Abstract
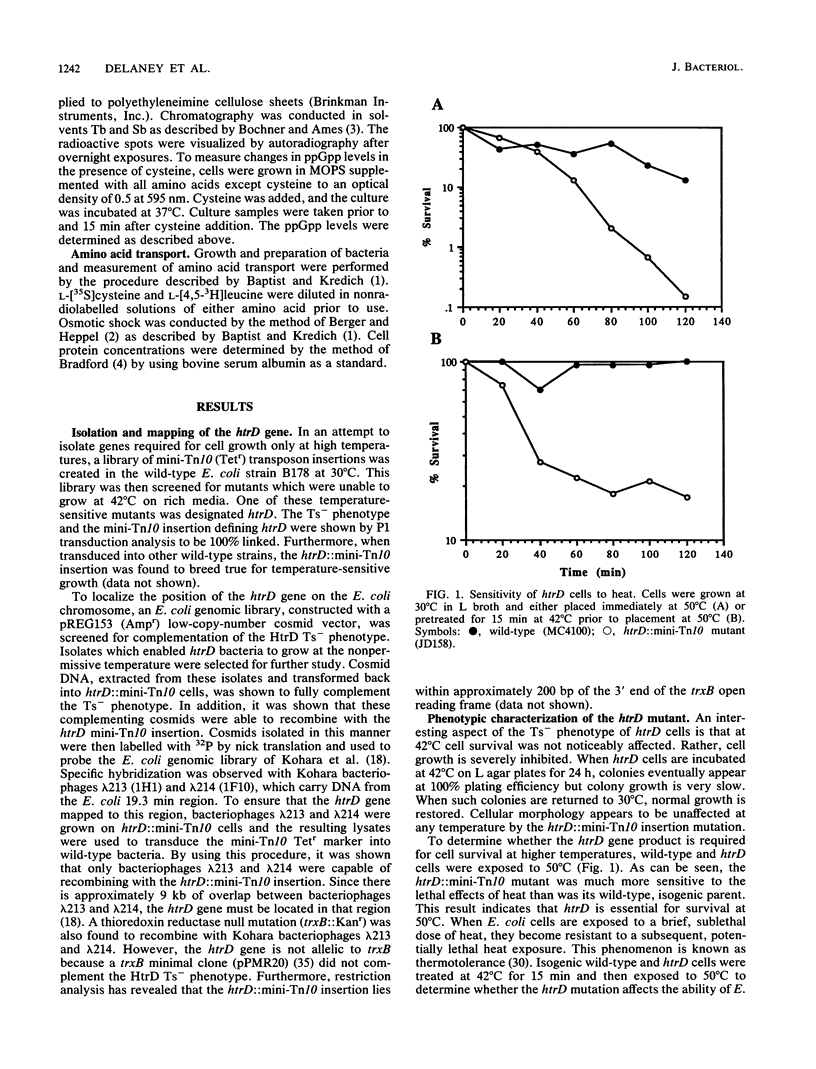
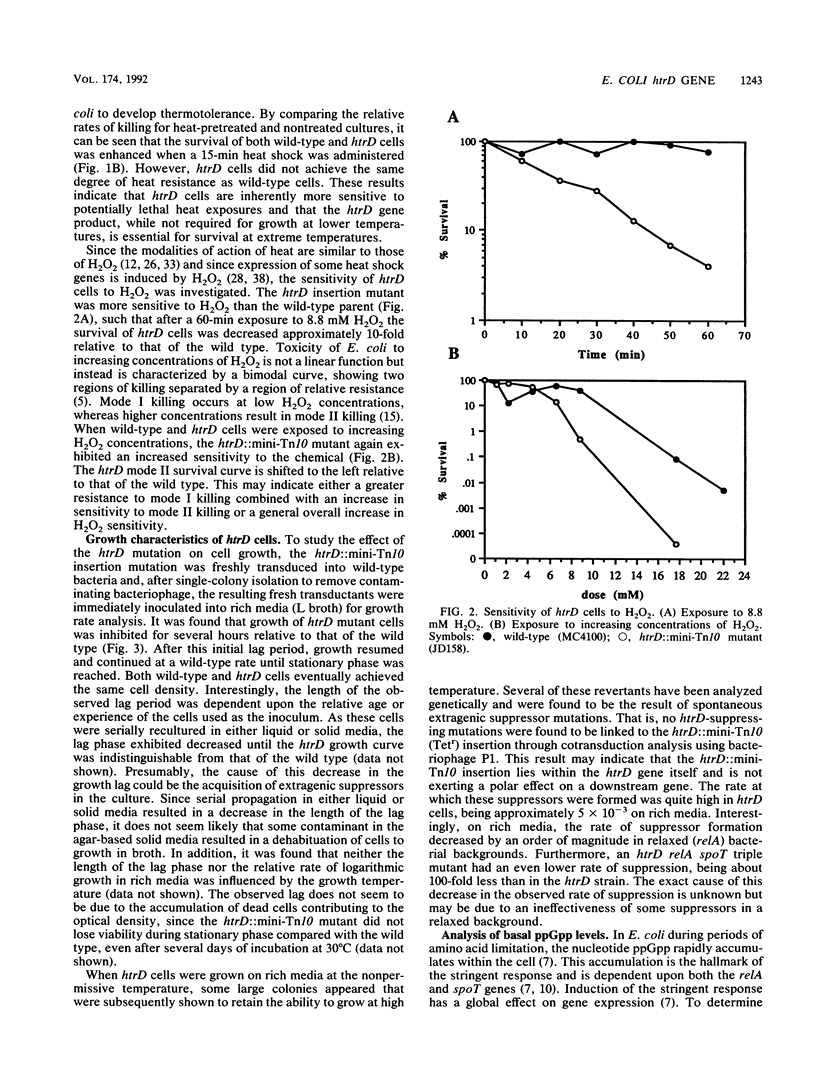
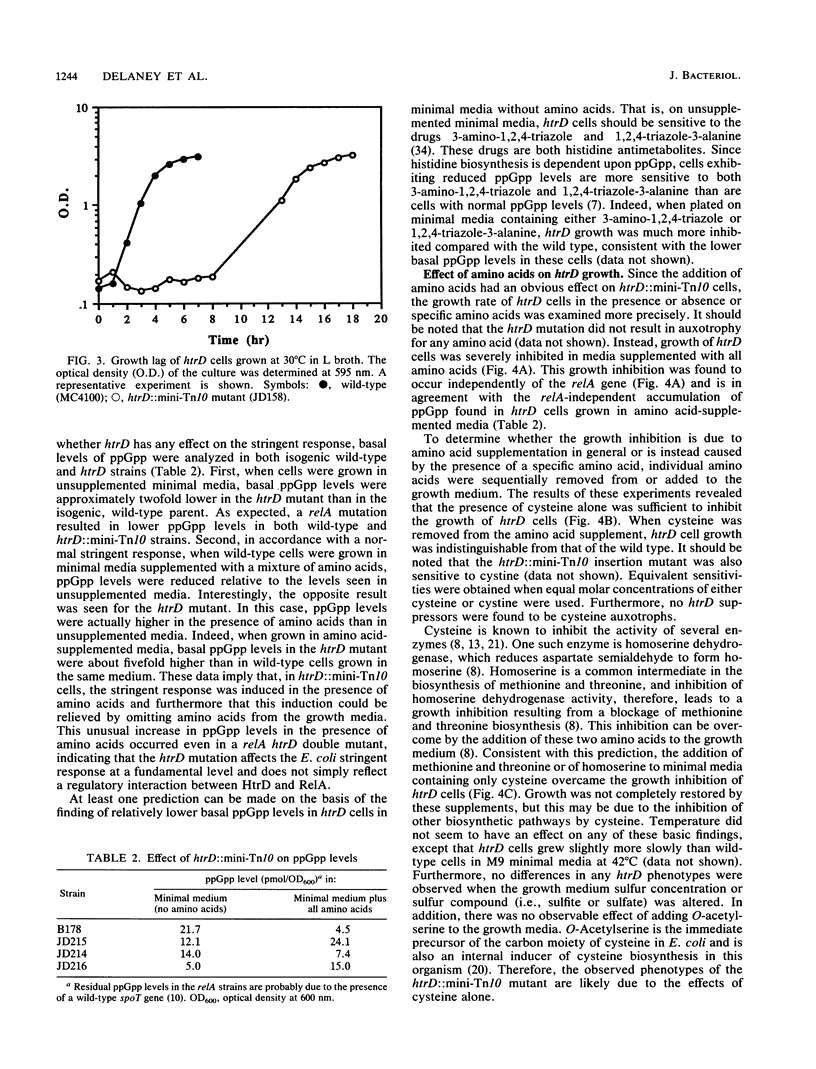
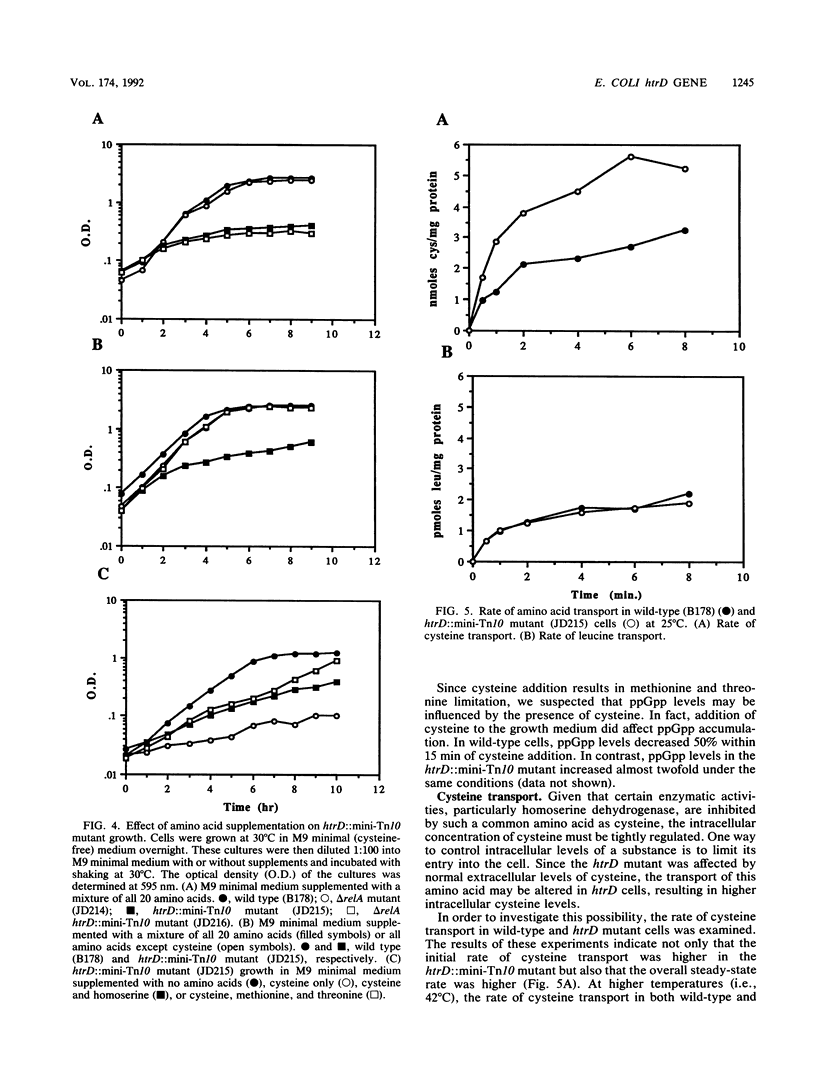
Those genes in Escherichia coli defined by mutations which result in an inability to grow at high temperatures are designated htr, indicating a high temperature requirement. A new htr mutant of E. coli was isolated and characterized and is designated htrD. The htrD gene has been mapped to 19.3 min on the E. coli chromosome. Insertional inactivation of htrD with a mini-Tn10 element resulted in a pleiotropic phenotype characterized by a severe inhibition of growth at 42 degrees C and decreased survival at 50 degrees C in rich media. Furthermore, htrD cells were sensitive to H2O2. Growth rate analysis revealed that htrD cells grow very slowly in minimal media supplemented with amino acids. This inhibitory effect has been traced to the presence of cysteine in the growth medium. Further studies indicated that the rate of cysteine transport is higher in htrD cells relative to the wild type. All of these results, taken together, indicate that the htrD gene product may be required for proper regulation of intracellular cysteine levels and that an increased rate of cysteine transport greatly affects the growth characteristics of E. coli.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baptist E. W., Kredich N. M. Regulation of L-cystine transport in Salmonella typhimurium. J Bacteriol. 1977 Jul;131(1):111–118. doi: 10.1128/jb.131.1.111-118.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger E. A., Heppel L. A. A binding protein involved in the transport of cystine and diaminopimelic acid in Escherichia coli. J Biol Chem. 1972 Dec 10;247(23):7684–7694. [PubMed] [Google Scholar]
- Bochner B. R., Ames B. N. Complete analysis of cellular nucleotides by two-dimensional thin layer chromatography. J Biol Chem. 1982 Aug 25;257(16):9759–9769. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brandi G., Sestili P., Pedrini M. A., Salvaggio L., Cattabeni F., Cantoni O. The effect of temperature or anoxia on Escherichia coli killing induced by hydrogen peroxide. Mutat Res. 1987 Apr;190(4):237–240. doi: 10.1016/0165-7992(87)90002-9. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976 Jul 5;104(3):541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- Datta P. Regulation of homoserine biosynthesis by L-cysteine, a terminal metabolite of a linked pathway. Proc Natl Acad Sci U S A. 1967 Aug;58(2):635–641. doi: 10.1073/pnas.58.2.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaal T., Gourse R. L. Guanosine 3'-diphosphate 5'-diphosphate is not required for growth rate-dependent control of rRNA synthesis in Escherichia coli. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5533–5537. doi: 10.1073/pnas.87.14.5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagensee M. E., Moses R. E. Repair response of Escherichia coli to hydrogen peroxide DNA damage. J Bacteriol. 1986 Dec;168(3):1059–1065. doi: 10.1128/jb.168.3.1059-1065.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris C. L. Cysteine and growth inhibition of Escherichia coli: threonine deaminase as the target enzyme. J Bacteriol. 1981 Feb;145(2):1031–1035. doi: 10.1128/jb.145.2.1031-1035.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herendeen S. L., VanBogelen R. A., Neidhardt F. C. Levels of major proteins of Escherichia coli during growth at different temperatures. J Bacteriol. 1979 Jul;139(1):185–194. doi: 10.1128/jb.139.1.185-194.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imlay J. A., Linn S. Mutagenesis and stress responses induced in Escherichia coli by hydrogen peroxide. J Bacteriol. 1987 Jul;169(7):2967–2976. doi: 10.1128/jb.169.7.2967-2976.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karow M., Fayet O., Cegielska A., Ziegelhoffer T., Georgopoulos C. Isolation and characterization of the Escherichia coli htrB gene, whose product is essential for bacterial viability above 33 degrees C in rich media. J Bacteriol. 1991 Jan;173(2):741–750. doi: 10.1128/jb.173.2.741-750.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohara Y., Akiyama K., Isono K. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987 Jul 31;50(3):495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- Kredich N. M., Foote L. J., Hulanicka M. D. Studies on the mechanism of inhibition of Salmonella typhimurium by 1,2,4-triazole. J Biol Chem. 1975 Sep 25;250(18):7324–7331. [PubMed] [Google Scholar]
- Lipinska B., Fayet O., Baird L., Georgopoulos C. Identification, characterization, and mapping of the Escherichia coli htrA gene, whose product is essential for bacterial growth only at elevated temperatures. J Bacteriol. 1989 Mar;171(3):1574–1584. doi: 10.1128/jb.171.3.1574-1584.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinska B., Zylicz M., Georgopoulos C. The HtrA (DegP) protein, essential for Escherichia coli survival at high temperatures, is an endopeptidase. J Bacteriol. 1990 Apr;172(4):1791–1797. doi: 10.1128/jb.172.4.1791-1797.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr A. G., Ingraham J. L. EFFECT OF TEMPERATURE ON THE COMPOSITION OF FATTY ACIDS IN ESCHERICHIA COLI. J Bacteriol. 1962 Dec;84(6):1260–1267. doi: 10.1128/jb.84.6.1260-1267.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massie H. R., Samis H. V., Baird M. B. The kinetics of degradation of DNA and RNA by H 2 O 2 . Biochim Biophys Acta. 1972 Jul 31;272(4):539–548. doi: 10.1016/0005-2787(72)90509-6. [DOI] [PubMed] [Google Scholar]
- Morgan R. W., Christman M. F., Jacobson F. S., Storz G., Ames B. N. Hydrogen peroxide-inducible proteins in Salmonella typhimurium overlap with heat shock and other stress proteins. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8059–8063. doi: 10.1073/pnas.83.21.8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Smith D. F. Culture medium for enterobacteria. J Bacteriol. 1974 Sep;119(3):736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F. C., VanBogelen R. A., Vaughn V. The genetics and regulation of heat-shock proteins. Annu Rev Genet. 1984;18:295–329. doi: 10.1146/annurev.ge.18.120184.001455. [DOI] [PubMed] [Google Scholar]
- O'Connor M., Gesteland R. F., Atkins J. F. tRNA hopping: enhancement by an expanded anticodon. EMBO J. 1989 Dec 20;8(13):4315–4323. doi: 10.1002/j.1460-2075.1989.tb08618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raina S., Georgopoulos C. A new Escherichia coli heat shock gene, htrC, whose product is essential for viability only at high temperatures. J Bacteriol. 1990 Jun;172(6):3417–3426. doi: 10.1128/jb.172.6.3417-3426.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripley L. S. Estimation of in-vivo miscoding rates. J Mol Biol. 1988 Jul 5;202(1):17–34. doi: 10.1016/0022-2836(88)90514-1. [DOI] [PubMed] [Google Scholar]
- Rudd K. E., Bochner B. R., Cashel M., Roth J. R. Mutations in the spoT gene of Salmonella typhimurium: effects on his operon expression. J Bacteriol. 1985 Aug;163(2):534–542. doi: 10.1128/jb.163.2.534-542.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russel M., Model P. Sequence of thioredoxin reductase from Escherichia coli. Relationship to other flavoprotein disulfide oxidoreductases. J Biol Chem. 1988 Jun 25;263(18):9015–9019. [PubMed] [Google Scholar]
- Sørensen M. A., Pedersen S. Cysteine, even in low concentrations, induces transient amino acid starvation in Escherichia coli. J Bacteriol. 1991 Aug;173(16):5244–5246. doi: 10.1128/jb.173.16.5244-5246.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- VanBogelen R. A., Kelley P. M., Neidhardt F. C. Differential induction of heat shock, SOS, and oxidation stress regulons and accumulation of nucleotides in Escherichia coli. J Bacteriol. 1987 Jan;169(1):26–32. doi: 10.1128/jb.169.1.26-32.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way J. C., Davis M. A., Morisato D., Roberts D. E., Kleckner N. New Tn10 derivatives for transposon mutagenesis and for construction of lacZ operon fusions by transposition. Gene. 1984 Dec;32(3):369–379. doi: 10.1016/0378-1119(84)90012-x. [DOI] [PubMed] [Google Scholar]
- Wilson G. G., Young K. Y., Edlin G. J., Konigsberg W. High-frequency generalised transduction by bacteriophage T4. Nature. 1979 Jul 5;280(5717):80–82. doi: 10.1038/280080a0. [DOI] [PubMed] [Google Scholar]


