers, which had only 16–22% viability (n = 3). Hence, the phenotypes of keratin-null or keratin mutant genotypes (summarized in Fig. 3 E) suggest that K8 G61C alters hepatocyte function differently than when K18 is mutated at R89C or K8/K18 proteins are absent. This is despite the finding that both K18 R89C and K8 G61C decrease keratin solubility and cause cross-linking during oxidative conditions (Fig. 3, A–C).
K8 G61C disrupts K8 S73 phosphorylation by stress-activated kinases
Human K8 includes three major in vivo phosphorylation sites (S23/S73/S431) that are conserved in mouse K8 (Omary et al., 1998). S23 is phosphorylated under basal conditions, and S73/S431 are phosphorylated by SAPKs, such as p38, JNK, and p42 MAPK. p38 phosphorylates only S73 and generates a unique, slightly slower-migrating K8 species (termed HK8) upon SDS-PAGE, whereas JNK and p42 phosphorylate S73/S431 and generate both HK8 and K8 phosphospecies (Fig. 4 A; He et al., 2002; Ku et al., 2002). Given that the natural hK8 G433S alters K8 S431 in vitro phosphorylation by p42 MAPK (Fig. 4 A; Ku et al., 2005; likely caused by proximity), we tested the effect of several K8 mutations on K8 phosphorylation by p38/JNK/p42. K8 G61C blocks S73 phosphorylation by purified SAPKs, but does not completely eliminate S73 in vivo phosphorylation (likely via other kinases), as determined by blotting of transfected-cell total lysates (Fig. 4 B) or transgenic mouse livers (Fig. 2 C) with anti-K8 pS73–specific Ab. Some of the other K8 mutants also inhibited K8 phosphorylation (R453C [all three kinases] and G433S [p42]), but the most prominent effect was noted in G61C (Fig. 4 B). Moreover, K8 I62V, which is a variant found at higher frequency in controls as compared with liver disease patients (Ku et al., 2005), has similar S73 in vitro phosphorylation by the SAPKs as WT K8 (unpublished data). We further substantiated the effect of K8 G61C by comparing K8 S73 phosphorylation in BHK cells cotransfected with WT or kinase-inactive p38. K8 G61C causes the near-complete absence of K8 S73 phosphorylation by transfected p38, which is similar to the near-absent phosphorylation of WT K8 upon kinase-inactive p38 transfection (Fig. 4 C). Hence, K8 G61C significantly inhibits K8 S73 phosphorylation in vivo by SAPKs.
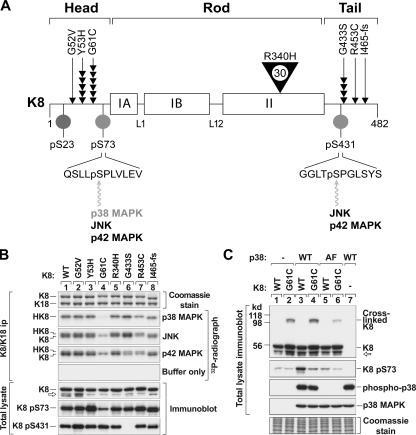
Figure 4.
K8 G61C mutation disrupts K8 S73 phosphorylation in vitro and in vivo. (A) Schematic of K8 protein and its in vivo phosphorylation sites (Omary et al., 1998), and the distribution and frequency of K8 mutations associated with human liver disease (Ku et al., 2005). The rod domain of all IF proteins is divided into the following subdomains: IA, linker 1 (L1), IB, L12, and II. Positions of K8 phosphorylation (S23/S73/S431) and the relevant kinases are shown. K8 variants are highlighted by arrowheads, with each arrowhead representing an independent patient from the 467 liver explants that were studied; the single large arrowhead for R340H indicates 30 individuals (Ku et al., 2005). I465-fs is a frame-shift mutation at Ile465 that generates a truncated 468–amino acid protein (instead of 482). (B) BHK cells were transiently cotransfected with K18 WT and one of the indicated K8 constructs, followed by immunoprecipitation of K8/K18, then in vitro phosphorylation by p38, JNK, or p42 kinases. Labeled immunoprecipitates (ip) were analyzed by SDS-PAGE and radiography. HK8 and K8 in the radiograph represent K8 S73 and K8 S431 phosphorylation, respectively. Total lysates were also prepared from the transfected cells then blotted with Abs to the indicated epitopes. No band corresponding to K8 S431 phosphorylation was detected in the pS431 immunoblot of K8 G433S (lane 6) because of an alteration of the Ab epitope in the mutant. (C) BHK cells were triple-transfected with K8/K18 WT or K8 G61C/K18 WT, and WT or kinase-inactive p38 (AF mutant; T180A/Y182F). Total lysates were prepared and blotted with Ab to the indicated epitopes. Open arrows in B and C represent degraded K8.
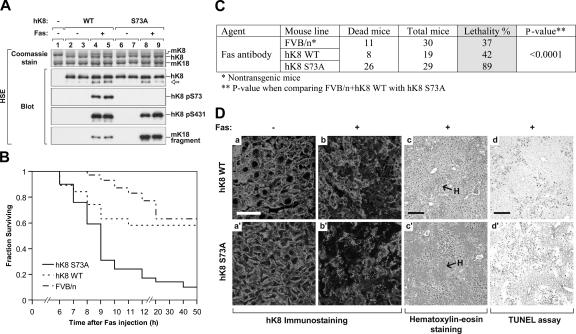
K8 S73A predisposes to Fas-induced liver injury in transgenic mice
The effect of K8 G61C on S73 phosphorylation led us to hypothesize that the inability to phosphorylate S73 in K8 G61C mice is a major trigger for their increased susceptibility to apoptosis. We tested this hypothesis by generating transgenic mice that overexpress hK8 S73A and tested their predisposition to apoptosis. Expression of hK8 S73A was verified by blotting using anti-hK8–specific Ab and by detection of K8 S73 phosphorylation, after Fas administration, in WT but not S73A hK8 livers (Fig. 5 A). Fas administration increases K8 S431 phosphorylation in WT and S73A, but generates the K18 apoptotic fragment more prominently in S73A livers (Fig. 5 A). This suggests that inhibition of K8 S73 phosphorylation increases susceptibility to Fas-mediated liver injury, which was confirmed by the marked lethality of S73A, as compared with WT K8 and nontransgenic mice (Fig. 5, B and C). The increased lethality of K8 S73A mice is likely attributable to increased hemorrhage and apoptosis (Fig. 5 D), which mimics findings in K8 G61C mice (Fig. 2 A). K8 S73A and G61C mice share other features, including normal-appearing keratin filaments under basal conditions (Fig. 5 D), and similar cell viability of the isolated hepatocytes after liver perfusion (84–92% viability; n = 5 livers).
Figure 5.
hK8 S73A transgenic mice have increased susceptibility to Fas-mediated hepatocyte apoptosis and injury. (A) Livers from nontransgenic (lane 1) and the indicated transgenic lines (lanes 2–9) were isolated from control or Fas-injected (± Fas) mice and analyzed as described in Fig. 2 C. Two independent transgenic lines per genotype (WT1 and WT2; S73A1 and S73A2) were used for the analysis (each of the lanes 2–9 represents one mouse from individual transgenic lines). (B and C) Nontransgenic FVB/n, hK8 WT, or S73A mice (19–30 age- and sex-matched mice per genotype) were injected with Fas and monitored as described in Fig. 1. Most deaths occurred within 12 h after Fas injection. The results are summarized in C. Mortality was significantly increased in hK8 S73A mice as compared with the combined (FVB/n + hK8 WT) mice. (D) Livers from Fas- or PBS-injected mice were analyzed by immunofluorescence staining (a–b′), by hematoxylin-eosin staining (c and c′), or TUNEL assay (d and d′). Hematoxylin-eosin staining and TUNEL assay of S73A livers under basal conditions (not depicted) were similar to those from the WT livers shown in Fig. 2 A (a and a′). H, hemorrhage. Bars: (a–b′) 40 μm (c–d′) 160 μm.
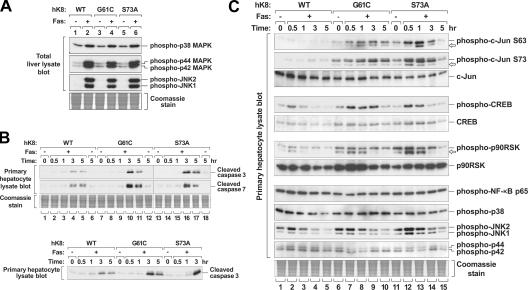
K8 G61C or S73A lead to sustained and enhanced phosphorylation of SAPK substrates after Fas-induced apoptosis
We hypothesized that G61C or S73A expression shunts phosphorylation from K8 S73 to other SAPK substrates after Fas stimulation. This hypothesis is based on the following: (a) cellular abundance of K8 (e.g., prominent Coomassie staining; Fig. 1 B), (b) its role as an in vivo substrate for SAPKs (He et al., 2002; Ku et al., 2002), (c) compensatory down-regulation of endogenous mK8 (i.e., the conserved SAPK K8 S73 substrate [S79 in mK8]) in response to mutant/WT hK8 overexpression (Fig. 1 B and Fig. 2 C), and (d) inhibition of K8 S73 phosphorylation in K8 G61C. We tested this hypothesis by first showing that p42/44, JNK1/2, and p38 phosphorylation (and, hence, activation) were markedly but similarly increased in response to Fas stimulation in WT and keratin mutant livers (Fig. 6 A), which supports previous reports that SAPKs play proapoptotic roles (Gallo and Johnson, 2002; Kaplowitz, 2002; Baines and Molkentin, 2005). We then compared primary hepatocyte cultures for their susceptibility to apoptosis by testing for the presence of caspase-generated products after Fas stimulation (Fig. 6 B). The use of primary hepatocyte cultures eliminates the potential variability among mice and makes it feasible to test multiple time points. Cleaved caspases 3/7 were detected between 3 and 5 h, but, as expected from the intact animal studies (Fig. 2 C and Fig. 5 A), the cleaved caspase products were more evident in K8 G61C and S73A compared with WT hepatocytes (Fig. 6 B).
Figure 6.
Effect of K8 G61C or S73A mutation on SAPK activation and substrate phosphorylation. (A) Liver homogenates were obtained from the indicated transgenic mice (± Fas injection), separated by SDS-PAGE, and visualized by Coomassie staining (shown for equal loading) or by blotting with the indicated Abs. (B) Hepatocytes were isolated by liver perfusion then treated with 0.5 μg/ml Fas Ab for the indicated times. Total cell lysates were then prepared and analyzed as in A. (top) Lanes 1–12 represent samples loaded on the same gel, whereas lanes 13–18 were loaded on a separate gel. (bottom) Representative samples from the top panel that were analyzed on the same gel to confirm the relative changes in caspase cleavage. (C) Total lysates of primary hepatocytes were prepared and analyzed as in B. Arrows likely represent degradation products. Note that phosphorylation of the SAPK substrates, c-Jun, CREB, and p90RSK, is increased and is more sustained in K8 G61C or S73A hepatocytes as compared with K8 WT hepatocytes.
Finally, we compared the phosphorylation of endogenous mouse proteins that are known to serve as SAPK substrates: c-Jun (S63/S73) by JNK, cAMP response element binding protein (CREB) and NF-κB p65 by p38, and p90RSK by p42/44 (Deak et al., 1998; Roux and Blenis, 2004; Liu and Lin, 2005). Although phosphorylation of NF-κB p65 was similar in K8 WT, G61C, or S73A hepatocytes after Fas treatment, phosphorylation of c-Jun, CREB, and p90RSK in K8 G61C and S73A hepatocytes was more pronounced and sustained when compared with WT hepatocytes (Fig. 6 C). The pattern of phosphorylated/activated SAPKs in K8 WT, G61C, or S73A hepatocytes after Fas treatment is relatively similar (Fig. 6 C), and activation by Fas is not as dramatic in primary cultures as it is in vivo (e.g., lanes 1 and 2 for phospho-JNK in Fig. 6 C, as contrasted with Fig. 6 A), which is likely caused by the stress incurred upon hepatocyte isolation. Our data support the conclusion that increased phosphorylation of nonkeratin SAPK substrates in G61C and S73A hepatocytes reflects a shunting of phosphorylation toward these other substrates in association with caspase activation.
Discussion
Disease relevance
The findings herein represent the first in vivo evidence that naturally occurring human K8 mutations can predispose to liver injury and apoptosis. Our results show that introducing hK8 G61C into mice predisposes to liver injury and apoptosis, and suggest the following sequence of events in response to stress: a G61C-mediated conformational change leads to the inability of K8 S73 to serve as a SAPK substrate, which creates an imbalance of kinase substrate availability, thereby predisposing to apoptosis and liver injury (Fig. 7). K8 G61C is the second most frequent liver disease–associated variant after R340H (Ku et al., 2005; Strnad et al., 2006a). Mutations of IF proteins, including keratins, desmin, neurofilaments, and lamins, among others, are associated with a wide range of tissue-specific human diseases (Fuchs and Cleveland, 1998; Omary et al., 2004). Compared with most other IF mutations, one unique characteristic of K8/K18 mutations is that they pose a risk of disease, rather than directly causing it (Omary et al., 2002; Porter and Lane, 2003; Ku et al., 2005). The results herein fill an important missing link by experimentally demonstrating that a human disease-associated keratin mutation can, indeed, cause disease when an animal carrying the mutation is challenged by oxidative and other stresses. Therefore, the findings in K8 G61C transgenic mice provide strong supporting evidence for the human association studies that have been performed to date.
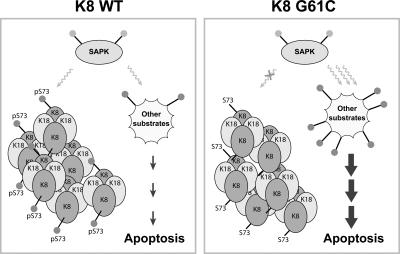
Figure 7.
K8 mutation shunts keratin phosphorylation by stress-activated kinases. During stress, activated kinases such as p38 MAPK phosphorylate K8 S73 and other proapoptotic substrates. However, K8 G61C mutation interferes with the ability of the highly abundant K8 S73 to serve as a substrate, which renders other substrates available for potential phosphorylation and consequent rapid progression of apoptosis. Filled dots indicate phosphorylated residues. The phosphate sponge model works in a hepatocyte-protective fashion in livers that express WT K8 and provides a function for K8 S73 phosphorylation that becomes unmasked upon K8 G61C mutation. This model predicts that keratins (and possibly other IFs) undergo hyperphosphorylation in part to absorb, in a “bulk” fashion given their abundance, cell kinase activation. Such sponge activity can be beneficial (e.g., K8 S73), or potentially detrimental, in a phosphorylation site-specific and context-dependent fashion. This model provides one (of several potential) nonmechanical functional capability for IFs and their phosphorylation and does not exclude the possibility that some kinase substrates may undergo dephosphorylation (e.g., via phosphatase activation by phosphorylation) and that other phosphorylation-dependent or -independent mechanisms for protection may be involved.
The mechanisms of liver injury predisposition by different K8/K18 variants may be variant/disease-specific or may have mechanistic overlap. This is supported by the inability of other (non-G61C) K8 natural mutants (e.g., G52V, Y53H, I465-fs [Fig. 4 B], and I62V [not depicted]) to interfere with SAPK phosphorylation of K8. Furthermore, K18 R89C (in mice) alters a K8/K18 mechanical function (i.e., fragility predisposition) that is not shared by K8 G61C (Fig. 3 E). K18 R89C also primes hepatocytes to undergo apoptosis (Ku et al., 2003) and oxidative injury (Zhou et al., 2005), which are likely mechanisms that are shared by K8 G61C and K8 S73A. A common denominator for imparting protection from liver injury is keratin phosphorylation, which correlates with progression from chronic to end-stage liver disease (Toivola et al., 2004; Zatloukal et al., 2004). This notion is supported by the finding that K18 S52A (S52 is a major hK18 phosphorylation site) expression predisposes to hepatotoxic injury in transgenic mice (Ku et al., 1998), and by the results herein.
Relationship between K8 G61 mutation and S73 phosphorylation
The conformational link between K8 S73 phosphorylation and G61 is supported by the similar findings in K8 G61C and S73A mice, and by the inhibition of K8 S73 phosphorylation in vitro upon G61C mutation. This link is also supported by inhibition of binding of an Ab directed to a K8 G61C-containing epitope when S73 is phosphorylated (Tao et al., 2006). Immunoblotting of liver homogenates from transgenic mice with this Ab showed its binding with WT and S73A, but not with G61C K8 homogenates (Fig. S1, available at http://www.jcb.org/cgi/content/full/jcb.200602146/DC1). K8 S73 phosphorylation also leads to a distinct retardation in migration during SDS-PAGE, which is specific to that phosphorylation site and is also seen when S73 is mutated to aspartate (Liao et al., 1997; Ku et al., 2002). Hence, the phenotype we observe appears to be caused by mutation-related interference with a kinase–substrate interaction though other direct/indirect interference with potential stable keratin and kinase–kinase regulator interactions is possible. For example, p38 and JNK associate with keratins, but this association appears to be stoichiometrically limited and is more consistent with a kinase–substrate association (He et al., 2002; Ku et al., 2002). In addition, both WT and S73A K8 coimmunoprecipitate with p38 MAPK, although we could not adequately test the K8 G61C mutant because of its limited solubility in mild detergents (unpublished data). Further support for a conformational link between K8 G61C and S73 phosphorylation is that the G61C mutation inhibits in vitro phosphorylation of K8 S73 by purified SAPKs, but does not significantly affect K8 S431 phosphorylation (Fig. 4 B).
Lack of accessibility of SAPKs to K8 S73 upon G61C mutation may also be impacted by the formation of K8 cross-links in response to oxidative stress (Ku et al., 2001; Owens et al., 2004). In this context, exposure to Fas or paraquat increases K8 cross-linked species that are otherwise barely detectable (Fig. 3 C). This reflects the change in the normally reducing cytoplasmic environment under stress, with production of reactive oxygen species during Fas-mediated apoptosis and other oxidative stresses as seen in rat hepatocytes (Reinehr et al., 2005) and T cell lines (Sato et al., 2004). The effect of G61C on keratin solubility (Fig. 3, A and B) may also independently contribute to kinase inaccessibility.
K8 S73 phosphorylation is likely to be associated with several functions because it occurs during stress, apoptosis, and mitosis (Liao et al., 1997; Ku et al., 2002; Toivola et al., 2002). Previous studies in transfected cells (Ku et al., 2002) showed that K8 S73 phosphorylation promotes keratin filament reorganization (e.g., Ala substitution blocked stimulus-induced filament reorganization, which was rescued by Asp substitution). The multiplicity of keratin phosphorylation sites raises the untested possibility that site-specific phosphorylation can have a domino effect that “opens” the filaments to additional phosphorylation/dephosphorylation events, with consequent functional implications.
The phosphate sponge model
The similarity of the K8 G61C and S73A transgenic mice phenotypes supports an important role for S73 as a phosphate sponge for SAPKs in normal tissues undergoing stress. Amongst the earliest descriptions of IFs potentially serving as “phosphate sinks” was the observation of significant vimentin and keratin hyperphosphorylation after short exposure of cells to okadaic acid (Lai et al., 1993). Subsequent studies suggested that neurofilaments may serve as phosphate sinks (Nguyen et al., 2001), which was supported by others, although in this case the sink model is not protective, as initially hypothesized (Lobsiger et al., 2005). We elected to use the term “sponge” instead of sink because it is more general, in that sponges are more easily transportable (i.e., dynamic) and not only collect spills but also allow their recovery (as free phosphates) with ready availability of fresh sponge capacity. We hypothesize that a phosphorylation sponge effect (Fig. 7) may be detrimental or beneficial, in a context and phosphorylation site-specific fashion, and that in the case of K8 S73 the phosphorylation role is beneficial. This model does not exclude the possibility that some antiapoptotic kinase substrates may in fact become hypophosphorylated, but predicts that overall phosphorylation is shunted, with the net effect being enhanced apoptosis.
The abundance of cytoplasmic keratins allows for plentiful sponge capacity. For example, K8/K18 can be easily seen by Coomassie staining upon HSE (Fig. 1 B), and they make up 5% of cultured colonocyte total proteins (Omary et al., 1998) and 0.2% of total mouse liver protein (Zhong et al., 2004; the 0.2% is an underestimate because it includes proteins from other resident nonkeratin-containing endothelial and Kupffer cells and some blood proteins). Also, K8 S73 is a unique and readily available SAPK substrate because it behaves as a switch that is either “on” or “off,” being completely unphosphorylated (off) under basal conditions but turning on via phosphorylation during apoptosis and cell injury (Liao et al., 1997).
SAPKs can play proapoptosis roles in several disorders, including liver (Kaplowitz, 2002), neuronal (Gallo and Johnson, 2002), and cardiac (Baines and Molkentin, 2005) disease. For example, disruption of JNK3 in mice results in resistance to excitotoxicity-induced neuronal apoptosis (Yang et al., 1997) and pharmacologic inhibition of p38 interferes with TNF-induced hepatocyte apoptosis (Pastorino et al., 2003). Most of the known SAPK substrates are signaling molecules, such as protein kinases (e.g., MSKs, RSKs, and MNKs), transcription factors (e.g., c-Jun, Elk-1, c-Fos, and NF-κB), or apoptosis-associated proteins (e.g., Bim and Bad; Roux and Blenis, 2004; Liu and Lin, 2005). Some protein kinases are phosphorylated and activated by SAPKs and then phosphorylate and activate transcription factors among other substrates. For example, p38 MAPK phosphorylates MSK1 that can phosphorylate the CREB transcription factor (Deak et al., 1998). The increased phosphorylation of cellular proteins after Fas stimulation was not universal in G61C and S73A hepatocytes (e.g., phospho–NF-κB p65 was not altered; Fig. 6 C). Several other substrates were tested, including MSKs, Elk-1, c-Fos, c-Myc, p53, and Bim, but the results were unrevealing because of the lack of cross reactivity of the respective Abs with mouse proteins (unpublished data). The K8 G61C/S73A-associated increase in c-Jun phosphorylation supports the enhanced susceptibility of G61C and S73A hepatocytes toward apoptosis, given that AP-1 members such as c-Jun are involved in proapoptotic or survival signaling depending on the cellular context and external stimulus. For example, stress-activated JNK phosphorylates c-Jun, which results in enhanced transcription of target genes (e.g., FasL) associated with apoptosis (Hess et al., 2004), and c-Jun S63/73 mutations in mice protects neurons from apoptosis (Behrens et al., 1999). The role of CREB and p90RSK is well studied in cell survival, but less so in proapoptotic pathways. However, serial analysis of chromatin occupancy supports the involvement of CREB in several proapoptotic gene products such as DEDD, TRADD, GADD45γ, and Bim (Impey et al., 2004).
Implications based on the relative conservation of K8 S73
Several type II keratins contain a unique LLS / TPL motif, which is 71LLSPL in hK8 (i.e., S73-containing) or LLTPL in K4/K5/K6 and hair keratins (Liao et al., 1997; Toivola et al., 2002). LLS / TPL is phosphorylated during apoptosis and other stresses by SAPKs in several epithelial tissues, including the liver and intestine (K8) and the esophagus and skin (K4/K5/K6; Liao et al., 1997; Ku et al., 2002; Toivola et al., 2002). Our findings suggest an important nonmechanical role for K8 in protecting hepatocytes from injury by serving as a phosphate sponge for SAPKs that can absorb some of their untoward effects (Fig. 7). This role may extend to keratins and their related diseases in other tissues where the K8 S73-containing LLS / TPL motif is conserved, and is negatively impacted by G61C mutation and possibly other K8 or K18 liver disease–associated mutations.
Materials and methods
Reagents and keratin-null mice
The reagents used include the following: MLR (Alexis Corp.), paraquat (Sigma-Aldrich), TdT-FragEL DNA fragmentation detection kit (Calbiochem), p38α MAPK (Upstate Biotechnology), JNK and p42 MAPK (Cell Signaling Technology), collagenase type II (Worthington Biochemical Corp.), and Lipofectamine (Invitrogen). All Abs to keratins and phosphokeratins were previously described (Ku et al., 2004). Other Abs used were directed to Fas for mouse injection (BD Biosciences); to phospho- or nonphospho-p38, CREB, CREB pS133, p90RSK, phospho-p90RSK (human T359/S363 and mouse T348/S352), phospho-NF-κB p65 (human S536 and mouse S534), phospho-p42, JNK, and c-Jun (Cell Signaling Technology); to Fas for immunoblotting, FADD, and Bax (Upstate Biotechnology); and to Fas-ligand, nonphospho–c-Jun (Santa Cruz Biotechnology, inc.). Two independent Abs were used to detect mouse c-Flip; one directed to an NH2-terminal region (SAEVIHQVEEALDTDE) that is 100% identical in human and mouse c-Flip (Upstate Biotechnology), and another directed to a mouse-specific COOH-terminal region (DKVYAWNSGVSSKEKYS) of c-Flip (Sigma-Aldrich). K8- and K18-null mice were provided by R. Oshima (The Burnham Institute, La Jolla, CA) and T. Magin (University of Bonn, Bonn, Germany), respectively.
Transgene constructs and generation of transgenic lines
A transformer kit (CLONTECH Laboratories) was used to introduce single point mutations into a human (h) WT 12-kb genomic K8 clone (Krauss and Franke, 1990). The K8 genomic clone (provided by W. Franke, German Cancer Research Center, Heidelberg, Germany) includes endogenous regulatory elements that maintain tissue expression. Two mutant genomic constructs were generated (hK8 G61C or S73A), and both strands of the mutated region were sequenced to confirm the mutation. Fidelity of the mutant and WT constructs was verified by testing its expression by transient transfection into BHK cells. The 12-kb Sal I fragments of mutant or WT genomic hK8 DNA were then injected into pronuclei of fertilized FVB/n mouse eggs. Progeny mice carrying the hK8 transgene were chosen after PCR screening of tail genomic DNA, which was followed by breeding to select for germline transmission (primers of a 250-bp PCR fragment; 5′-GGCGGCGGCTATGGTGGGGCC-3′ and 5′-AGATGTGCATAGGGACCGGGA-3′). Two independent heterozygous mouse lines per construct were established and expanded (K8: WT1 and WT2; G61C1 and G61C2; and S73A1 and S73A2), all in an FVB/n background, and then used for subsequent studies. The two transgenic lines for each construct had similar K8 expression and afforded near-identical results.
Toxin administration and hepatocyte isolation
For the lethality experiments, mice (age and sex matched) were fasted overnight, followed by intraperitoneal injection of Fas Ab (0.15 μg/g mouse body weight) or MLR (30 ng/g mouse body weight; Ku et al., 2004). Mice were killed by CO2 inhalation 4 h after Fas Ab injection, and their livers were isolated and processed for immunofluorescence, histology (HistoTec Laboratories), and TUNEL analyses (Ku et al., 2004). For induction of oxidative injury, mice were fasted overnight then injected intraperitoneally with paraquat (70 μg/g mouse body weight; Holzenberger et al., 2003), followed by harvesting of livers after 60 h. Hepatocyte isolation was performed by liver perfusion of three to six age- and sex-matched mice/genotype, using collagenase type II (Ku et al., 1995). Cultured hepatocytes were treated with Fas Ab (0.5 μg/ml for 0.5–5 h), followed by preparation of cell lysates for immunoblotting.
Biochemical and immunologic analyses
Keratins were isolated by HSE using liver pieces as previously described (Ku et al., 2004). Alternatively, total liver homogenates were prepared by solubilizing in SDS-containing buffer. Proteins were separated by SDS-PAGE, followed by staining with Coomassie blue or transferal to membranes; they were then immunoblotted and visualized by enhanced chemiluminescence. Quantitative immunoblotting was performed using serial dilutions of the two samples to be compared and analyzed on the same gel. Immunofluorescence staining was done as previously described (Ku et al., 2004), and fluorescence images were analyzed using a confocal microscope (MRC 1024ES; Bio-Rad Laboratories).
Solubility analysis and in vitro phosphorylation
BHK cells were transiently cotransfected (using Lipofectamine) with K18 WT and K8 (WT or mutant constructs; Ku et al., 2005) or K18 R89C and K8 WT (Ku et al., 1995). 3 d after transfection, the cells were further cultured (37°C) in the presence or absence of 20 mM H2O2 for 1 h, followed by isolation of a detergent-free, cytosolic soluble fraction or a total cell lysate (Ku et al., 2005). The triple transient transfections with kinase active/inactive p38α and keratins were also performed in BHK cells (Ku et al., 2002).
In vitro phosphorylation was done as previously described (Ku et al., 2004). K8/K18 immunoprecipitates, which were isolated from BHK-transfected cells, were washed with kinase-specific buffers (Cell Signaling Technology; Upstate Biotechnology), heated (90°C) to inactivate any bound kinase activity, and incubated with the kinases (p38, JNK, or p42) and γ-[32P]ATP (Ku et al., 2004). Kinase reactions were quenched by boiling in the presence of 2% SDS-containing buffer, which was followed by analysis by SDS-PAGE and autoradiography.
Online supplemental material
Fig. S1 shows blotting with anti-hK8 G61C Ab that binds with WT and S73A, but not G61C K8, in transgenic liver homogenates. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200602146/DC1.
Supplementary Material
Acknowledgments
We are grateful to the generous contributions of reagents and mice that were provided by Drs. Werner Franke (human K8 genomic clone), Robert Oshima (K8-null mice), and Thomas Magin (K18-null mice); to Steve Avolicino (HistoTec Laboratory) for the histochemical staining; to Evelyn Resurreccion for assistance with tissue staining; and to Kris Morrow for helping with figure preparation.
This work was supported by National Institutes of Health (NIH) DK47918 and VA Merit awards (M.B. Omary). N-O. Ku was supported, in part, by a Veterans Administration Research Enhancement Award Program, and NIH Digestive Disease Center grant DK56339 Pilot Award.
The authors declare no competing financial interests.
Abbreviations used in this paper: Ab, antibody; CREB, cAMP response element binding protein; EBS, epidermolysis bullosa simplex; HSE, high salt extraction; IF, intermediate filament; K, keratin; MLR, microcystin-LR; SAPK, stress-activated protein kinase; WT, wild type.
References
- Baines, C.P., and J.D. Molkentin. 2005. STRESS signaling pathways that modulate cardiac myocyte apoptosis. J. Mol. Cell. Cardiol. 38:47–62. [DOI] [PubMed] [Google Scholar]
- Baribault, H., J. Penner, R.V. Iozzo, and M. Wilson-Heiner. 1994. Colorectal hyperplasia and inflammation in keratin 8-deficient FVB/N mice. Genes Dev. 8:2964–2973. [DOI] [PubMed] [Google Scholar]
- Behrens, A., M. Sibilia, and E.F. Wagner. 1999. Amino-terminal phosphorylation of c-Jun regulates stress-induced apoptosis and cellular proliferation. Nat. Genet. 21:326–329. [DOI] [PubMed] [Google Scholar]
- Casanova, M.L., A. Bravo, A. Ramirez, G. Morreale de Escobar, F. Were, G. Merlino, M. Vidal, and J.L. Jorcano. 1999. Exocrine pancreatic disorders in transgenic mice expressing human keratin 8. J. Clin. Invest. 103:1587–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulin, C., C.F. Ware, T.M. Magin, and R.G. Oshima. 2000. Keratin-dependent, epithelial resistance to tumor necrosis factor–induced apoptosis. J. Cell Biol. 149:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulombe, P.A., and M.B. Omary. 2002. “Hard” and “soft” principles defining the structure, function and regulation of keratin intermediate filaments. Curr. Opin. Cell Biol. 14:110–122. [DOI] [PubMed] [Google Scholar]
- Deak, M., A.D. Clifton, L.M. Lucocq, and D.R. Alessi. 1998. Mitogen- and stress-activated protein kinase-1 (MSK1) is directly activated by MAPK and SAPK2/p38, and may mediate activation of CREB. EMBO J. 17:4426–4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs, E., and K. Weber. 1994. Intermediate filaments: structure, dynamics, function, and disease. Annu. Rev. Biochem. 63:345–382. [DOI] [PubMed] [Google Scholar]
- Fuchs, E., and D.W. Cleveland. 1998. A structural scaffolding of intermediate filaments in health and disease. Science. 279:514–519. [DOI] [PubMed] [Google Scholar]
- Gallo, K.A., and G.L. Johnson. 2002. Mixed-lineage kinase control of JNK and p38 MAPK pathways. Nat. Rev. Mol. Cell Biol. 3:663–672. [DOI] [PubMed] [Google Scholar]
- Gilbert, S., A. Loranger, N. Daigle, and N. Marceau. 2001. Simple epithelium keratins 8 and 18 provide resistance to Fas-mediated apoptosis. The protection occurs through a receptor-targeting modulation. J. Cell Biol. 154:763–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert, S., A. Loranger, and N. Marceau. 2004. Keratins modulate c-Flip/extracellular signal-regulated kinase 1 and 2 antiapoptotic signaling in simple epithelial cells. Mol. Cell. Biol. 24:7072–7081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, T., A. Stepulak, T.H. Holmstrom, M.B. Omary, and J.E. Eriksson. 2002. The intermediate filament protein keratin 8 is a novel cytoplasmic substrate for c-Jun N-terminal kinase. J. Biol. Chem. 277:10767–10774. [DOI] [PubMed] [Google Scholar]
- Herrmann, H., and U. Aebi. 2004. Intermediate filaments: molecular structure, assembly mechanism, and integration into functionally distinct intracellular scaffolds. Annu. Rev. Biochem. 73:749–789. [DOI] [PubMed] [Google Scholar]
- Hess, J., P. Angel, and M. Schorpp-Kistner. 2004. AP-1 subunits: quarrel and harmony among siblings. J. Cell Sci. 117:5965–5973. [DOI] [PubMed] [Google Scholar]
- Holzenberger, M., J. Dupont, B. Ducos, P. Leneuve, A. Geloen, P.C. Even, P. Cervera, and Y. Le Bouc. 2003. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 421:182–187. [DOI] [PubMed] [Google Scholar]
- Impey, S., S.R. McCorkle, H. Cha-Molstad, J.M. Dwyer, G.S. Yochum, J.M. Boss, S. McWeeney, J.J. Dunn, G. Mandel, and R.H. Goodman. 2004. Defining the CREB regulon: a genome-wide analysis of transcription factor regulatory regions. Cell. 119:1041–1054. [DOI] [PubMed] [Google Scholar]
- Kaplowitz, N. 2002. Biochemical and cellular mechanisms of toxic liver injury. Semin. Liver Dis. 22:137–144. [DOI] [PubMed] [Google Scholar]
- Krauss, S., and W.W. Franke. 1990. Organization and sequence of the human gene encoding cytokeratin 8. Gene. 86:241–249. [DOI] [PubMed] [Google Scholar]
- Ku, N.O., S. Michie, R.G. Oshima, and M.B. Omary. 1995. Chronic hepatitis, hepatocyte fragility, and increased soluble phosphoglycokeratins in transgenic mice expressing a keratin 18 conserved arginine mutant. J. Cell Biol. 131:1303–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku, N.O., S.A. Michie, R.M. Soetikno, E.Z. Resurreccion, R.L. Broome, R.G. Oshima, and M.B. Omary. 1996. Susceptibility to hepatotoxicity in transgenic mice that express a dominant-negative human keratin 18 mutant. J. Clin. Invest. 98:1034–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku, N.O., T.L. Wright, N.A. Terrault, R. Gish, and M.B. Omary. 1997. Mutation of human keratin 18 in association with cryptogenic cirrhosis. J. Clin. Invest. 99:19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku, N.O., S.A. Michie, R.M. Soetikno, E.Z. Resurreccion, R.L. Broome, and M.B. Omary. 1998. Mutation of a major keratin phosphorylation site predisposes to hepatotoxic injury in transgenic mice. J. Cell Biol. 143:2023–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku, N.O., X. Zhou, D.M. Toivola, and M.B. Omary. 1999. The cytoskeleton of digestive epithelia in health and disease. Am. J. Physiol. 277:G1108–G1137. [DOI] [PubMed] [Google Scholar]
- Ku, N.O., R. Gish, T.L. Wright, and M.B. Omary. 2001. Keratin 8 mutations in patients with cryptogenic liver disease. N. Engl. J. Med. 344:1580– 1587. [DOI] [PubMed] [Google Scholar]
- Ku, N.O., S. Azhar, and M.B. Omary. 2002. Keratin 8 phosphorylation by p38 kinase regulates cellular keratin filament reorganization: modulation by a keratin 1-like disease causing mutation. J. Biol. Chem. 277:10775–10782. [DOI] [PubMed] [Google Scholar]
- Ku, N.O., R.M. Soetikno, and M.B. Omary. 2003. Keratin mutation in transgenic mice predisposes to Fas but not TNF-induced apoptosis and massive liver injury. Hepatology. 37:1006–1014. [DOI] [PubMed] [Google Scholar]
- Ku, N.O., D.M. Toivola, Q. Zhou, G.Z. Tao, B. Zhong, and M.B. Omary. 2004. Studying simple epithelial keratins in cells and tissues. Methods Cell Biol. 78:489–517. [DOI] [PubMed] [Google Scholar]
- Ku, N.O., J.K. Lim, S.M. Krams, C.O. Esquivel, E.B. Keeffe, T.L. Wright, D.A. Parry, and M.B. Omary. 2005. Keratins as susceptibility genes for end-stage liver disease. Gastroenterology. 129:885–893. [DOI] [PubMed] [Google Scholar]
- Lai, Y.K., W.C. Lee, and K.D. Chen. 1993. Vimentin serves as a phosphate sink during the apparent activation of protein kinases by okadaic acid in mammalian cells. J. Cell. Biochem. 53:161–168. [DOI] [PubMed] [Google Scholar]
- Liao, J., N.O. Ku, and M.B. Omary. 1997. Stress, apoptosis, and mitosis induce phosphorylation of human keratin 8 at Ser-73 in tissues and cultured cells. J. Biol. Chem. 272:17565–17573. [DOI] [PubMed] [Google Scholar]
- Liu, J., and A. Lin. 2005. Role of JNK activation in apoptosis: a double-edged sword. Cell Res. 15:36–42. [DOI] [PubMed] [Google Scholar]
- Lobsiger, C.S., M.L. Garcia, C.M. Ward, and D.W. Cleveland. 2005. Altered axonal architecture by removal of the heavily phosphorylated neurofilament tail domains strongly slows superoxide dismutase 1 mutant-mediated ALS. Proc. Natl. Acad. Sci. USA. 102:10351–10356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loranger, A., S. Duclos, A. Grenier, J. Price, M. Wilson-Heiner, H. Baribault, and N. Marceau. 1997. Simple epithelium keratins are required for maintenance of hepatocyte integrity. Am. J. Pathol. 151:1673–1683. [PMC free article] [PubMed] [Google Scholar]
- Magin, T.M., R. Schroder, S. Leitgeb, F. Wanninger, K. Zatloukal, C. Grund, and D.W. Melton. 1998. Lessons from keratin 18 knockout mice: formation of novel keratin filaments, secondary loss of keratin 7 and accumulation of liver-specific keratin 8-positive aggregates. J. Cell Biol. 140:1441–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magin, T.M., J. Reichelt, and M. Hatzfeld. 2004. Emerging functions: diseases and animal models reshape our view of the cytoskeleton. Exp. Cell Res. 301:91–102. [DOI] [PubMed] [Google Scholar]
- Marceau, N., A. Loranger, S. Gilbert, N. Daigle, and S. Champetier. 2001. Keratin-mediated resistance to stress and apoptosis in simple epithelial cells in relation to health and disease. Biochem. Cell Biol. 79:543–555. [PubMed] [Google Scholar]
- Moll, R., W.W. Franke, D.L. Schiller, B. Geiger, and R. Krepler. 1982. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 31:11–24. [DOI] [PubMed] [Google Scholar]
- Nguyen, M.D., R.C. Lariviere, and J.P. Julien. 2001. Deregulation of Cdk5 in a mouse model of ALS: toxicity alleviated by perikaryal neurofilament inclusions. Neuron. 30:135–147. [DOI] [PubMed] [Google Scholar]
- Omary, M.B., N.O. Ku, J. Liao, and D. Price. 1998. Keratin modifications and solubility properties in epithelial cells and in vitro. Subcell. Biochem. 31:105–140. [PubMed] [Google Scholar]
- Omary, M.B., N.O. Ku, and D.M. Toivola. 2002. Keratins: guardians of the liver. Hepatology. 35:251–257. [DOI] [PubMed] [Google Scholar]
- Omary, M.B., P.A. Coulombe, and W.H. McLean. 2004. Intermediate filament proteins and their associated diseases. N. Engl. J. Med. 351:2087–2100. [DOI] [PubMed] [Google Scholar]
- Owens, D.W., N.J. Wilson, A.J. Hill, E.L. Rugg, R.M. Porter, A.M. Hutcheson, R.A. Quinlan, D. Van Heel, M. Parkes, D.P. Jewell, et al. 2004. Human keratin 8 mutations that disturb filament assembly observed in inflammatory bowel disease patients. J. Cell Sci. 117:1989–1999. [DOI] [PubMed] [Google Scholar]
- Pastorino, J.G., N. Shulga, and J.B. Hoek. 2003. TNF-alpha-induced cell death in ethanol-exposed cells depends on p38 MAPK signaling but is independent of Bid and caspase-8. Am. J. Physiol. Gastrointest. Liver Physiol. 285:G503–G516. [DOI] [PubMed] [Google Scholar]
- Porter, R.M., and E.B. Lane. 2003. Phenotypes, genotypes and their contribution to understanding keratin function. Trends Genet. 19:278–285. [DOI] [PubMed] [Google Scholar]
- Reinehr, R., S. Becker, A. Eberle, S. Grether-Beck, and D. Haussinger. 2005. Involvement of NADPH oxidase isoforms and Src family kinases in CD95-dependent hepatocyte apoptosis. J. Biol. Chem. 280:27179–27194. [DOI] [PubMed] [Google Scholar]
- Roux, P.P., and J. Blenis. 2004. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol. Mol. Biol. Rev. 68:320–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato, T., T. Machida, S. Takahashi, S. Iyama, Y. Sato, K. Kuribayashi, K. Takada, T. Oku, Y. Kawano, T. Okamoto, et al. 2004. Fas-mediated apoptosome formation is dependent on reactive oxygen species derived from mitochondrial permeability transition in Jurkat cells. J. Immunol. 173:285–296. [DOI] [PubMed] [Google Scholar]
- Strnad, P., T.C. Lienau, G.Z. Tao, L.C. Lazzeroni, F. Stickel, D. Schuppan, and M.B. Omary. 2006. a. Keratin variants associate with progression of fibrosis during chronic hepatitis C infection. Hepatology. 43:1354–1363. [DOI] [PubMed] [Google Scholar]
- Strnad, P., T.C. Lienau, G.Z. Tao, N.O. Ku, T.M. Magin, and M.B. Omary. 2006. b. Denaturing temperature selection may underestimate keratin mutation detection by DHPLC. Hum. Mutat. 27:444–452. [DOI] [PubMed] [Google Scholar]
- Tao, G.Z., I. Nakamichi, N.O. Ku, J. Wang, M. Frolkis, X. Gong, W. Zhu, R. Pytela, and M.B. Omary. 2006. Bispecific and human disease-related anti-keratin rabbit monoclonal antibodies. Exp. Cell Res. 312:411–422. [DOI] [PubMed] [Google Scholar]
- Toivola, D.M., Q. Zhou, L.S. English, and M.B. Omary. 2002. Type II keratins are phosphorylated on a unique motif during stress and mitosis in tissues and cultured cells. Mol. Biol. Cell. 13:1857–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toivola, D.M., N.O. Ku, E.Z. Resurreccion, D.R. Nelson, T.L. Wright, and M.B. Omary. 2004. Keratin 8 and 18 hyperphosphorylation is a marker of progression of human liver disease. Hepatology. 40:459–466. [DOI] [PubMed] [Google Scholar]
- Toivola, D.M., G.Z. Tao, A. Habtezion, J. Liao, and M.B. Omary. 2005. Beyond cellular integrity: organelle-related and protein-targeting functions of intermediate filaments. Trends Cell Biol. 15:608–617. [DOI] [PubMed] [Google Scholar]
- Yang, D.D., C.Y. Kuan, A.J. Whitmarsh, M. Rincon, T.S. Zheng, R.J. Davis, P. Rakic, and R.A. Flavell. 1997. Absence of excitotoxicity-induced apoptosis in the hippocampus of mice lacking the Jnk3 gene. Nature. 389:865–870. [DOI] [PubMed] [Google Scholar]
- Zatloukal, K., C. Stumptner, A. Fuchsbichler, P. Fickert, C. Lackner, M. Trauner, and H. Denk. 2004. The keratin cytoskeleton in liver diseases. J. Pathol. 204:367–376. [DOI] [PubMed] [Google Scholar]
- Zhong, B., Q. Zhou, D.M. Toivola, G.Z. Tao, E.Z. Resurreccion, and M.B. Omary. 2004. Organ-specific stress induces mouse pancreatic keratin overexpression in association with NF-κB activation. J. Cell Sci. 117:1709–1719. [DOI] [PubMed] [Google Scholar]
- Zhou, Q., X. Ji, L. Chen, H.B. Greenberg, S.C. Lu, and M.B. Omary. 2005. Keratin mutation primes mouse liver to oxidative injury. Hepatology. 41:517–525. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.