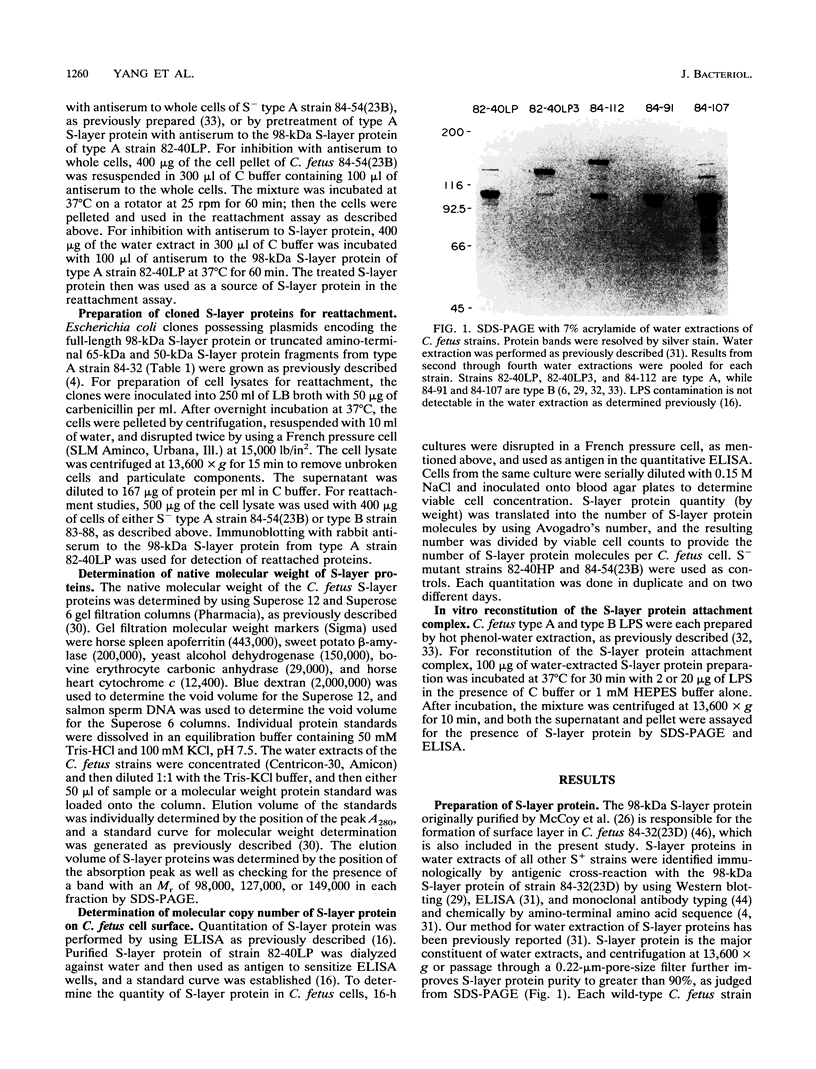
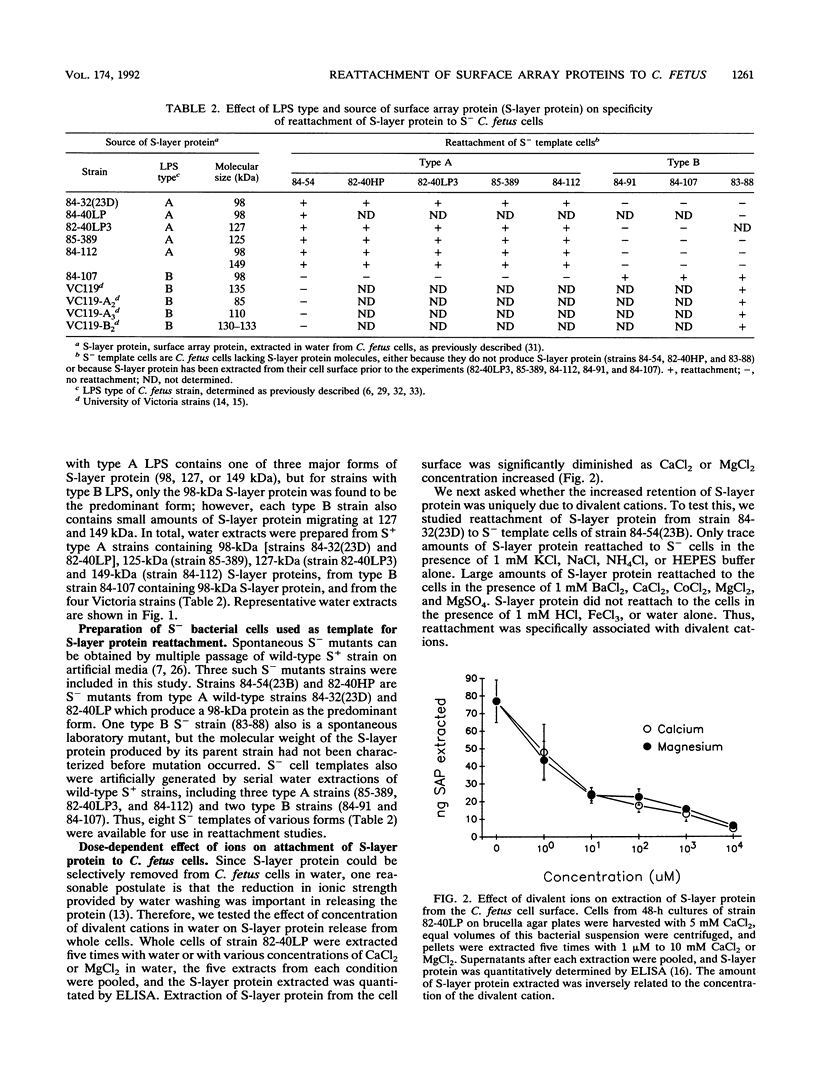
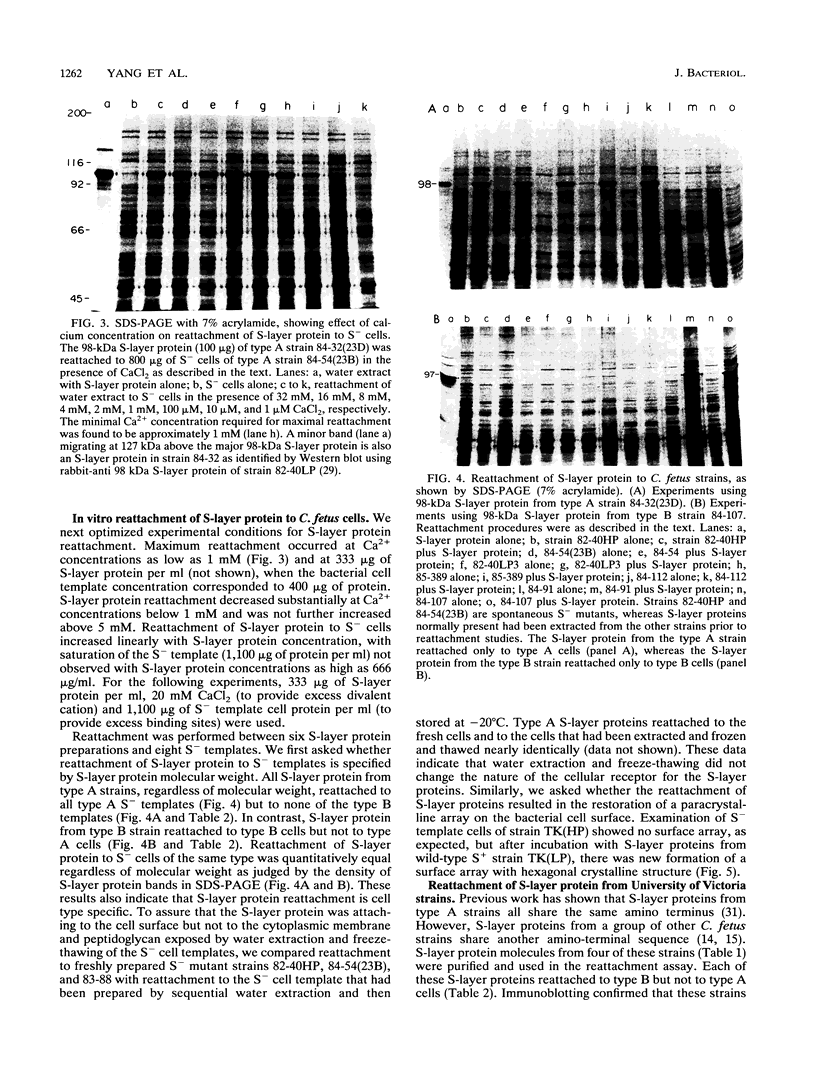
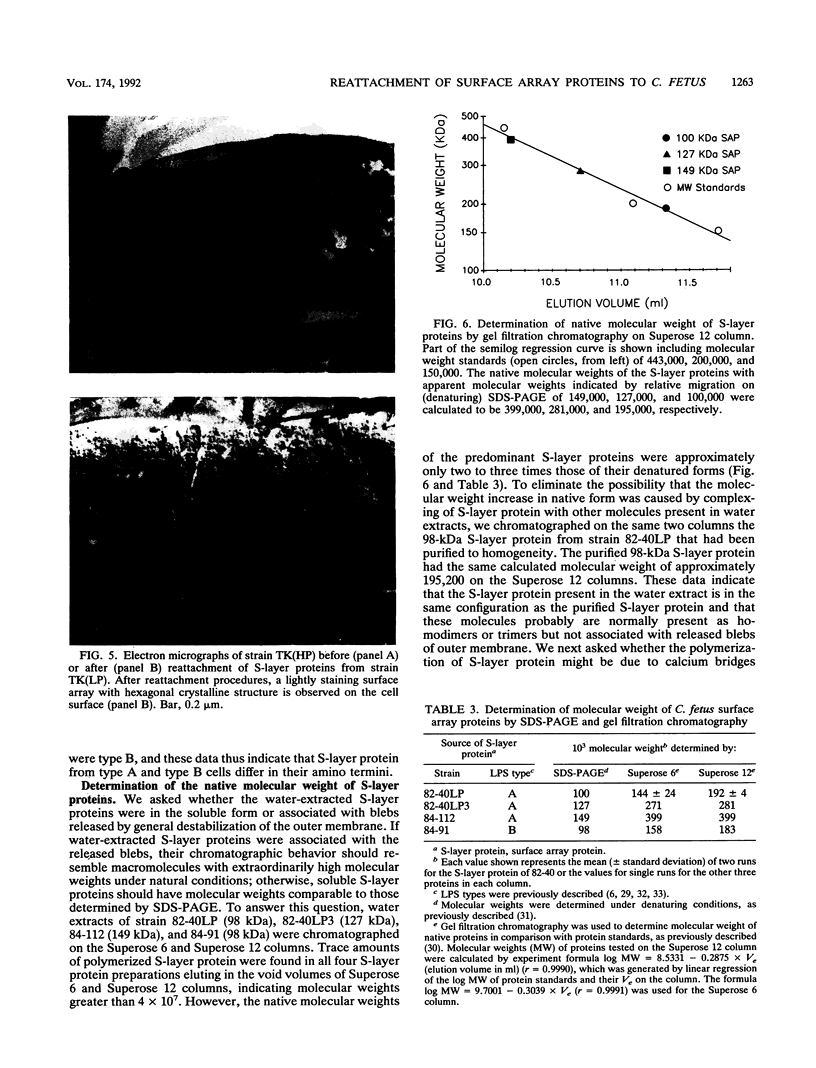
Abstract
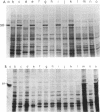
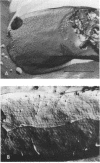
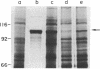
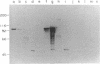
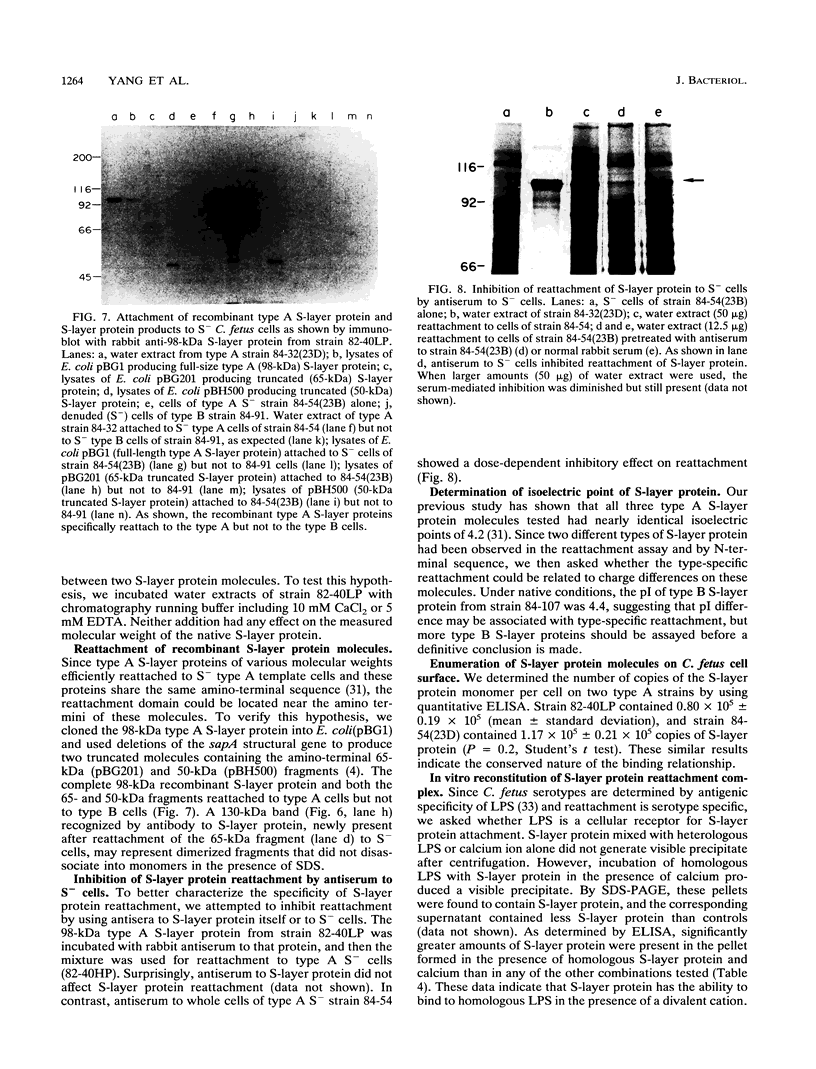
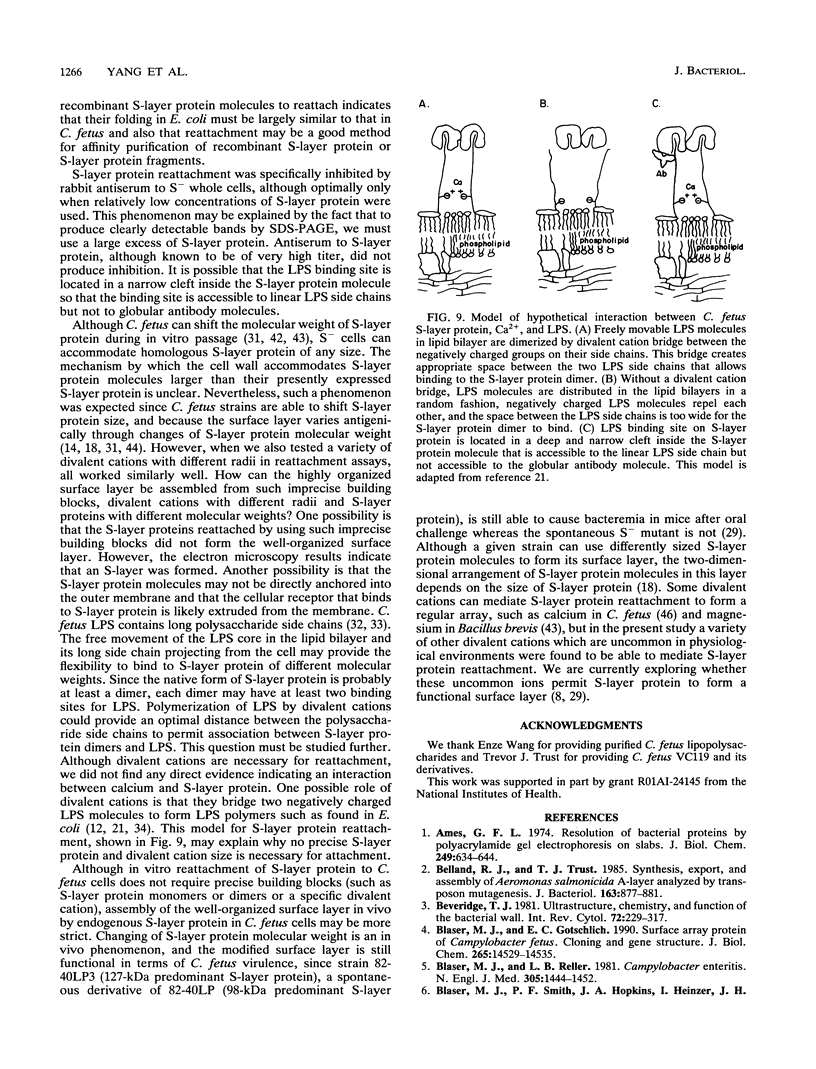
Campylobacter fetus strains may be of serotype A or B, a property associated with lipopolysaccharide (LPS) structure. Wild-type C. fetus strains contain surface array proteins (S-layer proteins) that may be extracted in water and that are critical for virulence. To explore the relationship of S-layer proteins to other surface components, we reattached S-layer proteins onto S- template cells generated by spontaneous mutation or by serial extractions of S+ cells with water. Reattachment occurred in the presence of divalent (Ba2+, Ca2+, Co2+, and Mg2+) but not monovalent (H+, NH4+, Na+, K+) or trivalent (Fe3+) cations. The 98-, 125-, 127-, and 149-kDa S-layer proteins isolated from strains containing type A LPS (type A S-layer protein) all reattached to S- template cells containing type A LPS (type A cells) but not to type B cells. The 98-kDa type B S-layer protein reattached to SAP- type B cells but not to type A cells. Recombinant 98-kDa type A S-layer protein and its truncated amino-terminal 65- and 50-kDa segments expressed in Escherichia coli retained the full and specific determinants for attachment. S-layer protein and purified homologous but not heterologous LPS in the presence of calcium produced insoluble complexes. By quantitative enzyme-linked immunosorbent assay, the S-layer protein copy number per C. fetus cell was determined to be approximately 10(5). In conclusion, C. fetus cells are encapsulated by a large number of S-layer protein molecules which may be specifically attached through the N-terminal half of the molecule to LPS in the presence of divalent cations.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Resolution of bacterial proteins by polyacrylamide gel electrophoresis on slabs. Membrane, soluble, and periplasmic fractions. J Biol Chem. 1974 Jan 25;249(2):634–644. [PubMed] [Google Scholar]
- Belland R. J., Trust T. J. Synthesis, export, and assembly of Aeromonas salmonicida A-layer analyzed by transposon mutagenesis. J Bacteriol. 1985 Sep;163(3):877–881. doi: 10.1128/jb.163.3.877-881.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge T. J. Ultrastructure, chemistry, and function of the bacterial wall. Int Rev Cytol. 1981;72:229–317. doi: 10.1016/s0074-7696(08)61198-5. [DOI] [PubMed] [Google Scholar]
- Blaser M. J., Gotschlich E. C. Surface array protein of Campylobacter fetus. Cloning and gene structure. J Biol Chem. 1990 Aug 25;265(24):14529–14535. [PubMed] [Google Scholar]
- Blaser M. J., Reller L. B. Campylobacter enteritis. N Engl J Med. 1981 Dec 10;305(24):1444–1452. doi: 10.1056/NEJM198112103052404. [DOI] [PubMed] [Google Scholar]
- Blaser M. J., Smith P. F., Hopkins J. A., Heinzer I., Bryner J. H., Wang W. L. Pathogenesis of Campylobacter fetus infections: serum resistance associated with high-molecular-weight surface proteins. J Infect Dis. 1987 Apr;155(4):696–706. doi: 10.1093/infdis/155.4.696. [DOI] [PubMed] [Google Scholar]
- Blaser M. J., Smith P. F., Kohler P. F. Susceptibility of Campylobacter isolates to the bactericidal activity of human serum. J Infect Dis. 1985 Feb;151(2):227–235. doi: 10.1093/infdis/151.2.227. [DOI] [PubMed] [Google Scholar]
- Blaser M. J., Smith P. F., Repine J. E., Joiner K. A. Pathogenesis of Campylobacter fetus infections. Failure of encapsulated Campylobacter fetus to bind C3b explains serum and phagocytosis resistance. J Clin Invest. 1988 May;81(5):1434–1444. doi: 10.1172/JCI113474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokkenheuser V. Vibrio fetus infection in man. I. Ten new cases and some epidemiologic observations. Am J Epidemiol. 1970 Apr;91(4):400–409. doi: 10.1093/oxfordjournals.aje.a121150. [DOI] [PubMed] [Google Scholar]
- Buckmire F. L., Murray R. G. Substructure and in vitro assembly of the outer, structured layer of Spirillum serpens. J Bacteriol. 1976 Jan;125(1):290–299. doi: 10.1128/jb.125.1.290-299.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester I. R., Murray R. G. Protein-lipid-lipopolysaccharide association in the superficial layer of Spirillum serpens cell walls. J Bacteriol. 1978 Feb;133(2):932–941. doi: 10.1128/jb.133.2.932-941.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin R. T., Tonsager S., McGroarty E. J. Quantitation of metal cations bound to membranes and extracted lipopolysaccharide of Escherichia coli. Biochemistry. 1983 Apr 12;22(8):2002–2007. doi: 10.1021/bi00277a041. [DOI] [PubMed] [Google Scholar]
- Dubreuil J. D., Kostrzynska M., Austin J. W., Trust T. J. Antigenic differences among Campylobacter fetus S-layer proteins. J Bacteriol. 1990 Sep;172(9):5035–5043. doi: 10.1128/jb.172.9.5035-5043.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubreuil J. D., Logan S. M., Cubbage S., Eidhin D. N., McCubbin W. D., Kay C. M., Beveridge T. J., Ferris F. G., Trust T. J. Structural and biochemical analyses of a surface array protein of Campylobacter fetus. J Bacteriol. 1988 Sep;170(9):4165–4173. doi: 10.1128/jb.170.9.4165-4173.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogg G. C., Yang L. Y., Wang E., Blaser M. J. Surface array proteins of Campylobacter fetus block lectin-mediated binding to type A lipopolysaccharide. Infect Immun. 1990 Sep;58(9):2738–2744. doi: 10.1128/iai.58.9.2738-2744.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francioli P., Herzstein J., Grob J. P., Vallotton J. J., Mombelli G., Glauser M. P. Campylobacter fetus subspecies fetus bacteremia. Arch Intern Med. 1985 Feb;145(2):289–292. [PubMed] [Google Scholar]
- Fujimoto S., Takade A., Amako K., Blaser M. J. Correlation between molecular size of the surface array protein and morphology and antigenicity of the Campylobacter fetus S layer. Infect Immun. 1991 Jun;59(6):2017–2022. doi: 10.1128/iai.59.6.2017-2022.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto S., Umeda A., Takade A., Murata K., Amako K. Hexagonal surface layer of Campylobacter fetus isolated from humans. Infect Immun. 1989 Aug;57(8):2563–2565. doi: 10.1128/iai.57.8.2563-2565.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrant R. L., Lahita R. G., Winn W. C., Jr, Roberts R. B. Campylobacteriosis in man: pathogenic mechanisms and review of 91 bloodstream infections. Am J Med. 1978 Oct;65(4):584–592. doi: 10.1016/0002-9343(78)90845-8. [DOI] [PubMed] [Google Scholar]
- Kist M. L., Murray R. G. Components of the regular surface array of Aquaspirillum serpens MW5 and their assembly in vitro. J Bacteriol. 1984 Feb;157(2):599–606. doi: 10.1128/jb.157.2.599-606.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koval S. F., Murray R. G. The isolation of surface array proteins from bacteria. Can J Biochem Cell Biol. 1984 Nov;62(11):1181–1189. doi: 10.1139/o84-152. [DOI] [PubMed] [Google Scholar]
- Luckevich M. D., Beveridge T. J. Characterization of a dynamic S layer on Bacillus thuringiensis. J Bacteriol. 1989 Dec;171(12):6656–6667. doi: 10.1128/jb.171.12.6656-6667.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy E. C., Doyle D., Burda K., Corbeil L. B., Winter A. J. Superficial antigens of Campylobacter (Vibrio) fetus: characterization of antiphagocytic component. Infect Immun. 1975 Mar;11(3):517–525. doi: 10.1128/iai.11.3.517-525.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Pei Z. H., Ellison R. T., 3rd, Blaser M. J. Identification, purification, and characterization of major antigenic proteins of Campylobacter jejuni. J Biol Chem. 1991 Sep 5;266(25):16363–16369. [PubMed] [Google Scholar]
- Pei Z., Blaser M. J. Pathogenesis of Campylobacter fetus infections. Role of surface array proteins in virulence in a mouse model. J Clin Invest. 1990 Apr;85(4):1036–1043. doi: 10.1172/JCI114533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei Z., Ellison R. T., 3rd, Lewis R. V., Blaser M. J. Purification and characterization of a family of high molecular weight surface-array proteins from Campylobacter fetus. J Biol Chem. 1988 May 5;263(13):6416–6420. [PubMed] [Google Scholar]
- Perez Perez G. I., Blaser M. J. Lipopolysaccharide characteristics of pathogenic campylobacters. Infect Immun. 1985 Feb;47(2):353–359. doi: 10.1128/iai.47.2.353-359.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Perez G. I., Blaser M. J., Bryner J. H. Lipopolysaccharide structures of Campylobacter fetus are related to heat-stable serogroups. Infect Immun. 1986 Jan;51(1):209–212. doi: 10.21236/ada265573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler M., Osborn M. J. Interaction of divalent cations and polymyxin B with lipopolysaccharide. Biochemistry. 1979 Oct 2;18(20):4425–4430. doi: 10.1021/bi00587a024. [DOI] [PubMed] [Google Scholar]
- Sleytr U. B., Messner P. Crystalline surface layers in procaryotes. J Bacteriol. 1988 Jul;170(7):2891–2897. doi: 10.1128/jb.170.7.2891-2897.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleytr U. B., Messner P. Crystalline surface layers on bacteria. Annu Rev Microbiol. 1983;37:311–339. doi: 10.1146/annurev.mi.37.100183.001523. [DOI] [PubMed] [Google Scholar]
- Sleytr U. B. Regular arrays of macromolecules on bacterial cell walls: structure, chemistry, assembly, and function. Int Rev Cytol. 1978;53:1–62. doi: 10.1016/s0074-7696(08)62240-8. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Thorne K. J. Regularly arranged protein on the surfaces of Gram-negative bacteria. Biol Rev Camb Philos Soc. 1977 May;52(2):219–234. doi: 10.1111/j.1469-185x.1977.tb01351.x. [DOI] [PubMed] [Google Scholar]
- Thorne K. J., Thornley M. J., Naisbitt P., Glauert A. M. The nature of the attachment of a regularly arranged surface protein to the outer membrane of an Acinetobacter sp. Biochim Biophys Acta. 1975 Apr 21;389(1):97–116. doi: 10.1016/0005-2736(75)90388-0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi A., Uchihi R., Engelhardt H., Hattori H., Shimizu S., Tsukagoshi N., Udaka S. In vitro reconstitution of a hexagonal array with a surface layer protein synthesized by Bacillus subtilis harboring the surface layer protein gene from Bacillus brevis 47. J Bacteriol. 1989 Dec;171(12):6747–6752. doi: 10.1128/jb.171.12.6747-6752.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby E. W., Lambert A. A sensitive silver stain for proteins in agarose gels. Anal Biochem. 1983 Apr 15;130(2):353–358. doi: 10.1016/0003-2697(83)90599-7. [DOI] [PubMed] [Google Scholar]
- Winter A. J., McCoy E. C., Fullmer C. S., Burda K., Bier P. J. Microcapsule of Campylobacter fetus: chemical and physical characterization. Infect Immun. 1978 Dec;22(3):963–971. doi: 10.1128/iai.22.3.963-971.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]