Abstract
Efficient delivery of cells to target tissues is a major problem in cell therapy. We report that enhancing delivery of mesoangioblasts leads to a complete reconstitution of downstream skeletal muscles in a mouse model of severe muscular dystrophy (α-sarcoglycan ko). Mesoangioblasts, vessel-associated stem cells, were exposed to several cytokines, among which stromal- derived factor (SDF) 1 or tumor necrosis factor (TNF) α were the most potent in enhancing transmigration in vitro and migration into dystrophic muscle in vivo. Transient expression of α4 integrins or L-selectin also increased several fold migration both in vitro and in vivo. Therefore, combined pretreatment with SDF-1 or TNF-α and expression of α4 integrin leads to massive colonization (>50%) followed by reconstitution of >80% of α-sarcoglycan–expressing fibers, with a fivefold increase in efficiency in comparison with control cells. This study defines the requirements for efficient engraftment of mesoangioblasts and offers a new potent tool to optimize future cell therapy protocols for muscular dystrophies.
Introduction
Cells can reach specific target tissues through the general circulation by different mechanisms. Homing has been studied extensively both in vitro and in vivo with different cell types, such as leukocytes or hematopoietic stem cells (Butcher, 1991; Fu and Liesveld, 2000); it is believed to rely on adhesion molecules and cytokines receptors by a multistep cascade, consisting of a rolling process followed by firm adhesion and transmigration into the surrounding tissue (Peled et al., 1999; Grabovsky et al., 2000). The repertoire and the level of cytokine expression by the target tissue, as well as expression of the relative receptors on endothelium, influence the efficiency of homing. For example, stromal-derived factor (SDF) 1 favors the arrest of progenitors on vascular endothelium, whereas interleukin (IL) 8 promotes stem cell mobilization from the marrow (Laterveer et al., 1996; Peled et al., 1999). Although these mechanisms have been elucidated to a large extent for leukocytes and hematopoietic stem cells, far less is known for other types of stem cells. Mesoangioblasts were recently characterized as a population of vessel-associated stem cells that differentiate into several mesoderm cell types, including skeletal muscle (Minasi et al., 2002). They have been shown to restore to a significant extent muscle structure and function in a mouse model of muscular dystrophy (Sampaolesi et al., 2003). One main reason for the partial effect of mesoangioblasts in this model is likely to be ascribed to the limited ability of these cells to reach and colonize the muscle, depending in turn on incomplete adhesion and extravasation.
Mesoangioblast extravasation must be directed by selective mesoangioblast–endothelial cell recognition. Microarray analysis revealed that mesoangioblasts express E-selectin, β7 integrin, AlCAM, several cytokines receptors, and CD44 but lack many of the leukocyte molecules implicated in transmigration (Tagliafico et al., 2004). To increase the efficiency of muscle repair by mesoangioblasts, it would be essential to increase their migration to skeletal muscle, with the additional benefit of reducing unspecific trapping in the capillary filters of the body, such as liver and lung. Here, we report that expression of α4 integrin and exposure of cells to SDF-1 or TNF-α improve up to fivefold migration of wild-type (WT) mesoangioblasts to the dystrophic muscles and consequent production of new fibers that express the normal copy of the mutated gene. These results elucidate the migration mechanism of mesoangioblasts and open a therapeutic opportunity for improving efficacy of cell therapy in muscular dystrophy.
Results
Differentiated myotubes and SDF-1 or TNF-α cytokines induce mesoangioblast transmigration in vitro
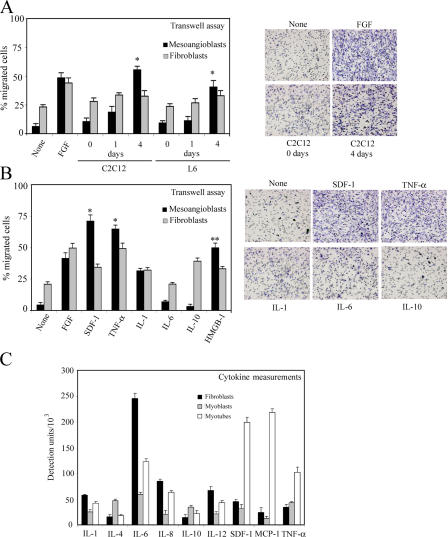
Mesoangioblasts (clone D16; Sampaolesi et al., 2003) were starved for 12 h in the absence of serum and then subjected to transmigration assays using transwell chambers, as described previously (Mohle et al., 1997). In a first series of experiments, we created an artificial environment where mesoangioblasts would face an activated endothelial layer separating them from differentiating muscle cells (much as it happens in regenerating muscle) or specific cytokines with known chemoattractive potency. As shown in Fig. 1 A (left), mesoangioblasts were unable to cross endothelium-coated filters in the absence of stimuli. However, multinucleated myotubes but not undifferentiated myoblasts induced active migration of mesoangioblasts but not primary fibroblasts. This effect was slightly stronger than that induced by FGF, used as a positive control. Fig. 1 A (right) shows a representative field of the lower side of the filter, fixed and stained.
Figure 1.
Induction of mesoangioblast transmigration in vitro by cytokines. (A) Mouse mesoangioblasts or fibroblasts were plated on endothelium-coated transwell filters and induced to migrate for 6 h in the presence of C2C12 or L6 myoblasts or differentiated myotubes. FGF was used as positive migration control. A representative out of five independent experiments run in duplicate is shown (left). Transmigrated mesoangioblasts were fixed, stained, and counted, and a representative image of different conditions is shown (right). *, α < 0.01. (B) Mesoangioblasts or fibroblasts were plated on endothelium-coated transwell filters and induced to migrate for 6 h the in presence of different cytokines. One representative out of five independent experiments run in duplicate is shown (left). A representative image of the transmigrated mesoangioblasts under the different conditions is also shown (right). *, α < 0.005; **, α < 0.01. (C) Supernatants from fibroblast, myoblast, or myotube cultures were collected after 4 d, and different cytokines were detected using the mouse multicytokine-detection system. One out of two experiments is shown.
We also performed the transmigration assay through endothelium-coated filters in the presence of a panel of cytokines for which mesoangioblasts have receptors (Tagliafico et al., 2004). The presence of SDF-1 and TNF-α in the lower chamber caused a tenfold increase of mesoangioblast migration, a significantly more robust effect than that elicited by FGF or high mobility group box (HMGB) 1, previously described as an enhancer of mesoangioblast migration (Palumbo et al., 2004); IL-1 had little effect, whereas IL-6 and -10 had no effect (Fig. 1 B, left). Mesoangioblasts stained at the lower side of the filter, confirming the positive transmigration effect of SDF-1 and TNF-α (Fig. 1 B, right).
This is in accordance with the fact that myotubes secrete these cytokines at a higher rate than myoblasts. As shown in Fig. 1 C, SDF-1 or TNF-α as well as IL-6 and monocyte chemotactic protein 1 were detected at a higher level in the supernatant from myotubes than in the corresponding supernatant from myoblasts. These data suggest that differentiated myotubes may secrete growth factors or cytokines such as SDF-1 or TNF-α that favor mesoangioblast transmigration by either a chemotactic effect and/or by modifying the endothelium barrier.
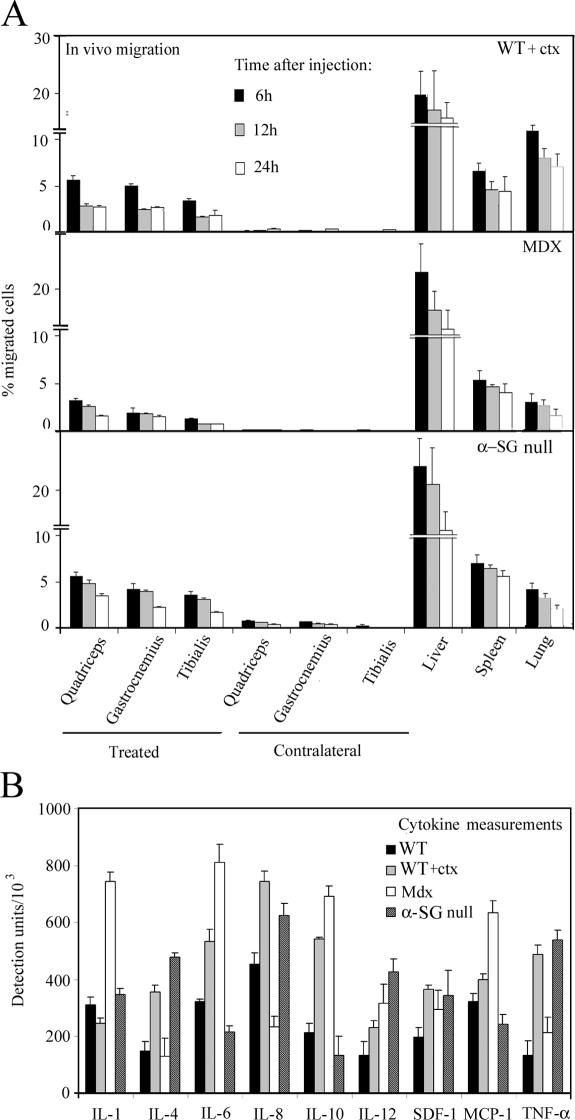
Mesoangioblast migration in vivo
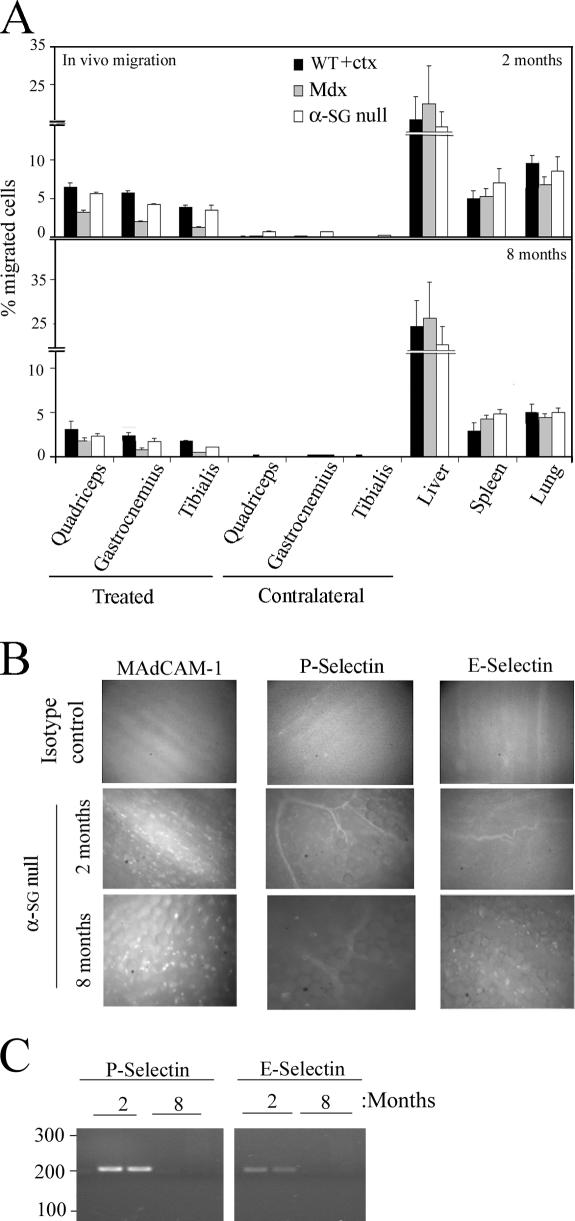
To assess the ability of mesoangioblasts to migrate in vivo from the vessel lumen to muscle interstitial tissue (see Fig. 8), we injected 5 × 105 GFP-labeled mesoangioblasts into the right femoral artery of WT C57 (previously injected intramuscularly with cardiotoxin [ctx]), X chromosome–linked muscular dystrophy (mdx), and α-SG–null mice. Mice were killed 6, 12, or 24 h after injection, the muscles (quadriceps, gastrocnemius, and tibialis) from treated (right) and contralateral legs as well as filter organs (liver, spleen, and lung) were collected, and RNA was extracted. The percentage of migrated mesoangioblasts in each recipient organ was calculated by real-time PCR for GFP expression as a percentage of the value corresponding to the total number of injected cells. As shown in Fig. 2 A, mesoangioblasts were able to migrate to the muscles of the treated legs; ∼10% of injected cells could be recovered, whereas most of injected cells were retained in the different filter organs without reaching the contralateral muscles. We set our time of analysis at 6 h after injection for the in vivo experiments, as we observed that with the exception of ctx-treated mice, the number of injected cells remains constant for the first 12 h after the injection and thereafter decreases to varying extents (up to 50% of the value observed at 6 h; Fig. 2 A) to increase again in the following days (see Fig. 9 A). Interestingly, mesoangioblasts migrate more efficiently to muscles of ctx-treated normal mice or of α-SG–null mice (Fig. 2 A, top and bottom) than to muscles of the mdx mouse (Fig. 2 A, middle). As shown in Fig. 2 B, the presence of a higher concentration of TNF-α (and to a minor extent SDF-1) in muscles from ctx-treated WT or α-SG–null mice than in mdx mice could explain the preferential migration of mesoangioblasts. Although other cytokines were found to be more abundant in the muscles of mdx mice, these did not affect in vitro transmigration of mesoangioblasts (IL-1, -6, and -10) or, alternatively, mesoangioblasts do not express the corresponding receptors (monocyte chemotactic protein 1 and IL-8).
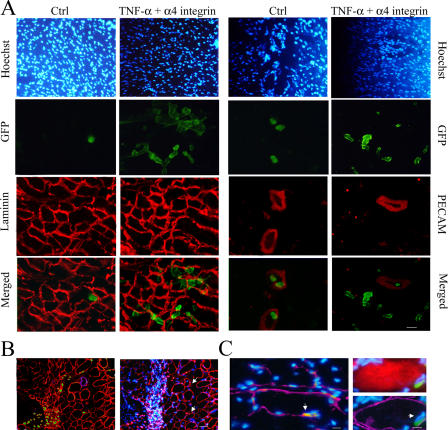
Figure 8.
Treated mesoangioblast cross-muscles vessels. (A) Muscles from α-SG–null mice killed 6 h after injection with TNF-α–pretreated mesoangioblasts transfected with α4 integrin–EGFP vectors or with control mesoangioblasts were frozen. Sections were immunostained with anti-GFP, anti-PECAM, and/or anti-laminin antibodies as indicated. DAPI was used for nuclear staining. Bar, 100 μm. (B) Muscles from α-SG–null mice killed 48 h after injection with treated mesoangioblasts were stained with anti-GFP (green), anti-laminin (red), and anti-PECAM (magenta) antibodies. Hoechst was used as nuclear staining. Bar, 100 μm. (C) Muscles from α-SG–null mice killed 2 wk after injection with treated mesoangioblasts were stained with anti-GFP (green), anti-myf5 (red; left), anti-myosin (red; right), and/or anti-laminin (magenta) antibodies. Hoechst was used as nuclear staining. Arrows indicate GFP-positive cells located underneath the muscle fiber basal lamina and expressing the satellite cell marker Myf5. Bars, 5 μm.
Figure 2.
In vivo mesoangioblast migration. (A) Mesoangioblasts were injected into the right femoral artery (treated muscles) of 2-mo-old WT (preinjected with ctx), mdx, or α-SG–null mice. After 6, 12, or 24 h, different organs were collected and the number of migrated cells was calculated by real-time PCR for GFP. A mean of three independent experiments run in triplicate is shown. (B) Muscles from 2-mo-old WT (either control or preinjected with ctx), mdx, or α-SG–null mice were homogenized and analyzed for cytokine expression with the mouse multicytokine-detection system. One out of two experiments is shown.
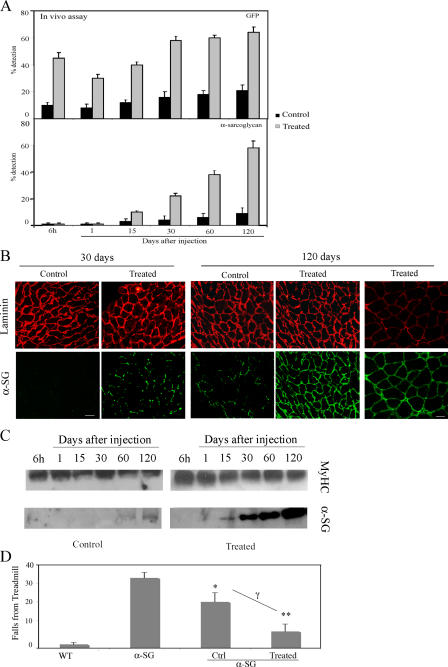
Figure 9.
Treated mesoangioblasts improve α-SG expression. (A) Muscles from α-SG–null mice injected with mesoangioblasts pretreated with TNF-α and transfected with the α4 integrin–EGFP vector or with control mesoangioblasts were collected after different days and subjected to real-time PCR for the presence of α-SG or GFP. A mean of two independent experiments run in triplicate is shown. (B) Immunostaining of tibialis anterior from α-SG–null mice collected 30 or 120 d after injection with control mesoangioblasts or with mesoangioblasts pretreated with TNF-α and transfected with α4. Laminin is shown in red, and α-SG or GFP protein is shown in green. Bars: (left) 100 μm; (right) 20 μm. (C) Western blot for α-SG protein of lysates from tibialis from α-SG–null mice collected at different times after injection with control mesoangioblasts or with mesoangioblasts pretreated with TNF-α and transfected with α4 integrin. Myosin heavy chain (MyHC) is shown as protein level control. One representative out of four independent experiments is shown. (D) α-SG–null mice injected or not with mesoangioblast control or pretreated with TNF-α and transfected with α4 integrin were exercised after 1 mo on a treadmill at 7 m/min. The number of times they fell onto the grid was recorded. C57 WT mice are presented as control. Bars represent mean and standard deviation of two independent tests (n = 4 mice for each group). *, α < 0.01; **, α < 0.005 (α-SG vs. WT); γ (nontreated vs. treated mesoangioblasts) = 0.01.
Mesoangioblast migration depends on age
Mesoangioblasts were injected in the femoral artery of WT (ctx injected), mdx, or α-SG–null mice at 2 or 8 mo of age, and different organs were collected after 6 h. As shown in Fig. 3 A, the number of mesoangioblasts that reached the damaged muscles was reduced to half when the injection was performed on old mice. This effect could be due to different adhesion molecules expressed by vessels in the muscles of young and old mice. Alexa 488–labeled mAbs anti–MAdCAM-1 and anti–E- or anti–P-selectin were injected in the tail vein of α-SG–null mice, and after 20 min, their accumulation was studied with intravital video microscopy. As shown in Fig. 3 B, E- and P-selectin were barely detected in the muscles of old α-SG–null mice. In contrast, in young α-SG–null mice, E- and P-selectin were clearly detected on capillaries and small vessels in the muscles. MAdCAM-1 and E- or P-selectin were not detected in muscles from old mdx mice, whereas only MAdCAM-1 was slightly expressed in young mdx mice (unpublished data). mRNA expression analysis confirmed that E- and P-selectins are highly expressed in muscles of 2-mo-old α-SG–null mice but barely detectable in 8-mo-old mice (Fig. 3 C). These results show that in addition to the reduction of the microvasculature that accompanies fat and connective tissue accumulation in aging, dystrophic muscles, reduced expression of adhesion molecules may further impair stem cell migration.
Figure 3.
Mesoangioblast migration depends on age. (A) GFP mesoangioblasts were injected into the right femoral artery (treated muscles) of 2- or 8-mo-old WT (preinjected with ctx), mdx, or α-SG–null mice, and after 6 h, organs were collected and the percentage of migrated cells was calculated by real-time PCR for GFP. A mean of three independent experiments run in triplicate is shown. (B) Intravital microscopy of Alexa 488 E- and P-selectin and MAdCAM-1 on the vascular endothelium from striated muscles were performed on α-SG–null mice of 2 and 8 mo. Isotype antibodies controls are also shown. A representative image out of three independent experiments is shown. (C) mRNA expression of P- and E-selectin in muscles taking from two α-SG–null mice of 2 or 8 mo analyzed by PCR.
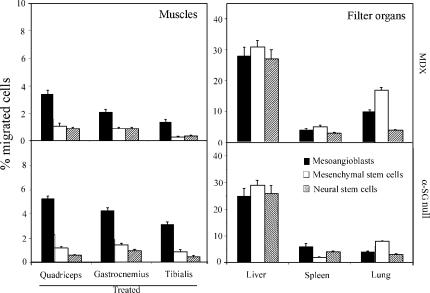
Mesoangioblasts migrate more efficiently to injured muscles than other types of stem cells
Different types of stem cells have been shown to circulate and to be able to differentiate into skeletal muscle, thus representing potential candidates for a cell therapy protocol. Therefore, we compared migration to muscles of mesoangioblasts with mesenchymal and neural stem cells. 2.5 × 105 EGFP mesenchymal, neural, or mesoangioblast stem cells were injected into the femoral artery of mdx or α-SG–null mice, and after 6 h, quadriceps, gastrocnemius, and tibialis from the treated leg and liver, spleen, and lung were collected, and the percentage of donor cells was calculated by real-time PCR for GFP. As shown in Fig. 4, mesoangioblasts reached the muscles of the injected leg in higher numbers than the other stem cell populations in both animal models (left). These data show that mesoangioblasts naturally migrate to dystrophic muscle in comparison with the other cells tested. Interestingly, mesenchymal stem cells were recovered preferentially in the lungs (Fig. 4, right). Experiments with acutely isolated, noncultivated EGFP-hematopoietic stem cells were also performed. Even though results are not directly comparable with those obtained with in vitro–expanded cells, we observed that most hematopoietic cells were retained in the liver and the spleen (unpublished data).
Figure 4.
Migration to dystrophic muscle of different types of stem cells. Mesoangioblasts, mesenchymal, or neural stem cells derived from homo-EGFP mice were injected into the femoral artery of α-SG–null mice, and after 6 h, treated muscles and filter organs were collected. Samples were subjected to real-time PCR for the presence of GFP. A mean of three independent experiments run in triplicate is shown.
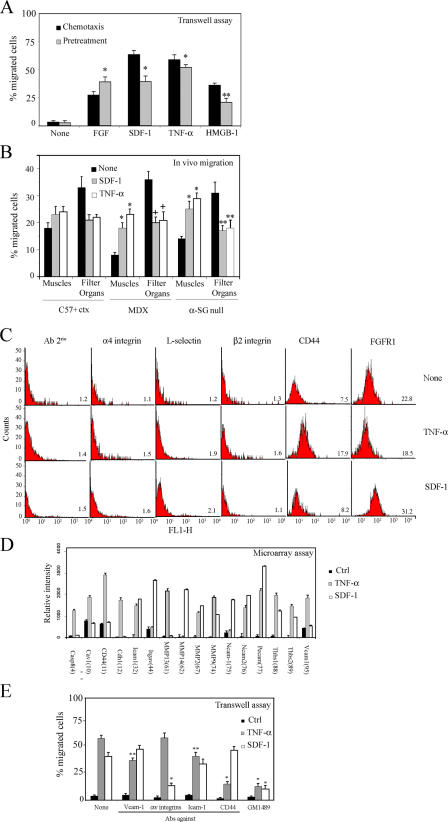
Pretreatment of mesoangioblasts with SDF-1 or TNF-α increases their migration in vitro and in vivo
In an attempt to increase the number of mesoangioblasts that migrate to skeletal muscles, we followed two approaches. First, we pretreated mesoangioblasts with the cytokines SDF-1 and TNF-α for 12 h before challenging them in the transwell assay. The results, shown in Fig. 5 A, clearly indicated that pretreatment with either SDF-1 and TNF-α stimulated mesoangioblast migration to the same extent observed when the same cytokines were present in the lower chamber. Therefore, SDF-1– or TNF-α–pretreated GFP mesoangioblasts were injected through the femoral artery of ctx-pretreated WT, mdx, or α-SG–null mice, and after 6 h, muscles and filter organs were collected and analyzed by real-time PCR for presence of GFP. As shown in Fig. 5 B, both SDF-1 and TNF-α had a modest effect on WT mice (probably because of the acute inflammation induced by ctx) but increased about twofold the migration of mesoangioblasts to the injured muscles of dystrophic mice. Consistently, the number of mesoangioblasts detected in the filter organs was reduced to almost half of control.
Figure 5.
SDF-1 and TNF-α favor mesoangioblast migration. (A) GFP mesoangioblasts pretreated with different cytokines (without pretreatment for chemotaxis assays) were plated on endothelium-coated filters and induced to migrate for 6 h (in the presence of different cytokines for chemotaxis assays). FGF was used as positive control. One representative out of five independent experiments run in duplicate is shown. * α < 0.005; **, α < 0.01. (B) GFP mesoangioblasts pretreated with 50 ng/ml SDF-1 or TNF-α were injected into the femoral artery of 2-mo-old WT (preinjected with ctx), mdx, or α-SG–null mice, and after 6 h, muscles and filter organs were analyzed by real-time PCR for the presence of migrated cells. *, α < 0.01; **, α < 0.05. (C) SDF-1– or TNF-α–pretreated mice mesoangioblasts were analyzed by flow cytometry for the presence of different surface molecules. One representative out of two independent experiments is shown. Mean fluorescence intensity is shown at the bottom right of each plot. (D) A gene array containing oligos corresponding to different mouse adhesion molecules was hybridized with cDNAs probes retrotranscribed from the RNA of mesoangioblasts pretreated with TNF-α or SDF-1. Relative RNA levels of selected mRNAs normalized to β-actin expression are shown. Data presented are the mean of two independent experiments. (E) SDF-1– or TNF-α–pretreated mesoangioblasts were incubated with MMP inhibitor GM1489 or with antibodies against different surface molecules and induced to migrate for 6 h through endothelium-coated filters. A representative out of three independent experiments run in duplicate is shown. *, α < 0.005; **, α < 0.01.
We then attempted to identify the surface molecules that may be responsible for the changes observed in mesoangioblast migration after pretreatment with SDF-1 or TNF-α. The possible change in the expression of several candidate surface molecules was first investigated by FACS analysis (Fig. 5 C). Many of the tested molecules were not changed by treatment with either SDF-1 or TNF-α. However, CD44 expression was increased more than twofold by TNF-α and not by SDF-1, whereas FGFR1 expression was increased only by SDF-1. Although these two molecules are involved in migration processes and could explain part of our effects, we performed a microarray analysis for 125 genes, including surface receptors, to look for more candidates genes induced in mesoangioblasts by SDF-1 or TNF-α. 16 genes were modified after pretreatment of mesoangioblasts with either SDF-1 or TNF-α (Fig. 5 D). Caspase-8 (Casp8), caveolin 1 (cav-1), CD44, cadherin 1 (Cdh1), metalloproteinase (MMP) 13, or vascular cell adhesion molecule (VCAM) 1 expression was increased two- to threefold only by TNF-α pretreatment, whereas Itgαv (αv integrin), MMP-14, or neural cell adhesion molecule (NCAM) 1 were induced approximately twofold only by SDF-1 pretreatment. Pretreatment with either TNF-α or SDF-1 increased expression of intercellular adhesion molecule (ICAM) 1, NCAM-2, different MMPs, platelet endothelial cell adhesion molecule (PECAM), and trombospondins. To verify the role in mesoangioblast transmigration of those molecules that appeared to be more potently induced by TNF-α or SDF-1, we repeated the transwell assay in presence of antibodies against VCAM-1, αv integrins, ICAM-1, or CD44 or MMP inhibitor GM1489. As shown in Fig. 5 E, antibodies against CD44 partially inhibited transmigration induced by TNF-α, whereas antibodies against αv integrin inhibited SDF-1–induced transmigration. Blocking MMP activity with GM1489 dramatically inhibited transmigration induced by both molecules. Together, these data suggest that TNF-α and SDF-1 increase mesoangioblast transmigration in vitro and migration to muscles in vivo by increasing expression of several molecules, among which CD44 and MMP appeared the most relevant in the case of TNF-α and αv integrin and MMP appeared most relevant in the case of SDF-1.
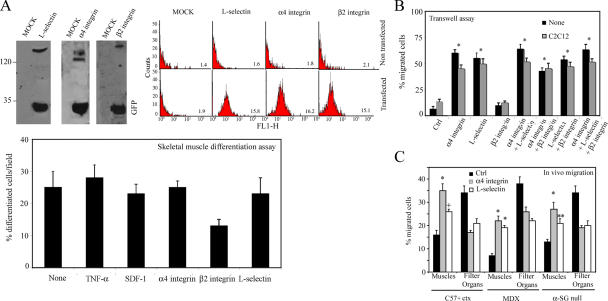
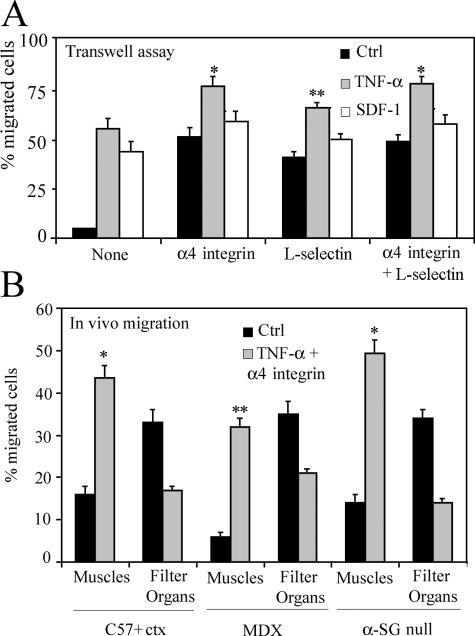
α4 integrin and L-selectin are necessary for efficient mesoangioblast migration in vitro and in vivo
As revealed by microarray analysis (Tagliafico et al., 2004), mesoangioblasts do not express some of the key molecules that control rolling and extravasation of leukocytes and different stem cells such as L-selectin, α4 integrin, or β2 integrin (Butcher, 1991). Therefore, we transfected mesoangioblasts with vectors expressing these molecules and EGFP, which allowed FACS selection of transfected cells. To verify the expression of these proteins, a Western blot for total lysates of transfected mesoangioblasts were performed (Fig. 6 A, left). Correct surface expression of these proteins was also verified by flow cytometry analysis (Fig. 6 A, right). Finally, mesoangioblasts transfected with the different constructs or pretreated with TNF-α or SDF-1 were subjected to differentiation assays. Expression of L-selectin or α4 integrin did not interfere with skeletal muscle differentiation of mesoangioblasts in vitro, whereas expression of β2 integrin inhibited differentiation (Fig. 6 A, bottom).
Figure 6.
α4 integrin increases mesoangioblast migration. (A) Mesoangioblasts were transfected with expression vectors encoding different surface molecules and EGFP and lysed with Laemmli buffer. Protein and GFP expression was checked by Western blot (left). Expression at the cell surface was also analyzed by flow cytometry (right). Mean fluorescence intensity is also shown in each plot. Transfected or pretreated mesoangioblasts were subjected to skeletal muscle differentiation assays. The mean of three different experiments is shown (bottom). (B) Mesoangioblasts transfected with one, two, or all three vectors expressing the different surface molecules and EGFP were plated on endothelium-coated filters and induced to migrate for 6 h. A representative out of five independent experiments run in duplicate is shown. *, α < 0.005. (C) Mesoangioblasts transfected with vectors expressing α4 integrin or L-selectin and GFP were injected into the right femoral artery of 2-mo-old α-SG–null mice, and after 6 h, muscles and filter organs were collected. Percentage of migrated cells was calculated after performing real-time PCR for GFP. A mean of three independent experiments run in triplicate is shown. * α < 0.005; **, α < 0.01.
To assess whether the expression of these molecules could improve mesoangioblast migration, cells transfected with one, two, or all three constructs were subjected to transmigration in vitro assay through activated endothelium. As shown in Fig. 6 B, α4 (but nor β2) integrin or L-selectin expression increased mesoangioblast transmigration six- to eightfold, whether or not C2C12-derived myotubes were present in the lower chamber. Thus, expression of the counterpart ligands for these molecules at the surface of the activated endothelium was sufficient to allow mesoangioblast transmigration independent of the presence of myotubes, with a chemoattractant-independent mechanism. Cotransfection of two or all constructs had only a slightly cumulative effect, which was not statistically significant. When α4 integrin– or L-selectin–expressing mesoangioblasts were injected into the femoral artery of ctx-treated WT, mdx, or α-SG–null mice, the number of cells that reached downstream muscles was increased approximately twofold with some variability in different mice (Fig. 6 C). In general, α4 integrin appeared to be more efficient than L-selectin. Therefore, expression of α4 integrins and L-selectin by mesoangioblasts improves their migration, possibly by favoring their interaction with the ligands expressed on the endothelium surface.
Pretreatment with TNF-α or SDF-1 of α4 integrin–expressing mesoangioblasts increased their migration to muscles five- to sixfold
We then tested the possibility of additive or synergistic effects of cytokines and adhesion molecules. Mesoangioblasts, previously transfected with α4 integrin or/and L-selectin constructs, were pretreated for 12 h with TNF-α or SDF-1 and then subjected to transmigration assay through endothelium. As shown in Fig. 7 A, some combinations were able to improve mesoangioblast transmigration in vitro, the most efficient of which was TNF-α treatment of α4 integrin–expressing cells, which migrated in vitro 15-fold more efficiently than control. Therefore, these cells were injected into the femoral artery of the different animal models: results showed that almost 50% of injected cells reached the injured muscles after 6 h, with a concomitant reduction in the number mesoangioblasts detected in filter organs (Fig. 7 B). To follow these modified mesoangioblasts, we performed immunohistology analysis of the tibialis anterior from an α-SG–null mouse whose femoral artery had been previously (6 h) injected with control or modified mesoangioblasts. As shown in Fig. 8 A (right), TNF-α–pretreated, α4 integrin–transfected mesoangioblasts (GFP, right) were able to cross the endothelial barrier of vessels (stained with PECAM, right) and localized in the surrounding muscle tissue (green, left) outside of and sometimes underneath laminin (red, left) in a position typical of satellite cells. Untreated mesoangioblasts were less efficient in crossing the vessel wall, and many fewer were found around the muscle fibers. To monitor the distribution of injected cells at 48 h after injection, muscle sections of transplanted mice were stained for GFP, laminin, and PECAM. As shown in Fig. 8 B, all GFP-modified mesoangioblasts were localized in the muscle tissue, mainly outside of the basal lamina and far from vessels, but in some case underneath it, in a position typical of satellite cells. Indeed, as shown in Fig. 8 C, 2 wk after injection, we detected GFP mesoangioblasts expressing the satellite cell Myf-5 (Beauchamp et al., 2000; Fig. 8 C, left) that occupied the typical position of satellite cells (Fig. 8 C, right).
Figure 7.
Combination of TNF-α and α4 integrin increases mesoangioblast homing. (A) TNF-α– or SDF-1–pretreated mesoangioblasts transfected with vectors expressing α4- and/or L-selectin and GFP were induced to migrate for 6 h through endothelium-coated filters. A mean of three independent experiments run in duplicate is shown. *, α < 0.005; **, α < 0.05. (B) TNF-α–pretreated mesoangioblasts transfected with vectors expressing α4 integrin–EGFP were injected through the femoral artery of WT (preinjected with ctx), mdx, or α-SG–null mice, and after 6 h, muscles and filter organs were collected and analyzed by real-time PCR for the presence of GFP. A mean of three independent experiments run in triplicate is shown. *, α < 0.01; **, α < 0.05.
TNF-α–pretreated, α4 integrin–expressing mesoangioblasts induce enhanced expression of α-SG
5 × 105 TNF-α–pretreated, α4 integrin–expressing mesoangioblasts were injected into the femoral artery of α-SG–null mice, and after 6 h or 1, 15, 30, 60, or 120 d, the tibialis anterior from treated legs was collected and analyzed by real-time-PCR for GFP or α-SG. As shown in Fig. 9 A, after an initial modest decline, the number of both control and modified mesoangioblasts began to increase by 15 d after the injection and reached a plateau by 1 mo. At all time points, modified mesoangioblasts were four- to sixfold more numerous than control mesoangioblasts. Likewise, α-SG became detectable ∼15 d after injection, when mesoangioblasts had been incorporated into regenerating fibers and continued to increase thereafter, again at a much higher level in muscles injected with modified mesoangioblasts. As a matter of fact, 4 mo after a single injection, the level of α-SG expression in the tibialis of injected α-SG–null mice was almost 60% of that detected in the corresponding tibialis from a WT mouse. At the same time, the level of expression of α-SG in the tibialis injected with control mesoangioblasts was only 10% of a WT mouse. In agreement with RT-PCR data, α-SG was detected in almost all (>90%) the muscle fibers of the tibialis injected with treated mesoangioblasts, whereas a lower percentage (∼20% with a weaker staining) of positive fibers was present in the tibialis injected with control mesoangioblasts (Fig. 9 B). The newly synthesized α-SG protein was also analyzed by Western blot. As shown in Fig. 9 C, after 4 mo, the quantity of protein detected was approximately sixfold higher in muscles injected with treated mesoangioblasts than with control cells. To test whether increased expression of α-SG would correspond to increased motility, mice were subjected to a running test on a treadmill. The number of times that WT mice, α-SG–null mice, either untreated or treated with control or modified (TNF-α–pretreated, α4 integrin–expressing) mesoangioblasts, fell into the grid when running was recorded. Fig. 9 D shows that α-SG–null mice injected with modified mesoangioblasts fell into the grid less frequently than noninjected or control mesoangioblast–injected α-SG–null mice. After this exercise test, many of the null mice appeared to be extremely fatigued and took a relatively long time to recover from the exercise, whereas treated mice seemed to recover from the effort much faster. Together, these data show that mesoangioblasts pretreated with TNF-α and transfected with α4 integrins are not only able to more efficiently reach the damaged muscle but also to reconstitute SG-positive fibers with much higher efficiency and improved muscle function.
TNF-α–pretreated, α4 integrin–expressing human mesoangioblasts reach damaged mdx-SCID muscles with higher efficiency
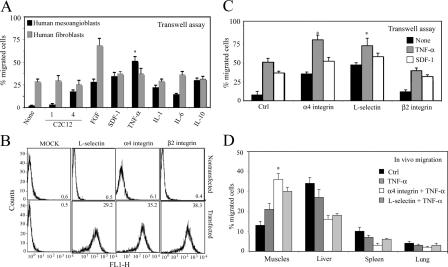
To test whether a similar protocol would also be suitable for human cells, we isolated human mesoangioblasts from small vessels of biopsies from normal human adult muscle. These cells have morphology and gene expression similar to their mouse counterparts, a finite lifespan, and a similar ability to differentiate into different types of muscle cells, osteoblasts, and adipocytes. Human mesoangioblasts were serum starved and subjected to transmigration assays using transwell chambers. As shown in Fig. 10 A, human mesoangioblasts were also unable to cross endothelium-coated filters in absence of stimuli. However, multinucleated myotubes or pretreatment of cells with TNF-α or SDF-1 increased human mesoangioblast migration four- to eightfold without significant enhancement of human fibroblast migration.
Figure 10.
Pretreatment of human mesoangioblasts improves migration to muscles. (A) Human mesoangioblasts or fibroblasts pretreated with different cytokines were plated on endothelium-coated filters and induced to migrate for 6 h in presence of C2C12 myoblasts or differentiated myotubes. FGF was used as positive migration control. One representative out of three independent experiments run in duplicate is shown. *, α < 0.005. (B) Human mesoangioblasts were transfected with expression vectors encoding different surface molecules and GFP, and their expression at the cell surface was analyzed by flow cytometry. Mean fluorescence intensity is shown in each plot. (C) Human mesoangioblasts transfected with vectors expressing different surface molecules and pretreated or not with different cytokines were plated on endothelium-coated filters and induced to migrate for 6 h. One representative out of three independent experiments run in duplicate is shown. *, α < 0.005. (D) Human mesoangioblasts transfected with vectors expressing α4 integrin or L-selectin and GFP and pretreated with TNF-α were injected into the right femoral artery of 2-mo-old mdx-SCID mice, and after 6 h, muscles and filter organs were collected. The percentage of migrated cells was calculated after performing real-time PCR for GFP with the different samples. A mean of three independent experiments run in triplicate is shown. *, α < 0.01.
We then transfected human mesoangioblasts (that also do not express these molecules; unpublished data) with vectors expressing L-selectin, α4 integrin, or β2 integrin and, after transfection, selected the GFP-positive cells with FACS. To verify the correct surface expression of these proteins, transfected human mesoangioblasts were also analyzed by flow cytometry analysis (Fig. 10 B). Fig. 10 C shows that pretreatment of α4 integrin– or L-selectin–expressing human mesoangioblasts with TNF-α or SDF-1 increased their ability to cross the endothelial barrier in transwell assays by ∼18-fold. Therefore, human mesoangioblasts, pretreated with TNF-α and transfected with vectors encoding for α4 integrin or L-selectin were injected into the femoral artery of mdx-SCID mice that do not reject human cells. Fig. 10 D shows that almost 40% of human modified mesoangioblasts reached the muscles 6 h after intrafemoral artery injection, with a concomitant reduction in the number of cells detected in filter organs. Together, these data show that it is possible to increase human mesoangioblast migration to dystrophic muscle with an experimental protocol similar to that developed for mouse mesoangioblasts.
Discussion
Until now, intraarterial delivery of mesoangioblasts (Sampaolesi et al., 2003) has appeared to be the most promising cell therapy protocol for muscular dystrophy because of a partial but significant restoration of muscle structure and function. However, before clinical trials, many hurdles have yet to be solved, among them efficient delivery to target tissue. In this work, we provide a new experimental strategy, based on pretreatment with cytokines and surface expression of certain adhesion molecules that dramatically improves migration of mesoangioblasts to the affected muscles.
Testing mesoangioblast migratory activity in vitro
We initially measured mesoangioblast migration with a transwell assay, already proven as a reliable indicator of the mechanisms that govern cellular trafficking in vivo (Mohle et al., 1997). Mesoangioblasts cross the endothelium and migrate toward multinucleated myotubes, an in vitro setting mimicking the microenvironment encountered by these cells after intraarterial injection, as myotubes roughly correspond to newly regenerated, immature fibers that may produce factors. Microarray analysis has also shown that mesoangioblasts express several receptors for cytokines, of which CXCR-4 and TNF-R raise the possibility that these cells may respond to SDF-1 and TNF-α. Indeed, we found that SDF-1 and TNF-α were the most potent inducers of mesoangioblast migration, more than HMGB-1, which was previously reported to be an enhancer of mesoangioblast migration (Palumbo et al., 2004).
Mesoangioblasts migrate into skeletal muscles of young mice in vivo more efficiently than in the muscles of old mice
Homing to skeletal muscle in vivo proceeds through multiple mechanisms, which are only partially mimicked by in vitro assays. When WT mesoangioblasts were injected through the femoral artery of ctx-treated WT or dystrophic mice, only a small percentage (∼10%) of injected cells reached downstream skeletal muscle, whereas most cells that had probably passed through the capillary network ended in the venous circulation and were trapped in filter organs. Indeed, a very low number of injected cells could be detected in the contralateral muscles. Interestingly, the percentage of donor cells that could be recovered from the muscles of α-SG–null or ctx-treated WT mice was higher than in mdx mice, possibly because of the different cytokine pattern expression in the muscles (Durbeej and Campbell, 2002). Moreover, age turned to be a critical factor for the success of cell transfer. Vessels present in the dystrophic muscle of young but not old mice expressed adhesion molecules necessary for the correct extravasation. The progression of the disease is characterized by reduced regeneration of muscles fibers, which are replaced by scar and fat. Microcirculation is also altered, thus reducing the possible delivery of cells or viral vectors. Our data show that, in addition, reduced expression of adhesion molecules further reduces migration of stem cells. This is an important issue, as it strongly suggests that cell therapy protocols in older patients would have much-reduced chances of success in comparison with younger patients.
Mesoangioblasts migrate to skeletal muscle in vivo more efficiently than other stem cells
Stem cells have the ability to migrate to their target tissue through different routes, including circulation. Neural stem cells are recruited to areas of brain injury even when injected in the general circulation (Pluchino et al., 2003); hematopoietic stem cells naturally home to bone marrow and other hematopoietic organs (Rosu-Myles et al., 2005); finally, mesenchymal stem cells can also circulate and engraft inside bone (Bensidhoum et al., 2004). We compared the natural ability of these different stem cells to colonize the damaged muscles of mdx or α-SG–null mice upon intraarterial injection. We found that mesoangioblasts are able to reach damaged muscles more efficiently than the other stem cells tested, including hematopoietic stem cells (although different experimental conditions prevent a direct comparison), which migrate preferentially to liver and spleen.
Enhancing mesoangioblast migratory activity
Previous work has shown that HMGB-1 stimulates proliferation and migration of mesoangioblasts to dystrophic muscles that expresses this molecule (Palumbo et al., 2004). We confirm those data; however, in a screen for factors and cytokines for which mesoangioblasts are known to express receptors (Tagliafico et al., 2004), we observed that SDF-1 and TNF-α are more potent as chemoattractants. Interestingly, our work shows that pretreatment of donor cells with these cytokines is sufficient to activate or increase expression of certain surface proteins necessary for the migration and extravasation process, immediately suggesting a simple ex vivo treatment of cells that does not require expression in target muscles. We further investigated the mechanisms involved in this migration increase through a low-density array hybridization performed with mesoangioblasts pretreated with TNF-α or SDF-1. The role of the different genes modified by the treatment was studied in the transmigration assay using inhibitors or blocking antibodies. This experiment revealed that TNF-α acts via an MMP- and CD44-dependent mechanism, whereas the effect of SDF-1 was mediated by MMPs and αv integrins.
Mesoangioblasts do not normally express many of the leukocytes molecules needed for an efficient rolling and arrest at blood vessels, which may explain why most of the injected cells do not bind efficiently, flow through the muscle capillary net, and are finally trapped inside filter organs. Therefore, L-selectin, α4 integrin, and β2 integrin were expressed in mesoangioblasts, forming dimers with other integrins (such as β1, β7 or αl, αm) already expressed by these cells. Expression of L-selectin and α4 integrin did not alter the myogenic differentiation of mesoangioblasts, whereas expression of β2 integrin did, a fact that precludes future use in vivo. The expression of α4 integrin or L-selectin resulted in an eightfold increase of mesoangioblast transmigration in vitro and a twofold increase in the number of mesoangioblasts that reached the damaged muscles, simultaneously reducing their trapping in filter organs. The combined strategies (i.e., pretreatment with TNF-α of mesoangioblasts expressing α4 integrin) resulted in a 15-fold increase of transmigration through endothelium in vitro and more than fivefold enhanced migration in vivo, with >50% of injected cells now present into the downstream muscles. Immunohistology confirmed these data, as many more modified mesoangioblasts could be detected in muscle interstitium and, later, within muscle fibers (as detected by expression of α-SG) or as satellite cells.
Increased migration corresponds to increased muscle repair and motility
Increasing migration to the target tissue has two positive outcomes: (1) it delivers more cells to repair the target tissue and (2) it reduces the number of cells that are trapped in filter organs. This number is large, and in the case of lungs, it may cause respiratory problems that are not evident in mice. In addition, human mesoangioblasts have a finite number of cell divisions, and this may in the end represent a limiting factor if most of them are wasted outside the target tissue. However, increased migration does not necessarily mean increased tissue repair. In principle, if more cells reach the target tissue, they may undergo a reduced number of divisions in vivo and thus give rise to a similar number of differentiated cells. Our data show that this is not the case.
We previously showed that mesoangioblasts are able to regenerate in part the damaged muscles after three injections and long periods (Sampaolesi et al., 2003). Other types of stem cells have also been shown to reconstitute the skeletal muscle of dystrophic mice to various extents (Corbel et al., 2003; De Bari et al., 2003), but a quantitative analysis was not reported. Mesoangioblasts treated with TNF-α and expressing α4 integrin were able to reach damaged muscles and to produce α-SG 30 d after injection with dramatically increased efficiency, allowing treated α-SG–null mice to improve the performance of exercise. 4 mo after a single injection of modified mesoangioblasts, α-SG–null mice expressed almost 60% of the α-SG detected in a WT mouse. Immunostaining of α-SG showed a partial recovery after 30 d and >90% of well-structured fibers expressing α-SG after 4 mo. This corresponded to a significant increase in the ability to run on a treadmill, despite a unilateral treatment.
In the perspective of a cell therapy in patients, it is important to test whether a similar protocol will also work with human cells. Human mesoangioblasts have been recently isolated from fragments of vessels inside biopsies of postnatal human skeletal muscle. Regarding the use of these cells in the present study, it is important to state that they have a morphology and marker expression similar to their murine counterparts, a finite lifespan (at variance with their murine counterparts), and a similar ability to differentiate into different types of solid mesoderm (unpublished data). Human mesoangioblasts were also exposed to TNF-α, which in the case of human cells was more potent than SDF-1, and transfected with either α4 integrin or L-selectin and, under these conditions, reached skeletal muscles of mdx-SCID mice threefold more efficiently than their corresponding untreated cells. Although these data suggest that further work may still be required to optimize human mesoangioblast migration to skeletal muscle, they clearly indicate that the basic migration mechanisms are similar in mouse and human mesoangioblasts and equally susceptible to enhancement by similar cytokines and adhesion molecules.
In conclusion, our results suggest that pretreatment of mesoangioblasts with TNF-α and the presence of α4 integrin are required for the efficient delivery of stem cells to injured muscles in the course of muscular dystrophy. Pretreatment of stem cells with cytokines is a simple procedure, and inclusion of a relatively small cDNA in a viral vector, designed for gene correction, is also a feasible procedure in the context of autologous cell therapy with engineered stem cells. Together, these strategies represent a novel and simple method for improving the migration of stem cells to damaged tissue and could be tailored in the future to different types of stem cells in different cell therapy protocols requiring migration to other target tissues such as the heart or the bone.
Materials and methods
Reagents
Anti-GFP antibody and anti-isotype control antibodies were purchased from Chemicon. Anti–α4, anti–αv, and anti–β2 integrins; anti–L-selectin; anti-CD44; anti-FGFR1; anti–VCAM-1; and anti–ICAM-1 antibodies were obtained from Serotec. Anti–L-selectin mAb Mel-14 was obtained from American Type Culture Collection. Anti–E- and anti–P-selectin mAbs (RME-1 and RMP-1) were obtained as previously described (Walter et al., 1997; Souza et al., 1999). Anti–MAdCAM-1 and anti-PECAM were provided by E. Butcher (Stanford University, Stanford, CA) and E. Dejana (FIRC Institute of Molecular Oncology, Milan, Italy). Anti–α-SG and anti-myf5 antibodies were purchased from NovoCastra.
Most reagents and some antibodies were purchased from Sigma-Aldrich, unless stated otherwise. All the cytokines used were obtained from PeproTech. GM1489, the MMP inhibitor, was obtained from Calbiochem. Alexa 488 and Alexa F 647 labeling kit were obtained from Invitrogen.
mdx and C57BL10 WT mice were purchased from Charles River Laboratories, and homo-EGFP mice were provided by A. Nagy (Mount Sinai Hospital, Toronto, Canada; Hadjantonakis et al., 1998). α-SG–null mice were a gift from K. Campbell (University of Iowa, Iowa City, IA; Sampaolesi et al., 2003). All mice were handled following institutional guidelines.
Cell cultures
C2C12 mouse myoblasts or L6 rat myoblasts were cultured in Dulbecco's minimal essential medium plus 20% FBS and induced to differentiate in Dulbecco's minimal essential medium plus 2% FBS. D16 mice mesoangioblasts were isolated from the dorsal aorta of mouse embryos (embryonic day 9.5), cloned, and expanded as described previously (Minasi et al., 2002). Mesoangioblasts transduced with a lentiviral vector encoding the GFP protein were used for some experiments. Murine microvascular endothelial H5V cells (a gift form E. Dejana, FIRC Institute of Molecular Oncology) and mouse embryonic fibroblasts 3T3 were grown in Dulbecco's minimal essential medium plus 10% FBS and starved for 12 h before functional experiments. Mesenchymal and hematopoietic stem cells were isolated from homo-EGFP mice using the SpinSep TM kit (StemCell Technologies, Inc.) and checked for purity according to the manufacturer's instructions. Neural stem cells were isolated from the forebrains of embryos (embryonic day 11.5) as previously described (Galli et al., 2000).
Constructs
Murine or human β2 integrin, L-selectin, and α4 integrin mRNA cloned in a pT7T3D-Pac1 vector were purchased from Research Genetics. Inserts were cloned into pIRES2-EGFP expression vector from CLONTECH Laboratories, Inc., and transfected into mice or human mesoangioblasts with Lipofectamine (Invitrogen). GFP-positive mesoangioblasts were sorted and used for the different experiments.
Differentiation assays
Treated mesoangioblasts were cocultured with L6 cells in low serum (2%). After 3 d, dishes were fixed with 4% PFA, and the number of mouse nuclei stained with DAPI inside myosin-positive cells was counted. The percentage of differentiation was calculated against the total number of cells.
Real-time PCR
Total RNA from different organs was isolated with TRIzol protocol (Invitrogen) and reverse transcribed by Taqman kit (Platinum Taq DNA polymerase; Invitrogen). RT-PCR was performed for E- and P-selectin (E-selectin primers: Fw, CCATGTTCCACGTCAAGGACC; Rw, GCCATGTGATAGGCCACAG; and P-selectin primers: Fw, CCAGTATCTAGACCCGAAGG; Rw, GCCATTCTCTCTCCTCGAAACAC), and mRNA expression was analyzed by agarose gel. Alternatively, real-time quantitative PCR was done on a real-time PCR system (Mx3000P; Stratagene). Each cDNA sample was amplified in duplicate by using the SYBR Green Supermix (Bio-Rad Laboratories) for GFP or ɛ chromosome detection (GFP primers: Fw, AAGTTCATCTGCACCACCG; Rw, TCCTTGAAGAAGATGGTGCG; and ɛ chromosome primers: Fw, AAGCGACCCATGAACGCATT; Rw, TTCGGGTATTTCTCTCTGTC) or the Taqman Universal PCR master mixture containing AmpliTaq Gold DNA with commercial primers (Applied Biosystems) for α-SG detection. Data are expressed as the percentage of migrated cells, which is calculated by comparing the concentration of target genes in our sample with the total input of injected cells. For the Taqman assay, the level of α-SG detected represents the specific signal detected in the sample (mesoangioblast- injected α-SG–null mouse tibialis anterior) compared with a positive control (WT mouse tibialis anterior; i.e., 100% of positive fibers).
In vitro transwell migration assay
8-μm transwell filters (Corning) were coated with 1% gelatin, and endothelial cells H5V (previously preactivated by 12 h of exposure to TNF-α) were plated to confluence on them for 24 h. Confluence of the endothelial monolayer was assessed by measuring the diffusion of BSA from the upper to the lower chamber. At the same time, C2C12 were grown on a p24w plate with or without differentiated medium for 0–4 d. 104 mice mesoangioblasts, WT or pretreated or transfected, were plated in Dulbecco's minimal essential medium containing 2% serum on the upper side of transwell chamber 24 h after plating H5V cells, and the chamber was then moved to the wells containing differentiated C2C12 or different cytokines. After 6 h of transmigration, migrated mesoangioblasts on the lower side of the filter were fixed in 4% paraformaldehyde, stained with crystal violet, and counted using an inverted microscope (five random fields of the lower face of the transwell membrane at 20× magnification). The results show migrated cells as a percentage of the total number of input cells.
In vivo migration assay
2- or 8-mo-old WT (injected slowly through all the anterior surface of the tibialis with a 27-gauge needle containing 100 μl of 5 μM ctx [Sigma-Aldrich] 12 h before treatment), mdx, or α-SG–null mice were injected through the right femoral artery with 5 × 105 mouse mesoangioblasts. After 6, 12, or 24 h, animals were killed, and different muscles (quadriceps, gastrocnemius, and tibialis) or filtered organs (liver, lung, and spleen) were collected. RNA was extracted, and a real-time PCR for GFP was performed in all the samples as described (see Real-time PCR). Data are represented as a percentage of migrated cells (percentage of GFP detected) to the different organs relative to the input value.
Intravital microscopy
50 μg of Alexa 488–labeled mAbs anti–E- or anti–P-selectin or anti–MAdCAM-1 were injected through the tail vein of α-SG–null mice. 20 min later, the animal was anesthetized and perfused through the left ventricle with cold PBS (Homeister et al., 1998). Blood vessels of striated muscles were visualized with a silicon-intensified target video camera (VE-1000 SIT; Dage-MTI) and monitor (SSM-125CE; Sony).
Microarray analysis
For mRNA analysis, total RNA was extracted from treated mesoangioblasts using TRIzol. cDNA was prepared by reverse-transcription reaction and hybridized to the GEArray gene expression array MM-010 (SuperArray) for extracellular matrix and adhesion molecules according to the manufacturer's instruction. Data were subjected to densitometric analysis using GEArray Expression Analysis Suite (SuperArray). RNA levels were expressed as the relative density after normalizing the hybridization signal to β-actin.
Western blot
Mesoangioblasts were transfected with the different constructs (see Constructs) and lysed directly in Laemmli buffer on ice. Lysates were resolved on 10% SDS-PAGE under reducing conditions, and proteins were transferred to nitrocellulose membrane (Hybond-ECL; GE Healthcare). Membranes were revealed with anti-GFP and anti–α4 or anti–β2 integrins or anti–L-selectin antibodies.
Flow cytometry
Mesoangioblasts pretreated with different cytokines or transfected with distinct constructs were detached with PBS plus 5 mM EDTA on ice and incubated with antibodies against the different surface molecules for 30 min at 4°C. Cells were then incubated with a Alexa 488–conjugated anti–mouse Ig. Finally, fluorescent samples were analyzed in a FACSCalibur flow cytometer (Becton Dickinson).
Cytokines measurements
Supernatants from fibroblasts, myoblasts, or myotube cultures were collected after 4 d of culture and analyzed with the Beadlyte mouse multicytokine-detection system (Upstate) for measuring cytokines according to the manufacturer's instructions. Muscles from different animal models were homogenized and cytokines were measured using the same kit protocol.
Inmunohistology
Tibialis anterior muscles of α-SG–null mice were removed from mice previously injected with mesoangioblasts and frozen in liquid N2 cooled isopentane. Serial muscle sections were fixed with 4% PFA, permeabilized, satured, and immunostained as previously described (Sampaolesi et al., 2003) with anti-GFP, anti–α-SG, anti-laminin, anti-myosin, anti-PECAM, or anti-myf5 antibodies. Alexa 488 or 594 or Cyane (Invitrogen) were used as secondary staining Ig and DAPI for nuclear staining. Isotype control antibodies were used as negative staining. Images were taken with a microscope (S100 TV; Carl Zeiss MicroImaging, Inc.).
Treadmill test
α-SG–null mice (n = 4 for each of the four groups) injected or not with control or treated mesoangioblasts were run on a treadmill (Columbus Instruments) set at 7 m/min for 2 min before and after 1 mo from injection. WT C57 mice are also presented as healthy control. The back of the treadmill was equipped with a grid that discharged a mild current, a stimulus designed to motivate the animal to keep running on the treadmill. Performance was measured by the number of times a mouse failed to stay on the running belt and fell into the stimulus grid. Running time was limited to 2 min because the majority of the α-SG–null mice could not run any longer.
Statistical analysis
Statistical significance of the differences between the percentage values was assessed by using the Kruskal-Wallis one-way analysis of variance by ranks test. α represents the significance in each independent case.
Acknowledgments
We thank E. Dejana, T. Lapidot, and R. Pardi for advice and help with the work; K. Campbell for the α-SG–null mice; and E. Butcher for the anti–MadCAM-1 mAb.
This work was supported by grants from Cassa di Risparmio Provincie Lombarde, Telethon, Association Francoise contra les Myopathies, Muscular Dystrophy Association, Duchenne Parent Project, Fondation Leducq, European Community, CariVerona, and Italian Ministries of Health and Research. Firb. B.G. Galvez was supported by a Programme 3+3 fellowship from the Centro Nacional de Investigaciones Cardiovasculares, Spain.
Abbreviations used in this paper: ctx, cardiotoxin; HMGB, high mobility group box; ICAM, intercellular adhesion molecule; IL, interleukin; mdx, X chromosome–linked muscular dystrophy; MMP, metalloproteinase; PECAM, platelet endothelial cell adhesion molecule; SDF, stromal-derived factor; SG, sarcoglycan; VCAM, vascular cell adhesion molecule; WT, wild-type.
References
- Beauchamp, J.R., L. Heslop, D.S.W. Yu, S. Tajbakhsh, R.G. Kelly, A. Wernig, M.E. Buckingham, T.A. Partridge, and P.S. Zammit. 2000. Expression of CD34 and Myf5 defines the majority of quiescent adult skeletal muscle satellite cells. J. Cell Biol. 151:1221–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensidhoum, M., A. Chapel, S. Francois, C. Demarquay, C. Mazurier, L. Fouillard, S. Bouchet, J. Bertho, P. Gourmelon, J. Aigueperse, et al. 2004. Homing of in vitro expanded Stro-1-or Stro-1+ human mesenchymal stem cells into the NOD/SCID mouse and their role in supporting human CD34 cell engraftment. Blood. 103:3313–3319. [DOI] [PubMed] [Google Scholar]
- Butcher, E. 1991. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 67:1033–1036. [DOI] [PubMed] [Google Scholar]
- Corbel, S., A. Lee, L. Yi, J. Duenas, T. Brazelton, H. Blau, and F. Rossi. 2003. Contribution of hematopoietic stem cells to skeletal muscle. Nat. Med. 9:1528–1532. [DOI] [PubMed] [Google Scholar]
- De Bari, C., F. Dell'Accio, F. Vandenabeele, J. Vermeesch, J. Raymackers, and F. Luyten. 2003. Skeletal muscle repair by adult human mesenchymal stem cells from synovial membrane. J. Cell Biol. 160:909–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durbeej, M., and K. Campbell. 2002. Muscular dystrophies involving the dystrophin-glycoprotein complex: an overview of current mouse models. Curr. Opin. Genet. Dev. 12:349–361. [DOI] [PubMed] [Google Scholar]
- Fu, S., and J. Liesveld. 2000. Mobilization of hematopoietic stem cells. Blood Rev. 14:205–218. [DOI] [PubMed] [Google Scholar]
- Galli, R., U. Borello, A. Gritti, M. Minasi, C. Bjornson, M. Coletta, M. Mora, M.D. Angelis, R. Fiocco, G. Cossu, and A. Vescovi. 2000. Skeletal myogenic potential of human and mouse neural stem cells. Nat. Neurosci. 3:986–991. [DOI] [PubMed] [Google Scholar]
- Grabovsky, V., S. Feigelson, C. Chen, D. Bleijs, A. Peled, G. Cinamon, F. Baleux, F. Arenzana-Seisdedos, T. Lapidot, Y. van Kooyk, et al. 2000. Subsecond induction of α4 integrin clustering by immobilized chemokines stimulates leukocyte tethering and rolling on endothelial vascular cell adhesion molecule 1 under flow conditions. J. Exp. Med. 192:495–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjantonakis, A., M. Gertsenstein, M. Ikawa, M. Okabe, and A. Nagy. 1998. Generating green fluorescent mice by germline transmission of green fluorescent ES cells. Mech. Dev. 76:79–90. [DOI] [PubMed] [Google Scholar]
- Homeister, J., M. Zhang, P. Frenette, R. Hynes, D. Wagner, J. Lowe, and R. Marks. 1998. Overlapping functions of E- and P-selectin in neutrophil recruitment during acute inflammation. Blood. 92:2345–2352. [PubMed] [Google Scholar]
- Laterveer, L., I. Lindley, D. Heemskerk, J. Camps, E. Pauwels, R. Willemze, and W. Fibbe. 1996. Rapid mobilization of hematopoietic progenitor cells in rhesus monkeys by a single intravenous injection of interleukin-8. Blood. 87:781–788. [PubMed] [Google Scholar]
- Minasi, M., M. Riminucci, L.D. Angelis, U. Borello, B. Berarducci, A. Innocenzi, A. Caprioli, D. Sirabella, M. Baiocchi, R.D. Maria, et al. 2002. The meso-angioblast: a multipotent, self-renewing cell that originates from the dorsal aorta and differentiates into most mesodermal tissues. Development. 129:2773–2783. [DOI] [PubMed] [Google Scholar]
- Mohle, R., M. Moore, R. Nachman, and S. Rafii. 1997. Transendothelial migration of CD34+ and mature hemopoietic cells: an in vitro study using a bone marrow endothelial cell line. Blood. 89:72–79. [PubMed] [Google Scholar]
- Palumbo, R., M. Sampaolesi, F.D. Marchis, R. Tonlorenzi, S. Colombetti, A. Mondino, G. Cossu, and M. Bianchi. 2004. Extracellular HMGB1, a signal of tissue damage, induces mesoangioblast migration and proliferation. J. Cell Biol. 164:441–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peled, A., V. Grabovsky, L. Habler, J.S. Arenzana-Seisdedos, I. Petit, H. Ben-Hur, T. Lapidot, and R. Alon. 1999. The chemokine SDF-1 stimulates integrin-mediated arrest of CD34+ cells on vascular endothelium under shear flow. J. Clin. Invest. 104:1199–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluchino, S., A. Quattrini, E. Brambilla, A. Gritti, G. Salani, G. Dina, R. Galli, U.D. Carro, S. Amadio, A. Bergami, et al. 2003. Injection of adult neurospheres induces recovery in a chronic model of multiple sclerosis. Nature. 422:688–694. [DOI] [PubMed] [Google Scholar]
- Rosu-Myles, M., E. Stewart, J. Trowbridge, C. Ito, P. Zandstra, and M. Bhatia. 2005. A unique population of bone marrow cells migrates to skeletal muscle via hepatocyte growth factor/c-met axis. J. Cell Sci. 118:4343–4352. [DOI] [PubMed] [Google Scholar]
- Sampaolesi, M., Y. Torrente, A. Innocenzi, R. Tonlorenzi, G. D'Antona, M. Pellegrino, R. Barresi, N. Bresolin, M.D. Angelis, K. Campbell, et al. 2003. Cell therapy of alpha-sarcoglycan null dystrophic mice through intra-arterial delivery of mesoangioblasts. Science. 301:487–492. [DOI] [PubMed] [Google Scholar]
- Souza, H., C. Elia, J. Spencer, and T. MacDonald. 1999. Expression of lymphocyte-endothelial receptor-ligand pairs, alpha4beta7/MAdCAM-1 and OX40/OX40 ligand in the colon and jejunum of patients with inflammatory bowel disease. Gut. 45:856–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliafico, E., S. Brunelli, A. Bergamaschi, L.D. Angelis, R. Scardigli, D. Galli, R. Battini, P. Bianco, S. Ferrari, G. Cossu, and S. Ferrari. 2004. TGFbeta/BMP activate the smooth muscle/bone differentiation programs in mesoangioblasts. J. Cell Sci. 117:4377–4388. [DOI] [PubMed] [Google Scholar]
- Walter, U., L. Ayer, A. Manning, P. Frenette, D. Wagner, R. Hynes, and B. Wolitzky. 1997. Generation and characterization of a novel adhesion function blocking monoclonal antibody recognizing both rat and mouse E-selectin. Hybridoma. 16:355–361. [DOI] [PubMed] [Google Scholar]