Abstract
Budding yeast chitin synthase 2 (Chs2p), which lays down the primary septum, localizes to the mother–daughter neck in telophase. However, the mechanism underlying the timely neck localization of Chs2p is not known. Recently, it was found that a component of the exocyst complex, Sec3p–green fluorescent protein, arrives at the neck upon mitotic exit. It is not clear whether the neck localization of Chs2p, which is a cargo of the exocyst complex, was similarly regulated by mitotic exit. We report that Chs2p was restrained in the endoplasmic reticulum (ER) during metaphase. Furthermore, mitotic exit was sufficient to cause Chs2p neck localization specifically by triggering the Sec12p-dependent transport of Chs2p out of the ER. Chs2p was “forced” prematurely to the neck by mitotic kinase inactivation at metaphase, with chitin deposition occurring between mother and daughter cells. The dependence of Chs2p exit from the ER followed by its transport to the neck upon mitotic exit ensures that septum formation occurs only after the completion of mitotic events.
Introduction
Cytokinesis and septum deposition in budding yeast occur only after exit from mitosis has taken place. Exit from mitosis, which is defined biochemically as the destruction of mitotic cyclins such as Clb2p, depends on ubiquitin-mediated proteolysis. The anaphase-promoting complex, an E3 ligase, together with its activators Cdc20p and Hct1p, act sequentially to cause the proteolysis of Clb2p upon which cells enter G1 of a new cell division cycle (Bardin and Amon, 2001; Yeong et al., 2002; Simanis, 2003). An early observation indicating that cytokinesis depends on mitotic exit was the failure of cells to proceed to cytokinesis in the presence of a nondestructible Clb2p (Surana et al., 1993). Furthermore, the mitotic exit network (MEN), which plays a role in the activation of Hct1p (Visintin et al., 1998; Jaspersen et al., 1999), is implicated in cytokinesis and septum formation (for review see Balasubramanian et al., 2004). The MEN comprises Tem1p (a GTPase), Lte1p (a GTP/GDP exchange factor), Cdc15p, Cdc5p, Dbf2p, and Dbf20p (Ser/Thr kinases), Mob1p (a kinase), and Cdc14p (a phosphatase; Jaspersen et al., 1998; Morgan, 1999; Lee et al., 2001). Tem1p, Cdc15p, Cdc5p, and Dbf2p have been observed at the mother–daughter neck, linking them to the process of cytokinesis and/or septation (for review see Balasubramanian et al., 2004). For instance, the overexpression of mutant constructs of either Cdc15p or Cdc5p can give rise to cytokinesis defects, whereas a deletion in MOB1 leads to a failure in cytokinesis. Both tem1-1 and cdc15-2 mutants are defective in actomyosin ring constriction, whereas the cdc15-2 mutant is also unable to form a proper septum. However, the exact roles of the MEN components at the neck with respect to cytokinesis and septum deposition remain undefined.
Septum deposition starts with the laying down of a primary septum of chitin between the mother and daughter cells by chitin synthase 2 (Chs2p) during cytokinesis (for review see Bi, 2001; Cabib et al., 2001; Walther and Wendland, 2003; Cabib, 2004). This occurs as the actomyosin ring contracts, which provides an inward force that leads to the invagination of the plasma membrane at the neck (Schmidt et al., 2002; Tolliday et al., 2003). Chs2p, which is localized to the plasma membrane at the neck during this time, deposits a layer of chitin between the mother and daughter cells after the line of constriction of the plasma membrane (Schmidt et al., 2002; Tolliday et al., 2003). The secondary septum is subsequently deposited around the primary septum from mother and daughter cells (for review see Bi, 2001; Cabib et al., 2001; Walther and Wendland, 2003; Cabib, 2004). It was previously documented that Chs2p was found at the neck only in telophase (Chuang and Schekman, 1996). Recently, it was shown that the stability of the Myo1p ring during cytokinesis is dependent on the presence of Chs2p (VerPlank and Li, 2005). Interestingly, the transcription of Chs2p peaks earlier on at G2/M phase (Pammer et al., 1992; Cho et al., 1998; Spellman et al., 1998). These observations suggest that Chs2p neck localization and chitin deposition could be tightly coordinated with late mitotic events, as Chs2p normally arrives at the neck before Myo1p ring constriction. To date, it is not clear what determines the timely neck localization of Chs2p to the neck at the end of mitosis. Given the localization of Chs2p to the neck late in telophase (Chuang and Schekman, 1996; VerPlank and Li, 2005), we asked whether there exists a link between the mitotic exit and neck localization of Chs2p that could explain the occurrence of septum deposition subsequent to mitotic exit.
In this study, we show that the translocation of Chs2p to the neck is dependent on mitotic kinase inactivation. Moreover, the inactivation of the mitotic kinase activity by Sic1p overexpression led to the localization of Chs2p to the neck in telophase in the absence of Cdc15p function, indicating that the MEN component of Cdc15p's role in Chs2p localization is through its function in promoting exit from mitosis. More importantly, premature inactivation of the mitotic kinase at metaphase was sufficient to send Chs2p to the neck. Overexpression of Sic1p resulted in the deposition of chitin in metaphase-arrested cells, implying that Chs2p prematurely localized at the neck was active. We further demonstrate that mitotic exit is required for the triggering of the COPII-dependent transport of Chs2p out of the ER. Our data provide evidence that the timely localization of Chs2p to the neck is regulated at the level of ER export by a decrease in mitotic kinase at the end of mitosis.
Results
Chs2p localization to the neck correlates with exit from mitosis
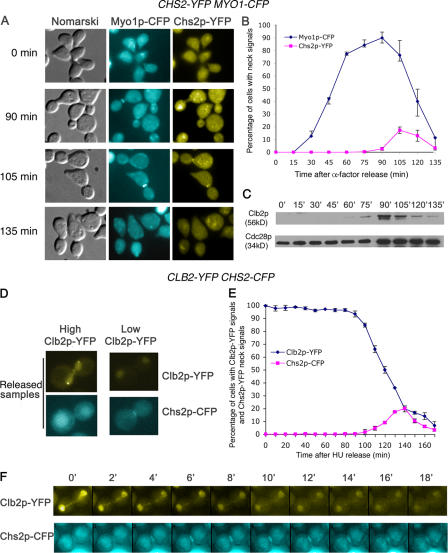
It was previously published that Chs2p localizes late in telophase to the mother–daughter neck (Chuang and Schekman, 1996) ∼2.4 ± 0.8 min before spindle disassembly (VerPlank and Li, 2005). To further understand the regulation of Chs2p at the end of mitosis, we examined the neck localization of Chs2p over one cell cycle in wild-type cells carrying CHS2-YFP MYO1-CFP (FM119). FM119 cells arrested in G1 using α-factor were released into fresh medium at 24°C, and samples were collected for observation of the GFP fusion proteins and Clb2p and Cdc28p levels. Chs2p-YFP can be seen at the mother–daughter necks (Fig. 1 A) at a peak of 17.33 ± 2.52% of the cells at 105 min after α-factor release (Fig. 1 B). This occurred upon Clb2p decrease from the 90- to 105-min time point (Fig. 1 C). The low percentage of cells exhibiting Chs2p-YFP signals was likely caused by the dynamics of Chs2p localization to the neck and its transport away for destruction shortly after. Indeed, from at least 10 time-lapsed sequences with 2-min intervals, it can be seen that Chs2p normally disappeared around 8 min (usually four frames after arriving at the neck) after going to the neck (unpublished data), which was in agreement with a previously published study (Roh et al., 2002).
Figure 1.
Chs2p-YFP localization to the mother–daughter neck in wild-type cells correlates with exit from mitosis. (A) Wild-type cells carrying CHS2P-YFP MYO1P-CFP were released from α-factor arrest. Samples were collected every 15 min for microscopy and Western blot analysis. (B) Percentage of cells showing Chs2p-YFP signals colocalizing with Myo1p-CFP neck signals. (C) Western blot analysis showing Clb2p and Cdc28p levels. (D) Chs2p-CFP translocated to the neck only when Clb2p-YFP levels were low. Images of CLB2-YFP CHS2-CFP cells released from HU arrest. (E) Percentage of cells showing Chs2p-CFP and Clb2-YFP signals. Error bars represent SD. (F) A typical montage of time-lapsed images at 2-min intervals showing Chs2p-CFP neck localization and Clb2p-YFP signals at 24°C. 0 min refers to 90 min after the release from HU arrest.
We also examined in cells carrying CLB2-YFP CHS2-CFP (FM224) the relative timings of Clb2p destruction and Chs2p localization to the mother–daughter necks. The patterns of Clb2-YFP localization in our strain to the nuclei, spindle pole bodies (SPBs), spindles, and necks (Fig. 1 D) are consistent with previously published data (Hood et al., 2001; Bailly et al., 2003) indicating that Clb2p driven from its endogenous promoter with only a single YFP fused to its C-terminal can be used for our subsequent studies. To enrich for cells going through mitosis, we examined FM224 cells that were synchronized in S phase by hydroxyurea (HU) arrest and were then released. Chs2p-YFP signals appeared only in cells in which Clb2p-YFP signals were low such that SPB, spindle, and neck signals were not visible but had low signals in the nuclei (Fig. 1 D). The correlation between Clb2p-YFP levels (Clb2p-YFP signals were counted as cells with clearly visible signals in nuclei, SPBs, spindle, and neck) and Chs2p-CFP signals supports this observation (Fig. 1 E). Furthermore, in at least 10 time-lapsed sequences, Chs2p-CFP was detected at the neck only upon a decrease in Clb2p-YFP signal intensity (Fig. 1 F; compare the intensity of Clb2p-YFP signals at 0 and 4 min, when faint Chs2p-CFP signals first appeared at the neck). This observation was consistent with the fixed samples, indicating that the decrease in Clb2p-YFP signals was not caused by bleaching during time-lapsed microscopy. Therefore, Chs2p neck localization correlates strongly with mitotic exit. This finding concurs with previous studies showing Chs2p neck localization in telophase (Chuang and Schekman, 1996; VerPlank and Li, 2005).
Localization of Chs2p at the neck is downstream of Cdc15p and depends solely on Cdc15p's function in promoting exit from mitosis
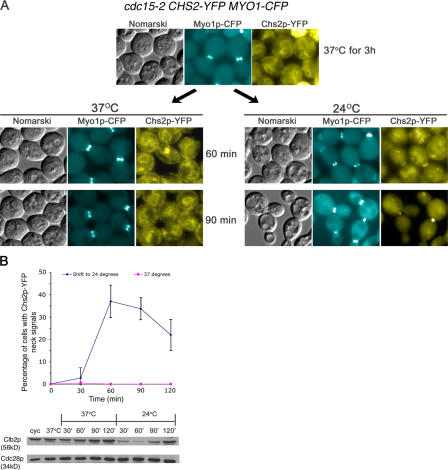
VerPlank and Li (2005) showed recently that Sec3p-GFP, a component of the exocyst complex, can be found at the mother–daughter neck 3.90 ± 1.90 min before spindle breakdown and that its localization to the neck depends on mitotic exit. Proper Chs2p neck localization depends on Sec10p, another component of the exocyst complex (VerPlank and Li, 2005). However, in a sec10 mutant, Chs2p was still able to translocate to a region near the neck, albeit forming a fuzzy ring around the neck. These observations indicate that although Chs2p localization to a discrete band at the plasma membrane of the neck requires the exocyst complex, the regulation of Chs2p transport itself to the neck depends on determinants other than the exocyst complex. Given our aforementioned data showing a strong correlation between mitotic exit and Chs2p neck localization (Fig. 1), we tested whether Chs2p localization to the neck was dependent on mitotic exit in the temperature-sensitive mutant cdc15-2 carrying CHS2-YFP MYO1-CFP (FM113). At 24°C, Chs2p-YFP in the cdc15-2 cells exhibited similar dynamics as in wild-type cells (unpublished data). When cdc15-2 cells (FM113) were arrested at 37°C in telophase, the cells could maintain Myo1p-CFP rings at the necks, although they were devoid of Chs2p-YFP neck signals (Fig. 2 A, top). When released from 37 to 24°C, a peak of 33.67 ± 4.93% (Fig. 2 B, top) of the cells exhibited Chs2p-YFP signals at the neck (Fig. 2 A, right; 60 min), upon Clb2p decrease (Fig. 2 B, bottom). This indicated that the translocation of Chs2p-YFP to the neck is an event downstream of Cdc15p function.
Figure 2.
Localization of Chs2p-YFP is downstream of Cdc15p function and depends on exit from mitosis. (A) cdc15-2 CHS2-YFP MYO1-CFP cells were arrested at 37°C. (left) cdc15-2 CHS2-YFP MYO1-CFP cells maintained at 37°C. (right) cdc15-2 CHS2-YFP MYO1-CFP cells released from 37 to 24°C. (B) Percentage of cells showing Chs2p-YFP neck signals when maintained at 37°C compared with cells released to 24°C. (bottom) Western blot analysis showing Clb2p and Cdc28p levels from cycling (cyc) cells and cells at selected time points after release from arrest at 37°C. Error bars represent SD.
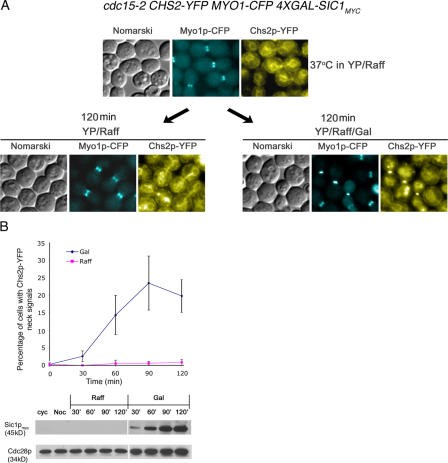
To directly test the role of mitotic exit in translocating Chs2p-YFP to the neck, cdc15-2 CHS2-YFP MYO1-CFP 4XGAL-SIC1myc cells (FM145) were cultured at 37°C in yeast extract peptone (YP)/raffinose (Raff) for 3 h to arrest the cells in telophase with high mitotic kinase. Galactose (Gal) was added to one half of the culture to induce Sic1p, whereas the other half remained in Raff. In the absence of Cdc15p function, Chs2p-YFP signals were detected at the necks (Fig. 3 A, right) only after Sic1p induction (Fig. 3 B, bottom). As there was a slight difficulty in triggering mitotic exit via Sic1p induction at 37°C (unpublished data), we saw a peak of 23.49 ± 7.71% of the Gal culture with Chs2p-YFP signals at the neck around 90 min (Fig. 3 B, top). Nonetheless, this was significantly higher than 0.61 ± 0.54% of the cells in the Raff culture with Chs2p-YFP neck signals (Fig. 3 B, top; 90 min). Our findings show that the localization of Chs2p to the neck does not depend on an active MEN component such as Cdc15p except for its role in triggering mitotic exit.
Figure 3.
Chs2p-YFP localizes to the mother–daughter neck in telophase-arrested cells overexpressing Sic1p. (A) cdc15-2 CHS2-YFP MYO1-CFP GAL-SIC1myc cells arrested at 37°C. Cells were maintained at 37°C without Gal addition (left) or with Gal (right) for 120 min. (B) Percentage of cells showing Chs2p-YFP neck signals in Raff versus Gal cultures. (bottom) Western blot analysis showing Sic1p induction and Cdc28p levels. Error bars represent SD.
Mitotic kinase reduction in metaphase can result in premature localization of Chs2p to the neck and chitin deposition
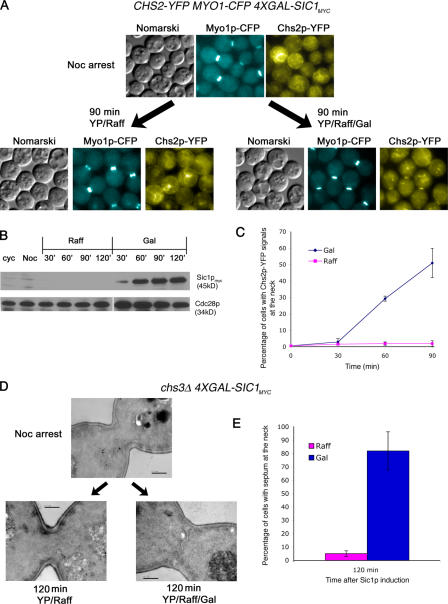
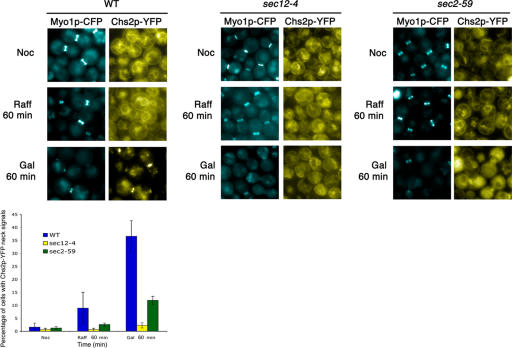
To further assess the role of mitotic kinase in the localization of Chs2p to the neck, we next tested whether the inactivation of mitotic kinase at a point when Chs2p normally does not localize to the neck can prematurely send Chs2p to the neck. We arrested CHS2-YFP MYO1-CFP 4XGAL-SIC1MYC (FM143) cells at metaphase in YP/Raff containing nocodazole (Noc) at 24°C. Once the cells were arrested, half of the culture was maintained in YP/Raff, whereas Gal was added to a final concentration of 2% in the other to induce Sic1p expression (Fig. 4 B). Interestingly, the cells arrested in Noc (Fig. 4 A, top) and telophase (Figs. 2 and 3) have a characteristic ringlike appearance, probably as a result of Chs2p-YFP's localization at the perinuclear ER (Chuang and Schekman, 1996; see next section). After 90 min of Sic1p induction (Fig. 4 B), Chs2p-YFP localized to the mother–daughter necks at a peak of 51.89 ± 8.89% of the cells (Fig. 4 C). This is significantly higher than that in the control culture, where only 2.00 ± 1.73% of cells exhibited Chs2p-YFP signals at the necks. That Chs2p-YFP can be sent to the neck prematurely at metaphase upon Sic1p induction clearly indicates that mitotic exit was indeed sufficient to cause the translocation of Chs2p to the neck.
Figure 4.
Premature localization of Chs2p-YFP at metaphase depends solely on exit from mitosis. (A) CHS2-YFP MYO1-CFP GAL-SIC1myc cells were maintained in Noc without Gal (left) or with Gal (right) induction of Sic1p for 90 min. (B) Western blot analysis showing Sic1p and Cdc28p levels. (C) Percentage of cells showing Chs2p-YFP neck signals. (D) Transmission electron micrographs showing that prematurely localized Chs2p is active for chitin deposition. chs3Δ 4xGAL-SIC1myc cells arrested at 24°C in Noc were maintained in Raff or with the addition of Gal. Bars, 0.5 μm. (E) Percentage of cells with septum deposition. 40 cells were counted for each time point for three experiments. Error bars represent SD.
Although it was possible to cause Chs2p to translocate to the neck by triggering mitotic exit in either telophase or metaphase (Figs. 3 and 4 A, respectively), it was not clear whether the prematurely localized Chs2p was in fact active at the neck. Using transmission EM, we examined the ultrastructural morphology of cells in which Sic1p was induced to assess whether Chs2p that prematurely localized can deposit a septum between the mother and daughter cells. Chs3p was previously shown to be involved in the formation of a remedial septum in the absence of proper Myo1p ring constriction (Schmidt et al., 2002; Cabib and Schmidt, 2003). To preclude the possible contribution of Chs3p in laying down a remedial septum in metaphase when the Myo1p ring is not known to undergo constriction, we generated a chs3Δ GAL-SIC1MYC strain (FM295). In experiments analogous to those described in the previous paragraph, FM295 cells were arrested in metaphase with Noc followed by the induction of Sic1p in one half of the culture, whereas the other half remained uninduced. In the Noc-arrested cells, the neck region was generally broad, with no septum laid across the neck (Fig. 4 D, top). In cells in which Sic1p was induced, electron-lucent septa formed across mother–daughter necks compared with the Raff control (Fig. 4 D, bottom; compare 120 min of Raff with Gal). 82.02 ± 14.31% of Sic1p-induced cells in three independent experiments had the thin septa compared with 5.11 ± 2.06% of uninduced cells (Fig. 4 E). Our results indicate that Sic1p induction during metaphase led to the deposition of chitin between the mother and daughter cells, most likely as a result of the premature neck localization of Chs2p (Fig. 4 A).
Chs2p requires Sec12p-mediated export from the ER
From our aforementioned data, Chs2p-YFP in telophase- (Figs. 2 and 3) and metaphase-arrested cells (Fig. 4) localized to perinuclear structures before its neck localization. To determine the localization of Chs2p before its neck localization, we examined wild-type CHS2-YFP SEC63-CFP MYO1-CFP cells (FM182) arrested in metaphase. Chs2p-YFP signals colocalized with the perinuclear signals of Sec63p-CFP (Fig. 5 A, Noc), an ER marker (Sadler et al., 1989). This is consistent with immunofluorescence experiments showing perinuclear staining of Chs2p with anti-Chs2p antibodies (Chuang and Schekman, 1996). Upon release from Noc, Chs2p-YFP signals dispersed from the ER and could be seen at the neck (Fig. 5 A, 100 min; and B). To exclude the possibility that the Chs2p-YFP we observed in Noc-arrested cells was an artifact of the metaphase arrest, we next tested whether Chs2p-YFP normally can be observed in the ER during progression through the cell division cycle. It was not easy to detect Chs2p-YFP in the ER in cells progressing through the cell division at 24°C, although the neck signals could be found in a few cells (unpublished data). This was most likely caused by the dynamic nature of Chs2p synthesis and export from the ER. To slow down the cell cycle, we first synchronized FM182 cells in S phase using HU, which is much earlier than metaphase, and then released the cells into YPD at the lower temperature of 16°C. As cells were released from the arrest, they progressed into the cell cycle at 16°C with an accumulation of cells with Chs2p-YFP signals that colocalized with Sec63p-CFP 270 min after the release (Fig. 5 A, bottom), confirming that in the normal cell cycle progression, Chs2p did in fact translocate into the ER and, thereafter, localized to the neck.
Figure 5.
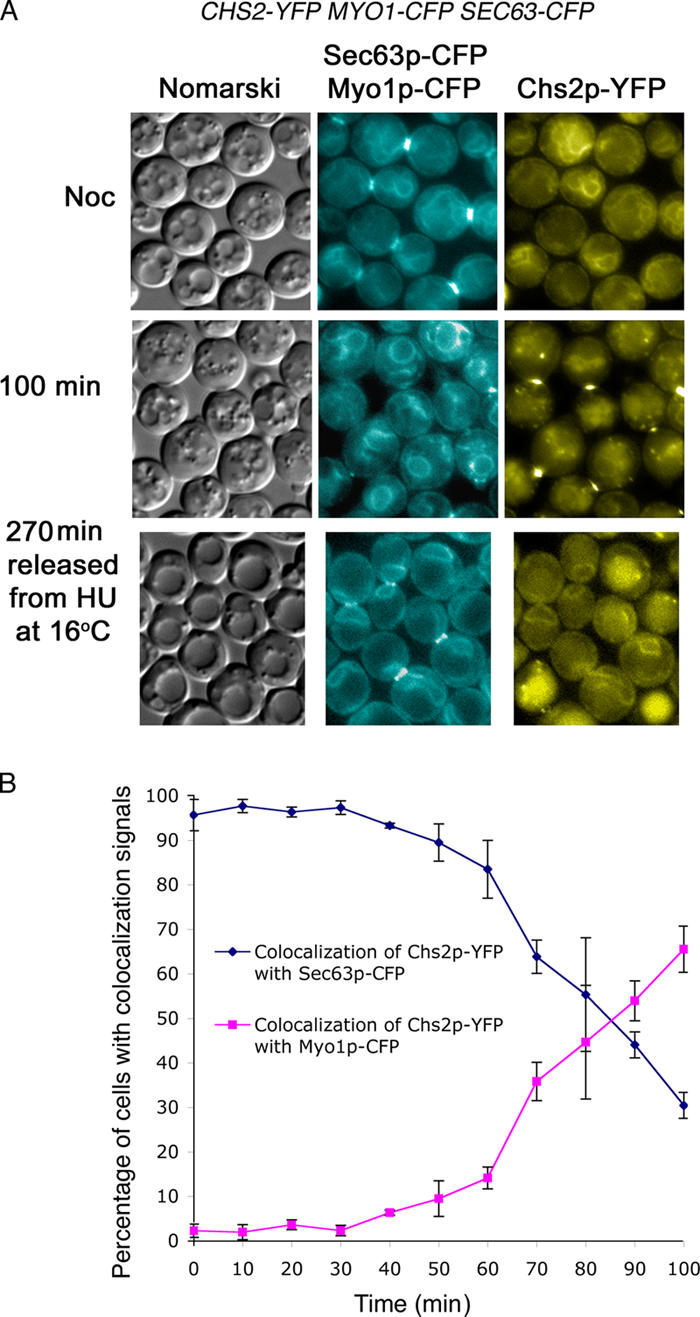
Chs2p-YFP is localized to the ER in metaphase. (A, top) CHS2-YFP SEC63-CFP MYO1-CFP cells were arrested in Noc at 24°C. (middle) Cells at 100 min after Noc release into 24°C. To observe the ER signals outside a Noc arrest, cells were synchronized with HU and released into 16°C for 270 min. (B) Percentage of cells showing Chs2p-YFP signals that colocalized with either Sec63p-CFP or Myo1p-CFP signals after Noc release into 24°C. Error bars represent SD.
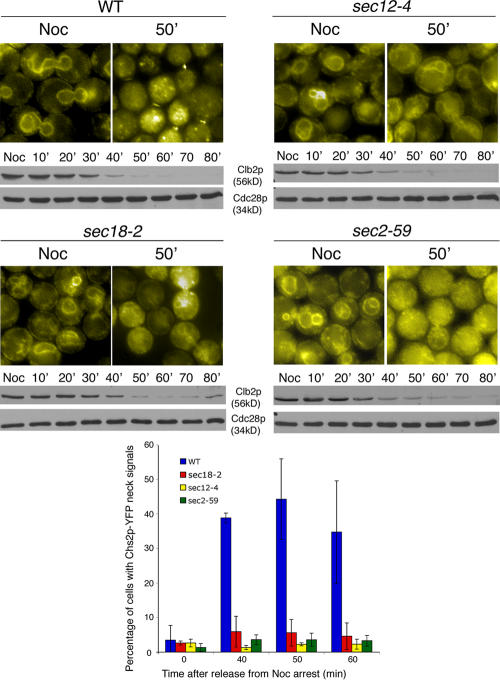
To further characterize the transport of Chs2p from the ER to the neck, various mutant strains of the secretory pathway were used (Bonifacino and Glick, 2004). Temperature-sensitive mutants sec12-4, sec18-4, and sec2-59 together with the wild-type strain each carrying CHS2-YFP MYO1-CFP were generated. Cells were released into YPD at 37°C from a Noc arrest. Chs2p-YFP localized to the perinuclear ER at the Noc-arrested stage in all of the strains (Fig. 6, top). Chs2p-YFP signals at the neck in wild-type cells (FM119) peaked at 44.26 ± 11.67% (Fig. 6, bottom) around 50 min after release from Noc arrest. This coincided with mitotic exit, as judged by the decrease in Clb2 (Fig. 6, wild type [WT] at 50 min). However, the sec mutants that were treated similarly failed to transport Chs2p-YFP to the neck, although Clb2 levels had decreased. In sec12-4 cells (FM262), the loss of function of the GTP exchange factor required for budding of COPII vesicles prevented Chs2-YFP from exiting the ER, as evident from the perinuclear YFP signals (Fig. 6, sec12-4 at 50 min). In sec18-2 (FM260), some Chs2p-YFP signals were found as spots in the cytoplasm, whereas some Chs2p-YFP remained in the ER (Fig. 6, sec18-2 at 50 min), perhaps because of the fact that Sec18p is needed for the recycling of membranes to the ER for the continued export of Chs2p from the ER. In sec2-59 cells (FM258), only a peak of 3.56 ± 1.40% of cells exhibited Chs2p-YFP neck signals (Fig. 6, bottom). However, Chs2p-YFP signals had dispersed from the ER, giving rise to a punctate appearance (Fig. 6, compare sec12-4 at 50 min with sec2-59 at 50 min). This suggested that Chs2p-YFP had exited from the ER but that the transport vesicles carrying Chs2p-YFP through the secretory pathway failed to fuse to the plasma membrane at the neck. Although it had previously been hinted at by biochemical analysis (Chuang and Schekman, 1996) that Chs2p enters the ER during synthesis, we show that Chs2p colocalized with Sec63p, an ER marker during mitosis. Furthermore, Chs2p depends on the secretory pathway for its transport from the ER to the neck during mitotic exit, which is consistent with previous data (Chuang and Schekman, 1996; VerPlank and Li, 2005).
Figure 6.
Chs2p-YFP neck localization depends on the secretory pathway. Wild-type (WT) and sec mutant cells were arrested in Noc and released into 37°C. (top) Images show Chs2p-YFP and corresponding Western blot analyses for Clb2p and Cdc28p levels. (bottom) Percentage of cells showing Chs2p-YFP neck signals at selected time points after release from Noc. Error bars represent SD.
To test whether the mitotic exit directly impinges upon the secretory pathway with respect to Chs2p transport to the neck, wild-type, sec12-4, and sec2-59 strains each carrying CHS2-YFP MYO1-CFP 4XGAL-SIC1myc were arrested in Noc in YP/Raff at 24°C. Each culture was then shifted to 37°C to inactivate the mutant sec components, and Sic1p was induced in one half of the culture for 60 min. At 37°C in the presence of Sic1p, the Chs2p-YFP signals could be detected at the neck (Fig. 7, top left) in 36.67 ± 6.03% of wild-type cells (FM143) compared with 9.00 ± 6.08% in control cells (Fig. 7, bottom). Although the inactivation of mitotic kinase activity was relatively inefficient at 37°C, the data are consistent with our aforementioned findings showing Chs2p localizing to the neck upon exit from mitosis (Figs. 1–4 ).
Figure 7.
Induction of Sic1p fails to cause neck localization in secretory pathway mutants. (top) Wild-type, sec12-4, or sec2-59 cells carrying CHS2-YFP MYO1-CFP GAL-SIC1myc cells were maintained in Noc with (Gal) or without Gal induction (Raff) of Sic1p for 60 min at 37°C. (bottom) Percentage of cells showing Chs2p-YFP neck signals. Error bars represent SD.
In sec12-4 mutants (FM272), the induction of Sic1p failed to cause the transport of Chs2p to the neck, as only 2.33 ± 1.53% of cells showed neck signals after Gal addition compared with 0.67 ± 0.58% in the Raff culture (Fig. 7, bottom). Consistent with the role of Sec12p in activating the assembly of COPII components (Bonifacino and Glick, 2004), the FM272 cells exhibited mostly ER signals even in the presence of Sic1p induction (Fig. 7, top middle). In sec2-59 (FM268) cells, there were 12.00 ± 2.00% of cells with neck signals upon Gal induction as compared with 2.67 ± 0.58% without (Fig. 7, bottom), although some of the Chs2p-YFP signals at the neck appeared diffused (not depicted). This could be caused by the incomplete inactivation of sec2-59p during the early part of Gal induction, leading to the translocation of some of the vesicles to a region near the neck (VerPlank and Li, 2005). However, unlike the sec12-4 cells, the sec2-59 cells in the Gal culture exhibited punctate staining and a decrease in clear Chs2p-YFP ER signals (Fig. 7, top right), indicating that Chs2p-YFP had exited from the ER upon a decrease in mitotic kinase but that Chs2p-YFP was trapped in vesicles as a result of the sec2-59 mutation. Our data showing that Sic1p-induced Chs2p-YFP neck localization was abolished in a sec12-4 mutant supported the notion that the mitotic exit normally served to trigger the export of Chs2p from the ER in a Sec12p-dependent manner.
Mitotic kinase destruction is necessary for triggering Sec12p-mediated exit of Chs2p from the ER
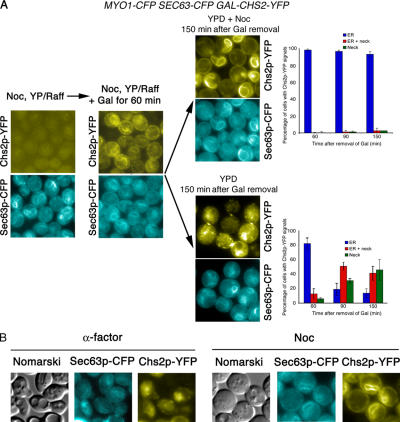
The experiments with Sic1p-induced mitotic exit suggested that Chs2p was retained in the ER during mitosis and continues along the secretory pathway only upon mitotic exit. However, an alternative interpretation is possible. CHS2 expression strongly peaks during mitosis (Choi et al., 1994). Thus, cells arrested in metaphase or in telophase could be overexpressing CHS2, and so the accumulation of Chs2p in the ER may be an artifact of overproduction. For example, it was previously shown that Chs3p, when overexpressed, remained in the ER unless the chaperone Chs7p was coinduced (Trilla et al., 1999; Kota and Ljungdahl, 2005). To exclude this possibility, we first examined the Chs2p-YFP ER signals after a limited induction of Chs2p-YFP in a wild-type strain carrying an integrated GAL-CHS2-YFP construct (FM317). A short pulse of CHS2-YFP expression from the GAL promoter would show a time-dependent decrease in the intensity of Chs2p-YFP signals in the ER if indeed there were continued export of Chs2p out of the ER in metaphase. FM317 cells were arrested in Noc and Gal induction performed for 1 h.
Fig. 8 A shows that after 60 min of Gal induction, Chs2p-YFP signals could be seen. Subsequently, the induction was shut off by washing half of the culture into YPD with Noc and the other half into YPD without Noc. At 60 min after Gal shut-off and Noc release, 82.00 ± 8.18% of cells had ER signals, but, after 150 min, only 13.33 ± 6.11% of the cells exhibited ER signals (Fig. 8 A, bottom right). Furthermore, these cells that were released from Noc showed punctate spots (Fig. 8 A, bottom right), which were most likely Chs2p being transported away from the neck in vesicles at the end of cytokinesis (Roh et al., 2002; Tolliday et al., 2003). However, in cells cultured continuously in the presence of Noc, 93.33 ± 3.06% of them exhibited characteristic ER signals even after 150 min of Gal shut-off and were devoid of the punctate spots (Fig. 8 A, top right). This observation supported the idea that Chs2p-YFP was retained in the ER in Noc-arrested cells.
Figure 8.
A limited pulse of Chs2p-YFP is retained in the ER in Noc-arrested cells, indicating an absence of constitutive Chs2p export. (A, left) Wild-type cells carrying GAL-CHS2-YFP SEC63-CFP were arrested in Noc, after which the cells were pulsed with Gal for 60 min. One half of the culture was washed to remove Noc, and the other half was maintained in Noc. (right) Percentage of cells showing Chs2p-YFP neck, ER or neck, and ER signals at selected time points after Gal removal. (B) CHS2-YFP driven from the GAL promoter is constitutively exported out of the ER in G1 cells. (left) Wild-type cells were arrested in G1 with α-factor and Chs2p-YFP induced from the GAL promoter. (right) Control cells are arrested in Noc, and Chs2p-YFP was induced similarly. Error bars represent SD.
Our data showing the accumulation of Chs2p in the ER in the presence of high mitotic kinase and the export of Chs2p from the ER upon mitotic exit would predict that the induction of Chs2p-YFP expression in α-factor–arrested cells in which the mitotic kinase is at the lowest would lead to the unrestrained export of Chs2p. To test this, FM317 cells were arrested in α-factor, and Chs2p-YFP was induced with a short pulse of Gal addition followed by shut-off by washing the cells in YPD. It was observed that several of the α-factor–arrested cells exhibited punctate signals in the cytoplasm that did not colocalize with Sec63p-CFP (Fig. 8 B, left). These were presumably Chs2p-YFP that had been induced but that was not retained in the ER. These spots were unlike the characteristic ER localization in the control cells that were arrested in Noc (Fig. 8 B, right). The spots in the cytoplasm in G1 cells were confirmed to have originated from the ER using a sec12-4 SEC63-CFP GAL-CHS2-YFP∷TRP strain (FM355; unpublished data). However, not all cells showed the cytoplasmic spots or Chs2p-YFP signals, indicating that perhaps the induction in G1 was not as uniform as in metaphase (Fig. 8 B, left). Nevertheless, collectively, our data showed that unlike in metaphase, the expression of Chs2p-YFP in G1 cells with low mitotic kinase led to unrestrained export from the ER.
We next wanted to confirm that Chs2p-YFP was not in fact slowly exiting the ER in Noc-arrested cells. This is because a decrease in mitotic kinase activity is necessary for targeted exocytosis to the neck (Pruyne and Bretscher, 2000), and, as such, if there were some Chs2p-YFP exiting from the ER in metaphase-arrested cells, the Chs2p-YFP could be delocalized and difficult to detect because of the absence of polarized transport. To eliminate this possibility, a sec2-59 CHS2-YFP MYO1-CFP (FM258) strain was arrested in Noc at 24°C, after which half of the culture was shifted to 37°C after washing out Noc. The other half was shifted to 37°C in the continued presence of Noc. If indeed Chs2p was continuously transported out of the ER during the metaphase arrest, the Chs2p-YFP signals from the ER would diminish, and the signals would be observed as punctate spots in the culture at 37°C in the continued presence of Noc as a result of the block at the sec2-59 stage.
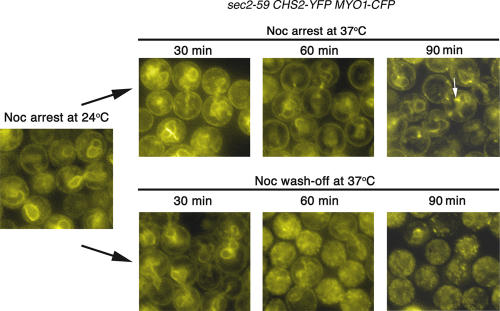
As can be seen in Fig. 9, in the control culture in which Noc was washed off at 37°C to allow for mitotic exit, a punctate appearance in the sec2-59 cells was evident at 60 min with a concomitant loss of ER signals (Fig. 9, bottom). By 90 min, most of the ER signals were no longer obvious, but punctate spots could be seen instead (Fig. 9, bottom). In the 37°C culture in which Noc was present throughout, ER signals were obvious (Fig. 9, top). This implied that there was no continuous export of Chs2p from the ER but that it was restrained in the ER during metaphase arrest. Intriguingly, there appeared to be areas of more intense YFP signals in the sec2-59 cells maintained at 37°C with Noc (Fig. 9, top; arrow). These spots of intensity were different from the punctate spots seen in the culture released from Noc (Fig. 9, bottom). They were unlikely to be localized in the Golgi compartments, as the patterns of localization did not coincide with Golgi markers such as Och1p-CFP or Sec7p-CFP (Fig. S1, top; available at http://www.jcb.org/cgi/content/full/jcb.200604094/DC1). However, these spots appeared to partially colocalize with Sec13p-CFP when the spinning disk confocal was used during image acquisition (Fig. S1, bottom right). This would suggest that Chs2p-YFP was accumulating, perhaps in potential ER exit sites where Sec13p had docked. The reason for the appearance of such spots in the sec2-59 background is unknown to us presently. Control experiments performed similarly with the wild-type strain (FM119) showed that cells maintained in the continued presence of Noc exhibited strong ER signals at 37°C throughout 90 min, whereas cells with Noc washed out had diminished ER signals with the appearance of neck signals at 30 min after Noc release. By 90 min, most of the ER signals had disappeared (Fig. S2). Collectively, these observations further confirmed the notion that Chs2p-YFP indeed remained in the ER during metaphase arrest.
Figure 9.
sec2-59 cells arrested in Noc retained Chs2p-YFP in the ER at 37°C. sec2-59 cells were arrested in Noc at 24°C and shifted to 37°C with Noc (top) or shifted to 37°C after washing off Noc (bottom). Areas of intense Chs2p-YFP signals could be observed in cells shifted to 37°C with Noc (top, arrow; see Results for details).
Mitotic kinase specifically restrains the export of Chs2p from the ER in metaphase but not other cargoes such as invertase
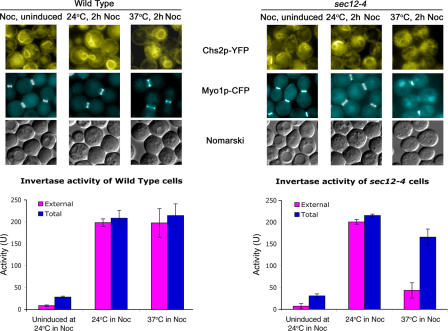
It had previously been documented that other cargoes of the secretory pathway such as α-factor and invertase are continuously transported via the secretory pathway during mitosis (Makarow, 1988; Nevalainen et al., 1989). To examine whether the regulation of Chs2p exit from the ER at metaphase was indeed specific to Chs2p in our set-up, we examined Chs2p-YFP localization and invertase activity in Noc-arrested cells. Both wild-type (FM119) and sec12-4 (FM262) cells were arrested in Noc in YPD, after which the cells were shifted to 0.05% glucose medium in the presence of 2% sucrose and Noc to induce invertase at metaphase. Fig. 10 (top left) shows Chs2p-YFP ER signals in the metaphase cells while invertase continued to be transported out of the ER, as observed from the periplasmic invertase activity (Fig. 10, bottom left; external). Transport of invertase to the periplasm was disrupted during metaphase only if SEC12 was inactivated (Fig. 10, bottom right; external at 37°C). Our data indicated that the high mitotic kinase in metaphase specifically retained Chs2p in the ER but not other cargoes of the secretory pathway such as invertase.
Figure 10.
Chs2p-YFP is restrained in the ER, but invertase is continuously secreted in metaphase cells. (top) Chs2p-YFP images showing ER localization of Chs2p-YFP in wild-type (left) and sec12-4 (right) cells. Cells were arrested in Noc at 24°C in YPD. After the arrest, invertase was induced by washing cells into YP/0.05% Glu/2% sucrose + Noc at either 24 or 37°C for 2 h. 24°C, 2 h Noc refers to Noc-arrested cells with 2 h of invertase induction at 24°C. 37°C, 2 h Noc refers to Noc-arrested cells with 2 h of invertase induction at 37°C. (bottom) Periplasmic (external) and total (total) invertase activities in wild-type (left) and sec12-4 (right) cells. Error bars represent SD.
Discussion
The timing of cytokinesis and septum formation in budding yeast, such that they occur late in mitosis, ensures that the mother and daughter cells are partitioned only after mitotic events are completed. For the completion of cytokinesis, cells from different organisms, including plant and higher eukaryotes, depend on membrane trafficking (Finger and White, 2002; Schweitzer and D'Souza-Schorey, 2004; Strickland and Burgess, 2004; Jurgens, 2005). The evidence in budding yeast suggests that secretory vesicles are targeted to the mother–daughter neck late in mitosis (Finger et al., 1998; VerPlank and Li, 2005), perhaps for the delivery of membranes and proteins such as Chs2p (VerPlank and Li, 2005). The secretory vesicles are associated with the class V myosin, Myo2p, tropomyosin, Tpm1p, and Tpm2p, all of which are needed for transporting the vesicles along the actin cables to their destinations (Finger and Novick, 1998; Pruyne et al., 2004). It is the orientation of actin cables that directs the secretory vesicles to their destinations (Finger and Novick, 1998; Pruyne et al., 2004). Indeed, the actin cytoskeleton, which is reoriented toward the neck at the end of mitosis (Pruyne and Bretscher, 2000), helps guide the post-Golgi secretory vesicles to the target membrane at the neck (Finger and Novick, 1998; Pruyne and Bretscher, 2000). Furthermore, the presence of the exocyst complex on the target membrane facilitates the docking of the vesicles onto the destination (TerBush et al., 1996; Guo et al., 1999) during targeted transport.
It is not clear what binds the timing of orientation of the actin cables toward the neck to the arrival of the exocyst complex at the neck at the end of mitosis such that secretory vesicles dock at the neck in time for cytokinesis (Finger and Novick, 1998). However, the orientation of the actin cables toward the neck (Pruyne and Bretscher, 2000) and the localization of Sec3p, one of the components of the exocyst complex (TerBush et al., 1996), to the mother–daughter neck upon mitotic exit (VerPlank and Li, 2005) point to the possibility that the status of the mitotic kinase activity contributes to the direction and timing of the arrival of the post-Golgi vesicles through its effects on both actin reorganization and Sec3p localization.
In this study, we investigated the regulation of Chs2p that could account for its timely arrival at the mother–daughter neck during cytokinesis. The precision needed for its timely neck localization reflects the role of Chs2p not just for primary septum deposition (Cabib et al., 2001; Klis et al., 2002) but also for successful cytokinesis. It has been shown that Chs2p translocates to the neck before Myo1p ring constriction (Schmidt et al., 2002), and a chs2Δ leads to abnormalities in Myo1p ring constriction (Schmidt et al., 2002). The basis of this requirement was recently revealed when it was found that a failure to transport Chs2p to the neck leads to a loss of Myo1p ring integrity during cytokinesis (VerPlank and Li, 2005). We found that Chs2p-YFP, a cargo of the secretory pathway (Fig. 6; Chuang and Schekman, 1996; VerPlank and Li, 2005), was regulated at the level of export from the ER (Figs. 6–10 ). This would imply that as cells progressed through metaphase to telophase and the mitotic kinase declined, Chs2p is triggered to exit from the ER via the COPII secretory pathway (Fig. 6). This, coupled with actin reorganization (Pruyne and Bretscher, 2000) and the arrival of Sec3p at the neck ∼5 min before cytokinesis (VerPlank and Li, 2005), leads to the localization of Chs2p to the neck 1 min after the exocyst complex (VerPlank and Li, 2005).
Our observations, which place the export of Chs2p from the ER as being contingent upon mitotic exit (Figs. 3 and 4), could explain the fact that Chs2p, which is transcribed in G2/M phase (Pammer et al., 1992; Cho et al., 1998; Spellman et al., 1998) and is synthesized in the ER in metaphase (Fig. 5 A), is localized to the neck only late in mitosis (Fig. 1; Chuang and Schekman, 1996; Schmidt et al., 2002; VerPlank and Li, 2005). The significance of the exact timing of Chs2p neck localization late in telophase is underscored by our finding that Sic1p overexpression in metaphase resulted in the premature Chs2p neck localization (Fig. 4 A) and deposition of chitin between the mother and daughter cells (Fig. 4 D). With respect to the activity of Chs2p, Sburlati and Cabib (1986) had previously shown that Chs2p is isolated in a zymogen form that requires partial proteolysis for activation in vitro. However, Uchida et al. (1996) found that Chs2p is in fact active in vitro before protease treatment, indicating that Chs2p may well be functional as an active form and that it is hyperactive upon proteolytic treatment. In vivo, it is not known whether Chs2p is activated before it reaches the mother–daughter neck, as no activators have been isolated to date. Our data showing chitin deposition between mother and daughter cells upon Sic1p induction (Fig. 4 D) would mean that Chs2p was active at the neck in the presence of low mitotic kinase, albeit in metaphase. Whether its activation occurred along its passage to the neck or only upon reaching the neck is unknown. Nonetheless, our study further extends the previous proposal that Chs2p function is regulated posttranslationally by synthesis and degradation (Choi et al., 1994). Indeed, an additional layer of control at Chs2p export from the ER, which is dependent on mitotic exit, is perhaps critical for ensuring that septum formation only occurs upon the completion of sister chromatid segregation and late mitotic events.
An interesting point to note from this experiment is that complete septa were formed across the mother–daughter neck in metaphase cells expressing Sic1p (Fig. 4 D, 120 min of YP/Raff/Gal). As the closure of a primary septum depends on concomitant actomyosin ring contraction (Schmidt et al., 2002; Tolliday et al., 2003), this would imply that actomyosin ring constriction had in fact occurred at metaphase. Indeed, we had observed Myo1p-CFP ring constriction in the Noc-arrested cells in which Sic1p was overexpressed (unpublished data). This could either be caused by the direct effect of the decrease in mitotic kinase or by the reduction in mitotic kinase that leads to the localization of MEN components to the neck, where they promote cytokinesis (for review see Balasubramanian et al., 2004). The tight coordination between mitotic exit, chitin deposition, and cytokinesis together with the interdependence of chitin deposition and actomyosin ring constriction underpin the proper partitioning of mother and daughter cells in a timely manner.
In mammalian cells, intracellular export from the ER is halted during mitosis, as evident from studies on the number of ER exit sites (Farmaki et al., 1999; Hammond and Glick, 2000). Cdc2 kinase has been implicated in the disassembly of ER exit sites in mammalian cells, thereby inhibiting the secretory pathway during mitosis (Kano et al., 2004). In the budding yeast, it remains to be seen how the export of Chs2p from the ER is affected by a decrease in mitotic kinase given that other cargoes such as invertase and α-factor are insensitive to mitotic kinase levels (Fig. 10; Makarow, 1988; Nevalainen et al., 1989). Interestingly, Chs2p has been found to be a potential substrate of the mitotic kinase (Loog and Morgan, 2005); however, the implication of that finding with respect to ER export has yet to be determined.
Materials and methods
Yeast culture reagents
Wild-type haploid W303a strain was used in this study. Cells were backcrossed to the W303a background at least three times. Cells were routinely grown in yeast extract peptone or selective medium supplemented with 2% dextrose at 24°C. For experiments requiring the Gal induction of Sic1p, cells were grown in yeast extract peptone supplemented with 2% Raff followed by the addition of Gal to a final concentration of 2%, unless otherwise stated.
Synchronization procedures
For experiments requiring synchronized cultures, exponential phase cells were diluted to 107 cells/ml in growth medium at 24°C and arrested using either α-factor at 0.4 μg/ml or HU at 0.2 M. After the cells were arrested, they were washed by filtration and resuspended in media at the required conditions as described in the various sections. For a typical Noc arrest, cells were first treated with 7.5 μg/ml Noc for 2 h followed by the further addition of 7.5 μg/ml for another 3 h. The drug was washed off by filtration or centrifugation of the cells. Cells were then sampled at intervals at the indicated times. Each experiment has been performed three times, and 100 cells were counted for each of the experiments unless otherwise stated. Graphs shown were plots of the mean values of three experiments with SD.
Strains and plasmids
A combination of standard molecular biology and molecular genetic techniques such as PCR-based tagging of endogenous genes and tetrad dissection were used to construct plasmids and strains with various genotypes (Table I). The plasmids for the CFP and YFP cassettes were obtained from EUROSCARF. A list of primers used for the tagging of endogenous genes with CFP or YFP cassettes is shown in Table II. Table III lists the primers used for checking the correct insertion of the CFP or YFP-tagged PCR products. Strains US1363 and US107 were provided by U. Surana (Institute of Molecular and Cell Biology, Singapore); strains RSY263, RSY279, and RSY319 were provided by R. Schekman (University of California, Berkeley, Berkeley, CA); and strain NY26 was provided by P. Novick (Yale University School of Medicine, New Haven, CT).
Table I.
List of strains
| Name | Genotype | Source |
|---|---|---|
| US1363 | MATa leu2-3,112 trp1-1 ura3-1 can1-100 ade2-1 his3-11,15 bar1Δ | U. Surana |
| US107 | MATα cdc15-2 leu2-3,112 trp1-1 ura3-1 can1-100 ade2-1 his3-11,15 bar1Δ | U. Surana |
| RSY263 | MATα sec12-4 ura3-52 his4-619 | R. Schekman |
| RSY319 | MATα sec18-2 | R. Schekman |
| RSY279 | MATα sec22-3 ura3-52 his4-619 | R. Schekman |
| NY26 | MATα sec2-59 ura3-52 | P. Novick |
| FM113 | MATa cdc15-2 CHS2-YFP-SpHIS5 a MYO1-CFP-CaURA3 b bar1Δ | This study |
| FM119 | MATa CHS2-YFP-SpHIS5 MYO1-CFP-CaURA3 bar1Δ | This study |
| FM143 | MATa CHS2-YFP-SpHIS5 MYO1-CFP-CaURA3 4XGAL-SIC1-cmyc3∷URA3 bar1Δ | This study |
| FM145 | MATa cdc15-2 CHS2-YFP-SpHIS5 MYO1-CFP-CaURA3 4XGAL-SIC1-cmyc3∷URA3 bar1Δ | This study |
| FM182 | MATa SEC63-CFP-SpHIS5 CHS2-YFP-SpHIS5 MYO1-CFP-CaURA3 bar1Δ | This study |
| FM224 | MATa CLB2-YFP-SpHIS5 CHS2-CFP-SpHIS5 | This study |
| FM258 | MATa sec2-59 CHS2-YFP-SpHIS5 MYO1-CFP-CaURA3 | This study |
| FM260 | MATa sec18-2 CHS2-YFP-SpHIS5 MYO1-CFP-CaURA3 | This study |
| FM262 | MATa sec12-4 CHS2-YFP-SpHIS5 MYO1-CFP-CaURA3 | This study |
| FM268 | MATa sec2-59 CHS2-YFP-SpHIS5 MYO1-CFP-CaURA3 4XGAL-SIC1-cmyc3∷URA3 | This study |
| FM272 | MATa sec12-4 CHS2-YFP-SpHIS5 MYO1-CFP-CaURA3 4XGAL-SIC1-cmyc3∷URA3 | This study |
| FM274 | MATa sec18-2 CHS2-YFP-SpHIS5 MYO1-CFP-CaURA3 4XGAL-SIC1-cmyc3∷URA3 | This study |
| FM295 | MATa chs3Δ∷KAN 4XGAL-SIC1-cmyc3∷URA3 | This study |
| FM311 | MATa sec2-59 CHS2-YFP-SpHIS5 SEC63-CFP-SpHIS5 | This study |
| FM317 | MATa SEC63-CFP-SpHIS5 GAL-CHS2-YFP∷TRP1 | This study |
| FM338 | MATa sec2-59 CHS2-YFP-SpHIS5 SEC7-CFP-CaURA3 | This study |
| FM339 | MATa sec2-59 CHS2-YFP-SpHIS5 OCH1-CFP-CaURA3 | This study |
| FM355 | MATa sec12-4 GAL-CHS2-YFP∷TRP1 SEC63-CFP-SpHIS5 | This study |
| FM358 | MATa sec2-59 CHS2-YFP-SpHIS5 SEC13-CFP-CaURA3 | This study |
SpHIS5 refers to Schizosaccharomyces pombe.
CaURA3 refers to Candida albicans.
Table II.
List of primers for generating YFP or CFP fusions
| Name | Sequence |
|---|---|
| CHS2F5 | 5′-GTCTCTAAATTAGACTTACCAAATGTTTTCCACAAAAAGGGCGGTGACGGTGCTGGTTTA-3′ |
| CHS2R3 | 5′-AAAAGAGGGAATGACGAGAAATTAGCTGAAAAATACTGGCATCGATGAATTCGAGCTCG-3′ |
| MYO1F5 | 5′-AAATATTGATAGTAACAATGCACAGAGTAAAATTTTCAGTGGTGACGGTGCTGGTTTA-3′ |
| MYO1R3 | 5′-ATGCATATTCTCATTCTGTATATACAAAACATCTCATCATTCGATGAATTCGAGCTCG-3′ |
| SEC63F5 | 5′-TCTTGAGGGTAAATGGGAACCCGCTGGTGAAGTTCATCAGGGTGACGGTGCTGGTTTA-3′ |
| SEC63R3 | 5′-TATACGTTACTGTACATTATGCGTTTATTTCCCTTCAAATTCGATGAATTCGAGCTCG-3′ |
| SEC7F5 | 5′-ATTTCTAAGCAGAGTTGGTGAATTATACCTTTCTACTGATGGTGACGGTGCTGGTTTA-3′ |
| SEC7R3 | 5′-ACTAAGCATATTTTAATCTGCTGGACCATTCAACAAAGCCTCGATGAATTCGAGCTCG-3′ |
| OCH1F5 | 5′-GAGCTGGAAAGAAGATGCTGATAAAAATGCAGGTCATAAAGGTGACGGTGCTGGTTTA-3′ |
| OCH1R3 | 5′-AAATTTTATTTAGAGAGGGTATGATGAAAGGAGAGCCTCGTCGATGAATTCGAGCTCG-3′ |
| SEC13F5 | 5′-TCTTGAGGGTAAATGGGAACCCGCTGGTGAAGTTCATCAGGGTGACGGTGCTGGTTTA-3′ |
| SEC13R3 | 5′-TATACGTTACTGTACATTATGCGTTTATTTCCCTTCAAATTCGATGAATTCGAGCTCG-3′ |
| CLB2F5 | 5′-GGTTAGAAAAAACGGCTATGATATAATGACCTTGCATGAAGGTGACGGTGCTGGTTTA-3′ |
| CLB2R3 | 5′-CGATTATCGTTTTAGATATTTTAAGCATCTGCCCCTCTTCTCGATGAATTCGAGCTCG-3′ |
Table III.
List of primers for checking for the integration of YFP or CFP cassettes
| Name | Sequence | Oligoa No. |
|---|---|---|
| CHS2gfpchkfor | 5′-AGCTGCCTTTAGGGTGGTTG-3′ | 30 |
| CHS2gfpchkrev | 5′-AACAGTGCGCTCTCTACCCA-3′ | 31 |
| CLB2GFPCHKFOR | 5′-TACAGTCTCGAACTCTTGCC-3′ | 34 |
| CLB2GFPCHKREV | 5′-AGGCGCTAAAATTGGGTGTG-3′ | 35 |
| MYO1gfpchkfor | 5′-AGAAGCGAATTTGAGGAAGC-3′ | 38 |
| MYO1gfpchkrev | 5′-CTATCGAAGGATACGGGGTG-3′ | 39 |
| SEC63GFPCHKFOR | 5′-GGCCAGTGTTTCTCAAGATC-3′ | 67 |
| SEC63GFPCHKREV | 5′-GCCGGTAGAATAATTTCGCC-3′ | 68 |
| SEC13GFPCHKFOR | 5′-GGCCAGTGTTTCTCAAGATC-3′ | 98 |
| SEC13GFPCHKREV | 5′-GCCGGTAGAATAATTTCGCC-3′ | 99 |
| OCH1GFPCHKFOR | 5′-ACCCAAACCTAAACAAGAAC-3′ | 217 |
| OCH1GFPCHKREV | 5′-TAGTTTTACCACGTTTGAGC-3′ | 218 |
| SEC7GFPCHKFOR | 5′-AACGATGATGAGAAGAAGGC-3′ | 219 |
| SEC7GFPCHKREV | 5′-GAGGACCAAAATGGCTAAAC-3′ | 220 |
Oligonucleotide.
In brief, to make the GAL-CHS2-YFP construct, CHS2-YFP was synthesized by PCR from the ATG of CHS2 to the ADH terminator sequence from the pKT series of plasmids (Sheff and Thorn, 2004) using genomic DNA from FM182 as the template. The PCR fragment was cut using HindIII and BamHI, which were incorporated into the primers and ligated to the EcoRI–BamHI-cut GAL1-10 promoter and YIplac204 cut open with EcoRI and HindIII.
Cell extracts and Western blot analyses
Western blot analyses were performed as previously described (Yeong et al., 2000). Anti-Cdc28p antibodies (Santa Cruz Biotechnology, Inc.) and anti-myc antibodies (Santa Cruz Biotechnology, Inc.) were used at a 1:1,000 dilution, and anti-Clb2 antibodies (Santa Cruz Biotechnology, Inc.) were used at a 1:5,000 dilution. An enhanced chemiluminescence kit (Pierce Chemical Co.) was used according to the manufacturer's recommendations.
Invertase assay
The invertase assay was performed as previously described (Goldstein and Lampen, 1975) with several modifications. In brief, an overnight culture in YPD was arrested in Noc at 24°C in YPD, and invertase was induced by washing the cells into YP/2% sucrose/0.05% glucose for 2 h. At the appropriate time points, cells were collected and washed once in stop mix (10 mM NaN3 in 25 mM Tris-Cl, pH 7.4) before resuspending in 5.5 ml of fresh stop mix. 0.5 ml of the cell suspension was rested on ice for use in assaying the external invertase activity. To prepare the lysate for the total invertase activity, the rest of the cell suspension was spun down and resuspended in 2 ml spheroplast medium (1.4 M sorbitol, 0.1 M KPi, pH 7.5, and 5 mM NaN3), and 80 U lyticase (Sigma-Aldrich) was added (3.34 μl from a 10-mg/ml stock). Spheroplasting was performed at 37°C for 45 min. 3 ml of lysis buffer (1.67% Triton X-100) was then added to lyse the cells. For sucrose conversion, 40 μl of intact cells or total lysate was combined with 35 μl sodium acetate (0.1 M, pH 5.0) and 25 μl of 0.5 M sucrose (Sigma-Aldrich), and the mixture was incubated at 37°C for 20 min. 150 μl of 0.2 M K2HPO4 was then added, and the reaction was put on ice before boiling for 3 min. 750 μl of sterile water was added to make up to 1 ml, and, of this, 250 μl was used for the glucose assay. Essentially, 0.5 ml glucose assay reagent (Glucose Assay Kit; Sigma-Aldrich) together with o-Dionisidine (Sigma-Aldrich) was added, and the mixture was incubated at 37°C for 30 min. The reaction was stopped by adding 0.5 ml of 12 N H2SO4. The absorbance at 540 nm was then measured. To calculate the amount of glucose produced from sucrose, the following equation was used: milligrams of glucose = (ΔA540 of test) × (milligrams of glucose in standard)/(ΔA540 of standard). To calculate invertase activity, the following equation was used: activity = (milligrams of glucose/180) × 106 × 4 × (500/40)/20.
Fluorescence microscopy
Cells carrying CFP and YFP fusions were fixed briefly in KPF (Surana et al., 1993) and washed in PBS or observed directly without fixation after washing in PBS. Samples were observed using a microscope (IX81; Olympus), 60× NA 1.4 oil lens, and 1.5× Opitvar. Filter sets for the fluorescence proteins were purchased from Omega and Semrock, and images were captured using a CCD camera (CoolSnap HQ; Photometrics). Image acquisition was controlled by MetaMorph software (Molecular Devices). Typically, the exposure time for YFP was ∼0.8–1 s and for CFP was ∼0.6–0.8 s. In most experiments, unless otherwise stated, 100 cells were counted for each time point for three independent experiments, and the mean values were plotted with SD. For time-lapse microscopy, cells were mounted in complete minimal media, and images were captured at room temperature at 2-min intervals. Image J (National Institutes of Health) and Adobe Photoshop were used for production of the figures.
Transmission EM
Cells were pelleted and fixed overnight at 4°C in primary fixative (2% PFA, 2% glutaraldehyde, 40 mM phosphate buffer, and 0.5 mM magnesium chloride, pH 6.5). Postfixation of the cells was performed in 2% osmium tetroxide (2% osmium, 40 mM phosphate buffer, and 0.1% ferro-cyanide) for 1 h at room temperature. Subsequently, the cells were washed twice with 40 mM phosphate buffer and dehydrated using 50% ethanol for 20 min at room temperature followed by 75% ethanol overnight at 4°C and 95% ethanol for 20 min at room temperature. Another three changes of absolute ethanol for 30 min each at room temperature were performed. The cells were infiltrated using a series of ethanol–LVER (Low Viscosity Epoxy Resin) mixtures (7:3 followed by 5:5 and 3:7 ratios of ethanol/ LVER). The cells were left in the first and second embedding mixtures for 1 h at room temperature and then overnight in a 3:7 ethanol/ LVER mixture at room temperature. The cells were passed through three changes of pure embedding media for a period of 90 min at each change. After the third change, cells were resuspended in ∼200 μl LVER and transferred into BEEM capsules. The BEEM capsules were topped up with embedding media and spun at 4,000 rpm for 10 min at 40°C. Polymerization was allowed to occur overnight at 60°C. Ultrathin sections were obtained using an ultramicrotome (Ultracut; Leica), and the sections were stained with uranyl acetate for 20 min followed by lead citrate for 30 min. The sections were viewed using an electron microscope (JEM-1220; JEOL) at 100 kV.
Online supplemental material
Fig. S1 shows that sec2-59 cells in prolonged Noc arrest at 37°C exhibit intense Chs2p-YFP spots that do not colocalize with Sec7p-CFP or Och1p-CFP. Fig. S2 shows that wild-type cells arrested in Noc at 37°C exhibit Chs2p-YFP ER signals. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200604094/DC1.
Supplementary Material
Acknowledgments
We thank U. Surana and R. Schekman for strains, constructs, and antibodies and K. Thorn for sharing technical information on GFP transformation. We also thank Mary Ng and B.L. Tang for other reagents. We are grateful to the EM Unit (National University of Singapore) for technical advice on EM techniques and to U. Surana, P.Y. Goh, and Alice Tay for their critical reading of the manuscript. We deeply appreciate the constructive and helpful comments from two anonymous reviewers.
F.M. Yeong is funded by the Singapore BioMedical Research Council (grant 04/1/21/19/325). G. Zhang is a recipient of the National University of Singapore's graduate scholarship.
G. Zhang, R. Kashimshetty, and K.E. Ng contributed equally to this paper.
Abbreviations used in this paper: Gal, galactose; HU, hydroxyurea; MEN, mitotic exit network; Noc, nocodazole; Raff, raffinose; SPB, spindle pole body; YP, yeast extract peptone.
References
- Bailly, E., S. Cabantous, D. Sondaz, A. Bernadac, and M.N. Simon. 2003. Differential cellular localization among mitotic cyclins from Saccharomyces cerevisiae: a new role for the axial budding protein Bud3 in targeting Clb2 to the mother-bud neck. J. Cell Sci. 116:4119–4130. [DOI] [PubMed] [Google Scholar]
- Balasubramanian, M.K., E. Bi, and M. Glotzer. 2004. Comparative analysis of cytokinesis in budding yeast, fission yeast and animal cells. Curr. Biol. 14:R806–R818. [DOI] [PubMed] [Google Scholar]
- Bardin, A.J., and A. Amon. 2001. Men and sin: what's the difference? Nat. Rev. Mol. Cell Biol. 2:815–826. [DOI] [PubMed] [Google Scholar]
- Bi, E. 2001. Cytokinesis in budding yeast: the relationship between actomyosin ring function and septum formation. Cell Struct. Funct. 26:529–537. [DOI] [PubMed] [Google Scholar]
- Bonifacino, J.S., and B.S. Glick. 2004. The mechanisms of vesicle budding and fusion. Cell. 116:153–166. [DOI] [PubMed] [Google Scholar]
- Cabib, E. 2004. The septation apparatus, a chitin-requiring machine in budding yeast. Arch. Biochem. Biophys. 426:201–207. [DOI] [PubMed] [Google Scholar]
- Cabib, E., and M. Schmidt. 2003. Chitin synthase III activity, but not the chitin ring, is required for remedial septa formation in budding yeast. FEMS Microbiol. Lett. 224:299–305. [DOI] [PubMed] [Google Scholar]
- Cabib, E., D.H. Roh, M. Schmidt, L.B. Crotti, and A. Varma. 2001. The yeast cell wall and septum as paradigms of cell growth and morphogenesis. J. Biol. Chem. 276:19679–19682. [DOI] [PubMed] [Google Scholar]
- Cho, R.J., M.J. Campbell, E.A. Winzeler, L. Steinmetz, A. Conway, L. Wodicka, T.G. Wolfsberg, A.E. Gabrielian, D. Landsman, D.J. Lockhart, and R.W. Davis. 1998. A genome-wide transcriptional analysis of the mitotic cell cycle. Mol. Cell. 2:65–73. [DOI] [PubMed] [Google Scholar]
- Choi, W.J., B. Santos, A. Duran, and E. Cabib. 1994. Are yeast chitin synthases regulated at the transcriptional or the posttranslational level? Mol. Cell. Biol. 14:7685–7694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang, J.S., and R.W. Schekman. 1996. Differential trafficking and timed localization of two chitin synthase proteins, Chs2p and Chs3p. J. Cell Biol. 135:597–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmaki, T., S. Ponnambalam, A.R. Prescott, H. Clausen, B.L. Tang, W. Hong, and J.M. Lucocq. 1999. Forward and retrograde trafficking in mitotic animal cells. ER-Golgi transport arrest restricts protein export from the ER into COPII-coated structures. J. Cell Sci. 112:589–600. [DOI] [PubMed] [Google Scholar]
- Finger, F.P., and P. Novick. 1998. Spatial regulation of exocytosis: lessons from yeast. J. Cell Biol. 142:609–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger, F.P., and J.G. White. 2002. Fusion and fission: membrane trafficking in animal cytokinesis. Cell. 108:727–730. [DOI] [PubMed] [Google Scholar]
- Finger, F.P., T.E. Hughes, and P. Novick. 1998. Sec3p is a spatial landmark for polarized secretion in budding yeast. Cell. 92:559–571. [DOI] [PubMed] [Google Scholar]
- Goldstein, A., and J.O. Lampen. 1975. Beta-D-fructofuranoside fructohydrolase from yeast. Methods Enzymol. 42:504–511. [DOI] [PubMed] [Google Scholar]
- Guo, W., D. Roth, C. Walch-Solimena, and P. Novick. 1999. The exocyst complex is an effector for Sec4p, targeting secretory vesicles to sites of exocytosis. EMBO J. 18:1071–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond, A.T., and B.S. Glick. 2000. Dynamics of transitional endoplasmic reticulum sites in vertebrate cells. Mol. Biol. Cell. 11:3013–3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood, J.K., W.W. Hwang, and P.A. Silver. 2001. The Saccharomyces cerevisiae cyclin Clb2p is targeted to multiple subcellular locations by cis- and trans-acting determinants. J. Cell Sci. 114:589–597. [DOI] [PubMed] [Google Scholar]
- Jaspersen, S.L., J.F. Charles, R.L. Tinker-Kulberg, and D.O. Morgan. 1998. A late mitotic regulatory network controlling cyclin destruction in Saccharomyces cerevisiae. Mol. Biol. Cell. 9:2803–2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaspersen, S.L., J.F. Charles, and D.O. Morgan. 1999. Inhibitory phosphorylation of the APC regulator Hct1 is controlled by the kinase Cdc28 and the phosphatase Cdc14. Curr. Biol. 9:227–236. [DOI] [PubMed] [Google Scholar]
- Jurgens, G. 2005. Cytokinesis in higher plants. Annu. Rev. Plant Biol. 56:281–299. [DOI] [PubMed] [Google Scholar]
- Kano, F., A.R. Tanaka, S. Yamauchi, H. Kondo, and M. Murata. 2004. Cdc2 kinase-dependent disassembly of the endoplasmic reticulum (ER) exit sites inhibits ER-to-Golgi vesicular transport during mitosis. Mol. Biol. Cell. 15:4289–4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klis, F.M., P. Mol, K. Hellingwerf, and S. Brul. 2002. Dynamics of cell wall structure in Saccharomyces cerevisiae. FEMS Microbiol. Rev. 26:239–256. [DOI] [PubMed] [Google Scholar]
- Kota, J., and P.O. Ljungdahl. 2005. Specialized membrane-localized chaperones prevent aggregation of polytopic proteins in the ER. J. Cell Biol. 168:79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S.E., L.M. Frenz, N.J. Wells, A.L. Johnson, and L.H. Johnston. 2001. Order of function of the budding-yeast mitotic exit-network proteins Tem1, Cdc15, Mob1, Dbf2, and Cdc5. Curr. Biol. 11:784–788. [DOI] [PubMed] [Google Scholar]
- Loog, M., and D.O. Morgan. 2005. Cyclin specificity in the phosphorylation of cyclin-dependent kinase substrates. Nature. 434:104–108. [DOI] [PubMed] [Google Scholar]
- Makarow, M. 1988. Secretion of invertase in mitotic yeast cells. EMBO J. 7:1475–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan, D.O. 1999. Regulation of the APC and the exit from mitosis. Nat. Cell Biol. 1:E47–E53. [DOI] [PubMed] [Google Scholar]
- Nevalainen, L.T., J. Louhelainen, and M. Makarow. 1989. Post-translational modifications in mitotic yeast cells. Eur. J. Biochem. 184:165–172. [DOI] [PubMed] [Google Scholar]
- Pammer, M., P. Briza, A. Ellinger, T. Schuster, R. Stucka, H. Feldmann, and M. Breitenbach. 1992. DIT101 (CSD2, CAL1), a cell cycle-regulated yeast gene required for synthesis of chitin in cell walls and chitosan in spore walls. Yeast. 8:1089–1099. [DOI] [PubMed] [Google Scholar]
- Pruyne, D., and A. Bretscher. 2000. Polarization of cell growth in yeast. J. Cell Sci. 113:571–585. [DOI] [PubMed] [Google Scholar]
- Pruyne, D., A. Legesse-Miller, L. Gao, Y. Dong, and A. Bretscher. 2004. Mechanisms of polarized growth and organelle segregation in yeast. Annu. Rev. Cell Dev. Biol. 20:559–591. [DOI] [PubMed] [Google Scholar]
- Roh, D.H., B. Bowers, M. Schmidt, and E. Cabib. 2002. The septation apparatus, an autonomous system in budding yeast. Mol. Biol. Cell. 13:2747– 2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler, I., A. Chiang, T. Kurihara, J. Rothblatt, J. Way, and P. Silver. 1989. A yeast gene important for protein assembly into the endoplasmic reticulum and the nucleus has homology to DnaJ, an Escherichia coli heat shock protein. J. Cell Biol. 109:2665–2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sburlati, A., and E. Cabib. 1986. Chitin synthetase 2, a presumptive participant in septum formation in Saccharomyces cerevisiae. J. Biol. Chem. 261:15147–15152. [PubMed] [Google Scholar]
- Schmidt, M., B. Bowers, A. Varma, D.H. Roh, and E. Cabib. 2002. In budding yeast, contraction of the actomyosin ring and formation of the primary septum at cytokinesis depend on each other. J. Cell Sci. 115:293–302. [DOI] [PubMed] [Google Scholar]
- Schweitzer, J.K., and C. D'Souza-Schorey. 2004. Finishing the job: cytoskeletal and membrane events bring cytokinesis to an end. Exp. Cell Res. 295:1–8. [DOI] [PubMed] [Google Scholar]
- Sheff, M.A., and K.S. Thorn. 2004. Optimized cassettes for fluorescent protein tagging in Saccharomyces cerevisiae. Yeast. 21:661–670. [DOI] [PubMed] [Google Scholar]
- Simanis, V. 2003. Events at the end of mitosis in the budding and fission yeasts. J. Cell Sci. 116:4263–4275. [DOI] [PubMed] [Google Scholar]
- Spellman, P.T., G. Sherlock, M.Q. Zhang, V.R. Iyer, K. Anders, M.B. Eisen, P.O. Brown, D. Botstein, and B. Futcher. 1998. Comprehensive identification of cell cycle-regulated genes of the yeast Saccharomyces cerevisiae by microarray hybridization. Mol. Biol. Cell. 9:3273–3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland, L.I., and D.R. Burgess. 2004. Pathways for membrane trafficking during cytokinesis. Trends Cell Biol. 14:115–118. [DOI] [PubMed] [Google Scholar]
- Surana, U., A. Amon, C. Dowzer, J. McGrew, B. Byers, and K. Nasmyth. 1993. Destruction of the CDC28/CLB mitotic kinase is not required for the metaphase to anaphase transition in budding yeast. EMBO J. 12:1969–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TerBush, D.R., T. Maurice, D. Roth, and P. Novick. 1996. The Exocyst is a multiprotein complex required for exocytosis in Saccharomyces cerevisiae. EMBO J. 15:6483–6494. [PMC free article] [PubMed] [Google Scholar]
- Tolliday, N., M. Pitcher, and R. Li. 2003. Direct evidence for a critical role of myosin II in budding yeast cytokinesis and the evolvability of new cytokinetic mechanisms in the absence of myosin II. Mol. Biol. Cell. 14:798–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trilla, J.A., A. Duran, and C. Roncero. 1999. Chs7p, a new protein involved in the control of protein export from the endoplasmic reticulum that is specifically engaged in the regulation of chitin synthesis in Saccharomyces cerevisiae. J. Cell Biol. 145:1153–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida, Y., O. Shimmi, M. Sudoh, M. Arisawa, and H. Yamada-Okabe. 1996. Characterization of chitin synthase 2 of Saccharomyces cerevisiae. II: Both full size and processed enzymes are active for chitin synthesis. J. Biochem. (Tokyo). 119:659–666 (Tokyo). [DOI] [PubMed] [Google Scholar]
- VerPlank, L., and R. Li. 2005. Cell cycle-regulated trafficking of Chs2 controls actomyosin ring stability during cytokinesis. Mol. Biol. Cell. 16:2529–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visintin, R., K. Craig, E.S. Hwang, S. Prinz, M. Tyers, and A. Amon. 1998. The phosphatase Cdc14 triggers mitotic exit by reversal of Cdk-dependent phosphorylation. Mol. Cell. 2:709–718. [DOI] [PubMed] [Google Scholar]
- Walther, A., and J. Wendland. 2003. Septation and cytokinesis in fungi. Fungal Genet. Biol. 40:187–196. [DOI] [PubMed] [Google Scholar]
- Yeong, F.M., H.H. Lim, C.G. Padmashree, and U. Surana. 2000. Exit from mitosis in budding yeast: biphasic inactivation of the Cdc28-Clb2 mitotic kinase and the role of Cdc20. Mol. Cell. 5:501–511. [DOI] [PubMed] [Google Scholar]
- Yeong, F.M., H.H. Lim, and U. Surana. 2002. MEN, destruction and separation: mechanistic links between mitotic exit and cytokinesis in budding yeast. Bioessays. 24:659–666. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.