Abstract
Neurites recognize their specific partners during the formation of interneuronal connections. In hippocampal pyramidal neurons, axons attach to dendrites for their synaptogenesis, but the dendrites do not form stable contacts with each other, suggesting the presence of a mechanism to allow their selective associations. Nectin-1 (N1), an immunoglobulin domain adhesive protein, is preferentially localized in axons, and its heterophilic partner, N3, is present in both axons and dendrites; we tested their potential roles in interneurite recognition. The overexpression of N1, causing its mislocalization to dendrites, induced atypical dendrodendritic as well as excessive axodendritic associations. On the contrary, the genetic deletion of N1 loosened the contacts between axons and dendritic spines. Those actions of nectins required cadherin–catenin activities, but the overexpression of cadherin itself could not accelerate neurite attachment. These results suggest that the axon-biased localization of N1 and its trans-interaction with N3 in cooperation with the cadherin machinery is critical for the ordered association of axons and dendrites.
Introduction
Neurons extend axons and dendrites, and these neurites select specific partners for establishing interneuronal connections. In the case of interactions between hippocampal pyramidal neurons, their axons are initially captured by filopodial protrusions from the dendrites of other neurons (Ziv and Smith, 1996; Fiala et al., 1998; Jontes and Smith, 2000), and the contact sites between the dendritic filopodia and axons gradually mature into synapses. At the same time, the filopodia are morphologically converted into the mushroom-shaped spines. Through these processes, stable axodendritic associations become established. In this type of neuron, dendrites do not appear to form functional contacts with other dendrites. Dendrites of some neurons even actively repel each other (Grueber et al., 2003; Sugimura et al., 2003) and avoid overlapping, which is called tiling (Gao and Bogert, 2003; Jan and Jan, 2003); multiple mechanisms seem to be involved in the tiling processes (Sestan et al., 1999; Emoto et al., 2004; Lin et al., 2004). On the other hand, in limited classes of neurons, dendrodendritic synapses (Peters et al., 1991; Kaba and Nakanishi, 1995) as well as dendrodendritic nonsynaptic junctions (Gulyas et al., 1996; Lohmann and Wong, 2001) can form. These observations suggest that there are neuron type–specific mechanisms to promote or suppress the interactions between a selected pair of neurites. However, the question of how axons or dendrites can preferentially bind their specific partners remains to be answered.
Formation of the contacts between axons and dendritic filopodia involves cadherin activities. Cadherins are homophilic adhesion molecules that function with their cytoplasmic (CP) partners, the catenins (Wheelock and Johnson, 2003). The cadherin–catenin complexes are accumulated at early axodendritic filopodial contacts and are retained in many of the mature synapses. Blockade of the cadherin–catenin system causes perturbation of synaptic differentiation (Togashi et al., 2002; Bozdagi et al., 2004). This adhesion system was also shown to be important for the assembly of synaptic subcellular structures (Bamji et al., 2003), stabilization of synaptic contacts (Abe et al., 2004), and activity-dependent synapse remodeling (Bozdagi et al., 2000; Murase et al., 2002). Based on their homophilic binding nature, cadherins theoretically can hold any combination of cells together, whether heterotypic or homotypic, if the cells express the same cadherin types (Hirano et al., 1987). In neurons, cadherins are localized in both axons and dendrites. Curiously, however, in many neurons such as hippocampal pyramidal neurons, although firm contacts between axons and dendritic spines are formed depending on cadherin activities, other types of contacts, such as dendrodendritic contacts, are not stabilized. We must ask why cadherins participate predominantly in the heterotypic (axodendritic) synaptic junctions but not in the homotypic dendrodendritic contacts even though this molecular family is in general used for linking the “like” cells. There should be some mechanisms for allowing cadherins to promote specifically axodendritic associations in these neurons.
Some classes of molecules that have cell-binding activities are localized only in axons or dendrites, and their partners are present on the counter-neurites. For example, neuroligin is expressed by dendrites, whereas its ligand, neurexin, is localized in axons (Scheiffele, 2003). Such receptor–ligand systems should be able to facilitate the selective contacts between axon and dendrite but not those between dendrite and dendrite or axon and axon. In the case of neuroligin and neurexin, their molecular interactions have been implicated in synaptic differentiation (Scheiffele et al., 2000; Dean et al., 2003; Graf et al., 2004). Although the neuroligin–neurexin interaction has been shown to promote cell adhesion (Ichtchenko et al., 1996; Nguyen and Sudhof, 1997), it remains to be defined whether these molecules are important for maintaining the physical associations between axons and dendrites.
Nectins, forming a small subfamily of Ig domain proteins, show an asymmetrical distribution in synapses (Mizoguchi et al., 2002). In the mossy fiber terminals of the hippocampus, nectin-1 (N1) is predominantly localized in the presynaptic membrane, and N3 is localized in the postsynaptic membrane, whereas their CP partner l-afadin is detected in both membranes (Mizoguchi et al., 2002). Both N1 and N3 show homophilic binding abilities and can promote cell aggregation (Takahashi et al., 1999; Satoh-Horikawa et al., 2000). However, importantly, they also can bind one another heterophilically, and this heterotypic binding is much stronger than the homophilic one (Fabre et al., 2002; Yasumi et al., 2003; Martinez-Rico et al., 2005). Furthermore, nectin interactions at cell–cell boundaries promote the recruitment of cadherin molecules to these sites (Tachibana et al., 2000; Honda et al., 2003). These unique distributions and properties of nectins suggest that they may play an active role in the preferential contacts between axons and dendrites. In this study, we tested this idea and found that the trans-interaction of N1 and N3 indeed controlled the adhesion between these heterotypic neurites. Our results also explain why cadherins are active only for axodendritic connections.
Results
Differential distribution of N1 and N3 in neurites
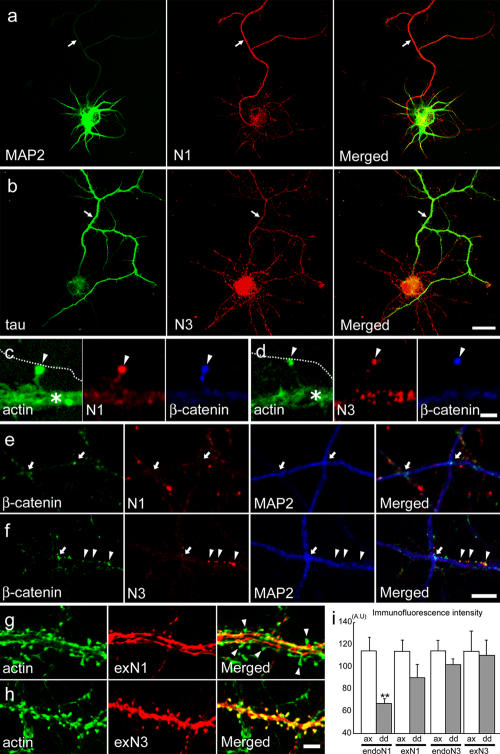
We first examined the distribution of N1 and N3 in rat hippocampal pyramidal neurons cultured for 4–6 d in vitro (DIV). Their axons and dendrites were identified by immunostaining for dephosphorylated tau (Binder et al., 1985) and MAP2 (Caceres et al., 1984), respectively. In isolated single neurons, both N1 and N3 were detected diffusely along their neurites, but they displayed distinct patterns of distribution: N1 was always more abundant in axons than in dendrites, whereas N3 was equally present in axons and dendrites (Fig. 1, a, b, and i). When axodendritic contact formation began, both N1 and N3 became concentrated at early synaptic contacts formed on the tip of dendritic filopodia, overlapping with β-catenin, a representative of the cadherin–catenin complex. Concomitantly, the diffuse nectin signals disappeared from the axons (Fig. 1, c and d). Dendrites from different neurons did not form firm contacts to each other, but they occasionally happened to cross. At these dendrodendritic crossing points, N1 was not detectable (Fig. 1 e), and N3 was present around the dendrodendritic interfaces but was not particularly concentrated there (compare the faint N3 signals on these sites with those highly up-regulated at synaptic contacts on the same dendrite in Fig. 1 f). β-Catenin was detected on some of the dendrodendritic crossing points (Fig. 1, e and f) but not on all of them (<40% at 10 DIV). These dendrodendritic β-catenin accumulations became hardly detectable at later stages (e.g., at 14 DIV). In summary, N1 and N3 were preferentially concentrated at axodendritic interfaces.
Figure 1.
Differential distribution of nectins in neurites of the hippocampal neuron. (a and b) Double immunostaining for nectins and MAP2 (dendrite marker) or tau (axon marker) in isolated neurons at 5 DIV. Nectin-1 (N1) is more abundant in the axon than in dendrites (a). N3 is detectable throughout the neurites (b). Arrows point to branches of the axon. (c and d) Close-up views of early contacts between a dendritic filopodium and axon (indicated by arrowheads) that were triple stained for F-actin, β-catenin, and N1 (c) or N3 (d) at 6 DIV. Each nectin is sharply concentrated at the contact sites, colocalizing with β-catenin, and is absent from the free surfaces of the axon. Axons are indicated by the dotted lines, as the original actin stain was faint. Asterisks indicate dendrites. (e and f) N1 is not localized at the dendrodendritic crossing points (indicated by arrows). N3 is detectable around the dendrodendritic crossing points but is not particularly concentrated there, although this molecule is highly concentrated at early synapses present on the same dendrite (indicated by arrowheads). β-Catenin is localized at some of the dendrodendritic crossing points. Neurons were examined at 10 DIV. (g and h) Distribution of exogenous N1 (exN1) or exN3 in mature neurons at 21 DIV. In close-up views of their dendrites, exN1 is detected on fibers running on the dendritic shaft (g); the majority of spines on the same dendrite, detected by F-actin staining, do not have exN1, some of which are indicated by arrowheads. On the contrary, exN3 is evenly detected on all spines as well as on the shaft portion of the dendrite (h). See Fig. S1 for lower magnification views (available at http://www.jcb.org/cgi/content/full/jcb.200601089/DC1). (i) Relative intensity of immunofluorescence signals emanating from endogenous (endo) or exogenous (ex) nectins on the axons (ax) or dendrites (dd; mean ± SEM [error bars]). **, P < 0.001 versus the axon; n = 16 for dendrites, and n = 6 for axons. AU, arbitrary unit. Bars (a and b), 10 μm; (c and d) 2 μm; (e–h) 5 μm.
Once the nectin signals had been concentrated in the synapses, it became difficult to define whether these signals were derived from axons or dendrites. To determine their localization in mature neurons accurately, we transfected neurons with N1 or N3 cDNA, cultured them for 3 wk, and observed the distributions of the exogenously introduced nectins. Because of the overexpression, excess nectin signals were not restricted to synapses but were diffusely detected along neurites, allowing us to determine which neurites expressed these nectins. In N1-transfected cultures, N1 immunofluorescence signals were detected emanating from thin neurites that migrated on the culture plate as well as from those associated with dendritic processes (Fig. S1 a, available at http://www.jcb.org/cgi/content/full/jcb.200601089/DC1). The former population of neurites was identified as axonal because of their MAP2 negativity. Closer observations of the latter revealed that the N1 signals were localized along spine-free neurites running on the dendritic shaft (Fig. 1 g), suggesting that these also were axons. The majority of the dendritic spines in these cultures were N1 negative. On the other hand, N3 immunofluorescence signals evenly delineated the entire dendritic process, showing typical arrays of spines (Fig. 1 h and Fig. S1 b), and their signals were barely detectable on MAP2-negative neurites. These findings indicate that N1 prefers to localize in axons at any developmental stage and that N3 localization becomes biased toward dendrites during development, which is consistent with the in vivo observation (Mizoguchi et al., 2002).
Effects of the overexpression of nectins on neurite patterning
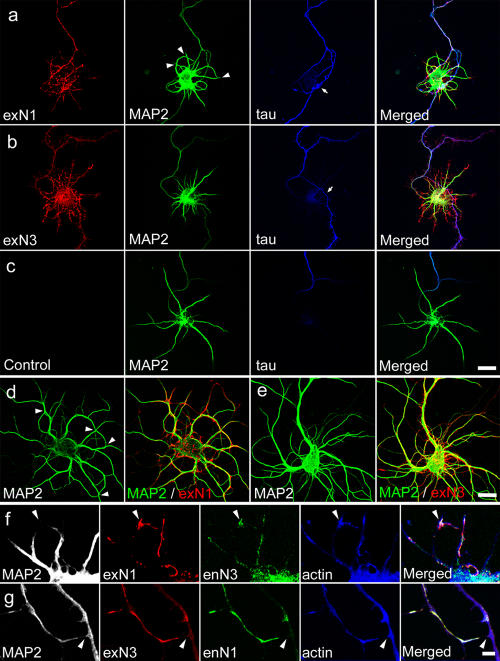
To study the role of N1 and N3 showing the aforementioned differential distribution, we examined the effects of N1 or N3 overexpression in more detail by observing neurons earlier after their transfection. Neurons transfected with N1 or N3 cDNA were cultured for 5–6 d and analyzed for the expression of exogenous nectins. Western blot analysis showed that the total level of N1 or N3 in these cultures significantly increased (Fig. S1 c). Immunostaining analysis revealed that exogenous N1 (exN1) was abundant in axons, but, as a result of overexpression, its relative level in dendrites appeared to have increased (Figs. 1 i and 2 a). Intriguingly, this N1 overexpression caused abnormal neurite patterning. Nontransfected pyramidal neurons, in principle, extended axons and dendrites radially from their soma (Fig. 2 c). In N1-overexpressing neurons, however, their axons often entwined around their own dendrites (Fig. 2 a, tau). Furthermore, many of their dendrites aberrantly touched each other, giving a looplike appearance (Fig. 2 a, MAP2; see Fig. 3 f for quantification). On the other hand, axons of neurons with exN3, which was distributed evenly among neurites (as seen for the endogenous N3 [enN3]), did not show such abnormal migration: when their axons happened to migrate onto their own bodies, they crossed them with a simple track (Fig. 2 b, arrow). A similar crossing was observed in nontransfected neurons, suggesting that this behavior was not caused by N3 overexpression. At 14 DIV, neurons expressing exN1 again exhibited extensive intraneuronal dendritic attachments, whereas those expressing N3 did so only at a minimum level (Fig. 2, d and e). We could not accurately trace axons in these older cultures, as tau distribution lost its continuity along the axons. These observations indicate that the overexpression of N1 but not N3 induced atypical sticking between neurites.
Figure 2.
Effects of the overexpression of nectins on neurite patterning. (a–c) Neurons transfected with N1 (a) or N3 (b) and nontransfected (c) at 6 DIV. Cultures were triple stained for nectin, MAP2, and tau. In N1 transfectants (a), the relative level of N1 in dendrites is increased, MAP2-positive dendrites aberrantly touch each other (indicated by arrowheads), and tau-positive axons have become irregularly entangled around their own dendrites. In N3 transfectants (b), dendrites extend radially, as seen in the nontransfected control (c), and axon extension is not disturbed by the dendrites of the same neuron. Arrows point to axon branches in contact with dendrites. The reactivity of anti-MAP2 antibodies toward axons tended to increase in nectin transfectants for some unknown reason. (d and e) Neurons transfected with N1 or N3 and double stained for nectin and MAP2 at 14 DIV. Dendrodendritic attachment inducing their looping appearance occurs extensively in N1 transfectants (d, arrowheads) but much less in N3 ones (e). (f and g) Close-up views of dendrodendritic contacts under nectin overexpression. Cells were quadruple stained for MAP2, exogenous (ex) and endogenous (en) nectins, and F-actin at 5 DIV. In N1 transfectants (f), exN1 and enN3 are concentrated together at dendrodendritic contact sites, as indicated by arrowheads. In N3 transfectants (g), such concentration does not occur even when dendrodendritic contacts are formed (arrowheads). In these dendrites, exN3 and endoN1 colocalize at noncontact portions. Bars (a–e), 20 μm; (f and g) 5 μm.
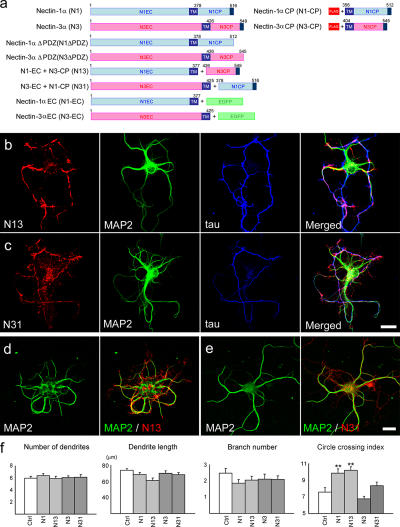
Figure 3.
Effects of the expression of chimeric nectin molecules. (a) Diagram of nectin constructs used. TM, trans-membrane region. The N1-derived regions are light blue, and N3-derived regions are pink. + indicates where the two regions are fused. (b and c) Neurons transfected with N13 or N31 and triple stained for the chimeric nectin MAP2 and tau at 5 DIV. In N13 transfectants (b), N13 molecules are clustered in various regions, and dendrites and axons are strongly entangled. In N31 transfectants (c), N31 molecules are localized most abundantly in axons, and the atypical association of neurites is less extensive than in the case of N13 transfectants. (d and e) Neurons transfected with N13 or N31 and double stained for the chimeric nectin and MAP2 at 14 DIV. N13 strongly induces dendrodendritic attachments (d), and N31 only induces these weakly (e). (f) Statistical analysis of dendritic arbor pattern. Number of dendrites, dendrite length, and dendrite branch number in neurons nontransfected (Ctrl) or transfected with N1, N13, N3, or N31 were measured at 7 DIV. Histogram shows the mean plus SEM (error bars) for each sample (n = 20 for dendrite and branch number; n = 40 for dendrite length). No significant difference was found between these samples. For the right histogram, n = 20. **, P < 0.001 versus control, N3, and N31. The circle-crossing index represents the mean number of dendrites that cross the circle (40 μm in diameter) superimposed on the soma of each neuron. This index is expected to increase when dendrites turn and form loops as a result of dendrodendritic attachments. Bars, 20 μm.
Analysis of nectin domains responsible for abnormal neurite patterning
To determine which domain (the extracellular [EC] or CP) was critical for the aforementioned activity of nectins, we expressed the EC domain of N1 (N1-EC) or N3 (N3-EC; Fig. 3 a) in neurons and found that both constructs were not particularly effective in inducing aberrant neurite patterning (Fig. S2, a and b; available at http://www.jcb.org/cgi/content/full/jcb.200601089/DC1). Both of these molecules were detected on axons and dendrites, although N3-EC was homogenously distributed, whereas N1-EC tended to be clustered. We also expressed the CP domain of N1 (N1-CP) and N3 (N3-CP) but found no effects on neurite patterning. Both of these constructs tended to accumulate in the cell body regions, but a fraction of them also spread into neurites. Importantly, although N3-CP was widely distributed into MAP2-positive neurites, N1-CP was uniquely condensed along a single neurite extending from the soma of each neuron; these neurites had been identified as axons, as they did not react with anti-MAP2 antibodies except at the proximal region (Fig. S2, c and d). These results suggest that the CP domain of N1 was responsible for its axon-biased localization and also that this domain of N1 or its EC domain alone was not sufficient to exhibit the biological activities.
We also examined whether the aforementioned activities of nectins required the COOH-terminal afadin-binding site by using a COOH terminus–truncated construct of N1 (N1ΔPDZ) or N3 (N3ΔPDZ) and found that their expression in neurons had no effect on dendrite patterning (Fig. S2, e and f). On the other hand, N1ΔPDZ was still preferentially condensed in axon fibers (Fig. S2 e). These results suggest that the afadin-binding site is necessary for the activity of N1 to promote interneurite attachment, but the sorting signals for axonal localization reside elsewhere in the CP domain.
To further investigate the roles of the EC or CP domains of nectins, we constructed chimeric molecules of N1 and N3, N13 and N31, by swapping their EC and CP domains (Fig. 3 a). When their transfectants were examined at 5–6 DIV, N13 (N1-EC + N3-CP) was detected on both axons and dendrites as clustered signals (Fig. 3 b), whereas the N31, having N3-EC + N1-CP, was localized more abundantly in axons (Fig. 3 c). This was reminiscent of the enN1 localization. These results support the idea that the CP domain is responsible for the axon-biased distribution of N1.
The expression of N13 caused the severe entangling of axons along their own dendrites (Fig. 3 b), as in the case of N1 overexpression. In neurons expressing N31, their axons also showed a tendency to attach to their own dendrites (Fig. 3 c), but to a lesser extent as compared with the N13 expression (i.e., those axons simply crossed dendrites in most cases in contrast with the firm tangling of axons with dendrites in N13-expressing neurons). Aberrant attachment between dendritic processes was also induced by N13 expression but not N31 (Fig. 3, d and e) and was confirmed by quantitative analysis (Fig. 3 f). These findings suggest that misexpression of the N1-EC domain, whether it is linked with its own CP domain or with the N3-CP domain, induces atypical neurite associations.
Trans-interaction between N1 and N3 in aberrant neurite association
We sought to understand how N1 misexpression induced the abnormal neurite interactions. Double immunostaining for N1 and N3 revealed that in N1-transfected neurons exhibiting atypical dendrodendritic contacts, exN1 molecules were concentrated together with enN3 at their contact sites (Fig. 2 f), suggesting that their heterophilic interactions were involved in inducing these phenotypes. In neurons transfected with N3, exN3 was unable to condense at the sites where their dendrites had happened to touch each other (Fig. 2 g). In these dendrites, exN3 was deposited along their noncontacting portions together with enN1. These observations suggest that only excess N1 molecules were able to accumulate themselves and their partner molecules into ectopic neurite contact sites and sustain their atypical associations. Whether exN1 also recruited the same nectin type remains to be determined because our antibodies to detect endogenous nectins could not distinguish between the exogenous and endogenous molecules.
To test further whether the interaction between N1 and N3 was important for the aberrant neurite sticking, we mixed N1- and N3-transfected neurons in the same cultures. When transfectants with the different nectins happened to reside next to each other, their dendritic branches became deeply intermingled (Fig. 4 a). At their contact points, the two molecules were closely colocalized. Thus, the trans-interactions between overexpressed N1 and N3 molecules induced interneuronal dendrodendritic associations, which are not generally observed in hippocampal cultures except for their simple crossing (Fig. 1, e and f). Similar cocondensation of N1 and N3 was also found at axodendritic contact sites formed between these transfectants. For example, when an axon expressing exN1 migrates on other neurons with exN3, the exN3 molecules have sharply been concentrated along the axon (Fig. 4 b). In many such cases, noncontacting portions of the recipient neuron lost the exN3 signals, suggesting that the majority of N3 molecules expressed by the cell had been accumulated at the axodendritic contact sites. All of these results support the hypothesis that the N1–N3 interaction facilitates interneurite adhesions. In addition, we examined whether the nectin overexpression also affected synapse formation by immunostaining the aforementioned mixed cultures for synaptic markers and found that at the contact sites between N1-overexpressing axons and N3-overexpressing dendrites, the distribution pattern of synaptotagmin, a presynaptic marker, was not particularly altered (Fig. S3, available at http://www.jcb.org/cgi/content/full/jcb.200601089/DC1). This result suggests that the N1–N3 interactions enhance only the affinities between neurites but not their synaptogenesis, which probably requires additional machineries such as the neuroligin–neurexin interactions.
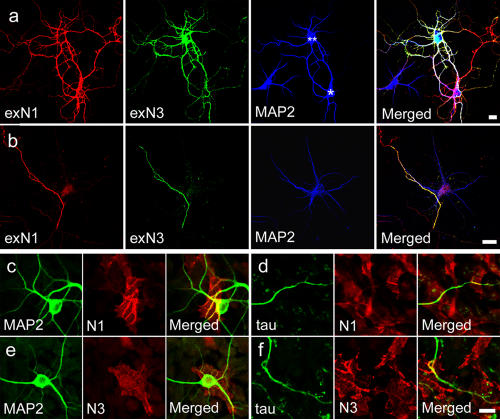
Figure 4.
Nectin interactions at neurite contact sites. (a) Dendrodendritic associations observed between different neurons and induced by nectin overexpression. Neurons were independently transfected with N1 or N3, and these cells were mixed at a 1:1 ratio, cultured for 14 d, and triple stained for exN1, exN3, and MAP2. In the pair of neurons situated next to each other (one overexpressing exN1 [single asterisk] and the other overexpressing exN3 [double asterisks]), their dendrites have almost entirely intermingled. The nontransfected neuron located at the bottom left is less extensively associated with them. Neurons with exN1 or exN3 were identified by the abundance of the respective molecules at the cell body regions. (b) Heterophilic nectin interaction at axodendritic interfaces. An axon extending from a remote neuron with exN1, located outside at the top left corner, has attached to dendrites of a neuron with exN3, which is visualized by MAP2 staining, in the culture prepared as in panel a. At their contact sites, exN3 is exclusively concentrated along the exN1-positive axon. Faint fluorescence on the neuronal body in the exN1 panel is likely a result of the nonspecific reaction of the antibodies. (c–f) Interaction of nectins at the interfaces between neurites and 293 cells. Neurons were plated onto mixed cultures of 293 cells nontransfected or transfected with N1 (c and d) or N3 (e and f), incubated for 5 d, and double stained for exN1 or exN3 and MAP2 or tau. Dendrites have strongly recruited N1 molecules derived from 293 transfectants to their contact sites (c) but have N3 ones only weakly (e). Axons recruited these nectins indiscriminately (d and f). Bars (a and b), 20 μm; (c–f) 10 μm.
Preferential trans-recruitment of N1 by dendrites
We hypothesized that the role of enN1 localized in axons might be to promote the attachment of the axons to dendrites through its trans-interactions with dendritic N3 molecules. As a step to test this idea, we asked with which of the nectin types dendrites or axons preferred to interact. As neurite–neurite interfaces do not provide sufficient resolution for this analysis, we constructed a model system: we prepared HEK293 (293) cells transfected with N1 (N1-293) or N3 (N3-293). These cells endogenously express N-cadherin, and the respective nectins were concentrated at their cell–cell boundaries (Fig. S4, available at http://www.jcb.org/cgi/content/full/jcb.200601089/DC1). We seeded neurons onto monolayers of these 293 transfectants and observed the distribution of each nectin at the interfaces between neurites and 293 cells. For accurate assessment of the specific effects of nectin expression, we used mosaic cultures of transfected and nontransfected 293 cells. Neurons extended their axons and dendrites onto the surfaces of these transfectants or nontransfectants.
Immunostaining of these samples showed that when dendrites had attached onto N1-293 cells, N1 molecules derived from the N1-293 cells became intensely concentrated along the dendritic processes (Fig. 4 c). On N3-293 cells, the dendrites also recruited N3 molecules, but only faintly (Fig. 4 e; our antibodies against N1 and N3 could detect the antigens from these transfectants with similar fluorescence intensity; see Fig. S4). Thus, dendrites preferentially recruited N1 that was present on the counter–cell membranes. On the other hand, when axons had attached to N1- or N3-293 cells, these nectins were similarly concentrated along the axons, although not uniformly (Fig. 4, d and f), which is consistent with the finding that early axons expressed both N1 and N3. This result suggests that axonal N1 and N3 are ready to interact with the counter-nectins if these molecules are expressed on the surfaces of the adjacent cells. However, dendrites, which are the actual partners for axons, did not equally express these nectins and responded to them differentially, as shown above. Thus, as a result of the nonuniform distribution of N1 in neurites, a biased interacting system between dendritic N3 and axonal N1 seems to have been established (see Fig. 7).
Figure 7.
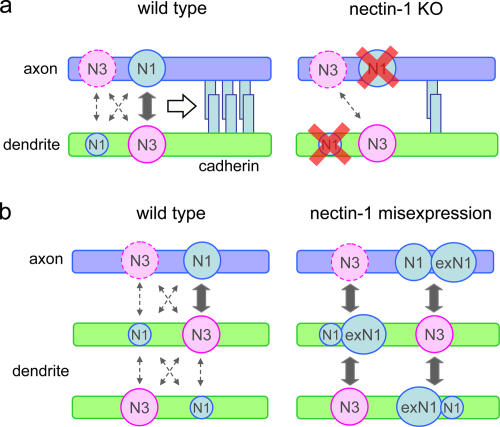
Working models to explain the role of nectins in neurite interactions. (a) In wild-type neurons, N1 abundant in the axon interacts with N3 in the dendrite, and this trans-heterophilic interaction of N1 and N3 promotes homophilic cadherin–cadherin interactions to strengthen synaptic junctions. In the absence of N1, only a basic level of cadherin interactions would take place. Homophilic interactions between N3 and N3 would not be strong enough to sustain normal axodendritic contacts. Dotted arrows indicate possible weaker interactions between nectins. The axonal N3 level appears to decrease with the maturation of neurons. (b) Dendrodendritic interactions are not stable because the N1 level in dendrites is low and N3–N3 interaction is not strong enough. However, when N1 is overexpressed, the misexpressed N1 molecules not only induce atypical dendrodendritic adhesions but also overstabilize axodendritic contacts. KO, knockout.
Effects of the genetic deletion of N1 on axodendritic association
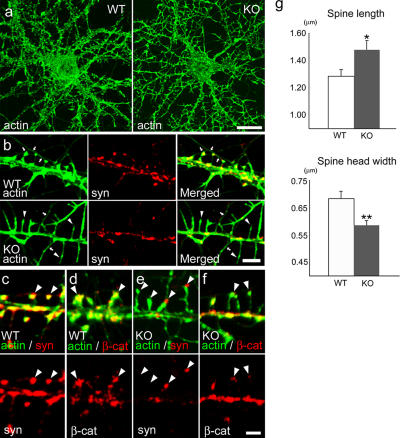
To verify the hypothesis that the N1–N3 interaction regulates axodendritic association, we examined the effects of the genetic deficiency of N1 on neurite patterning by culturing hippocampal neurons isolated from N1 knockout mice (Inagaki et al., 2005). Radial extension of axons and dendrites normally occurred in the mutant pyramidal neurons, and they did not display any aberrant patterning. However, when axodendritic contacts had begun, the mutant neurons came to exhibit atypical morphologies. Actin staining at 14–17 DIV revealed that their dendritic spines were unusually elongated or deformed, resulting in a smaller spine head (Fig. 5, a and g). In the cultures of wild-type neurons, their spine heads swelled, firmly attaching to axon fibers (Fig. 5 b, top). In contrast, in N1-deficient neurons, many of their dendritic spines, which exhibited filopodia-like morphology, did not associate with axons that could have been traced with their diffuse synaptotagmin signals (Fig. 5 b, bottom). All of these results suggest that the adhesive affinity between axons and dendritic spines, the major structures on pyramidal neurons to receive axonal input, was significantly reduced as a result of N1 deficiency. In more mature stages, synaptotagmin or β-catenin became concentrated onto their spine heads even in mutant neurons. Nevertheless, their signals were generally reduced, corresponding to the reduction in head size, and many of the spine or filopodial heads lost their association with synaptotagmin signals (Fig. 5, c–f). These results indicate that although N1 is dispensable for synapse formation, its absence impairs the normal process of axodendritic spine contacts and keeps them looser than usual even after their maturation.
Figure 5.
Effects of N1 deficiency on axodendritic interactions. (a) Hippocampal neurons obtained from wild-type (WT) and N1-deficient (knockout; KO) brains stained for F-actin at 14 DIV. Note the elongated morphology of the mutant dendritic spines. (b) Close-up views of a wild-type (top) or N1-deficient (bottom) neuron at 14 DIV double stained for F-actin and synaptotagmin (syn). Arrows point to axons, which are identified by their association with spine heads or reactivity to antisynaptotagmin antibodies. In the mutant sample, although some spines are in contact with axons, others (indicated by arrowheads) appear to be free from the axons. (c–f) Synaptotagmin and β-catenin localization in wild-type (c and d) and N1-deficient (e and f) synapses at 21 DIV. These proteins are seen at synaptic sites in both samples, but the β-catenin condensation has decreased, and synaptotagmin puncta have become reduced in size and have even lost from some of the spine heads (the left two arrowheads in panel e) in the mutant samples. Arrowheads point to representative spine heads. (g) Statistical analysis of dendritic spine morphology. Spine length and spine head width were significantly changed in N1 mutants (mean ± SEM [error bars]; n = 50 for each sample). *, P < 0.05 versus N1 +/+; **, P < 0.005 versus N1 +/+. The data were collected from 10 neurons in two independently prepared cultures at 17 DIV. Bars (a), 20 μm; (b) 5 μm; (c–f) 2 μm.
Cooperative action of nectins and the cadherin–catenin complex
Because a role of nectins is to recruit cadherins, we tested whether the aforementioned activities of nectins involved cadherin actions. We found that whenever N1 and N3 were concentrated together at neurite contact sites, β-catenin was also recruited to these sites (see example in Fig. 6 a), and N-cadherin showed a similar response (not depicted), confirming that nectin interactions promote cadherin-mediated adhesion. To examine how much the heterophilic N1–N3 and homophilic N1–N1 or N3–N3 interactions differ in their abilities to recruit β-catenin, we prepared mixed cultures of N1- and N3-293. In the original transfectants, either N1 or N3 was concentrated at cell–cell boundaries, although β-catenin was less tightly colocalized with N3 than with N1 (Fig. S4). In the mixed cultures, three types of interfaces—N1–N1, N3–N3, and N1–N3—were formed. Triple immunostaining for N1, N3, and β-catenin in these cultures showed the clear tendency that N1 and N3 were more intensely condensed together at the heterotypic boundaries between N1- and N3-293 cells than at the homotypic boundaries, and the β-catenin level proportionally increased in those heterotypic contact sites (Fig. 6 b and Fig. S5, available at http://www.jcb.org/cgi/content/full/jcb.200601089/DC1). As a consequence, the junctional accumulation of these molecules was relatively decreased at N1–N1 or N3–N3 cell interfaces and, surprisingly, even disappeared from certain homotypic boundaries, causing the local separation of cells at these boundaries (Fig. S5). These results indicate that the homotypic and heterotypic cell boundaries compete for β-catenin recruitment and that the latter prevails.
Figure 6.
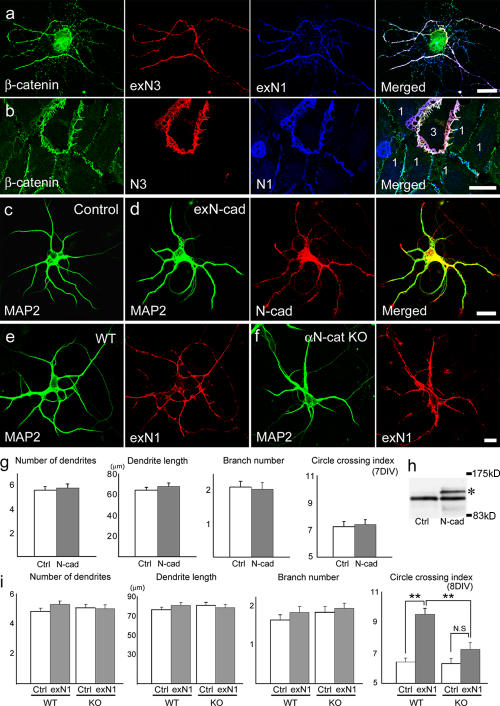
Cooperative action of nectins and N-cadherin/catenins. (a) A mixed culture of neurons transfected with N1 or N3 prepared as in Fig. 4 B and triple stained for β-catenin, exN3, and exN1 at 10 DIV. Axons expressing exN3 have migrated on a neuron with exN1, and β-catenin is concentrated together with N3 and N1 along the axons. (b) A mixed culture of 293 cells transfected with N1 or N3 triple stained for exN1, exN3, and β-catenin. Each transfectant was identified by comparing the localization of these two molecules and are marked as 1 (for N1) or 3 (for N3). A single N3 transfectant is surrounded by multiple N1 transfectants. (c and d) Neurons nontransfected (c) or transfected with Flag-tagged N-cadherin cDNA (d; Nakagawa and Takeichi, 1998) were cultured for 7 d and double stained for MAP2 and the Flag tag. (e and f) Hippocampal neurons derived from wild-type (e) or αN-catenin knockout (KO) mice (f) were transfected with N1 and double stained for MAP2 and exN1 at 8 DIV. (g) Statistical analysis of the experiments in panels c and d. n = 20 for dendrite number and dendrite branch number; n = 40 for dendrite length. (h) Immunoblots for N-cadherin expressed in nontransfected (Ctrl) and N-cadherin–transfected (N-cad) cultures at 8 DIV. EGFP-tagged N-cadherin (asterisk; Horikawa and Takeichi, 2001) was used for transfection. The amount of the exogenous N-cadherin is about equal to or slightly lower than that of the endogenous one. As ∼50% of cells were transfected, we can estimate that the level of total N-cadherin per neuron increased two to three times in the transfected neurons. (i) Statistical analysis of the experiments in panels e and f. Error bars represent SEM. **, P < 0.001 versus control. n = 20 for dendrite number and dendrite branch number; n = 40 for dendrite length. 20 neurons were used for this assay. Bars (a–d), 20 μm; (e and f) 10 μm.
The aforementioned observations in a model system confirmed that the trans-interaction between N1 and N3 most effectively recruited cadherin–catenin complexes to cell contact sites. We also noticed that in N1 or N3-transfected neurons, the total level of N-cadherin slightly increased approximately two times per neuron in each transfectant (Fig. S1 c), suggesting that both nectins can stabilize cadherins irrespective of their abilities to induce excessive interneurite contacts. Therefore, we tested whether an increase in cadherin-dependent adhesiveness was sufficient to induce excessive neurite interactions by overexpressing N-cadherin in neurons. The total N-cadherin level per neuron increased two to three times in these cultures compared with untransfected cultures (Fig. 6 h). However, this N-cadherin overexpression had no effect on neurite patterning (Fig. 6, c, d, and g) even though cadherins had previously been shown to generate much stronger adhesiveness than nectins (Martinez-Rico et al., 2005), indicating that a simple increase in cadherin level or surface adhesiveness was not sufficient for inducing the atypical neurite interactions.
Finally, we asked whether the cadherin–catenin adhesion system was required for the aforementioned actions of nectins. To test this possibility, we isolated hippocampal neurons from αN-catenin–deleted mutant mice in which cadherin activities were impaired (Abe et al., 2004) and transfected them with N1 cDNA. We first confirmed that mouse pyramidal neurons responded to N1 overexpression in a way similar to the rat ones (Fig. 6, e and i). Notably, when αN-catenin–deleted neurons had been used for transfection, their dendritic morphology was little affected by N1 overexpression (Fig. 6, f and i). We also found that the N-cadherin level was kept lower in αN-catenin–deficient neurons than in wild-type ones after the N1 transfection (Fig. S1 d). Thus, these results demonstrate that the cadherin–αN-catenin system was required for the actions of the nectins.
Discussion
We showed that N1 was preferentially localized in axons and that perturbation of its distribution by overexpression induced atypical associations between neurites. On the other hand, N3 was equally detected on both axons and dendrites, although this molecule appeared to prefer localizing on dendrites in mature neurons. Upon synaptogenesis, both N1 and N3 became concentrated together at axodendritic contact sites, whereas such condensation did not occur at the sites where dendrites crossed each other. In the 293 cell model system, the N1–N3 heterophilic interaction prevailed over the homophilic one, with more recruitment of β-catenin by the former. We also showed that dendrites more efficiently recruited N1 than N3 onto the counter–cell membranes, implying that the dendrites dominantly use their N3 molecules to interact with axons. Furthermore, N1-deleted neurons exhibited loosened associations between axons and dendritic spines. All of these results support the idea that the axon-biased localization of N1 and its trans-interaction with dendritic N3 plays a critical role in sustaining the normal association between axons and dendrites (Fig. 7 a). These actions of nectins required cadherin–catenin activities. Intriguingly, however, the overexpression of N-cadherin itself had no effect on neurite patterning. Thus, a cooperation of these heterophilic and homophilic adhesion systems is required for exerting their full activities, possibly generating unique mechanisms for linking the heterotypic pair of axonal and dendritic plasma membranes.
The overexpression of N1 resulted in the excessive association of axons and dendrites derived from the same neuron. The formation of synapses by neurons onto themselves occurs normally, whose structures are known as autapses (Bekkers and Stevens, 1991; Lubke et al., 1996). However, the overexpressed N1 appeared to have overly attracted axons and dendrites and, furthermore, induced atypical dendrodendritic contacts. Under these conditions, N1 molecules were leaked out to dendrites and ectopically condensed at dendrodendritic interfaces, recruiting enN3 to theses sites. This suggests that the mislocalization of excess N1 and its interaction with N3 was a primary cause for the induction of the dendrodendritic adhesions (Fig. 7 b). Once the level of N1 has increased in dendrites, this molecule should also be able to undergo substantial interactions with axonal N3, accounting for the excessive axodendritic associations (Fig. 7 b). This idea is supported by the observation that N13 exhibited similar effects. As N13 has the N3-CP domain, this chimeric molecule should have followed the N3 distribution, ensuring ectopic localization of the N1-EC domain to dendrites. Together, our results indicate that the proper localization of N1 is important for the correct neurite interactions.
On the other hand, the overexpression of N3 had little effect. exN3 molecules did not accumulate at dendrodendritic interfaces, suggesting that these molecules cannot actively hold their attachments. It should be noted that the N3–N3 homophilic interactions are less effective in inducing cell aggregation than those of N1 (Martinez-Rico et al., 2005). We also noticed that N3 less efficiently recruited β-catenin to cell–cell contact sites compared with N1 in 293 cells; nevertheless, the total level of N-cadherin increased not only in N1- but also in N3-transfected neurons. Thus, we can speculate that this nectin can interact with the cadherin–catenin complex by itself but is unable to efficiently bring the complex into cell contact sites for some reason. Based on these observations, we suspect that N3 itself may not be a strong adhesion molecule and that it functions only significantly as a heterophilic partner for N1. Once the level of N3 has reached saturation with respect to N1, excess N3 molecules may not be able to exert additional biological effects.
Although these two nectins were differentially distributed in axons and dendrites, they were not strictly confined to either of these neurites, particularly in early neurons. Thus, we can suppose that nectins or cadherins can also be used to promote dendrodendritic adhesion (Fig. 7). However, this form of adhesion was not observable unless N1 had been overexpressed. Is there any mechanism to exclude stable dendrodendritic attachment in the normal situation? We found that in mixed cultures of N1- and N3-expressing 293 cells, the N1–N3 boundaries collected greater amounts of N1 and N3 than their homophilic interfaces, sometimes causing cell separation at the latter interfaces. Such competition between the heterophilic and homophilic interactions of nectins also likely occurs in neurons, and the N1–N3 interactions at axodendritic interfaces could sweep away N1 or N3 from dendrodendritic interfaces, prohibiting their associations.
Nectin interactions have been proposed to facilitate the accumulation of cadherins at cell–cell contact sites (Tachibana et al., 2000; Honda et al., 2003). Consistent with this idea, β-catenin was always highly concentrated at the nectin-condensed sites in cultured neurons. At dendrodendritic crossing points where nectins were not concentrated, β-catenin was only transiently localized. Our results also showed that nectin overexpression could not induce aberrant neurite associations if αN-catenin–deficient neurons were used for transfection and, in addition, that for exhibiting the overexpression phenotype, N1 required the COOH-terminal domain that was the binding site for l-afadin, a mediator for the interaction between N1 and α-catenin (Tachibana et al., 2000). These results suggest that nectins alone cannot function but that they need to interact with cadherin via the l-afadin–α-catenin complex. We further demonstrated that nectin overexpression up-regulated the N-cadherin level, but this effect was suppressed in αN-catenin–deficient neurons, suggesting the possibility that the role of N1 is to stabilize or up-regulate cadherin via binding with α-catenin. Importantly, however, the overexpression of N-cadherin itself had no effect on neurite patterning. Moreover, not only N1 but also N3 could up-regulate the N-cadherin level in their transfectants even though only N1 was active in altering neurite adhesiveness. These suggest that the real role of nectins was not simply to up-regulate the level of cadherins, although cadherin up-regulation might have been a prerequisite for the nectin actions.
It is known that trans-nectin interactions activate small GTPases in their CP domain–dependent manners and also that these small GTPases can facilitate cadherin activities (Kawakatsu et al., 2002; Fukuhara et al., 2003; Sato et al., 2006). Thus, nectins may cooperate with cadherin through such physiological cross talks in addition to its up-regulation. A similar cooperation of cadherin and an Ig domain protein, echinoid, was found to occur in cell–cell adhesion in Drosophila imaginal discs (Wei et al., 2005). Intriguingly, the CP domain of echinoid resembles that of nectins, as both can bind l-afadin. Transfection experiments thus far published suggest that the cadherin–catenin complex can solely function for cell–cell adhesion (Martinez-Rico et al., 2005), but its activity seems to be modulated by these Ig domain molecules.
Our in vitro analysis of N1-deleted neurons provides loss of function evidence that N1 is required for proper axodendritic interactions. In the absence of N1, the attachment of axons to dendritic spines appeared significantly loosened. In vivo analysis of the hippocampus in N1 knockout mice demonstrated that a population of axons from the dentate gyrus failed to terminate at the correct portions of CA3 neurons (Honda et al., 2006), supporting the idea that N1 is required for axons to properly recognize and attach to their target dendrites. On the other hand, synaptic protein assembly more or less occurred in the N1-deleted neurons both in vitro and in vivo, indicating that nectins are dispensable for synapse formation itself. As a certain level of β-catenin is still detectable on the N1-deficient synapses, residual cadherin–catenin complexes or other cell adhesion molecules may serve or compensate for maintaining their remnant synaptic contacts. It would be intriguing to test the effects of the double knockout of the cadherin and nectin systems on synapse formation in future studies. Nectins are widely but not ubiquitously expressed in the brain (Haarr et al., 2001). Other ligand receptors may also play a role in regulating axodendritic associations. It is important to note that some classes of neurons can form dendrodendritic adherens junctions or synapses (Peters et al., 1991; Kaba and Nakanishi, 1995). Therefore, our final goal should be to identify neuron type–specific mechanisms, which control the adhesive affinities between neurites, for a deeper understanding of interneuronal recognition mechanisms.
Materials and methods
Mice
Mice in which exon 2 of the N1 gene had been replaced with the neomycin resistance gene (Inagaki et al., 2005) were maintained on a C57/BL6 background. The genotyping methods for αN-catenin knockout mice were described previously (Togashi et al., 2002).
Cell culture and transfection
Rat hippocampal neuronal cultures were prepared from embryonic day (E) 18 rat embryos by using previously described methods (Brewer et al., 1993) with some modifications. In brief, hippocampi were dissociated by trypsinization and trituration and were plated at 5,000–10,000 cells/cm2 onto poly-l-lysine–coated glass coverslips. Cultures were maintained in DME F-12 with 2% B27 supplements (Invitrogen) and 5% horse serum or in neurobasal medium (Invitrogen) with 2% B27 supplements. Cytosine arabinoside was added after 3 d to inhibit glial proliferation. Hippocampal cultures of N1 −/− or wild-type mice were prepared from E16–17 embryos according to the methods used for the rat hippocampus. Cultures of neurons from αN-catenin knockout mice were prepared separately from individual mouse embryos at E16–17, and those of mutant neurons were selected after genotyping of the original embryos. Neurons were transfected with various DNA constructs by using an electroporation device (Nucleofector 1; Amaxa). For transfection, neurons were suspended at 250,000–3,000,000 cells/transfection in 40–100 μl of the Amaxa nucleofector solution and electroporated with 1–2.5 μg DNA.
293 cells expressing exogenous mouse N1 or N3 were generated by standard transfection methods. For the preparation of cocultures of 293 cells and neurons, nectin-transfected 293 cells were seeded on collagen-coated glass coverslips at semiconfluent densities in neurobasal medium with 2% B27 supplements. After 24 h, hippocampal neurons were seeded onto these 293 cell layers, and the cultures were then incubated for 5 d in the same medium before examination.
Plasmid construction
cDNAs for mouse N1α and N3α were used throughout the experiments. For the construction of N13, cDNA fragments encoding amino acids 1–379 of mouse N1α and amino acids 429–549 of mouse N3α were amplified by PCR by using primer sets of 5′-ATGGCTCGGATGGGGCTTGCCG-3′ and 5′-GTCGACGCAGGGCCACTATGATCCCTCCGAC-3′ as well as 5′-GTCGACGACGGACGTTTCGTGGAGA-3′ and 5′-TTAGACATACCACTCCCTCC-3′, respectively. For the construction of N31, cDNA fragments encoding amino acids 1–427 of mouse N3α and amino acids 381–516 of mouse N1α were amplified by PCR using primer sets of 5′-ATGGCGCGGACCCCGGG-3′ and 5′-GTCGACGATAGCAGAATACCCCAGCTAAAA-3′ as well as 5′-GTCGACGCCGGCACACCTTCAAG-3′ and 5′-CTACACATACCACTCTTTCTTG-3′, respectively. Obtained fragments were ligated through the underlined SalI sites. To construct the expression vector for N13 and N31, we subcloned each cDNA fragment into pCA-pA using a HindIII and NheI linker. The Flag-tagged nectin CP region of N1α (pCA-N1CP; 356–512 residues) and N3α (pCA-N3CP; 404–545 residues) were constructed by using pCA-Sig-pA. For the construction of pCA-N1EC-EGFP and pCA-N3EC-EGFP, cDNA fragments encoding amino acids 1–377 of mouse N1α and 1–425 of N3α were ligated into pCA-EGFP-pA, respectively. The generation of other constructs was described previously (Tanoue and Takeichi, 2004).
Immunocytochemistry
Cells on coverslips were fixed in 2–4% PFA in HBSS with 4% sucrose for 10–15 min at room temperature or 37°C. After treatment with 0.25% Triton X-100 in TBST (TBS with 0.005% Tween-20) for 5 min at room temperature, the cells were blocked with 5% skim milk in TBST at 37°C and exposed for 2 h to primary antibodies in 5% skim milk in TBST at room temperature or 37°C. Primary antibodies were visualized with goat fluorochrome-conjugated secondary antibodies. The fluorochromes used were AlexaFluor350, -488, -555, -647 (Invitrogen), and Cy3 (Chemicon). F-actin was visualized by use of AlexaFluor488-conjugated phalloidin (Invitrogen).
Antibodies
Rabbit anti-N1 and anti-N3 antibodies were raised against the CP portion of mouse N1α and N3α proteins, respectively, and were affinity purified by using standard protocols. These antibodies cross reacted with rat nectins and were used to detect endogenous rat nectins. Rat monoclonal anti–mouse N1 (clone 48–12; MBL International Corporation) and anti–mouse N3 (clone 103-A1; MBL International Corporation) antibodies, which recognized the EC regions of N1 and N3, respectively, were used to detect exogenously introduced mouse nectins. These monoclonal antibodies did not immunocytochemically detect rat endogenous nectins in cultured neurons. Other antibodies used were mouse monoclonal anti-MAP2 antibody (clone HM-2; Sigma-Aldrich), rabbit anti-MAP2 antibody (Chemicon), mouse monoclonal anti–tau-1 antibody (clone PC1C6; Chemicon), mouse monoclonal anti–β-catenin antibody (clone 5H10; a gift from M.J. Wheelock, University of Nebraska, Omaha, NE), rabbit anti–β-catenin antibody (Sigma-Aldrich), rat anti-GFP antibody (Nacalai Tesque), rabbit anti-Flag antibody (Sigma-Aldrich), mouse monoclonal anti–N-cadherin antibody (Transduction Laboratories), and mouse antisynaptotagmin antibody (Chemicon).
Western blotting
Neuronal cultures were prepared in 35-mm petri dishes, and their lysates were analyzed by SDS-PAGE in which the total protein concentration had been adjusted to be equal for each lane. Proteins were transferred to a nitrocellulose membrane, the membrane was blocked with 5% skim milk for 1 h, and membranes were incubated overnight at 4°C with anti-nectin or anti–N-cadherin antibodies in Can Get Signal solution (Toyobo). Blots were washed with TBS, incubated for 1 h in HRP-conjugated goat anti–mouse antiserum (1:5,000; Jackson ImmunoResearch Laboratories), and visualized by exposing to X-ray films after treatment with ECL Plus Substrate (GE Healthcare). The signals on the films were digitally scanned and analyzed by using Scion Image densitometric analysis.
Image acquisition and quantification of dendrite morphology
Images of neurons were obtained with a confocal microscope (LSM510; Carl Zeiss MicroImaging, Inc.) equipped with a 63× NA 1.4 or a 40× NA 1.3 lens using LSM510 software (Carl Zeiss MicroImaging, Inc.), and their morphology was analyzed with the same software and with Adobe Photoshop. For quantification of the dendritic arbor pattern, the number of dendrites that elongated directly from the cell body was first counted, in which measurement dendrites shorter than the diameter of neuronal soma were omitted. The dendrite processes were then manually traced to measure their length by LSM software; the two longest dendrites were chosen for this measurement. The number of branches protruding from these dendrites was also manually counted. To obtain the circle-crossing index of dendritic arbors, we superimposed a circle of 40 μm in diameter on the center of the cell body of each neuron. Then, the number of dendrites crossing the circle was counted and plotted; subsequently, Welch's t test was performed. In general, several neurons were randomly chosen from multiple culture plates for each assay. Neurons at 7–8 DIV were used for these analyses.
Online supplemental material
Fig. S1 shows the localization of exogenous nectins in nectin-transfected mature neurons as well as the effects of nectin overexpression on the N-cadherin level. Fig. S2 shows the effects of expression of nectin mutants on neurite patterning. Fig. S3 shows the effects of nectin overexpression on synaptotagmin distribution. Fig. S4 shows nectin and β-catenin distribution in nectin-transfected 293 cells. Fig. S5 shows nectin and β-catenin distribution in a mixed culture of N1- and N3-transfected 293 cells. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200601089/DC1.
Supplementary Material
Acknowledgments
We are grateful to S. Hirano, S. Nakagawa, T. Tanoue, S.C. Suzuki, and all of the other members of our laboratory for helpful discussions. We also thank H. Ishigami, M. Harata, and C. Yoshii for technical assistance.
This work was supported by a grant from the program Grants-in-Aid for Specially Promoted Research of the Ministry of Education, Science, Sports and Culture of Japan to M. Takeichi and by a grant-in-aid for scientific research from the Japan Society for the Promotion of Science for Junior Scientists to H. Togashi.
Abbreviations used in this paper: CP, cytoplasmic; DIV, days in vitro; EC, extracellular.
References
- Abe, K., O. Chisaka, F. Van Roy, and M. Takeichi. 2004. Stability of dendritic spines and synaptic contacts is controlled by alpha N-catenin. Nat. Neurosci. 7:357–363. [DOI] [PubMed] [Google Scholar]
- Bamji, S.X., K. Shimazu, N. Kimes, J. Huelsken, W. Birchmeier, B. Lu, and L.F. Reichardt. 2003. Role of beta-catenin in synaptic vesicle localization and presynaptic assembly. Neuron. 40:719–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekkers, J.M., and C.F. Stevens. 1991. Excitatory and inhibitory autaptic currents in isolated hippocampal neurons maintained in cell culture. Proc. Natl. Acad. Sci. USA. 88:7834–7838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder, L.I., A. Frankfurter, and L.I. Rebhun. 1985. The distribution of tau in the mammalian central nervous system. J. Cell Biol. 101:1371–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozdagi, O., W. Shan, H. Tanaka, D.L. Benson, and G.W. Huntley. 2000. Increasing numbers of synaptic puncta during late-phase LTP: N-cadherin is synthesized, recruited to synaptic sites, and required for potentiation. Neuron. 28:245–259. [DOI] [PubMed] [Google Scholar]
- Bozdagi, O., M. Valcin, K. Poskanzer, H. Tanaka, and D.L. Benson. 2004. Temporally distinct demands for classic cadherins in synapse formation and maturation. Mol. Cell. Neurosci. 27:509–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer, G.J., J.R. Torricelli, E.K. Evege, and P.J. Price. 1993. Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. J. Neurosci. Res. 35:567–576. [DOI] [PubMed] [Google Scholar]
- Caceres, A., G. Banker, O. Steward, L. Binder, and M. Payne. 1984. MAP2 is localized to the dendrites of hippocampal neurons which develop in culture. Brain Res. 315:314–318. [DOI] [PubMed] [Google Scholar]
- Dean, C., F.G. Scholl, J. Choih, S. DeMaria, J. Berger, E. Isacoff, and P. Scheiffele. 2003. Neurexin mediates the assembly of presynaptic terminals. Nat. Neurosci. 6:708–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emoto, K., Y. He, B. Ye, W.B. Grueber, P.N. Adler, L.Y. Jan, and Y.N. Jan. 2004. Control of dendritic branching and tiling by the Tricornered-kinase/Furry signaling pathway in Drosophila sensory neurons. Cell. 119:245–256. [DOI] [PubMed] [Google Scholar]
- Fabre, S., N. Reymond, F. Cocchi, L. Menotti, P. Dubreuil, G. Campadelli-Fiume, and M. Lopez. 2002. Prominent role of the Ig-like V domain in trans-interactions of nectins. Nectin3 and nectin 4 bind to the predicted C-C′-C″-D beta-strands of the nectin1 V domain. J. Biol. Chem. 277:27006–27013. [DOI] [PubMed] [Google Scholar]
- Fiala, J.C., M. Feinberg, V. Popov, and K.M. Harris. 1998. Synaptogenesis via dendritic filopodia in developing hippocampal area CA1. J. Neurosci. 18:8900–8911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuhara, A., K. Shimizu, T. Kawakatsu, T. Fukuhara, and Y. Takai. 2003. Involvement of nectin-activated Cdc42 small G protein in organization of adherens and tight junctions in Madin-Darby canine kidney cells. J. Biol. Chem. 278:51885–51893. [DOI] [PubMed] [Google Scholar]
- Gao, F.B., and B.A. Bogert. 2003. Genetic control of dendritic morphogenesis in Drosophila. Trends Neurosci. 26:262–268. [DOI] [PubMed] [Google Scholar]
- Graf, E.R., X. Zhang, S.X. Jin, M.W. Linhoff, and A.M. Craig. 2004. Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins. Cell. 119:1013–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueber, W.B., B. Ye, A.W. Moore, L.Y. Jan, and Y.N. Jan. 2003. Dendrites of distinct classes of Drosophila sensory neurons show different capacities for homotypic repulsion. Curr. Biol. 13:618–626. [DOI] [PubMed] [Google Scholar]
- Gulyas, A.I., N. Hajos, and T.F. Freund. 1996. Interneurons containing calretinin are specialized to control other interneurons in the rat hippocampus. J. Neurosci. 16:3397–3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haarr, L., D. Shukla, E. Rodahl, M.C. Dal Canto, and P.G. Spear. 2001. Transcription from the gene encoding the herpesvirus entry receptor nectin-1 (HveC) in nervous tissue of adult mouse. Virology. 287:301–309. [DOI] [PubMed] [Google Scholar]
- Hirano, S., A. Nose, K. Hatta, A. Kawakami, and M. Takeichi. 1987. Calcium-dependent cell-cell adhesion molecules (cadherins): subclass specificities and possible involvement of actin bundles. J. Cell Biol. 105:2501–2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda, T., K. Shimizu, T. Kawakatsu, M. Yasumi, T. Shingai, A. Fukuhara, K. Ozaki-Kuroda, K. Irie, H. Nakanishi, and Y. Takai. 2003. Antagonistic and agonistic effects of an extracellular fragment of nectin on formation of E-cadherin-based cell-cell adhesion. Genes Cells. 8:51–63. [DOI] [PubMed] [Google Scholar]
- Honda, T., T. Sakisaka, T. Yamada, T. Hoshino, M. Kajita, T. Kayahara, H. Ishizaki, M. Tanaka-Okamoto, A. Mizoguchi, J. Miyoshi, and Y. Takai. 2006. Involvment of nectins in the formation of puncta adherentia junctions and the mossy fiber trajectory at the mouse hippocampus. Mol. Cell. Neurosci. 31:315–325. [DOI] [PubMed] [Google Scholar]
- Horikawa, K., and M. Takeichi. 2001. Requirement of the juxtamembrane domain of the cadherin cytoplasmic tail for morphogenetic cell rearrangement during myotome development. J. Cell Biol. 155:1297–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichtchenko, K., T. Nguyen, and T.C. Sudhof. 1996. Structures, alternative splicing, and neurexin binding of multiple neuroligins. J. Biol. Chem. 271:2676–2682. [DOI] [PubMed] [Google Scholar]
- Inagaki, M., K. Irie, H. Ishizaki, M. Tanaka-Okamoto, K. Morimoto, E. Inoue, T. Ohtsuka, J. Miyoshi, and Y. Takai. 2005. Roles of cell-adhesion molecules nectin 1 and nectin 3 in ciliary body development. Development. 132:1525–1537. [DOI] [PubMed] [Google Scholar]
- Jan, Y.N., and L.Y. Jan. 2003. The control of dendrite development. Neuron. 40:229–242. [DOI] [PubMed] [Google Scholar]
- Jontes, J.D., and S.J. Smith. 2000. Filopodia, spines, and the generation of synaptic diversity. Neuron. 27:11–14. [DOI] [PubMed] [Google Scholar]
- Kaba, H., and S. Nakanishi. 1995. Synaptic mechanisms of olfactory recognition memory. Rev. Neurosci. 6:125–141. [DOI] [PubMed] [Google Scholar]
- Kawakatsu, T., K. Shimizu, T. Honda, T. Fukuhara, T. Hoshino, and Y. Takai. 2002. Trans-interactions of nectins induce formation of filopodia and lamellipodia through the respective activation of Cdc42 and Rac small G proteins. J. Biol. Chem. 277:50749–50755. [DOI] [PubMed] [Google Scholar]
- Lin, B., S.W. Wang, and R.H. Masland. 2004. Retinal ganglion cell type, size, and spacing can be specified independent of homotypic dendritic contacts. Neuron. 43:475–485. [DOI] [PubMed] [Google Scholar]
- Lohmann, C., and R.O. Wong. 2001. Cell-type specific dendritic contacts between retinal ganglion cells during development. J. Neurobiol. 48:150–162. [PubMed] [Google Scholar]
- Lubke, J., H. Markram, M. Frotscher, and B. Sakmann. 1996. Frequency and dendritic distribution of autapses established by layer 5 pyramidal neurons in the developing rat neocortex: comparison with synaptic innervation of adjacent neurons of the same class. J. Neurosci. 16:3209–3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Rico, C., F. Pincet, E. Perez, J.P. Thiery, K. Shimizu, Y. Takai, and S. Dufour. 2005. Separation force measurements reveal different types of modulation of E-cadherin-based adhesion by nectin-1 and -3. J. Biol. Chem. 280:4753–4760. [DOI] [PubMed] [Google Scholar]
- Mizoguchi, A., H. Nakanishi, K. Kimura, K. Matsubara, K. Ozaki-Kuroda, T. Katata, T. Honda, Y. Kiyohara, K. Heo, M. Higashi, et al. 2002. Nectin: an adhesion molecule involved in formation of synapses. J. Cell Biol. 156:555–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murase, S., E. Mosser, and E.M. Schuman. 2002. Depolarization drives beta-Catenin into neuronal spines promoting changes in synaptic structure and function. Neuron. 35:91–105. [DOI] [PubMed] [Google Scholar]
- Nakagawa, S., and M. Takeichi. 1998. Neural crest emigration from the neural tube depends on regulated cadherin expression. Development. 125:2963–2971. [DOI] [PubMed] [Google Scholar]
- Nguyen, T., and T.C. Sudhof. 1997. Binding properties of neuroligin 1 and neurexin 1beta reveal function as heterophilic cell adhesion molecules. J. Biol. Chem. 272:26032–26039. [DOI] [PubMed] [Google Scholar]
- Peters, A., S.L. Palay, and H.deF. Webster. 1991. The fine structure of the nervous system: neurons and their supporting cells. Oxford University Press, New York. 494 pp.
- Sato, T., N. Fujita, A. Yamada, T. Ooshio, R. Okamoto, K. Irie, and Y. Takai. 2006. Regulation of the assembly and adhesion activity of E-cadherin by nectin and afadin for the formation of adherens junctions in Madin-Darby canine kidney cells. J. Biol. Chem. 281:5288–5299. [DOI] [PubMed] [Google Scholar]
- Satoh-Horikawa, K., H. Nakanishi, K. Takahashi, M. Miyahara, M. Nishimura, K. Tachibana, A. Mizoguchi, and Y. Takai. 2000. Nectin-3, a new member of immunoglobulin-like cell adhesion molecules that shows homophilic and heterophilic cell-cell adhesion activities. J. Biol. Chem. 275:10291–10299. [DOI] [PubMed] [Google Scholar]
- Scheiffele, P. 2003. Cell-cell signaling during synapse formation in the CNS. Annu. Rev. Neurosci. 26:485–508. [DOI] [PubMed] [Google Scholar]
- Scheiffele, P., J. Fan, J. Choih, R. Fetter, and T. Serafini. 2000. Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell. 101:657–669. [DOI] [PubMed] [Google Scholar]
- Sestan, N., S. Artavanis-Tsakonas, and P. Rakic. 1999. Contact-dependent inhibition of cortical neurite growth mediated by notch signaling. Science. 286:741–746. [DOI] [PubMed] [Google Scholar]
- Sugimura, K., M. Yamamoto, R. Niwa, D. Satoh, S. Goto, M. Taniguchi, S. Hayashi, and T. Uemura. 2003. Distinct developmental modes and lesion-induced reactions of dendrites of two classes of Drosophila sensory neurons. J. Neurosci. 23:3752–3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana, K., H. Nakanishi, K. Mandai, K. Ozaki, W. Ikeda, Y. Yamamoto, A. Nagafuchi, S. Tsukita, and Y. Takai. 2000. Two cell adhesion molecules, nectin and cadherin, interact through their cytoplasmic domain-associated proteins. J. Cell Biol. 150:1161–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, K., H. Nakanishi, M. Miyahara, K. Mandai, K. Satoh, A. Satoh, H. Nishioka, J. Aoki, A. Nomoto, A. Mizoguchi, and Y. Takai. 1999. Nectin/PRR: an immunoglobulin-like cell adhesion molecule recruited to cadherin-based adherens junctions through interaction with Afadin, a PDZ domain-containing protein. J. Cell Biol. 145:539–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanoue, T., and M. Takeichi. 2004. Mammalian Fat1 cadherin regulates actin dynamics and cell–cell contact. J. Cell Biol. 165:517–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togashi, H., K. Abe, A. Mizoguchi, K. Takaoka, O. Chisaka, and M. Takeichi. 2002. Cadherin regulates dendritic spine morphogenesis. Neuron. 35:77–89. [DOI] [PubMed] [Google Scholar]
- Wei, S.Y., L.M. Escudero, F. Yu, L.H. Chang, L.Y. Chen, Y.H. Ho, C.M. Lin, C.S. Chou, W. Chia, J. Modolell, and J.C. Hsu. 2005. Echinoid is a component of adherens junctions that cooperates with DE-Cadherin to mediate cell adhesion. Dev. Cell. 8:493–504. [DOI] [PubMed] [Google Scholar]
- Wheelock, M.J., and K.R. Johnson. 2003. Cadherins as modulators of cellular phenotype. Annu. Rev. Cell Dev. Biol. 19:207–235. [DOI] [PubMed] [Google Scholar]
- Yasumi, M., K. Shimizu, T. Honda, M. Takeuchi, and Y. Takai. 2003. Role of each immunoglobulin-like loop of nectin for its cell-cell adhesion activity. Biochem. Biophys. Res. Commun. 302:61–66. [DOI] [PubMed] [Google Scholar]
- Ziv, N.E., and S.J. Smith. 1996. Evidence for a role of dendritic filopodia in synaptogenesis and spine formation. Neuron. 17:91–102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.