Abstract
Focal adhesion kinase (FAK) transduces cell adhesion to the extracellular matrix into proliferative signals. We show that FAK overexpression induced proliferation in endothelial cells, which are normally growth arrested by limited adhesion. Interestingly, displacement of FAK from adhesions by using a FAK−/− cell line or by expressing the C-terminal fragment FRNK also caused an escape of adhesion-regulated growth arrest, suggesting dual positive and negative roles for FAK in growth regulation. Expressing kinase-dead FAK-Y397F in FAK−/− cells prevented uncontrolled growth, demonstrating the antiproliferative function of inactive FAK. Unlike FAK overexpression–induced growth, loss of growth control in FAK−/− or FRNK-expressing cells increased RhoA activity, cytoskeletal tension, and focal adhesion formation. ROCK inhibition rescued adhesion-dependent growth control in these cells, and expression of constitutively active RhoA or ROCK dysregulated growth. These findings demonstrate the ability of FAK to suppress and promote growth, and underscore the importance of multiple mechanisms, even from one molecule, to control cell proliferation.
Introduction
Cell proliferation in the multicellular organism is tightly controlled through the cooperative efforts of numerous microenvironmental cues, including soluble growth factors and adhesion to the ECM. One potential point of integration between growth factor and adhesive signaling is in the focal adhesion (Schwartz and Ginsberg, 2002). Focal adhesions are structures that arise during the binding and clustering of integrins and serve to physically link the actin cytoskeleton to the underlying ECM. Because they also contain numerous growth factor receptors and signaling proteins, focal adhesions have been proposed as localized sites where growth factor and adhesion signaling converge (for reviews see Schwartz and Ingber, 1994; Sastry and Horwitz, 1996). FAK is a key effector in focal adhesion signaling and a potential integrator of integrin- and growth factor–mediated proliferative signaling. It is rapidly phosphorylated after integrin ligation (Guan et al., 1991; Burridge et al., 1992; Kornberg et al., 1992), which stimulates its kinase activity (Guan and Shalloway, 1992; Lipfert et al., 1992) and triggers the activation of signaling pathways involved in modulating focal adhesions and their surrounding cytoskeletal structures (Parsons et al., 2000; Geiger et al., 2001).
Given its central role in adhesion signaling, it is not surprising that numerous studies have demonstrated a regulatory role for FAK in cell cycle progression (Gilmore and Romer, 1996; Zhao et al., 1998; Oktay et al., 1999). Such studies have shown that FAK overexpression drives G1/S phase cell cycle progression, whereas dominant–negative FAK mutants, such as FRNK, or anti-FAK antibodies block the cell cycle at the G1/S phase boundary (Gilmore and Romer, 1996; Zhao et al., 1998; Nolan et al., 1999; Oktay et al., 1999). Mechanistically, FAK overexpression appears to enhance the transcriptional activation of cyclin D1 (Zhao et al., 1998). FAK appears to regulate the G1 cell cycle machinery through numerous signaling pathways. In endothelial cells (EC), FAK is required for sustained ERK activity downstream of VEGF stimulation (Hood et al., 2003). Additionally, FAK regulates the activity of the Rho GTPase RhoA, which is also required for sustained ERK signaling (Danen et al., 2000; Ren et al., 2000; Welsh et al., 2001). Importantly, although FAK signaling clearly modulates cell cycle progression, it does not appear to be required, as FAK−/− cells and cells treated with FAK RNAi still proliferate (Ilic et al., 1995; Duxbury et al., 2003). Thus, the role of FAK in adhesion-regulated proliferation is likely to be multifaceted, and may depend on the adhesive context in which FAK signaling occurs.
To conceptually dissect how FAK might regulate adhesion-dependent proliferation, it is necessary to define adhesion more precisely. Although cell adhesion is initiated by integrin binding to ECM ligands, it involves numerous other processes, such as integrin clustering, focal adhesion maturation, and cell spreading and flattening against the substrate, each of which appears to be involved in regulating proliferation. Integrin ligation and clustering, although necessary for the proliferation of adherent cells, is not sufficient to support cell cycle progression. Proliferation also requires that the ECM allows cells to physically spread against the substrate; cells that are prevented from spreading or flattening against the ECM are growth arrested (Chen et al., 1997). Interestingly, these changes in cell spreading appear to be required for RhoA-mediated cytoskeletal tension and focal adhesions to develop (Chen et al., 2003; Tan et al., 2003), and inhibiting cytoskeletal tension and focal adhesion formation appear to abolish proliferation in spread cells (Bohmer et al., 1996; Huang et al., 1998). Thus, changes in integrin ligation, cell spreading, cytoskeletal tension, and focal adhesion formation are clearly interdependent, and have all been implicated in growth regulation. Because of the prominent role of FAK in multiple aspects of the adhesive processes, including focal adhesion development (Lewis and Schwartz, 1995), spreading (Gilmore and Romer, 1996; Richardson et al., 1997), and mechanical tension (Burridge and Chrzanowska-Wodnicka, 1996), FAK may serve as a critical point of integration for transducing each of these adhesive processes into a coordinated biological response, such as proliferation. However, despite the involvement of FAK in the various aspects of adhesion, how FAK functions to regulate proliferation under different adhesive contexts is ill defined.
By examining the proliferative effects of modulating FAK in different adhesive contexts, we have found that FAK plays a dual role in regulating growth. In contexts of high adhesion, FAK activity and proliferation are high. In low ECM ligand or low cell-spreading contexts, normally growth-arrested cells can be induced to proliferate by activating FAK. Surprisingly, the growth inhibition in these low adhesive states is mediated by inactive FAK, as loss of FAK in either FAK−/− cells or FRNK-expressing cells dysregulated adhesion-dependent growth control. Full-length, kinase-dead FAK-Y397F, in contrast to FRNK, rescued adhesion-dependent growth regulation, suggesting the possibility that the N terminus of FAK may mediate the growth inhibitory function. The uncontrolled growth after loss of FAK was mediated through an increase in RhoA signaling and cytoskeletal tension. Thus, FAK appears to transduce both high adhesive signals, to stimulate proliferation, and low adhesive signals, to arrest growth. This dual nature highlights FAK as a central control point for growth regulation, and underscores its critical role in integrating the multiple adhesive, mechanical, and biochemical functions of focal adhesions.
Results
FAK regulates adhesion-mediated proliferation
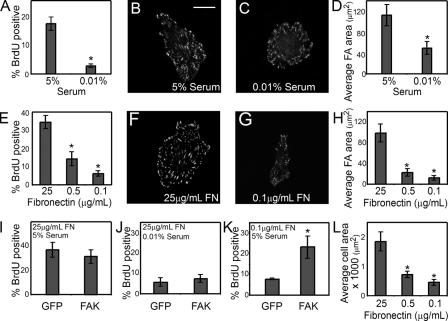
To begin to explore the role of FAK in regulating proliferation, we first established the dependence of bovine pulmonary artery EC proliferation on growth factors and adhesion. Cells were G0 synchronized at confluence, replated under various growth factor or adhesive conditions, and assayed for proliferation by tracking BrdU incorporation as a marker of S phase entry. As expected, when ECs were exposed to low serum (0.01%) or grown on surfaces coated with a low density of fibronectin (0.1 μg/ml), cell proliferation was inhibited compared with cells grown in high serum (5%) or on surfaces coated with a high density of fibronectin (25 μg/ml; Fig. 1, A and E). To examine whether the serum or fibronectin concentrations affected focal adhesion formation, we analyzed vinculin distribution by immunofluorescence. Whereas cells grown in high serum formed large, well-defined focal adhesions (Fig. 1 B), cells cultured in low serum showed reduced focal adhesion number and area (Fig. 1, C and D). Fibronectin concentration affected focal adhesion formation to an even greater extent (Fig. 1, F–H). This correlation between proliferation and focal adhesion area in both serum- and adhesion-regulated growth suggested the possibility that FAK might be involved in both growth factor– and adhesion-mediated proliferation. To begin to explore this possibility, we examined whether overexpression of FAK could overcome the proliferation block caused by either low serum or low-density fibronectin. G0-synchronized ECs were transduced with a recombinant adenovirus containing wild-type FAK, resulting in FAK overexpression and constitutive autophosphorylation. FAK overexpression did not rescue the growth arrest caused by low serum and did not affect proliferation induced by high serum (Fig. 1, I and J). In contrast, cells plated on low-density fibronectin dramatically increased proliferation upon FAK overexpression (Fig. 1 K). These findings suggest the possibility that FAK mediates the proliferative signals initiated by adhesion, but not by growth factors.
Figure 1.
FAK regulates adhesion-mediated proliferation. (A–D) Graph of the percentage of ECs in S phase, measured by the incorporation of BrdU (A), immunofluorescence images of vinculin (B and C), and a graph of the average focal adhesion area per cell (D) in cells cultured for 24 h in 5 or 0.01% serum, on surfaces coated with 25 μg/ml fibronectin. (E–H) Graph of the percentage of ECs in S phase (E), immunofluorescence images of vinculin (F and G), and graph of average focal adhesion area per cell (H) in cells cultured in 5% serum on surfaces coated with 25, 0.5, or 0.1 μg/ml fibronectin. (I–K) Graph of the percentage of GFP- or FAK-overexpressing ECs that enter S phase when cultured on 25 μg/ml fibronectin-coated surfaces in 5% serum (I), on 25 μg/ml fibronectin-coated surfaces in 0.01% serum (J), or on 0.1 μg/ml fibronectin-coated surfaces in 5% serum (K). (L) Average area of cells when cultured in 5% serum and on surfaces coated with 25, 0.5, or 0.1 μg/ml fibronectin. Data is expressed ± SEM for three independent experiments. *, P < 0.05. Bar, 20 μm.
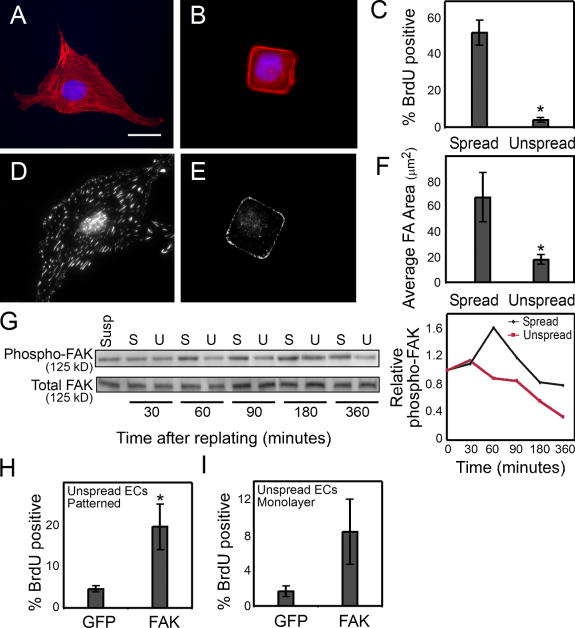
Cell adhesion involves many different steps, including integrin ligation and clustering and cell spreading and flattening against the substrate (Chen et al., 1997). Decreasing fibronectin density not only decreased integrin clustering and focal adhesion formation, but also impaired cell spreading (Fig. 1 L). Because changes in cell spreading can directly regulate cell proliferation, despite the presence of excess extracellular matrix, we examined whether FAK is also involved in the regulation of cell proliferation by changes in cell shape. To specifically modulate cell shape without altering fibronectin density and integrin clustering, we used microcontact printing to generate micrometer-scale islands coated with a high density of fibronectin, separated by nonadhesive regions such that the size of the islands dictated the degree of cell spreading. ECs seeded onto small, square islands (625 μm2) remained relatively unspread, whereas ECs seeded onto uniformly coated surfaces spread to an average of 2,000 μm2 (Fig. 2, A and B). Measurement of S phase entry under these conditions demonstrated that the unspread cells could not proliferate (Fig. 2 C). Substantially fewer and smaller focal adhesions formed in the growth-arrested unspread cells compared with spread controls (Fig. 2, D–F), suggesting the possibility that alterations in focal adhesion architecture and/or signaling may also underlie proliferative regulation by cell spreading. To examine whether cell spreading specifically affected FAK activity, we measured FAK phosphorylation at tyrosine 397 in these cells. At early time points after replating, attachment, spreading, and FAK phosphorylation at Y397 was similar between spread and unspread cells (Fig. 2 G). At later time points, unspread cells showed progressively lower FAK phosphorylation while spread cells transiently increased FAK activation (Fig. 2 G). These data suggested the possibility that FAK signaling may be fundamentally different in spread versus unspread cells and that FAK may be directly involved in the proliferation response of cells to changes in cell spreading.
Figure 2.
FAK regulates shape-mediated proliferation. (A and B) F-actin (red) and DAPI (blue) stain of ECs cultured on surfaces uniformly coated with 25 μg/ml fibronectin (Spread; A) or onto 625-μm2 islands of fibronectin (Unspread; B). (C) Graph of the percentage of spread versus unspread cells in S phase measured by the incorporation of BrdU. (D and E) Immunofluorescence images of vinculin in cells cultured on surfaces uniformly coated with 25 μg/ml fibronectin (D) or onto 625-μm2 islands of fibronectin (E). (F) Graph of the average focal adhesion area per spread versus unspread cell. (G) Western blot of phospho–Y397-FAK and total FAK in spread (S) versus unspread (U) cells at 30, 60, 90, 180, and 360 min after replating, and a graph showing phospho-FAK normalized to total FAK. (H and I) Graph of the percentage of GFP- or FAK-overexpressing ECs that enter S phase when cultured on 625-μm2 islands of fibronectin (H) or in a monolayer (I). Data is expressed ± SEM for three independent experiments. *, P < 0.05. Bar, 20 μm.
To explore this possibility, cells were transduced with wild-type FAK adenovirus and cultured on the micropatterned substrates. FAK overexpression increased proliferation as compared with a GFP control (Fig. 2 H). Because FAK overexpression appears to rescue proliferation that was inhibited both by low-density fibronectin and by reduced cell spreading, but not by low serum, FAK appears to be specifically involved in proliferative signals mediated by adhesive cues. In physiologic settings, however, the primary mode of adhesion-mediated arrest in ECs is mediated by confluence of the monolayer, not through changes in ligand density or cell area. To test whether FAK signaling is involved in confluence-induced arrest, we expressed FAK in monolayer cultures. FAK overexpression increased proliferation in cells arrested by traditional contact inhibition (Fig. 2 I). Together these studies suggest that FAK may be involved in several of the means by which adhesion regulates proliferation.
Loss of FAK signaling causes constitutive cell proliferation
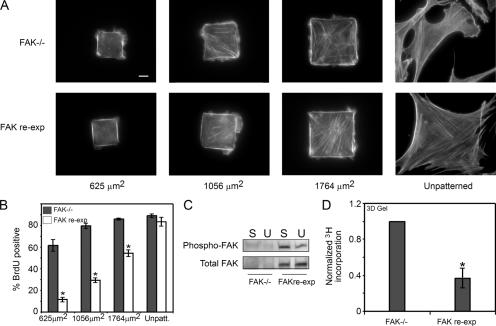
The stimulation of proliferation by FAK overexpression suggests at least two possible models for adhesion-regulated proliferation. The first, and predominantly accepted, model is that FAK activity triggered by adhesion stimulates proliferation (Gilmore and Romer, 1996; Zhao et al., 1998). A second, equally plausible model is that inactive FAK in cells with limited adhesion or spreading inhibits proliferation. To begin to address these possibilities, we examined the proliferative response of cells completely lacking FAK. G0-synchronized FAK−/− mouse embryo fibroblasts were seeded onto micropatterned islands of various sizes or onto unpatterned surfaces, where the cells ranged in size from 625 μm2 to fully spread (∼2,500 μm2; Fig. 3 A). Well-spread FAK−/− cells proliferated maximally, as expected (Fig. 3 B). Surprisingly, unspread FAK−/− cells also proliferated (Fig. 3 B), indicating that loss of FAK may have eliminated adhesion-dependent proliferative control mechanisms. To address this, we examined the effect of reexpressing FAK on proliferation. FAK reexpression to endogenous levels, which resulted in the rescue of the spreading-dependent FAK autophosphorylation seen in ECs (Fig. 3 C), inhibited proliferation only in unspread cells, rescued normal adhesion-dependent growth control, and confirmed that the loss of growth control was specific to loss of FAK (Fig. 3 B). The constitutive proliferation in FAK−/− cells suggests that one important and previously undescribed function of FAK is to limit proliferation in low adhesive conditions. However, although the micropatterned substrates provide a precise quantitative method to control adhesion, fibroblasts are typically adhesion-regulated in a 3D microenvironment. In this context, we cultured the FAK−/− and FAK-reexpressing fibroblasts in 3D collagen gels, where cell proliferation is often suppressed. Consistent with the micropatterning studies, FAK−/− cells continued to proliferate at higher levels in the collagen gel, whereas FAK reexpression rescued growth suppression (Fig. 3 D). As with ECs, highly overexpressing FAK to severalfold above endogenous levels in the FAK-reexpressing fibroblasts increased proliferation in unspread conditions (unpublished data). Thus, it appears that a delicate balance of FAK expression is needed for proliferative control.
Figure 3.
FAK has a growth inhibitory role. (A and B) F-actin stain (A) and graph of the percentage of cells in S phase (B) for FAK−/− cells and FAK-reexpressing cells cultured on different-sized islands of fibronectin. (C) Western blot of phospho–Y397-FAK and total FAK in FAK−/− and FAK-reexpressing cells in spread (substrates coated with 25 μg/ml fibronectin; S) or unspread (substrates patterned with 625-μm2 islands of fibronectin; U) conditions. (D) Graph of the percentage of FAK−/− and FAK-reexpressing cells in S phase when cultured in a 3D collagen gel. All data is expressed as ± SEM for three independent experiments. *, P < 0.05 between FAK−/− or FAK-reexpressing cells. Bar, 10 μm.
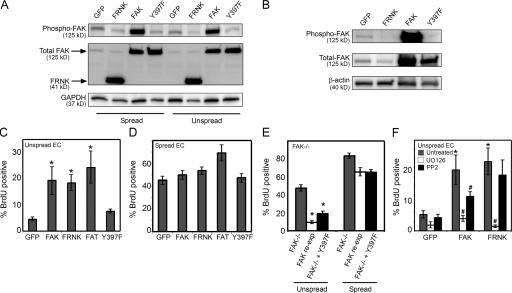
Because the FAK−/− and FAK-reexpressing cell lines are immortalized, and known compensatory changes in signaling pathways might have affected our interpretation of the proliferative effect, we next examined whether the same inhibitory role of FAK in proliferation might operate in normal nonimmortalized cells. To address this question, we generated recombinant adenoviruses to express the well-characterized dominant–negative FAK construct FRNK; consisting of amino acids 668–1,053 of wild-type FAK (Schaller et al., 1993), as well as a shorter C-terminal construct of FAK containing only the focal adhesion–targeting (FAT) domain (amino acids 919–1,053; Prutzman et al., 2004). We also generated an autophosphorylation-defective FAK mutant (FAK-Y397F) in adenovirus (Schaller et al., 1994). Infecting cells with the FAK adenovirus causes overexpression of FAK that is highly phosphorylated (Fig. 4 A), whereas expression of FRNK, FAT, and FAK-Y397F down-regulates endogenous FAK phosphorylation (Fig. 4 A). Previous studies have shown that FRNK and FAT displace endogenous FAK from adhesions (Richardson and Parsons, 1996). We have confirmed these findings in our system. Cells expressing GFP, FRNK, FAK, and FAK-Y397F were fractionated into Triton X-100–soluble and –insoluble pools and blotted for FAK. FRNK decreased total FAK in the insoluble pool and phosphorylated FAK to nearly undetectable levels (Fig. 4 B). Similarly, FRNK expression also decreased the amount of total FAK (and phosphorylated FAK) that coimmunoprecipitated with paxillin (unpublished data). Because FRNK, FAT, and FAK-Y397F all contain the C-terminal FAT region, lack kinase activity, compete to displace endogenous full-length FAK from the focal adhesion, and thereby decrease endogenous FAK phosphorylation, we postulated that expression of these dominant–negative mutants might have the same proliferative effects as seen in the FAK−/− cells. To examine this possibility, ECs were transduced with recombinant adenoviruses to express FRNK, FAT, or FAK-Y397F, cultured on small islands of fibronectin, and assayed for proliferation by BrdU incorporation. As compared with GFP and FAK, as negative and positive controls, respectively, FRNK increased proliferation (Fig. 4 C). Expressing the FAT construct also relieved the proliferation arrest induced by restricted adhesion. Interestingly, FAK-Y397F did not induce cell proliferation. FAK or FRNK expression also released cells from growth arrest in monolayer cultures, but did not rescue proliferation in cells placed in suspension (unpublished data). Although the various FAK constructs increased proliferation relative to a GFP control in conditions of low adhesion, cell proliferation in a highly adhesive environment was not dramatically affected by expression of the FAK constructs (Fig. 4 D). Although the stimulatory effects of wild-type FAK expression on proliferation is consistent with previous studies (Gilmore and Romer, 1996; Zhao et al., 1998), the loss of adhesion-dependent proliferative control in FAK−/−, FRNK-, or FAT-expressing cells suggests that, in addition, inactive FAK might function to actively inhibit proliferation. FRNK and FAT may relieve this inhibition by displacing inactive FAK from the adhesion, whereas FAK phosphorylation might do so via a different mechanism. In support of this hypothesis, overexpressing the inactive FAK-Y397F in FAK−/− cells, like wild-type FAK, rescued adhesion-mediated growth control (Fig. 4 E). Together, these results uncover a previously undescribed function of FAK as a negative growth regulator, and, in particular, support a model whereby inactive FAK within adhesions inhibits proliferation.
Figure 4.
FRNK stimulates proliferation in low adhesive contexts. (A) Western blots of GFP-, FAK-, FRNK-, or FAK-Y397F-overexpressing ECs in spread (substrates coated with 25 μg/ml fibronectin) or unspread (substrates patterned with 625-μm2 islands of fibronectin) conditions and probed for phospho–Y397-FAK, total FAK, or GAPDH. (B) Western blot of phospho–Y397-FAK and total FAK in the Triton X-100–insoluble fraction of unspread ECs expressing GFP (control), FRNK, FAK, or FAK-Y397F. β–actin is shown as a loading control. (C and D) Graph of the percentage of GFP-, FAK-, FRNK-, FAT-, or FAK-Y397F–overexpressing ECs entering S phase when cultured on 625-μm2 islands of fibronectin (C) or on substrates coated with 25 μg/ml fibronectin (D). (E) Graph of the percentage of FAK−/− cells, FAK-reexpressing cells, and FAK−/− cells overexpressing FAK-Y397F in S phase when cultured in spread or unspread conditions. (F) Graph of the percentage of GFP-, FAK-, or FRNK-overexpressing ECs entering S phase when cultured on 625-μm2 islands of fibronectin and treated with either 10 μM UO126 or 1 μM PP2. All data is expressed as ± SEM for three independent experiments. *, P < 0.05 with GFP control or FAK−/− cells. #, P < 0.05 with untreated control.
As an initial characterization of the proliferative mechanisms induced by FAK or FRNK, we examined the role of downstream MAPK and Src signaling pathways. Although most extracellular signals regulate proliferation through the regulation of MAPK-dependent signals in the G1 phase of the cell cycle, others have been reported to occur at different levels (Brooks et al., 1997). Because FAK is known to have a very close association with the nonreceptor tyrosine kinase Src, which is another important proliferative signaling protein, we also examined whether FAK- or FRNK-induced proliferation were Src dependent. G0-synchronized cells were transduced with adenoviruses to express FAK, FRNK, or GFP, seeded onto 625-μm2 islands of fibronectin, and treated with 10 μM of the MEK inhibitor UO126, 25 μM JNK inhibitor I, 1 μM of the p38 inhibitor SB203580, or 1 μM of the Src inhibitor PP2. Although inhibiting MEK or JNK activity completely blocked FAK- and FRNK-induced proliferation, the p38 inhibitor had no effect (Fig. 4 F and not depicted). Interestingly, FAK- and FRNK-expressing cells responded differently to PP2 treatment. The FAK-mediated increase in cell proliferation was blocked by inhibiting Src, but FRNK-mediated proliferation was not (Fig. 4 F). These findings suggest a divergence of signaling pathways between the proliferative effects mediated by FAK activation and those mediated by loss of FAK. Because the dysregulation of adhesion-dependent growth control by FAK down-regulation has not been previously described, we chose to further investigate the molecular mechanisms underlying this process.
FAK regulates proliferation through RhoA
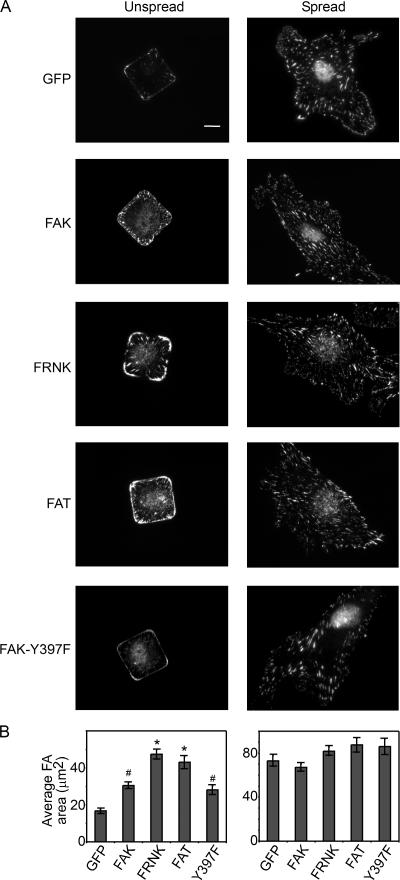
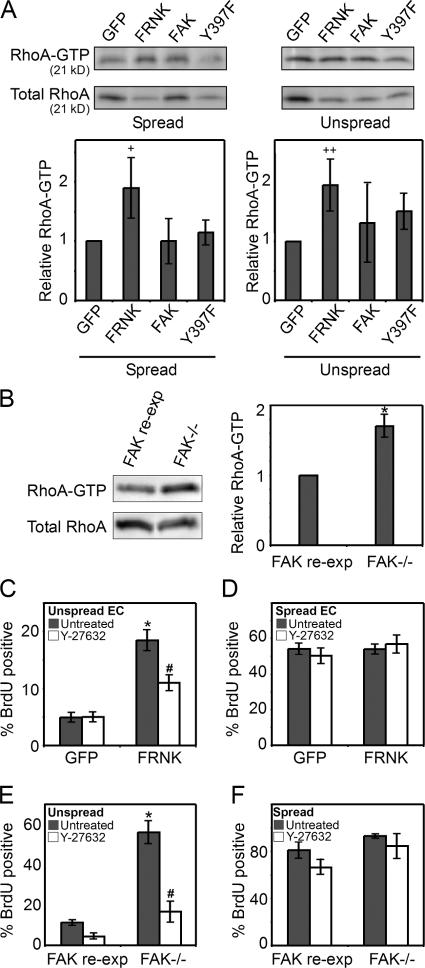
Our initial studies indicated that focal adhesions are significantly larger in conditions that promoted proliferation than in those that arrested growth. Therefore, we explored whether the size of focal adhesions in spread and unspread cells was also affected by the expression of FAK, FRNK, FAT, and FAK-Y397F. FRNK and FAT expression both dramatically increased focal adhesion area in unspread cells, but not in well-spread cells (Fig. 5, A and B), mirroring their effects on proliferation. FAK and the Y397F mutant increased focal adhesion size, but to a lesser extent. Focal adhesion size has been shown to depend on RhoA signaling (Ridley and Hall, 1992; Nobes and Hall, 1995), suggesting that changes in FAK signaling may modulate RhoA activity. To test this possibility, we examined RhoA activity in FRNK-, FAK-, or FAK-Y397F–expressing ECs. Cells were transduced with recombinant adenoviruses, replated onto 625-μm2 square patterns or onto surfaces uniformly coated with fibronectin, and lysed 6 h after replating. Using the RhoA pull-down assay to measure GTP-bound RhoA, we found that FRNK expression increased RhoA activity compared with GFP-expressing control cells both in spread and unspread conditions, whereas FAK or FAK-Y397F expression had little to no effect (Fig. 6 A). Likewise, the FAK−/− cells showed higher RhoA activity than FAK-reexpressing cells (Fig. 6 B). To address whether RhoA was directly involved in the dysregulation of proliferative control induced by loss of FAK signaling, we examined the effects of inhibiting the RhoA effector ROCK in FRNK-expressing cells. ROCK inhibition with 50 μM Y-27632 blocked the FRNK-induced increase in proliferation in unspread cells (Fig. 6 C). This effect was specific to the release of growth inhibition by FRNK, as Y-27632 treatment did not inhibit proliferation rates in well-spread cells (Fig. 6 D). Similarly, FAK−/− cells treated with Y-27632 also regained adhesion-dependent growth control. That is, cell proliferation was low in unspread cells and high in spread cells in the presence of the ROCK inhibitor (Fig. 6, E and F). Collectively, these data suggest a signaling pathway whereby lack of FAK or displacing endogenous FAK from focal adhesions causes an increase in RhoA activity, and this increase, in turn, is required for loss of the growth control normally observed in low adhesive conditions.
Figure 5.
FRNK and FAT induce focal adhesion growth in unspread cells. (A) Immunofluorescence images of vinculin in GFP-, FAK-, FRNK-, FAT-, or FAK-Y397F–overexpressing ECs cultured for 24 h onto 625-μm2 islands of fibronectin (Unspread) or surfaces uniformly coated with 25 μg/ml fibronectin (Spread). Graph of the average focal adhesion area of GFP-, FAK-, FRNK-, FAT-, or FAK-Y397F–expressing ECs when cultured in spread versus unspread conditions (B). Data is expressed ± SEM. Approximately 150 cells were analyzed in each condition; *, P < 0.05 with GFP control; #, P < 0.05 as compared with FRNK or FAT conditions. Bar, 10 μm.
Figure 6.
FRNK expression increases RhoA activity. (A) RhoA-GTP and total RhoA levels in GFP-, FRNK-, FAK-, or FAK-Y397F–expressing ECs. (B) RhoA-GTP and total RhoA levels in FAK−/− or FAK-reexpressing cells. (C and D) Graph of the percentage of GFP- or FRNK-overexpressing ECs that enter S phase when cultured on 625-μm2 islands of fibronectin (C) or on surfaces coated with 25 μg/ml fibronectin (D) and either untreated or treated with 50 μM Y-27632. (E and F) Graph of the percentage of FAK−/− or FAK-reexpressing cells that enter S phase when cultured on 625-μm2 islands of fibronectin (E), or on surfaces coated with 25 μg/ml fibronectin (F) and either untreated or treated with 50 μM Y-27632. Data is expressed ± SEM for three independent experiments. +, P < 0.08 compared with control; ++, P < 0.06 compared with control; *, P < 0.05 between FRNK-overexpressing condition versus GFP control, or FAK−/− versus FAK-reexpressing cells; #, P < 0.05 between FRNK-induced proliferation or FAK−/− proliferation in untreated versus drug-treated samples.
FAK regulates proliferation through RhoA-mediated changes in cytoskeletal tension
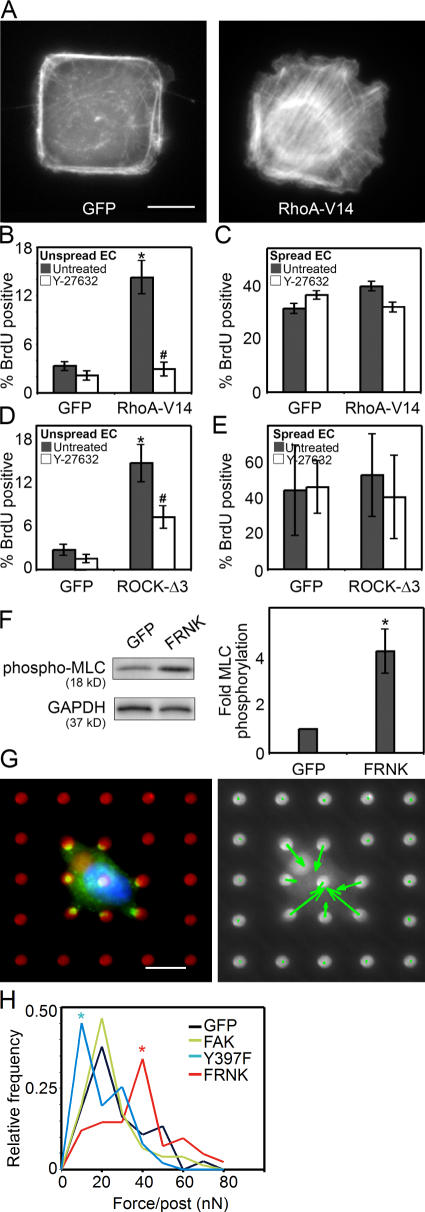
To determine whether changes in RhoA signaling are sufficient to directly affect proliferation, we overexpressed a constitutively active form of RhoA (RhoA-V14) in unspread ECs. RhoA-V14 dramatically increased stress fiber formation (Fig. 7 A) and was sufficient to overcome the spreading-regulated block in proliferation (Fig. 7 B). High RhoA also released cells from proliferation arrest induced by confluence (unpublished data). This effect was mediated through the RhoA effector ROCK, as treatment with Y-27632 abrogated the RhoA-V14–induced proliferation (Fig. 7 B). This ROCK activity was not only necessary but also sufficient to induce proliferation, as expression of a constitutively active ROCK (ROCK-Δ3) also bypassed the shape-dependent control mechanism (Fig. 7 D). As with RhoA-V14 overexpression, ROCK-Δ3 overexpression had no effect in well-spread cells (Fig. 7, C and E).
Figure 7.
RhoA-mediated contractility rescues proliferation in unspread cells. (A) F-actin stain of GFP-, or RhoA-V14–overexpressing ECs cultured on 625-μm2 islands of fibronectin. (B and C) Graph of the percentage of GFP- or RhoA-V14–overexpressing ECs that enter S phase when cultured on 625-μm2 islands of fibronectin (B), or on surfaces uniformly coated with 25 μg/ml fibronectin (C) and either untreated or treated with 50 μM Y-27632. (D and E) Graph of the percentage of GFP- or ROCK-Δ3–overexpressing ECs that enter S phase when cultured on 625-μm2 islands of fibronectin (D), or on surfaces that were uniformly coated with 25 μg/ml fibronectin (E), and either untreated or treated with 50 μM Y-27632. (F) Western blot and graph of phosphorylated myosin light chain in GFP- versus FRNK-expressing ECs, normalized to GAPDH. (G) A representative GFP-expressing EC cultured on the mPAD force sensors (red, fibronectin; green, GFP; blue, nucleus) and accompanying vector plot (green arrows indicate magnitude and the direction of force exerted on each underlying post). (H) Distribution plot of the magnitude of traction forces exerted by GFP-, FAK-, FRNK-, or FAK-Y397F–expressing ECs on mPADs. Data is expressed ± SEM for at least three independent experiments for proliferation and myosin phosphorylation data. For proliferation graphs, * denotes P < 0.05 between RhoA-V14 or ROCK-Δ3 versus GFP control and # denotes P < 0.05 between RhoA-V14 or ROCK-Δ3–induced proliferation in untreated versus drug-treated samples. For force distribution plot, * denotes P < 0.05 between adenovirus condition as compared with GFP control. Bars, 10 μm.
One important consequence of RhoA and ROCK signaling is in mediating changes in myosin-regulated cytoskeletal tension (Amano et al., 1996; Kimura et al., 1996; Ishizaki et al., 1997). To address whether FRNK-induced signaling altered focal adhesion structure and proliferation via RhoA-mediated changes in cytoskeletal tension, we assessed myosin phosphorylation in cells expressing FRNK. FRNK expression dramatically increased the amount of phosphomyosin compared with GFP controls (Fig. 7 F). Although this suggests that FRNK might be functioning to increase cytoskeletal tension in unspread cells, myosin phosphorylation is not always associated with the development of tension. To directly measure the tension transmitted across the focal adhesion onto the underlying substrate, we used a previously described microfabricated force sensor (Tan et al., 2003), consisting of an array of vertically placed elastomeric microneedles. These microneedles report the traction force exerted by cells on the underlying substrate. Thus, we directly measured the tension generated in unspread cells expressing FAK, FRNK, FAK-Y397F, or a GFP control. Notably, only FRNK expression increased traction force (Fig. 7, G and H). FAK expression showed no differences in tension, whereas expression of FAK-Y397F decreased tension. Collectively, these data support a novel role for FAK in growth control, in which loss of FAK signaling can induce RhoA-mediated cytoskeletal tension, leading to the loss of adhesion-dependent control of cell proliferation.
Discussion
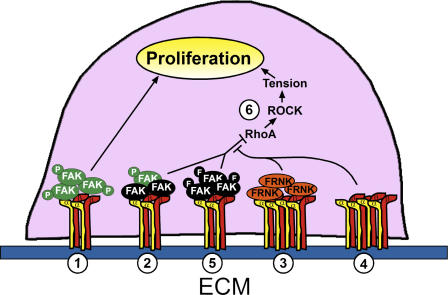
In this study, we demonstrate that FAK plays a key role in the regulation of proliferation by cell adhesion, whether modulated by ECM density, cell spreading, confluence, or 3D culture. FAK overexpression has been shown to increase proliferation in previous studies (Gilmore and Romer, 1996; Zhao et al., 1998). We find that FAK exerts not only stimulatory but also inhibitory effects on proliferation. The inhibitory function of FAK is lost in FAK−/− cells and, importantly, rescued when FAK is reexpressed. Interestingly, expressing the C-terminal fragments of FAK (FRNK or FAT) also dysregulated the inhibitory function of FAK, whereas the full-length, kinase-dead mutant (FAK-Y397F) could rescue growth inhibition. These data suggest that the inhibitory function of FAK lies in its N-terminal domain. Given that we and others find that FRNK and FAT displace endogenous full-length FAK from focal adhesions (Richardson and Parsons, 1996), these C-terminal constructs might interfere with FAK function by competitively inhibiting the targeting of cellular FAK to the focal adhesion, suggesting the interesting possibility that a pool of inactive FAK may normally function to inhibit proliferation through these interactions, and suggests a model whereby FAK acts within adhesions as a graded sensor that transduces adhesive signals to regulate the cell cycle (Fig. 8). High FAK activation caused by high adhesion or by high FAK expression stimulates proliferation, whereas minimal adhesion prevents FAK activation and yields inactive complexes that inhibit proliferation. Interestingly, Moissoglu and Gelman (2003) observed an unexpected enhancement of soft agar colony formation in v-Src–transformed cells lacking FAK that was subsequently prevented by FAK reexpression, suggesting the possibility that FAK may play a negative regulatory role in transformation. Notably, this occurred in a low adhesive environment. An alternative model for the proliferative response to both up- and down-regulation of FAK is the possibility that dynamic cycling of FAK activation and deactivation is required for growth inhibition. Repeated cycles of FAK phosphorylation and dephosphorylation appear to be important for cell migration, as both decreasing and increasing FAK activity reduce migration (Yu et al., 1998; Angers-Loustau et al., 1999). Thus, both stimulatory and inhibitory roles for FAK may be an inherent feature of its function in numerous cellular processes.
Figure 8.
Model for FAK modulation of adhesion-regulated proliferation. (1) In conditions that activate FAK (green FAK circles), such as high adhesive contexts or FAK overexpression, FAK plays a stimulatory role in proliferation. (2) Endogenous FAK in low adhesive contexts, including low cell spreading, low fibronectin density, and 3D gels, is largely inactive (black FAK circles) and inhibits proliferation. When inactive full-length FAK is displaced by FRNK or FAT (3), or is eliminated as in FAK−/− cells (4), RhoA is activated, leading to ROCK activation and the development of cytoskeletal tension, creating a condition that is permissive for proliferation even in low adhesive conditions. (5) The dominant–negative FAK-Y397F (black FAK circles with F) is sufficient to rescue the inhibitory function of FAK, but not its stimulatory role, in proliferation. Expression of constitutively active RhoA or ROCK (6) alone can induce proliferation in low adhesive contexts.
RhoA is a critical regulator of focal adhesion formation (Ridley and Hall, 1992; Nobes and Hall, 1995). Our results also demonstrate that RhoA plays a role in the dysregulation of growth control in cells lacking FAK. Both FRNK-expressing cells and FAK−/− cells exhibit high RhoA activity that appears to be both necessary and sufficient for the observed proliferative effect (Fig. 8), supporting studies suggesting that RhoA promotes cell cycle progression (Olson et al., 1998). Although we show that the RhoA effector ROCK is important in our system, RhoA-mediated mDia signaling also appears to be sufficient to induce proliferation (Mammoto et al., 2004), suggesting that numerous RhoA signals may regulate growth. The mechanism by which FRNK and loss of FAK might up-regulate RhoA remains to be defined, although a simple mechanism may be that FRNK opposes the suppression of RhoA activity by endogenous FAK. The ability of FAK to down-regulate RhoA activity is well documented (Ren et al., 2000), and it has been shown that FAK may interact with the Rho GTPase-activating protein (GAP) GRAF (Hildebrand et al., 1996) and phosphorylate p190RhoGAP (Holinstat et al., 2006). It is possible that under different adhesive contexts, such as high or low ECM ligand density or high or low cell spreading, FAK may alter its interaction with Rho GAPs or Rho GEFs and, thus, modulate RhoA activity and proliferation.
It has long been known that changes in cell shape and the associated changes in cytoskeletal tension are required for proliferation (Folkman and Moscona, 1978; Ingber, 1990; Chen et al., 1997; Huang et al., 1998). We show that FAK transduces cell shape into proliferative signals. Interestingly, although FAK has been implicated as a mechanosensor where increasing tension leads to FAK activation (Wang et al., 2001), we show that FAK also alters the cytoskeletal tension and forces experienced at the adhesion. Expression of FRNK, through its effects on RhoA, increases myosin-based cytoskeletal tension, confirming earlier suggestions from the Parsons group that FRNK might increase cellular contractility (Martin et al., 2002). It has been previously observed that FRNK also increases focal adhesion size (Giannone et al., 2002). Our findings would suggest that these changes in focal adhesions are actually mediated by increased cytoskeletal tension, as focal adhesion maturation is induced by mechanical stress (Choquet et al., 1997; Balaban et al., 2001; Riveline et al., 2001). Thus, it appears that FAK both responds to and causes changes in mechanical force, and the latter links changes in cell adhesion to changes in cell mechanics and proliferation. These two reciprocal functions likely provide the mechanochemical feedback that is required for tightly integrating the mechanical and biochemical dynamics of cell adhesion.
The role of FAK in cell proliferation has implications for human physiology and pathology, where FAK protein overexpression has been found in invasive human tumors (Owens et al., 1995; Kornberg, 1998). This has led to the suggestion that targeting FAK might reduce cancer proliferation, migration, and invasion. However, it is now clear that the model whereby FAK is strictly a stimulatory molecule for proliferation is oversimplified. In fact, FAK down-regulation can increase tumor cell motility, invasion, and metastasis (Ayaki et al., 2001; Lu et al., 2001), and we speculate that it may also extend to include increased proliferation. Thus, simply eliminating FAK function in cancer settings may be detrimental, and recognizing these additional layers in FAK function may reveal how cells can interpret complex adhesive contexts into a well-adapted response.
For many adherent cell types, both integrin ligation and cell spreading are required to support proliferation. Because focal adhesion architecture and, likely, the focal adhesion character are different in spread and unspread cells, it is probable that focal adhesions formed under these various adhesive or mechanical contexts transmit different signals, leading to potentially divergent cellular behaviors. Importantly, FAK appears to be a central regulator of adhesion-mediated proliferation, whether signaled by spreading, confluence, ligand density, or 3D matrix architecture, where it can transduce both stimulatory and inhibitory proliferative signals. Understanding how this single molecule can play such a central role in many complex interactions will uncover important insights into how cells navigate and respond to their adhesive and mechanical environments in physiologically meaningful ways.
Materials and methods
Cell culture and reagents
Bovine pulmonary artery ECs (VEC Technologies, Inc.) were cultured in low glucose DME containing 2 mM glutamine, 100 units/ml penicillin, 100 μg/ml streptomycin, and 5% bovine serum (all from Invitrogen). ECs were maintained in a humidified 10% CO2 incubator. FAK−/− and FAK-reexpressing mouse embryo fibroblasts were a gift from S. Hanks (Vanderbilt University, Nashville, TN) and were cultured in DME containing 4,500 mg of D-glucose/ml, 2 mM glutamine, 100 units/ml penicillin, 100 μg/ml streptomycin, 0.25 μg of amphotericin B/ml (all from Invitrogen), and 10% fetal bovine serum (Atlanta Biologicals) and were maintained at 37°C in a humidified 5% CO2 incubator. The following reagents were purchased from the given suppliers: human fibronectin (Invitrogen); Y-27632 (Calbiochem), PP2 (Calbiochem), JNK inhibitor I, UO126 (Calbiochem), anti-vinculin clone hVin-1 (Sigma-Aldrich), TRITC-conjugated phalloidin (Sigma-Aldrich), anti-RhoA (Santa Cruz Biotechnology, Inc.), phospho-Y397-FAK antibody (BioSource International), total FAK antibody (Cell Signaling Technology), phospho-S18/S19 MLC antibody (Cell Signaling Technology), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody (Abcam).
Immunocytochemistry, image analysis, and quantitative analysis of focal adhesions
For F-actin stains, cells were fixed with 4% paraformaldehyde in PBS. F-actin was visualized by incubating samples with fluorophore-conjugated phalloidin (Invitrogen). Quantitative analysis of focal adhesions was performed as previously described (Nelson et al., 2004). In brief, cells were incubated for 1 min in ice-cold cytoskeleton buffer (50 mM NaCl, 150 mM sucrose, 3 mM MgCl2, 1 μg/ml aprotinin, 1 μg/ml leupeptin, 1 μg/ml pepstatin, and 2 mM PMSF), followed by 1 min in cytoskeleton buffer supplemented with 0.5% Triton X-100. Detergent-extracted cells were fixed in 4% paraformaldehyde in PBS, washed, and incubated with a primary antibody to vinculin (Sigma-Aldrich). After incubation with Alexa Fluor 594–conjugated secondary antibodies (Invitrogen), quantitative microscopy of focal adhesion proteins was performed using a charge-coupled device camera (Orca; Hamamatsu) attached to an inverted microscope (model TE2000; Nikon) using a 100×, 1.4 NA, oil immersion objective with a 400-ms exposure time at RT. Images were obtained and processed using IPLab software (Scanalytics); original images were filtered and binarized to subtract background fluorescence, and then segmented with a threshold of 0.25 μm2 to quantify the area of individual adhesions. Approximately 100–150 cells were analyzed per experimental condition.
Cell fractionation
Triton X-100 soluble and insoluble pools were generated by washing cells with ice-cold TBS, followed by a 5-min wash with Triton extraction buffer (50 mM NaCl, 150 mM sucrose, 3 mM MgCl2, 0.5% Triton X-100, 1 μg/ml aprotinin, 1 μg/ml leupeptin, 1 μg/ml pepstatin, and 2 mM PMSF). The soluble fraction was collected, mixed with Laemmli sample buffer, and boiled. The remaining Triton-insoluble fraction was collected by scraping directly into 1× Laemmli sample buffer and then boiled. Soluble and insoluble fractions were run on SDS-PAGE gels and blotted.
Culture and proliferation measurement of cells in collagen gel
3D collagen I gels were prepared by mixing M199 (Invitrogen), NaHCO3 (0.035% wt/vol; Sigma-Aldrich), 10 mM Hepes buffer (Invitrogen), rat tail collagen I (BD Biosciences), and distilled water with the pH adjusted to 7.4. Synchronized FAK−/− and FAK-reexpressing cells were seeded into a 2.4-mg/ml collagen gel at a concentration of 16,000 cells/ml followed by gelation at 37°C for 30 min. Cells were incubated for 22 h in the presence of radiolabeled thymidine (MP Biomedicals), after which the cells were lysed and DNA was precipitated with 16 M NaOH containing 0.25% Triton X-100. Radioactivity counts were measured using a scintillation counter (Beckman Coulter). Blank collagen gels were used to measure background residual thymidine.
Micropatterned substrates
To generate stamps for microcontact printing of proteins, a prepolymer of poly(dimethylsiloxane) (PDMS; Sylgard 184; Dow Corning) was poured over a photolithographically generated master, as previously described (Chen et al., 1997). Stamps were immersed for 1 h in 50 μg/ml fibronectin, washed three times in water, and blown dry under nitrogen. Coated stamps were placed in conformal contact with a surface-oxidized PDMS-coated glass coverslip. Stamped coverslips were immersed in 0.2% Pluronic F127 (BASF) in PBS for 1 h and washed.
Adenovirus production
FAK, FRNK, FAT, FAK-Y397F, RhoA-V14, ROCK-Δ3, and GFP recombinant adenoviruses were constructed using the AdEasy XL system (Stratagene) according to manufacturer's instructions. RhoA cDNAs were obtained from M. Philips (New York University Medical Center, New York, NY) and P. Burbelo (Georgetown University, Washington, DC). ROCK cDNAs were obtained from S. Narumiya (Kyoto University, Kyoto, Japan). In brief, cDNAs were subcloned into the pShuttle-IRES-GFP1 vector, and then cotransformed with the pADEASY1 plasmid. After homologous recombination, plasmids were used to transfect human embryonic kidney 293 cells. High titer preparations of recombinant adenovirus were generated by CsCl2 density gradient centrifugation. In viral infection experiments, viral MOI resulting in a transduction efficiency of at least 80% was added to cells.
Proliferation assays
ECs were G0 synchronized by holding the cells at confluence for 2 d. FAK−/− and FAK-reexpressing cells were synchronized by 60-h serum starvation. Cells were then trypsinized and replated in the presence of BrdU (GE Healthcare). Cells were fixed at 22 h and stained for BrdU incorporation using a monoclonal antibody directed against BrdU (GE Healthcare). Cells were counterstained with Hoechst 33342 (Invitrogen).
RhoA activity assays
RhoA-GTP levels were measured by pull-down assay (Ren and Schwartz, 2000). In brief, cells were washed with cold TBS, scraped into lysis buffer (25 mM Hepes, pH 7.5, 15 mM NaCl, 1% Igepal CA-630, 5 mM MgCl2, 1 mM EDTA, 10% glycerol, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 10 μg/ml pepstatin, and 2 mM PMSF). Cleared lysates were incubated with 30 μg GST–rhotekin-binding domain–agarose beads (Upstate Biotechnology) for 45 min at 4°C, centrifuged, washed, and eluted by boiling in SDS-PAGE buffer containing 5% β-mercaptoethanol for 5 min. RhoA was detected by Western blotting using a monoclonal antibody to RhoA (Santa Cruz Biotechnology, Inc.). The level of RhoA activity in different samples was determined by normalizing the amount of rhotekin-binding domain–bound RhoA to the total amount of RhoA in cell lysates.
Western blots
Cells were washed in TBS and lysed in cold modified RIPA buffer (50 mM Tris-HCl, pH 7.4, 1% Igepal CA-630, 0.25% deoxycholate, 150 mM NaCl, 1 mM EDTA, 1 mM PMSF, 1 mM orthovanadate, 1 mM NaF, and 1 μg/ml each aprotinin, leupeptin, and pepstatin). Proteins were separated by denaturing SDS-PAGE electroblotted onto PVDF, blocked with 5% milk in TBS, immunoblotted with specific primary antibodies, and detected using horseradish peroxidase–conjugated secondary antibodies (Jackson ImmunoResearch Laboratories) and SuperSignal West Dura (Pierce Chemical Co.) as a chemiluminescent substrate. Densitometric analysis was performed using a VersaDoc imaging system with QuantityOne software (Bio-Rad Laboratories).
Microfabricated post array detectors
Microfabricated post array detectors (mPADs) were fabricated as previously described (Lemmon et al., 2005; Tan et al., 2003). mPADs used in these studies were 11 μm tall and 3 μm in diameter, with 9 μm center–center spacing. To control cell spreading on microneedle tips, the tips were stamped with fibronectin using microcontact printing (Tan et al., 2003), and nonstamped regions were blocked with 0.2% Pluronic F127 (BASF). ECs expressing either GFP, FRNK, FAK, or FAK-Y397F were cultured on the mPADs for 22 h, after which the samples were fixed with 4% paraformaldehyde in PBS. Fibronectin was stained with goat anti-fibronectin antibody (ICN Biomedicals) and the nuclei were stained with Hoechst 33342. The samples were imaged using an Axiovert 200M (Carl Zeiss MicroImaging, Inc.) with the Apotome module, equipped with 63× Plan-Apochromat, 1.4 NA, oil immersion objective, an Axiocam camera, and Axiovision software (Carl Zeiss MicroImaging, Inc.). A Matlab program (The MathWorks) was used to obtain tractional force from the acquired images. At least six cells were used in force measurements in each condition.
Acknowledgments
The authors are grateful to Dr. Shuh Narumiya for the ROCK cDNAs, to Dr. Steve Hanks for providing the FAK−/− and FAK-reexpressing cells, to Dr. Martin Schwartz for advice on the Rho GTPase activity assay, and to Dr. Mark Philips and Dr. Peter Burbelo for providing the RhoA cDNAs. We also thank Kiran Bhadriraju and John Tan for helpful discussions.
This work was supported by National Institutes of Health grants HL073305 and EB00262 (C. Chen) and AI061042 and HL058064 (L. Romer), a Ruth L. Kirschstein NRSA fellowship (D. Pirone), and Funds for Medical Discovery from Johns Hopkins University (L. Romer and C. Chen).
Abbreviations used in this paper: EC, endothelial cell; FAT, focal adhesion targeting; GAP, GTPase-activating protein; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; mPAD, microfabricated post array detector; PDMS, poly(dimethylsiloxane).
References
- Amano, M., M. Ito, K. Kimura, Y. Fukata, K. Chihara, T. Nakano, Y. Matsuura, and K. Kaibuchi. 1996. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J. Biol. Chem. 271:20246–20249. [DOI] [PubMed] [Google Scholar]
- Angers-Loustau, A., J.F. Cote, A. Charest, D. Dowbenko, S. Spencer, L.A. Lasky, and M.L. Tremblay. 1999. Protein tyrosine phosphatase–PEST regulates focal adhesion disassembly, migration, and cytokinesis in fibroblasts. J. Cell Biol. 144:1019–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayaki, M., K. Komatsu, M. Mukai, K. Murata, M. Kameyama, S. Ishiguro, J. Miyoshi, M. Tatsuta, and H. Nakamura. 2001. Reduced expression of focal adhesion kinase in liver metastases compared with matched primary human colorectal adenocarcinomas. Clin. Cancer Res. 7:3106–3112. [PubMed] [Google Scholar]
- Balaban, N.Q., U.S. Schwarz, D. Riveline, P. Goichberg, G. Tzur, I. Sabanay, D. Mahalu, S. Safran, A. Bershadsky, L. Addadi, and B. Geiger. 2001. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat. Cell Biol. 3:466–472. [DOI] [PubMed] [Google Scholar]
- Bohmer, R.M., E. Scharf, and R.K. Assoian. 1996. Cytoskeletal integrity is required throughout the mitogen stimulation phase of the cell cycle and mediates the anchorage-dependent expression of cyclin D1. Mol. Biol. Cell. 7:101–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks, S.C., III, R. Sturgill, J. Choi, and A. Yen. 1997. An RXR-selective analog attenuates the RAR alpha-selective analog-induced differentiation and non-G1-restricted growth arrest of NB4 cells. Exp. Cell Res. 234:259–269. [DOI] [PubMed] [Google Scholar]
- Burridge, K., C.E. Turner, and L.H. Romer. 1992. Tyrosine phosphorylation of paxillin and pp125FAK accompanies cell adhesion to extracellular matrix: a role in cytoskeletal assembly. J. Cell Biol. 119:893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge, K., and M. Chrzanowska-Wodnicka. 1996. Focal adhesions, contractility, and signaling. Annu. Rev. Cell Dev. Biol. 12:463–518. [DOI] [PubMed] [Google Scholar]
- Chen, C.S., M. Mrksich, S. Huang, G.M. Whitesides, and D.E. Ingber. 1997. Geometric control of cell life and death. Science. 276:1425–1428. [DOI] [PubMed] [Google Scholar]
- Chen, C.S., J.L. Alonso, E. Ostuni, G.M. Whitesides, and D.E. Ingber. 2003. Cell shape provides global control of focal adhesion assembly. Biochem. Biophys. Res. Commun. 307:355–361. [DOI] [PubMed] [Google Scholar]
- Choquet, D., D.P. Felsenfeld, and M.P. Sheetz. 1997. Extracellular matrix rigidity causes strengthening of integrin-cytoskeleton linkages. Cell. 88:39–48. [DOI] [PubMed] [Google Scholar]
- Danen, E.H., P. Sonneveld, A. Sonnenberg, and K.M. Yamada. 2000. Dual stimulation of Ras/mitogen-activated protein kinase and RhoA by cell adhesion to fibronectin supports growth factor–stimulated cell cycle progression. J. Cell Biol. 151:1413–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duxbury, M.S., H. Ito, E. Benoit, M.J. Zinner, S.W. Ashley, and E.E. Whang. 2003. RNA interference targeting focal adhesion kinase enhances pancreatic adenocarcinoma gemcitabine chemosensitivity. Biochem. Biophys. Res. Commun. 311:786–792. [DOI] [PubMed] [Google Scholar]
- Folkman, J., and A. Moscona. 1978. Role of cell shape in growth control. Nature. 273:345–349. [DOI] [PubMed] [Google Scholar]
- Geiger, B., A. Bershadsky, R. Pankov, and K.M. Yamada. 2001. Transmembrane crosstalk between the extracellular matrix–cytoskeleton crosstalk. Nat. Rev. Mol. Cell Biol. 2:793–805. [DOI] [PubMed] [Google Scholar]
- Giannone, G., P. Ronde, M. Gaire, J. Haiech, and K. Takeda. 2002. Calcium oscillations trigger focal adhesion disassembly in human U87 astrocytoma cells. J. Biol. Chem. 277:26364–26371. [DOI] [PubMed] [Google Scholar]
- Gilmore, A.P., and L.H. Romer. 1996. Inhibition of focal adhesion kinase (FAK) signaling in focal adhesions decreases cell motility and proliferation. Mol. Biol. Cell. 7:1209–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan, J.L., and D. Shalloway. 1992. Regulation of focal adhesion-associated protein tyrosine kinase by both cellular adhesion and oncogenic transformation. Nature. 358:690–692. [DOI] [PubMed] [Google Scholar]
- Guan, J.L., J.E. Trevithick, and R.O. Hynes. 1991. Fibronectin/integrin interaction induces tyrosine phosphorylation of a 120-kDa protein. Cell Regul. 2:951–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand, J.D., J.M. Taylor, and J.T. Parsons. 1996. An SH3 domain-containing GTPase-activating protein for Rho and Cdc42 associates with focal adhesion kinase. Mol. Cell. Biol. 16:3169–3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holinstat, M., N. Knezevic, M. Broman, A.M. Samarel, A.B. Malik, and D. Mehta. 2006. Suppression of RhoA activity by focal adhesion kinase-induced activation of p190RhoGAP: role in regulation of endothelial permeability. J. Biol. Chem. 281:2296–2305. [DOI] [PubMed] [Google Scholar]
- Hood, J.D., R. Frausto, W.B. Kiosses, M.A. Schwartz, and D.A. Cheresh. 2003. Differential αv integrin–mediated Ras-ERK signaling during two pathways of angiogenesis. J. Cell Biol. 162:933–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, S., C.S. Chen, and D.E. Ingber. 1998. Control of cyclin D1, p27(Kip1), and cell cycle progression in human capillary endothelial cells by cell shape and cytoskeletal tension. Mol. Biol. Cell. 9:3179–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilic, D., Y. Furuta, S. Kanazawa, N. Takeda, K. Sobue, N. Nakatsuji, S. Nomura, J. Fujimoto, M. Okada, and T. Yamamoto. 1995. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 377:539–544. [DOI] [PubMed] [Google Scholar]
- Ingber, D.E. 1990. Fibronectin controls capillary endothelial cell growth by modulating cell shape. Proc. Natl. Acad. Sci. USA. 87:3579–3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaki, T., M. Naito, K. Fujisawa, M. Maekawa, N. Watanabe, Y. Saito, and S. Narumiya. 1997. p160ROCK, a Rho-associated coiled-coil forming protein kinase, works downstream of Rho and induces focal adhesions. FEBS Lett. 404:118–124. [DOI] [PubMed] [Google Scholar]
- Kimura, K., M. Ito, M. Amano, K. Chihara, Y. Fukata, M. Nakafuku, B. Yamamori, J. Feng, T. Nakano, K. Okawa, et al. 1996. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science. 273:245–248. [DOI] [PubMed] [Google Scholar]
- Kornberg, L., H.S. Earp, J.T. Parsons, M. Schaller, and R.L. Juliano. 1992. Cell adhesion or integrin clustering increases phosphorylation of a focal adhesion-associated tyrosine kinase. J. Biol. Chem. 267:23439–23442. [PubMed] [Google Scholar]
- Kornberg, L.J. 1998. Focal adhesion kinase expression in oral cancers. Head Neck. 20:634–639. [DOI] [PubMed] [Google Scholar]
- Lemmon, C.A., N.J. Sniadecki, S.A. Ruiz, J.L. Tan, L.H. Romer, and C.S. Chen. 2005. Shear force at the cell-matrix interface: enhanced analysis for microfabricated post array detectors. Mech Chem Biosyst. 2:1–16. [PMC free article] [PubMed] [Google Scholar]
- Lewis, J.M., and M.A. Schwartz. 1995. Mapping in vivo associations of cytoplasmic proteins with integrin beta 1 cytoplasmic domain mutants. Mol. Biol. Cell. 6:151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipfert, L., B. Haimovich, M.D. Schaller, B.S. Cobb, J.T. Parsons, and J.S. Brugge. 1992. Integrin-dependent phosphorylation and activation of the protein tyrosine kinase pp125FAK in platelets. J. Cell Biol. 119:905–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, Z., G. Jiang, P. Blume-Jensen, and T. Hunter. 2001. Epidermal growth factor-induced tumor cell invasion and metastasis initiated by dephosphorylation and downregulation of focal adhesion kinase. Mol. Cell. Biol. 21:4016–4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammoto, A., S. Huang, K. Moore, P. Oh, and D.E. Ingber. 2004. Role of RhoA, mDia, and ROCK in cell shape-dependent control of the Skp2-p27kip1 pathway and the G1/S transition. J. Biol. Chem. 279:26323–26330. [DOI] [PubMed] [Google Scholar]
- Martin, K.H., S.A. Boerner, and J.T. Parsons. 2002. Regulation of focal adhesion targeting and inhibitory functions of the FAK related protein FRNK using a novel estrogen receptor “switch.” Cell Motil. Cytoskeleton. 51:76–88. [DOI] [PubMed] [Google Scholar]
- Moissoglu, K., and I.H. Gelman. 2003. v-Src rescues actin-based cytoskeletal architecture and cell motility and induces enhanced anchorage independence during oncogenic transformation of focal adhesion kinase-null fibroblasts. J. Biol. Chem. 278:47946–47959. [DOI] [PubMed] [Google Scholar]
- Nelson, C.M., D.M. Pirone, J.L. Tan, and C.S. Chen. 2004. Vascular endothelial-cadherin regulates cytoskeletal tension, cell spreading, and focal adhesions by stimulating RhoA. Mol. Biol. Cell. 15:2943–2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobes, C.D., and A. Hall. 1995. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 81:53–62. [DOI] [PubMed] [Google Scholar]
- Nolan, K., J. Lacoste, and J.T. Parsons. 1999. Regulated expression of focal adhesion kinase-related nonkinase, the autonomously expressed C-terminal domain of focal adhesion kinase. Mol. Cell. Biol. 19:6120–6129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oktay, M., K.K. Wary, M. Dans, R.B. Birge, and F.G. Giancotti. 1999. Integrin-mediated activation of focal adhesion kinase is required for signaling to Jun NH2-terminal kinase and progression through the G1 phase of the cell cycle. J. Cell Biol. 145:1461–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson, M.F., H.F. Paterson, and C.J. Marshall. 1998. Signals from Ras and Rho GTPases interact to regulate expression of p21Waf1/Cip1. Nature. 394:295–299. [DOI] [PubMed] [Google Scholar]
- Owens, L.V., L. Xu, R.J. Craven, G.A. Dent, T.M. Weiner, L. Kornberg, E.T. Liu, and W.G. Cance. 1995. Overexpression of the focal adhesion kinase (p125FAK) in invasive human tumors. Cancer Res. 55:2752–2755. [PubMed] [Google Scholar]
- Parsons, J.T., K.H. Martin, J.K. Slack, J.M. Taylor, and S.A. Weed. 2000. Focal adhesion kinase: a regulator of focal adhesion dynamics and cell movement. Oncogene. 19:5606–5613. [DOI] [PubMed] [Google Scholar]
- Prutzman, K.C., G. Gao, M.L. King, V.V. Iyer, G.A. Mueller, M.D. Schaller, and S.L. Campbell. 2004. The focal adhesion targeting domain of focal adhesion kinase contains a hinge region that modulates tyrosine 926 phosphorylation. Structure (Camb). 12:881–891. [DOI] [PubMed] [Google Scholar]
- Ren, X.D., and M.A. Schwartz. 2000. Determination of GTP loading on Rho. Methods Enzymol. 325:264–272. [DOI] [PubMed] [Google Scholar]
- Ren, X.D., W.B. Kiosses, D.J. Sieg, C.A. Otey, D.D. Schlaepfer, and M.A. Schwartz. 2000. Focal adhesion kinase suppresses Rho activity to promote focal adhesion turnover. J. Cell Sci. 113:3673–3678. [DOI] [PubMed] [Google Scholar]
- Richardson, A., and T. Parsons. 1996. A mechanism for regulation of the adhesion- associated proteintyrosine kinase pp125FAK. Nature. 380:538–540. [DOI] [PubMed] [Google Scholar]
- Richardson, A., R.K. Malik, J.D. Hildebrand, and J.T. Parsons. 1997. Inhibition of cell spreading by expression of the C-terminal domain of focal adhesion kinase (FAK) is rescued by coexpression of Src or catalytically inactive FAK: a role for paxillin tyrosine phosphorylation. Mol. Cell. Biol. 17:6906–6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley, A.J., and A. Hall. 1992. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 70:389–399. [DOI] [PubMed] [Google Scholar]
- Riveline, D., E. Zamir, N.Q. Balaban, U.S. Schwarz, T. Ishizaki, S. Narumiya, Z. Kam, B. Geiger, and A.D. Bershadsky. 2001. Focal contacts as mechanosensors: externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. J. Cell Biol. 153:1175–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastry, S.K., and A.F. Horwitz. 1996. Adhesion-growth factor interactions during differentiation: an integrated biological response. Dev. Biol. 180:455–467. [DOI] [PubMed] [Google Scholar]
- Schaller, M.D., C.A. Borgman, and J.T. Parsons. 1993. Autonomous expression of a noncatalytic domain of the focal adhesion-associated protein tyrosine kinase pp125FAK. Mol. Cell. Biol. 13:785–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller, M.D., J.D. Hildebrand, J.D. Shannon, J.W. Fox, R.R. Vines, and J.T. Parsons. 1994. Autophosphorylation of the focal adhesion kinase, pp125FAK, directs SH2-dependent binding of pp60src. Mol. Cell. Biol. 14:1680–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz, M.A., and D.E. Ingber. 1994. Integrating with integrins. Mol. Biol. Cell. 5:389–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz, M.A., and M.H. Ginsberg. 2002. Networks and crosstalk: integrin signalling spreads. Nat. Cell Biol. 4:E65–E68. [DOI] [PubMed] [Google Scholar]
- Tan, J.L., J. Tien, D.M. Pirone, D.S. Gray, K. Bhadriraju, and C.S. Chen. 2003. Cells lying on a bed of microneedles: an approach to isolate mechanical force. Proc. Natl. Acad. Sci. USA. 100:1484–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J.G., M. Miyazu, E. Matsushita, M. Sokabe, and K. Naruse. 2001. Uniaxial cyclic stretch induces focal adhesion kinase (FAK) tyrosine phosphorylation followed by mitogen-activated protein kinase (MAPK) activation. Biochem. Biophys. Res. Commun. 288:356–361. [DOI] [PubMed] [Google Scholar]
- Welsh, C.F., K. Roovers, J. Villanueva, Y. Liu, M.A. Schwartz, and R.K. Assoian. 2001. Timing of cyclin D1 expression within G1 phase is controlled by Rho. Nat. Cell Biol. 3:950–957. [DOI] [PubMed] [Google Scholar]
- Yu, D.H., C.K. Qu, O. Henegariu, X. Lu, and G.S. Feng. 1998. Protein-tyrosine phosphatase Shp-2 regulates cell spreading, migration, and focal adhesion. J. Biol. Chem. 273:21125–21131. [DOI] [PubMed] [Google Scholar]
- Zhao, J.H., H. Reiske, and J.L. Guan. 1998. Regulation of the cell cycle by focal adhesion kinase. J. Cell Biol. 143:1997–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]