Abstract
Adult skeletal muscle is able to repeatedly regenerate because of the presence of satellite cells, a population of stem cells resident beneath the basal lamina that surrounds each myofiber. Little is known, however, of the signaling pathways involved in the activation of satellite cells from quiescence to proliferation, a crucial step in muscle regeneration. We show that sphingosine-1-phosphate induces satellite cells to enter the cell cycle. Indeed, inhibiting the sphingolipid-signaling cascade that generates sphingosine-1-phosphate significantly reduces the number of satellite cells able to proliferate in response to mitogen stimulation in vitro and perturbs muscle regeneration in vivo. In addition, metabolism of sphingomyelin located in the inner leaflet of the plasma membrane is probably the main source of sphingosine-1-phosphate used to mediate the mitogenic signal. Together, our observations show that sphingolipid signaling is involved in the induction of proliferation in an adult stem cell and a key component of muscle regeneration.
Introduction
The syncytial myofiber is the functional unit of skeletal muscle. As with many other adult tissues, skeletal muscle contains resident stem cells, termed satellite cells because of their location on the periphery of myofibers under the surrounding basal lamina (Mauro, 1961; for review see Zammit and Beauchamp, 2001). Satellite cells proliferate to provide myonuclei to growing myofibers before becoming quiescent in mature muscle (Schultz et al., 1978). Fulfillment of satellite cell functions of maintenance, hypertrophy, and repair requires that they first be activated to enter the cell cycle and produce large numbers of myoblast progeny. Most of these subsequently differentiate and either fuse with existing myofibers or form new myotubes (Snow, 1978), but others adopt an alternative fate and self-renew to maintain the satellite cell pool (Zammit et al. 2004; Collins et al., 2005).
What controls the crucial transition of satellite cells from quiescence to proliferation remains largely unknown. Various stimuli released from crushed myofibers, invading macrophages, and connective tissue have been proposed to trigger satellite cell activation (for review see Chargé and Rudnicki, 2004). One of these signals is hepatocyte growth factor (HGF; Tatsumi et al., 1998), and members of the FGF family have also been proposed to recruit satellite cells to division (Bischoff, 1986; Yablonka-Reuveni et al., 1999). Because the receptors for both HGF (c-met) and FGF are members of the tyrosine kinase family of receptors, attention has been directed toward classical kinase-mediated signaling in control of satellite cell activation (Yablonka-Reuveni et al., 1999; Shefer et al., 2001; Jones et al., 2005) and proliferation in myogenic cells (Jones et al., 2001; Tortorella et al., 2001).
Over the last few years, the importance of sphingolipid signaling has begun to be understood (for review see Spiegel and Milstien, 2003). Sphingosine-1-phosphate (S1P) and homologous phosphorylated long-chain sphingoids act in diverse organisms such as mammals, worms, flies, slime mold, yeast, and plants. In mammals, S1P is mitogenic for several cell types, including fibroblasts and endothelial cells (Zhang et al., 1991; Olivera and Spiegel, 1993; Olivera et al., 1999). In addition to this role in cell division and survival, S1P is involved in processes such as calcium homeostasis and cell migration during angiogenesis and cardiac development, as well as in the adult immune system (for review see Spiegel and Milstien, 2003). In contrast, other sphingolipids such as ceramide and sphingosine are associated with cell growth arrest, stress responses, and apoptosis (for review see Hannun and Obeid, 2002).
Recent studies have revealed that sphingolipids are also active in muscle. Defects in muscle development and integrity in Drosophila melanogaster are observed when the level of S1P is perturbed by targeted deletion of S1P lyase, an enzyme responsible for the irreversible degradation of S1P (Herr et al., 2003). Sphingosine can also affect muscle contraction by modulating the function of certain calcium channels (for review see Sabbadini et al., 1999), whereas ceramide has an inhibitory effect on insulin-like growth factor I–induced protein synthesis in mouse myogenic C2C12 cells (Strle et al., 2004). S1P affects Ca2+ homeostasis and cytoskeletal rearrangement (Formigli et al., 2002) in C2 cells and stimulates differentiation in this cell line (Donati et al., 2005).
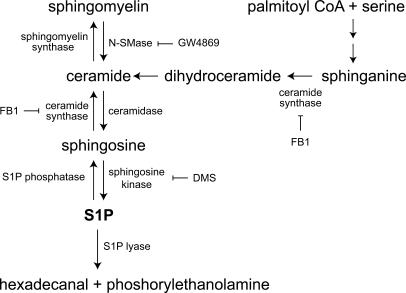
Sphingomyelin is a precursor of ceramide, sphingosine, and S1P, which are all components of the same metabolic pathway (Fig. 1). Sphingomyelin is one of the major lipid components of cell membranes in animals, with significant amounts in the plasma membrane (for review see van Meer and Holthuis, 2000). Although the location of sphingomyelin used to generate signaling molecules such as S1P is unclear, the inner leaflet of the plasma membrane has been suggested as a source (Kent et al., 1974; Linardic and Hannun, 1994; Andrieu et al., 1996; Zhang et al., 1997).
Figure 1.
Simplified overview of sphingolipid metabolism. De novo synthesis of sphingolipids begins with the serine palmitoyltransferase catalyzed condensation of serine with palmitoyl CoA. The resulting 3-ketosphinganine is reduced to sphinganine and then subsequently acylated to form dihydroceramide by ceramide synthase (inhibited by FB1). Ceramide is then produced from dihydroceramide by the insertion of the trans 4,5 double bond by a desaturase. Ceramide can be reversibly converted to either sphingomyelin by sphingomyelin synthase or deacylated to sphingosine by ceramidase. S1P is then generated from sphingosine by the phosphorylation of the primary hydroxyl group by sphingosine kinase (inhibited by DMS). Sphingomyelin can act as a reservoir for sphingolipids and can be cleaved to ceramide by sphingomyelinase (inhibited by GW4869). S1P degradation is mediated by either the reversible dephosphorylation back to sphingosine by specific S1P phosphatases or the irreversible degradation by S1P lyase to hexadecenal and phosphorylethanolamine.
We have recently demonstrated the presence of high concentrations of sphingomyelin in the plasma membrane of quiescent, but not proliferating, satellite cells (Nagata et al., 2006). Here, we focus on the role of S1P as a novel regulator of satellite cells and muscle regeneration. S1P can induce satellite cells to enter the cell cycle, whereas inhibiting the sphingolipid signaling cascade that generates S1P significantly reduces the number of satellite cells able to divide in response to mitogen stimulation. Sphingomyelin is hydrolyzed by neutral sphingomyelinase (N-SMase) and can then be further metabolized to S1P, to mediate the mitogenic signal. By use of a novel combination of the sphingomyelin binding protein lysenin (Sekizawa et al. 1997; Yamaji et al. 1998; Kobayashi et al., 2004) as a cytochemical probe, together with selective sphingomyelin digestion, we demonstrate that the main source of S1P is sphingomyelin located in the inner leaflet of the plasma membrane. Crucially, inhibiting S1P production after muscle damage greatly perturbs subsequent muscle regeneration. Together, our observations show the central role that sphingolipid signaling plays in mediating the entry of satellite cells into the cell cycle and muscle regeneration.
Results
We initially used the reserve cell model to investigate the role of lipid signaling pathways in myogenic cell activation, before examining its role in primary satellite cells and muscle regeneration in vivo. The kinetics of myogenesis in mouse C2 cells is well defined (Yablonka-Reuveni and Rivera, 1997). When C2 cells are cultured in mitogen-poor conditions, the majority differentiate and fuse to form multinucleated myotubes. Some, however, stop dividing, down-regulate MyoD, and escape immediate differentiation to form reserve cells, but retain the ability to reenter the cell cycle when stimulated, thus providing a model of myogenic cell activation (Yoshida et al., 1998).
Myogenic reserve cells can be stimulated to divide by S1P
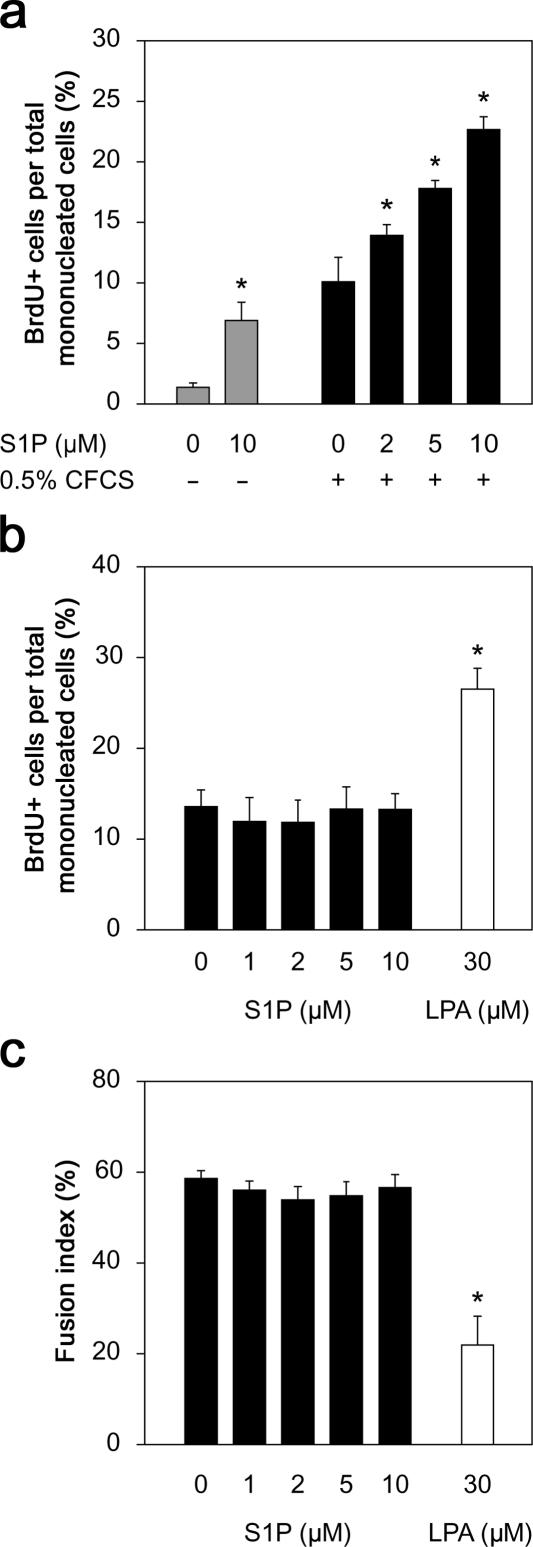
Proliferating C2C12 cells were switched to DME containing 1% horse serum for 5 d, after which few cells incorporated BrdU (0.9 ± 1.4%; Fig. 2 a). To first determine whether lipid signaling was involved in reserve cell activation, 10 μM S1P was added to differentiation medium on day 5 for 24 h, which resulted in an approximately sevenfold increase in the number of reserve cells entering the cell cycle (Fig. 2 a). The use of defined medium has previously been used to analyze satellite cell activation (Yablonka-Reuveni and Rivera, 1994). To deplete lipids in the culture medium, FCS was incubated with charcoal (Lee et al., 1998). Incubation with the charcoal-stripped FCS (CFCS) resulted in an increase of ∼10-fold in BrdU incorporation, but addition of 2–10 μM S1P further increased the number of cells synthesizing DNA, in a dose-dependent manner (Fig. 2 a). When cycling C2C12 cells were switched to differentiation medium, the level of BrdU incorporation dropped significantly, and the presence of 1–10 μM S1P made no difference (Fig. 2 b). The ability of these cells to differentiate in response to serum withdrawal was similarly unaffected by S1P (Fig. 2 c), consistent with a recent study (Donati et al., 2005). Therefore, S1P is important for entry of myogenic cells into the cell cycle but does not maintain division or prevent differentiation in the absence of other mitogens. In contrast, lysophosphatidic acid did not induce reserve cells to proliferate (unpublished data), but this lipid did perpetuate cell division (Fig. 2 b) and inhibit differentiation (Fig. 2 c) when serum was withdrawn (Yoshida et al., 1996).
Figure 2.
Exogenous S1P can directly induce DNA synthesis in quiescent myogenic cells. In the absence of serum stimulation, only ∼1% of reserve cells incorporate BrdU, but the addition of 10 μM S1P increases the number of dividing cells approximately sevenfold (a). The addition of 0.5% CFCS also stimulates cell division, but the proportion of cells incorporating BrdU is significantly increased by S1P in a dose-dependent manner (a). To determine whether S1P also perpetuated proliferation in a manner similar to other lipids, such as lysophosphatidic acid (LPA), proliferating C2C12 cells were switched to differentiation medium containing 0–10 μM S1P or 30 μM LPA and cultured with daily medium changes for 3 d. After a 3-h BrdU pulse, either cells were immunostained for BrdU (b) or the percentage of nuclei incorporated in myotubes was determined (fusion index; c). S1P was unable to perpetuate division after mitogen withdrawal or prevent differentiation, in contrast to LPA. Data presented are the mean percentage ± SEM from four independent experiments. Asterisks indicate that data are statistically significant using a t test (P < 0.01).
S1P production is required for reserve cells to enter the cell cycle
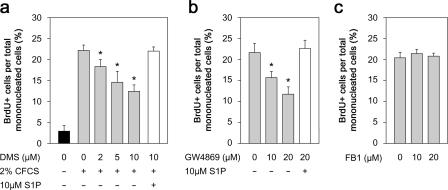
The cellular level of S1P is low and controlled by the balance between synthesis and degradation (Fig. 1). S1P is generated from sphingosine by phosphorylation of the primary hydroxyl group by sphingosine kinase. To examine the role of S1P in myogenic activation, we first blocked its synthesis from sphingosine using N,N-dimethylsphingosine (DMS; Yatomi et al., 1996; Edsall et al., 1998), which inhibits both mammalian isozymes of sphingosine kinase. BrdU incorporation by reserve cells in response to CFCS was inhibited by DMS in a dose-dependent manner, such that ∼50% failed to enter the cell cycle in the presence of 10 μM DMS (Fig. 3 a). Importantly, addition of 10 μM S1P counteracted the inhibitory effect of the highest concentration of DMS (Fig. 3 a), demonstrating that the reduction in the number of cells incorporating BrdU arose specifically from the block on S1P generation and that restoration of S1P levels was sufficient to stimulate division.
Figure 3.
S1P is generated from sphingomyelin. When reserve cells were pretreated with DMS to inhibit sphingosine kinase activity before stimulation with 2% CFCS and pulsed with BrdU, the proportion of proliferating cells was significantly reduced, indicating that S1P was being produced from sphingosine during entry to the cell cycle (a). 10 μM S1P overcame the DMS-induced block on S1P synthesis and restored proliferation, showing that the DMS was specifically inhibiting sphingosine kinases (a). Sphingosine is derived from ceramide, of which there are two main sources, sphingomyelin cleavage by SMases or de novo formation (Fig. 1). Reserve cells were treated with GW4869 for 45 min to inhibit the N-SMase catalyzed synthesis from sphingomyelin and then stimulated with 2% CFCS and pulsed with BrdU. Immunostaining for BrdU showed that the number of proliferating cells was significantly reduced after GW4869 treatment (b). The inhibition by GW4869 was specific to the generation of S1P, as S1P overcame the block (b). In contrast, blocking de novo synthesis of ceramide by inhibiting ceramide synthase with FB1 had no effect on reserve cell activation (c). Mean percentage ± SEM of BrdU-positive cells per total mononucleated cells from four independent experiments. Asterisks indicate that data are statistically significant using a t test (P < 0.05).
Sphingomyelin is the origin of sphingosine for mediating reserve cell proliferation
We next examined the mechanism of generation of sphingosine in reserve cells in response to CFCS stimulation. Sphingosine is produced by deacylation of ceramide by ceramidase (Fig. 1). We used pharmacological inhibitors to target the two main sources of ceramide, namely, sphingomyelin cleavage by sphingomyelinases and de novo formation from sphinganine by ceramide synthase (Fig. 1). N-SMase is a regulator of lipid signaling (for review see Levade and Jaffrezou, 1999) and is specifically inhibited by GW4869 (Luberto et al., 2002). Incorporation of BrdU by reserve cells in response to CFCS stimulation was as effectively diminished by GW4869 as by DMS (Fig. 3 b). This was again counteracted by addition of S1P, indicating that the inhibition was specific to S1P production and was not significantly affecting other signaling pathways (Fig. 3 b). Therefore, inhibiting the metabolic pathway responsible for generating S1P at two separate steps resulted in a similar reduction in the number of cells entering the cell cycle.
Synthesis of ceramide by the de novo pathway was not a major source of S1P because the ceramide synthase inhibitor fumonisin B1 (FB1; Merrill et al., 1996) had no effect on the number of reserve cells induced to incorporate BrdU in response to CFCS exposure (Fig. 3 c). Moreover, when reserve cells were exposed to both FB1 and GW4869 before CFCS stimulation, the proportion of reserve cells induced to synthesize DNA was reduced to the same level as with GW4869 alone (unpublished data). This indicates that S1P synthesis from sphingomyelin, not prevention of S1P degradation to ceramide, was the predominant pathway responsible for induction of cell division. Collectively, these results demonstrate that reserve cell activation requires the cleavage of sphingomyelin to ultimately produce S1P, which mediates the mitogenic signal.
Separate visualization of sphingomyelin in the outer and inner leaflet of the plasma membrane
Sphingomyelin is predominantly distributed at the outer leaflet of the plasma membrane (Koval and Pagano, 1991), but a distinct signaling pool has been postulated to be located in the inner leaflet (Linardic and Hannun, 1994; Andrieu et al., 1996; Zhang et al., 1997). Because the recently cloned N-SMase2 locates to the plasma membrane (Hofmann et al., 2000; Karakashian et al., 2004), it is possible that most stimulus-induced sphingomyelin degradation is processed at this site.
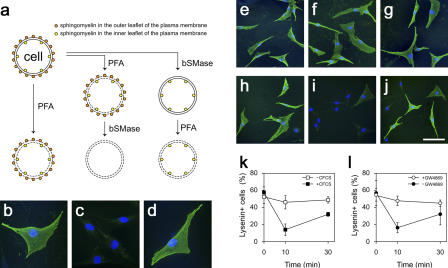
To directly investigate the dynamics of sphingomyelin levels in individual reserve cells, we used the sphingomyelin-specific binding protein lysenin as a cytochemical marker (Sekizawa et al., 1997; Yamaji et al., 1998; Kobayashi et al., 2004) in combination with selective sphingomyelin digestion by bacterial sphingomyelinase (bSMase; Fig. 4 a). As we showed previously (Nagata et al., 2006), when reserve cells are fixed/permeabilized with 4% PFA (Sugii et al., 2003), incubated with 0.2 or 10 μg/ml lysenin, and immunostained using lysenin-specific antisera, the surface of most reserve cells is strongly labeled, revealing high levels of sphingomyelin in the plasma membrane (Fig. 4 b). Complete loss of such lysenin immunostaining after incubation of fixed/permeabilized cells in 100 mU/ml bSMase confirms that binding of lysenin to sphingomyelin is specific (Fig. 4 c). However, when live reserve cells were incubated for 2 h in bSMase, to prevent the enzyme from entering the cells, subsequent fixation/permeabilization before probing with lysenin revealed that sphingomyelin had been protected in the inner leaflet under the entire cell surface (Fig. 4 d). Such inner leaflet staining required 10 μg/ml lysenin, though, suggesting that there was a lower concentration of sphingomyelin in the inner leaflet than in the outer leaflet of the plasma membrane. In this way, the combination of digestion of specific sphingomyelin pools and probing with lysenin permits us to visualize, for the first time, two distinct pools of sphingomyelin in reserve cells, one located in the outer leaflet of the plasma membrane on the cell surface and a second on the inside of the cell in the inner leaflet (Fig. 4, a–d).
Figure 4.
Sphingomyelin in the inner leaflet of the plasma membrane is the source of S1P. The dynamics of sphingomyelin during reserve cell activation was examined using bSMase to selectively remove sphingomyelin from either the outer or inner leaflet while monitoring sphingomyelin levels with lysenin (a). Reserve cells were fixed/permeabilized with 4% PFA, probed with 0.2 μg/ml lysenin, and immunostained (green), and all nuclei were identified using DAPI (blue). The vast majority of reserve cells bound lysenin, showing sphingomyelin on their surface (b). When reserve cells were fixed/permeabilized and then treated with 100 mU/ml bSMase to digest sphingomyelin on both the outer and inner leaflet, sphingomyelin was no longer detectable (c). To distinguish between sphingomyelin on the inner and outer surfaces of the cell membrane, live reserve cells were treated with bSMase so that only external sphingomyelin was accessible to digestion. Lysenin (10 μg/ml) binding then revealed sphingomyelin located only in the inner leaflet (d). To determine whether sphingomyelin in the outer leaflet was metabolized to generate S1P, reserve cells were activated with 2% CFCS and either fixed/permeabilized immediately (e) or after 10 (f) or 30 min (g). No change in lysenin immunostaining was observed (e–g). To specifically assay sphingomyelin in the inner leaflet, live reserve cells were incubated with bSMase for 2 h to digest sphingomyelin in the outer leaflet before activation with 2% CFCS. Cells were then fixed/permeabilized, probed with lysenin, and immunostained immediately (h) or after 10 (i) or 30 min (j) of stimulation. There was a transient drop in the number of reserve cells with lysenin immunostaining after 10 min of stimulation (i, quantified in k [closed squares]), whereas unstimulated reserve cells exhibited no such drop (k, open squares). Live reserve cells treated with bSMase for 2 h and then incubated with 20 μM GW4869 before activation with 2% CFCS (l) showed that inhibition of N-SMase prevented the transient loss of sphingomyelin in the inner leaflet by blocking its cleavage (l, open circles). Data were obtained from three independent experiments and are expressed as mean percentage ± SEM of lysenin-positive cells per total mononucleated cells. Bar: (b–d) 50 μm; (e–j) 100 μm.
Sphingomyelin in the inner leaflet of the plasma membrane is metabolized to generate S1P
The ability to separately visualize these two pools of sphingomyelin at the plasma membrane enabled us to determine which was involved in the lipid-signaling cascade controlling reserve cell activation. Sphingomyelin turnover in response to extracellular stimuli is usually rapid: N-SMase catalyzed sphingomyelin cleavage, occurring within 10 min (Andrieu-Abadie and Levade, 2002). To explore this event, sphingomyelin levels were monitored in reserve cells for 30 min after stimulation with CFCS. No changes in overall lysenin immunostaining were observed, indicating that sphingomyelin on the cell surface remained constant (Fig. 4, e–g).
Detection of changes of sphingomyelin content of the inner leaflet, however, would be masked by the stable amounts in the outer leaflet of the plasma membrane, which we were able to eliminate by incubation of live reserve cells with bSMase for 2 h before stimulation with CFCS. Such removal of sphingomyelin solely from the outer leaflet of the plasma membrane did not prevent cell cycle entry, as these cells incorporated BrdU as efficiently as untreated cells (unpublished data). After 10 min of CFCS stimulation, however, a marked drop was found in the proportion of cells with high inner leaflet sphingomyelin levels, which largely recovered by 30 min (Fig. 4, h–k). This transient decrease in sphingomyelin in the inner leaflet during activation (Fig. 4, h–k) was prevented by the addition of GW4869 (Fig. 4 l). This shows that the fall in sphingomyelin levels at the inner leaflet of the plasma membrane in response to CFCS stimulation is caused by the cleavage of sphingomyelin by N-SMase. Because GW4869 significantly reduces the number of cells entering the cell cycle after stimulation (Fig. 3 b), this shows that sphingomyelin in the inner leaflet is a major source of mitogenic S1P.
Entry of satellite cells into the cell cycle requires sphingolipid signaling
Having established that S1P signaling plays a role in the induction of proliferation in C2 reserve cells, we next investigated the relevance of this pathway in primary satellite cells. Viable myofibers can be isolated from muscle, complete with their retinue of quiescent satellite cells (Rosenblatt et al., 1995). If these myofibers are not exposed to mitogens and are maintained in basal medium (e.g., DME/BSA), then the vast majority of satellite cells do not enter the cell cycle. When these myofibers are cultured in mitogen-rich medium, however, their associated satellite cells proliferate and then differentiate, modeling the early events of muscle regeneration (Yablonka-Reuveni and Rivera, 1994; Beauchamp et al., 2000; Zammit et al., 2004).
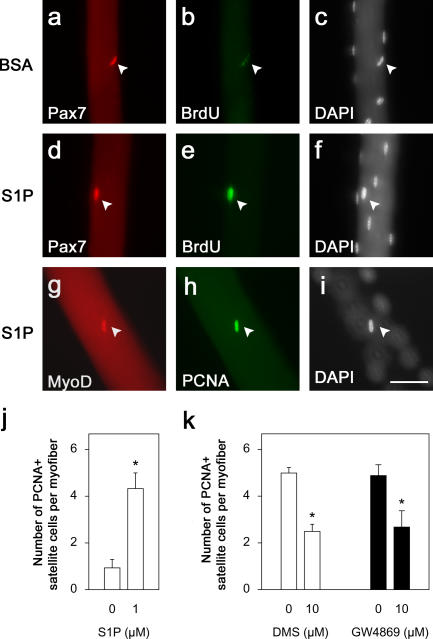
To determine whether exposure to S1P can directly elicit proliferation in satellite cells, myofibers were cultured in either DME/BSA (Fig. 5, a–c) or DME/BSA/1 μM S1P (Fig. 5, d–f) plus BrdU for 48 h. Coimmunostaining for Pax7 (Seale et al., 2000) and BrdU (Fig. 5, d–f) or proliferating cell nuclear antigen (PCNA) and MyoD (Fig. 5, g–j) showed that S1P significantly increased the number of satellite cells entering the cell cycle.
Figure 5.
S1P is required for inducing entry of satellite cells into the cell cycle. Isolated myofibers were cultured in DME/BSA (a–c) or DME/BSA/ 1 μM S1P (d–f) in the presence of BrdU. Myofibers were fixed 48 h later, and their associated satellite cells were coimmunostained for Pax7 (red) and BrdU (green; arrowheads in a–f). Similarly, immunostaining for MyoD (red) and PCNA (green; arrowheads in g–i) showed that there were significantly more satellite cells entering the cell cycle in the presence of S1P than in DME/BSA (j). All nuclei present were identified with DAPI (c, f, and i). Bar, 50 μm. To determine whether S1P is generated in response to mitogen stimulation during satellite cell activation, isolated myofibers were treated with 10 μM DMS in DME for 45 min before their associated satellite cells were stimulated with CEE/serum and analyzed for the expression of PCNA (k). In the absence of DMS, satellite cells were readily activated and practically all entered the cell cycle, but after exposure to the inhibitor, significantly less did so (k). To determine whether sphingomyelin was the source of S1P, isolated myofibers were incubated with 10 μM GW4869 to inhibit N-SMase activity before mitogen stimulation and then immunostained. Again, there was a significant reduction in the number of satellite cells entering the cell cycle (k), showing that S1P is derived from sphingomyelin. Data represent the mean ± SEM of immunostained cells per myofiber from three independent experiments (20 myofibers per experiment). Asterisks indicate that data are statistically significant using a t test (P < 0.05).
Generation of S1P is central to the induction of satellite cell proliferation
To examine whether S1P was generated as part of the response of satellite cells to mitogen stimulation, we again used the sphingosine synthase inhibitor DMS. Myofibers and their associated satellite cells were immunostained with Pax7 to identify the total number of satellite cells present. Others were stimulated with serum/chick embryo extract for 24 h and then immunostained for PCNA, which showed that virtually all satellite cells had entered the cell cycle. However, when myofibers were stimulated in the presence of 10 μm DMS, the number of PCNA-positive satellite cells was reduced by ∼50% (Fig. 5 k).
To examine whether the ultimate source of S1P was sphingomyelin, we again applied GW4869 to suspensions of isolated myofibers and associated satellite cells stimulated with serum/chick embryo extract. Specific inhibition of N-SMase with GW4869 (Luberto et al., 2002) again reduced the number of dividing PCNA-positive cells by ∼50% (Fig. 5 k), confirming that entry into cell cycle is mediated by S1P generated from sphingomyelin.
Inhibition of sphingolipid signaling perturbs muscle regeneration
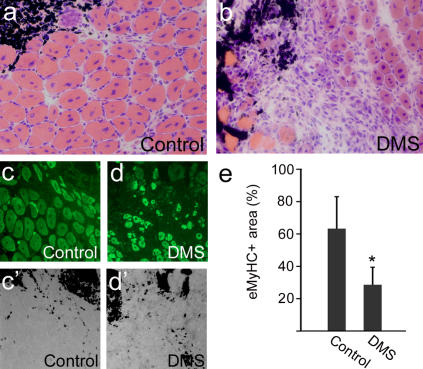
Having established that sphingolipid signaling is involved in the induction of proliferation in satellite cells in vitro, we tested whether muscle regeneration in vivo was affected by inhibition of this pathway. Muscle damage was induced by injection of cardiotoxin into both tibialis anterior (TA) muscles of adult mice, and DMS was administered to the right TA to inhibit sphingosine kinase and, thus, S1P production, with the left serving as a control. 7 d later, the muscles were removed, cryosectioned, and either stained with hematoxylin and eosin or immunostained for embryonic myosin heavy chain (eMyHC). Control muscles contained numerous nascent myotubes identified by central nucleation and expression of eMyHC (Fig. 6, a and c). In contrast, muscles forced to regenerate in the presence of DMS to inhibit S1P production showed poor repair with fewer myotubes (Fig. 6, b and d). Quantification of the area of muscle occupied by regenerating myofibers showed that the presence of the sphingosine kinase inhibitor significantly reduced the amount of regenerated muscle (Fig. 6 e).
Figure 6.
Inhibition of S1P synthesis perturbs muscle regeneration. TA muscles were induced to regenerate by injection of cardiotoxin together with India ink to mark the injection site. At the same time, the right TA muscle was also injected with DMS to inhibit sphingosine kinase activity, thus preventing S1P synthesis. The left TA muscle served as a control, and only carrier DMSO was injected. 7 d later, both muscles were removed, cryosectioned, and analyzed. Hematoxylin and eosin staining showed that control left TA muscles had regenerated successfully (a), with many large new myofibers identified by the central location of their myonuclei, in the vicinity of the injection site, as shown by the presence of the black India ink (a, top right). In contrast, even 7 d after injection, right TA muscles in which DMS had been administered exhibited severely retarded regeneration (b). To quantify regenerated muscle, sections were immunostained for eMyHC to identify new myofibers (c and d) in the vicinity of the injection site (c′ and d′ bright field image included to show location of India ink). The area occupied by regenerating myofibers was determined from several such experiments, and data were pooled and expressed as a percentage ± SEM of the total area assayed (e). This showed that administration of DMS resulted in significantly less regenerating myofibers. Asterisks indicate that data are statistically significant using a t test (P < 0.05).
Together, these observations show that S1P signaling is clearly involved in muscle regeneration. Although administration of this pharmacological inhibitor may not only have affected S1P synthesis in satellite cells, the resulting perturbation in myofiber regeneration is consistent with the observations that inhibiting the generation of S1P significantly reduces the number of satellite cells able to enter the cell cycle in response to stimulation in vitro (Fig. 5).
Discussion
Precise control of skeletal muscle size, along with rapid and appropriate repair and maintenance, is predominantly a function of the satellite cell population and demands a well-controlled transition between quiescence and proliferation. Although several factors involved in this process during muscle regeneration have been described, the process is far from fully understood (for review see Chargé and Rudnicki, 2004). Here, we show that this mitogenic signal is in part mediated in satellite cells by the cleavage of sphingomyelin at the inner leaflet of the plasma membrane that is ultimately metabolized to generate S1P, which in turn stimulates entry into the cell cycle. As would be expected from these observations, inhibition of S1P synthesis perturbs muscle regeneration in vivo.
S1P can function in two ways: either as an agonist for specific cell surface receptors or as a second messenger. S1P was shown to be a ligand for the EDG-1 (S1P1) receptor (Lee et al., 1998) and is now recognized as an agonist for five (S1P1 through S1P5) G-protein–coupled cell surface receptors. S1P1 through S1P5 are coupled to different G-proteins and so the relative abundance of specific receptors and G-proteins allows S1P to have heterogeneous effects. The C2 myogenic cell line was originally derived from regenerating adult muscle (Yaffe and Saxel, 1977) and has been shown to express S1P1 through S1P3 (Meacci et al., 1999). S1P, acting through S1P2, induces a small decrease in the number of cells proliferating in response to serum and stimulates the appearance of proteins associated with differentiation (Donati et al., 2005). Our results are consistent with those of Donati et al. (2005) where they overlap because, in our hands, S1P did not prevent differentiation in cycling C2C12 cells, but we did not determine whether it actively promoted it. Although the S1P receptors present on satellite cells are presently unknown, analysis has shown that S1P3 mRNA is present in quiescent satellite cells, but the levels are dramatically lower in dividing cells (Montarras, D., and Buckingham, M., personal communication). Intriguingly, overexpression of S1P3 in cycling C2C12 cells suppresses markers of myogenic differentiation elicited by S1P (Donati et al., 2005).
S1P has also been implicated as a second messenger for cell growth and survival independently of its receptors (Olivera and Spiegel 1993; Van Brocklyn et al., 1998; Olivera et al., 2003). For example, it has recently been shown that VEGF binding to VEGF receptor 2 stimulates endothelial cell growth through protein kinase C, which leads to the activation of sphingosine kinase 1 and the generation of S1P. The extracellular signal–regulated kinase (ERK)–MAPK pathway is then activated by S1P, resulting in cell division (Shu et al., 2002). Because HGF also binds a tyrosine kinase type receptor, it is possible that the activation of sphingosine kinase 1 and subsequent production of S1P may also stimulate ERK–MAPK, already shown to be involved in satellite cell activation (Yablonka-Reuveni et al., 1999; Shefer et al., 2001; Jones et al., 2005). In addition to HGF (Tatsumi et al., 1998), TNF-α has recently been proposed to be able to activate satellite cells, as systemically delivered TNF-α enhances BrdU incorporation into skeletal muscles, presumably by the intermediary of satellite cells (Li, 2003). Although TNF-α can work through serum response factor, it also activates sphingolipid signaling in various cell types and induces sphingomyelin cleavage (Levade and Jaffrezou, 1999; Spiegel and Milstien, 2003). Therefore, lipid signaling may act as a second messenger or via receptor-mediated pathways, or a combination of the two, in induction of satellite cell proliferation.
In contrast to the mitogenic effects of S1P, ceramide is associated with cell growth arrest, stress responses, and apoptosis in several cell types (for review see Hannun and Obeid, 2002). In muscle, ceramide has been shown to suppress the hypertrophic effects of insulin-like growth factor I (Strle et al., 2004) as well as that of insulin in C2C12 cells (Schmitz-Peiffer et al., 1999). As it has been proposed that the relative levels of these interconvertible metabolites can determine cell fate (Spiegel and Milstien, 2003), it is possible that S1P and ceramide also exert contrary effects in satellite cells. Higher ceramide levels may be involved in the maintenance of satellite cell quiescence, whereas increasing S1P levels would act to overcome this block, inducing activation and subsequent proliferation.
Although both DMS and GW4869 effectively reduce the mitogenic effect of serum, neither of them was able to totally prevent the activation of all myogenic cells. There is increasing evidence that satellite cells may represent a heterogeneous population (Beauchamp et al., 2000), so it is possible that they have different requirements for sphingolipid signaling. Another possible explanation is that the pharmacological inhibitors do not completely block the target enzyme, which would have a significant influence when the ratio of S1P to ceramide, rather than their absolute amounts, is proposed to be the crucial factor in the cellular response (Spiegel and Milstien, 2003). It is also probable that there are other pathways that control satellite cells in addition to S1P-mediated signaling (Shefer et al., 2001; Jones et al., 2005) and that signal redundancy and cross talk between pathways may reduce the effects of specifically inhibiting a particular signaling route.
Sphingolipid metabolites can be generated from two main sources: de novo synthesis of ceramide or cleavage of sphingomyelin. The site of the subcellular pool of sphingomyelin that is used for signaling, however, is unclear. Although sphingomyelin is mostly located at the outer leaflet of the plasma membrane (Koval and Pagano, 1991), several lines of evidence suggest that there is a distinct pool of signaling sphingomyelin in the inner leaflet, which acts as a reservoir for generating sphingolipids (Linardic and Hannun, 1994; Andrieu et al., 1996; Zhang et al., 1997). In particular, labeling sphingomyelin in various compartments with 3H before stimulation and determining the location and amount of labeled metabolites (Andrieu et al., 1996) or using subcellular fractionation to locate membranes depleted of sphingomyelin (Linardic and Hannun, 1994) both indicate the inner leaflet as the source. The enzyme mainly responsible for N-SMase activity outside the CNS, sphingomyelin phosphodiesterase 2 (Stoffel et al. 2005), is primarily located in the plasma membrane (Karakashian et al. 2004). Furthermore, N-SMase activity predominantly occurs in the plasma membrane fraction, and receptor-coupled sphingomyelin degradation has been shown to mostly occur at this site (Levade and Jaffrezou, 1999).
The novel technique that we have used, combining probing with the sphingomyelin binding protein lysenin (for review see Kobayashi et al., 2004) with selective sphingomyelin digestion, permits separate microscopic visualization of the dynamics of sphingomyelin in either the outer or inner leaflets on an individual cell basis. Using this method, we show that the inner leaflet of the plasma membrane contains a pool of sphingomyelin that can be recruited for signaling during induction of myogenic cell proliferation. This reduction in sphingomyelin levels in the inner leaflet in response to CFCS stimulation was caused by N-SMase catalyzed cleavage, as the N-SMase inhibitor GW4869 (Luberto et al., 2002) both prevented the transient drop in sphingomyelin and drastically reduced the number of cells entering the cell cycle. These observations strongly indicate that the inner leaflet is the source of the mitogenic S1P, consistent with and extending previous studies (Linardic and Hannun, 1994; Andrieu et al., 1996; Zhang et al., 1997).
In conclusion, we demonstrate that sphingolipid signaling plays a central role in adult stem cell biology. Upon stimulation, sphingomyelin in the inner leaflet of the plasma membrane is cleaved by N-SMase, and subsequent metabolism results in an increased level of S1P, which acts as a mitogen for muscle satellite cells. As would be predicted from these observations, inhibition of S1P generation perturbs muscle regeneration. Indeed, a recently described mutation in choline kinase β gene, central to phospholipid biosynthesis, results in a rapidly progressive muscular dystrophy (Sher et al., 2006). Coordinated control of satellite cell function is crucial to the regenerative responsiveness of muscle both during aging (Conboy et al., 2005) and in hereditary myopathies. Lipid signaling potentially offers new targets for therapeutic intervention that could augment/restore satellite cell function and may well be involved in the control of related stem cell systems.
Materials and methods
Cell culture and drug treatment
The C2 (Yaffe and Saxel, 1977) subclone C2C12 was maintained in DME containing 20% FCS, 4 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin at 37°C in 5% CO2. To prepare reserve cells, proliferating C2C12 cells were switched to DME containing 1% horse serum for 5 d, after which few cells were still dividing as shown by BrdU incorporation. CFCS was prepared by incubating 10 ml FCS with 1 g of activated charcoal (Sigma-Aldrich) overnight at 4°C before the charcoal was removed by centrifugation and filtration. Cells were stimulated with either 0.5–2% CFCS or S1P (BIOMOL Research Laboratories, Inc.) in DME containing 4 mg/ml fatty acid–free BSA (Sigma-Aldrich) for 18 h, and 10 μM BrdU was added for 6 h before fixation with 4% PFA/PBS. Cells were preincubated with inhibitors for 45 min before serum stimulation. DMS (BIOMOL Research Laboratories, Inc.) stock solution was prepared in DMSO and used at a final concentration of 2–10 μM. GW4869 (Calbiochem) was stored as a 1.5 mM suspension in DMSO and solubilized by the addition of methane sulphonic acid to a final concentration of 0.25%, immediately before use at 10–20 μM. FB1 (BIOMOL Research Laboratories, Inc.) was dissolved in distilled water and used at 10–20 μM.
Single-fiber isolation and culture
Adult (>8 wk of age) C57Bl/6 or C57B16/DBA2 mice were killed by cervical dislocation before the extensor digitorum longus muscles were carefully removed. Myofibers were isolated in DME as described previously (Rosenblatt et al., 1995) and cultured in either DME/BSA or DME with 10% [vol/vol] horse serum and 0.5% [vol/vol] CEE [MP Biomedicals]) at 37°C in 5% CO2. Drug treatments were performed in the same way as for C2C12 cells, except that BrdU was added to the medium from the beginning of culture.
Visualization of sphingomyelin using lysenin
Cells were treated with 100 mU/ml bSMase from Bacillus cereus (Sigma-Aldrich) for 2 h at 37°C in 5% CO2 either before or after fixation with 4% PFA. When GW4869 was used, the cells were washed in DME after bSMase treatment. Fixed cells were blocked with 0.5% BSA in PBS and incubated with 0.2–10 μg/ml lysenin (provided by S. Kawashima and N. Ohta, Zenyaku Kogyo, Tokyo, Japan; Peptide Institute, Inc.) in 0.5% BSA in PBS for 1 h. Lysenin was then visualized with an anti-lysenin antibody (Peptide Institute, Inc.).
Muscle regeneration
To elicit muscle damage, both TA muscles were injected with 20 μl of the snake venom cardiotoxin (Sigma-Aldrich) together with India ink to mark the injection site. The right TA also received DMS (1 μmol/kg of body weight) dissolved in DMSO, whereas the left served as a control, receiving only DMSO. 7 d later, both muscles were removed and cryosectioned, and separate sections were analyzed by hematoxylin and eosin staining or immunostaining for eMyHC. The area occupied by regenerating myofibers was then determined by using Scion Image (Scion Corp.) from several such experiments, and the data were pooled and expressed as a percentage ± SEM of the total area assayed.
Immunostaining
For BrdU detection, fixed C2C12 cells or myofibers were permeabilized with 0.5% (vol/vol) Triton X-100 and treated with 3 N hydrochloric acid for 10 min at room temperature. For immunostaining for PCNA, myofibers were treated with 100% methanol. Frozen sections were fixed with 10% formalin/PBS for 30 min at room temperature and then autoclaved for 10 min in 20 mM Tris-HCl buffer, pH 9.0. After these treatments, samples were incubated for 2 h at room temperature with primary antibodies in 0.5% BSA/PBS, followed by fluorochrome (Alexafluor 488 with Alexafluor 594 or TRITC)–conjugated secondary antibodies (Invitrogen) before mounting in Fluoromount fluorescent mounting medium (DakoCytomation) containing 100 ng/ml DAPI. Primary antibodies used were monoclonal rat anti-BrdU (clone BU1/75; Abcam), monoclonal mouse anti-PCNA (clone PC10; DakoCytomation), monoclonal Pax7 (Developmental Studies Hybridoma Bank), monoclonal eMyHC (clone F1.652; American Type Culture Collection), monoclonal MyoD (clone 5.8A; DakoCytomation), polyclonal rabbit anti-lysenin (Peptide Institute, Inc.), and polyclonal MyoD (Santa Cruz Biotechnology, Inc.).
Image capture
Immunostained cells and myofibers were viewed on an epifluorescence microscope (model Axiophot; Carl Zeiss MicroImaging, Inc.) using 10×/0.30 Ph1-, 20×/0.50 Ph2-, and 40×/0.75 Ph2-PlanNeofluar lenses. Digital images were acquired with a charge-coupled device camera (model RTE/CCD-1300-Y; Princeton Instruments, Inc.) at −10°C using MetaMorph software version 4.5r5 (Universal Imaging Corp.). Images were optimized globally for contrast and brightness and assembled into figures using Photoshop 7.0.1 (Adobe).
Acknowledgments
We would like to thank Dr. J. Beauchamp for critical reading of the manuscript and Dr. S. Kawashima and Mr. N. Ohta at Zenyaku Kogyo for advice and lysenin. P.S. Zammit wishes to acknowledge the invaluable assistance of John Peel.
This work was supported by The Medical Research Council. Y. Nagata was funded by the Japan scholarship foundation. R. Matsuda is the recipient of research grant 17A-10 for nervous and mental disorders from the Ministry of Health, Labour and Welfare.
Abbreviations used in this paper: bSMase, bacterial sphingomyelinase; CFCS, charcoal-stripped FCS; DMS, N,N-dimethylsphingosine; eMyHC, embryonic myosin heavy chain; FB1, fumonisin B1; HGF, hepatocyte growth factor; N-SMase, neutral sphingomyelinase; PCNA, proliferating cell nuclear antigen; S1P, sphingosine-1-phosphate; TA, tibialis anterior.
References
- Andrieu, N., R. Salvayre, and T. Levade. 1996. Comparative study of the metabolic pools of sphingomyelin and phosphatidylcholine sensitive to tumor necrosis factor. Eur. J. Biochem. 236:738–745. [DOI] [PubMed] [Google Scholar]
- Andrieu-Abadie, N., and T. Levade. 2002. Sphingomyelin hydrolysis during apoptosis. Biochim. Biophys. Acta. 1585:126–134. [DOI] [PubMed] [Google Scholar]
- Beauchamp, J.R., L. Heslop, D.S. Yu, S. Tajbakhsh, R.G. Kelly, A. Wernig, M.E. Buckingham, T.A. Partridge, and P.S. Zammit. 2000. Expression of CD34 and Myf5 defines the majority of quiescent adult skeletal muscle satellite cells. J. Cell Biol. 151:1221–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff, R. 1986. Proliferation of muscle satellite cells on intact myofibers in culture. Dev. Biol. 115:129–139. [DOI] [PubMed] [Google Scholar]
- Chargé, S.B., and M.A. Rudnicki. 2004. Cellular and molecular regulation of muscle regeneration. Physiol. Rev. 84:209–238. [DOI] [PubMed] [Google Scholar]
- Collins, C.A., I. Olsen, P.S. Zammit, L. Heslop, A. Petrie, T.A. Partridge, and J.E. Morgan. 2005. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell. 122:289–301. [DOI] [PubMed] [Google Scholar]
- Conboy, I.M., M.J. Conboy, A.J. Wagers, E.R. Girma, I.L. Weissman, and T.A. Rando. 2005. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 433:760–764. [DOI] [PubMed] [Google Scholar]
- Donati, C., E. Meacci, F. Nuti, L. Becciolini, M. Farnararo, and P. Bruni. 2005. Sphingosine 1-phosphate regulates myogenic differentiation: a major role for S1P2 receptor. FASEB J. 19:449–451. [DOI] [PubMed] [Google Scholar]
- Edsall, L.C., J.R. Van Brocklyn, O. Cuvillier, B. Kleuser, and S. Spiegel. 1998. N,N-dimethylsphingosine is a potent competitive inhibitor of sphingosine kinase but not of protein kinase C: modulation of cellular levels of sphingosine 1-phosphate and ceramide. Biochemistry. 37:12892–12898. [DOI] [PubMed] [Google Scholar]
- Formigli, L., F. Francini, E. Meacci, M. Vassalli, D. Nosi, F. Quercioli, B. Tiribilli, C. Bencini, C. Piperio, P. Bruni, and S.Z. Orlandini. 2002. Sphingosine 1-phosphate induces Ca2+ transients and cytoskeletal rearrangement in C2C12 myoblastic cells. Am. J. Physiol. Cell Physiol. 282:C1361–C1373. [DOI] [PubMed] [Google Scholar]
- Hannun, Y.A., and L.M. Obeid. 2002. The ceramide-centric universe of lipid-mediated cell regulation: stress encounters of the lipid kind. J. Biol. Chem. 277:25847–25850. [DOI] [PubMed] [Google Scholar]
- Herr, D.R., H. Fyrst, V. Phan, K. Heinecke, R. Georges, G.L. Harris, and J.D. Saba. 2003. Sply regulation of sphingolipid signaling molecules is essential for Drosophila development. Development. 130:2443–2453. [DOI] [PubMed] [Google Scholar]
- Hofmann, K., S. Tomiuk, G. Wolff, and W. Stoffel. 2000. Cloning and characterization of the mammalian brain-specific, Mg2+-dependent neutral sphingomyelinase. Proc. Natl. Acad. Sci. USA. 97:5895–5900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, N.C., Y.V. Fedorov, R.S. Rosenthal, and B.B. Olwin. 2001. ERK1/2 is required for myoblast proliferation but is dispensable for muscle gene expression and cell fusion. J. Cell. Physiol. 186:104–115. [DOI] [PubMed] [Google Scholar]
- Jones, N.C., K.J. Tyner, L. Nibarger, H.M. Stanley, D.D. Cornelison, Y.V. Fedorov, and B.B. Olwin. 2005. The p38α/β MAPK functions as a molecular switch to activate the quiescent satellite cell. J. Cell Biol. 169:105–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakashian, A.A., N.V. Giltiay, G.M. Smith, and M.N. Nikolova-Karakashian. 2004. Expression of neutral sphingomyelinase-2 (NSMase-2) in primary rat hepatocytes modulates IL-beta-induced JNK activation. FASEB J. 18:968–970. [DOI] [PubMed] [Google Scholar]
- Kent, C., S.D. Schimmel, and P.R. Vagelos. 1974. Lipid composition of plasma membranes from developing chick muscle cells in culture. Biochim. Biophys. Acta. 360:312–321. [DOI] [PubMed] [Google Scholar]
- Kobayashi, H., N. Ohta, and M. Umeda. 2004. Biology of lysenin, a protein in the coelomic fluid of the earthworm Eisenia foetida. Int. Rev. Cytol. 236:45–99. [DOI] [PubMed] [Google Scholar]
- Koval, M., and P.E. Pagano. 1991. Intracellular transport and metabolism of sphingomyelin. Biochim. Biophys. Acta. 1082:113–125. [DOI] [PubMed] [Google Scholar]
- Lee, M.J., J.R. Van Brocklyn, S. Thangada, C.H. Liu, A.R. Hand, R. Menzeleev, S. Spiegel, and T. Hla. 1998. Sphingosine-1-phosphate as a ligand for the G protein-coupled receptor EDG-1. Science. 279:1552–1555. [DOI] [PubMed] [Google Scholar]
- Levade, T., and J.P. Jaffrezou. 1999. Signalling sphingomyelinases: which, where, how and why? Biochim. Biophys. Acta. 1438:1–17. [DOI] [PubMed] [Google Scholar]
- Li, Y.P. 2003. TNF-α is a mitogen in skeletal muscle. Am. J. Physiol. Cell Physiol. 285:C370–C376. [DOI] [PubMed] [Google Scholar]
- Linardic, C.M., and Y.A. Hannun. 1994. Identification of a distinct pool of sphingomyelin involved in the sphingomyelin cycle. J. Biol. Chem. 269:23530–23537. [PubMed] [Google Scholar]
- Luberto, C., D.F. Hassler, P. Signorelli, Y. Okamoto, H. Sawai, E. Boros, D.J. Hazen-Martin, L.M. Obeid, Y.A. Hannun, and G.K. Smith. 2002. Inhibition of tumor necrosis factor-induced cell death in MCF7 by a novel inhibitor of neutral sphingomyelinase. J. Biol. Chem. 277:41128–41139. [DOI] [PubMed] [Google Scholar]
- Mauro, A. 1961. Satellite cell of skeletal muscle fibers. J. Biophys. Biochem. Cytol. 9:493–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meacci, E., V. Vasta, C. Donati, M. Farnararo, and P. Bruni. 1999. Receptor-mediated activation of phospholipase D by sphingosine 1-phosphate in skeletal muscle C2C12 cells. A role for protein kinase C. FEBS Lett. 457:184–188. [DOI] [PubMed] [Google Scholar]
- Merrill, A.H., Jr., D.C. Liotta, and R.T. Riley. 1996. Fumonisins: fungal toxins that shed light on sphingolipid function. Trends Cell Biol. 6:218–223. [DOI] [PubMed] [Google Scholar]
- Nagata, Y., H. Kobayashi, M. Umeda, N. Ohta, S. Kawashima, P. Zammit, and R. Matsuda. 2006. Sphingomyelin levels in the plasma membrane correlate with the activation state of muscle satellite cells. J. Histochem. Cytochem. 54:375–384. [DOI] [PubMed] [Google Scholar]
- Olivera, A., and S. Spiegel. 1993. Sphingosine-1-phosphate as second messenger in cell proliferation induced by PDGF and FCS mitogens. Nature. 365:557–560. [DOI] [PubMed] [Google Scholar]
- Olivera, A., T. Kohama, L. Edsall, V. Nava, O. Cuvillier, S. Poulton, and S. Spiegel. 1999. Sphingosine kinase expression increases intracellular sphingosine-1-phosphate and promotes cell growth and survival. J. Cell Biol. 147:545–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivera, A., H.M. Rosenfeldt, M. Bektas, F. Wang, I. Ishii, J. Chun, S. Milstien, and S. Spiegel. 2003. Sphingosine kinase type 1 induces G12/13-mediated stress fiber formation, yet promotes growth and survival independent of G protein-coupled receptors. J. Biol. Chem. 278:46452–46460. [DOI] [PubMed] [Google Scholar]
- Rosenblatt, J.D., A.I. Lunt, D.J. Parry, and T.A. Partridge. 1995. Culturing satellite cells from living single muscle fiber explants. In Vitro Cell. Dev. Biol. Anim. 31:773–779. [DOI] [PubMed] [Google Scholar]
- Sabbadini, R.A., D. Danieli-Betto, and R. Betto. 1999. The role of sphingolipids in the control of skeletal muscle function: a review. Ital. J. Neurol. Sci. 20:423–430. [DOI] [PubMed] [Google Scholar]
- Schmitz-Peiffer, C., D.L. Craig, and T.J. Biden. 1999. Ceramide generation is sufficient to account for the inhibition of the insulin-stimulated PKB pathway in C2C12 skeletal muscle cells pretreated with palmitate. J. Biol. Chem. 274:24202–24210. [DOI] [PubMed] [Google Scholar]
- Schultz, E., M.C. Gibson, and T. Champion. 1978. Satellite cells are mitotically quiescent in mature mouse muscle: an EM and radioautographic study. J. Exp. Zool. 206:451–456. [DOI] [PubMed] [Google Scholar]
- Seale, P., L.A. Sabourin, A. Girgis-Gabardo, A. Mansouri, P. Gruss, and M.A. Rudnicki. 2000. Pax7 is required for the specification of myogenic satellite cells. Cell. 102:777–786. [DOI] [PubMed] [Google Scholar]
- Sekizawa, Y., T. Kubo, H. Kobayashi, T. Nakajima, and S. Natori. 1997. Molecular cloning of cDNA for lysenin, a novel protein in the earthworm Eisenia foetida that causes contraction of rat vascular smooth muscle. Gene. 191:97–102. [DOI] [PubMed] [Google Scholar]
- Shefer, G., U. Oron, A. Irintchev, A. Wernig, and O. Halevy. 2001. Skeletal muscle cell activation by low-energy laser irradiation: a role for the MAPK/ERK pathway. J. Cell. Physiol. 187:73–80. [DOI] [PubMed] [Google Scholar]
- Sher, R.B., C. Aoyama, K.A. Huebsch, S. Ji, J. Kerner, Y. Yang, W.N. Frankel, C.L. Hoppel, P.A. Wood, D.E. Vance, and G.A. Cox. 2006. A rostrocaudal muscular dystrophy caused by a defect in choline kinase beta, the first enzyme in phosphatidylcholine biosynthesis. J. Biol. Chem. 281:4938–4948. [DOI] [PubMed] [Google Scholar]
- Shu, X., W. Wu, R.D. Mosteller, and D. Broek. 2002. Sphingosine kinase mediates vascular endothelial growth factor-induced activation of Ras and mitogen-activated protein kinases. Mol. Cell. Biol. 22:7758–7768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow, M.H. 1978. An autoradiographic study of satellite cell differentiation into regenerating myotubes following transplantation of muscles in young rats. Cell Tissue Res. 186:535–540. [DOI] [PubMed] [Google Scholar]
- Spiegel, S., and S. Milstien. 2003. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat. Rev. Mol. Cell Biol. 4:397–407. [DOI] [PubMed] [Google Scholar]
- Stoffel, W., B. Jenke, B. Blöck, M. Zumbansen, and J. Koebke. 2005. Neutral sphingomyelinase 2 (smpd3) in the control of postnatal growth and development. Proc. Natl. Acad. Sci. USA. 102:4554–4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strle, K., S.R. Broussard, R.H. McCusker, W.H. Shen, R.W. Johnson, G.G. Freund, R. Dantzer, and K.W. Kelley. 2004. Proinflammatory cytokine impairment of insulin-like growth factor I-induced protein synthesis in skeletal muscle myoblasts requires ceramide. Endocrinology. 145:4592–4602. [DOI] [PubMed] [Google Scholar]
- Sugii, S., P.C. Reid, N. Ohgami, Y. Shimada, R.A. Maue, H. Ninomiya, Y. Ohno-Iwashita, and T.Y. Chang. 2003. Biotinylated theta-toxin derivative as a probe to examine intracellular cholesterol-rich domains in normal and Niemann-Pick type C1 cells. J. Lipid Res. 44:1033–1041. [DOI] [PubMed] [Google Scholar]
- Tatsumi, R., J.E. Anderson, C.J. Nevoret, O. Halevy, and R.E. Allen. 1998. HGF/SF is present in normal adult skeletal muscle and is capable of activating satellite cells. Dev. Biol. 194:114–128. [DOI] [PubMed] [Google Scholar]
- Tortorella, L.L., D.J. Milasincic, and P.F. Pilch. 2001. Critical proliferation-independent window for basic fibroblast growth factor repression of myogenesis via the p42/p44 MAPK signaling pathway. J. Biol. Chem. 276:13709–13717. [DOI] [PubMed] [Google Scholar]
- Van Brocklyn, J.R., M.J. Lee, R. Menzeleev, A. Olivera, L. Edsall, O. Cuvillier, D.M. Thomas, P.J. Coopman, S. Thangada, C.H. Liu, et al. 1998. Dual actions of sphingosine-1-phosphate: extracellular through the Gi-coupled receptor Edg-1 and intracellular to regulate proliferation and survival. J. Cell Biol. 142:229–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meer, G., and J.C. Holthuis. 2000. Sphingolipid transport in eukaryotic cells. Biochim. Biophys. Acta. 1486:145–170. [DOI] [PubMed] [Google Scholar]
- Yablonka-Reuveni, Z., and A.J. Rivera. 1994. Temporal expression of regulatory and structural muscle proteins during myogenesis of satellite cells on isolated adult rat fibers. Dev. Biol. 164:588–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yablonka-Reuveni, Z., and A.J. Rivera. 1997. Influence of PDGF-BB on proliferation and transition through the MyoD-myogenin-MEF2A expression program during myogenesis in mouse C2 myoblasts. Growth Factors. 15:1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yablonka-Reuveni, Z., R. Seger, and A.J. Rivera. 1999. Fibroblast growth factor promotes recruitment of skeletal muscle satellite cells in young and old rats. J. Histochem. Cytochem. 47:23–42. [DOI] [PubMed] [Google Scholar]
- Yaffe, D., and O. Saxel. 1977. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature. 270:725–727. [DOI] [PubMed] [Google Scholar]
- Yamaji, A., Y. Sekizawa, K. Emoto, H. Sakuraba, K. Inoue, H. Kobayashi, and M. Umeda. 1998. Lysenin, a novel sphingomyelin-specific binding protein. J. Biol. Chem. 273:5300–5306. [DOI] [PubMed] [Google Scholar]
- Yatomi, Y., F. Ruan, T. Megidish, T. Toyokuni, S. Hakomori, and Y. Igarashi. 1996. N,N-dimethylsphingosine inhibition of sphingosine kinase and sphingosine 1-phosphate activity in human platelets. Biochemistry. 35:626–633. [DOI] [PubMed] [Google Scholar]
- Yoshida, S., A. Fujisawa-Sehara, T. Taki, K. Arai, and Y. Nabeshima. 1996. Lysophosphatidic acid and bFGF control different modes in proliferating myoblasts. J. Cell Biol. 132:181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida, N., S. Yoshida, K. Koishi, K. Masuda, and Y. Nabeshima. 1998. Cell heterogeneity upon myogenic differentiation: down-regulation of MyoD and Myf-5 generates ‘reserve cells’. J. Cell Sci. 111:769–779. [DOI] [PubMed] [Google Scholar]
- Zammit, P., and J. Beauchamp. 2001. The skeletal muscle satellite cell: stem cell or son of stem cell? Differentiation. 68:193–204. [DOI] [PubMed] [Google Scholar]
- Zammit, P.S., J.P. Golding, Y. Nagata, V. Hudon, T.A. Partridge, and J.R. Beauchamp. 2004. Muscle satellite cells adopt divergent fates: a mechanism for self-renewal? J. Cell Biol. 166:347–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H., N.N. Desai, A. Olivera, T. Seki, G. Brooker, and S. Spiegel. 1991. Sphingosine-1-phosphate, a novel lipid, involved in cellular proliferation. J. Cell Biol. 114:155–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, P., B. Liu, G.M. Jenkins, Y.A. Hannun, and L.M. Obeid. 1997. Expression of neutral sphingomyelinase identifies a distinct pool of sphingomyelin involved in apoptosis. J. Biol. Chem. 272:9609–9612. [DOI] [PubMed] [Google Scholar]