Abstract
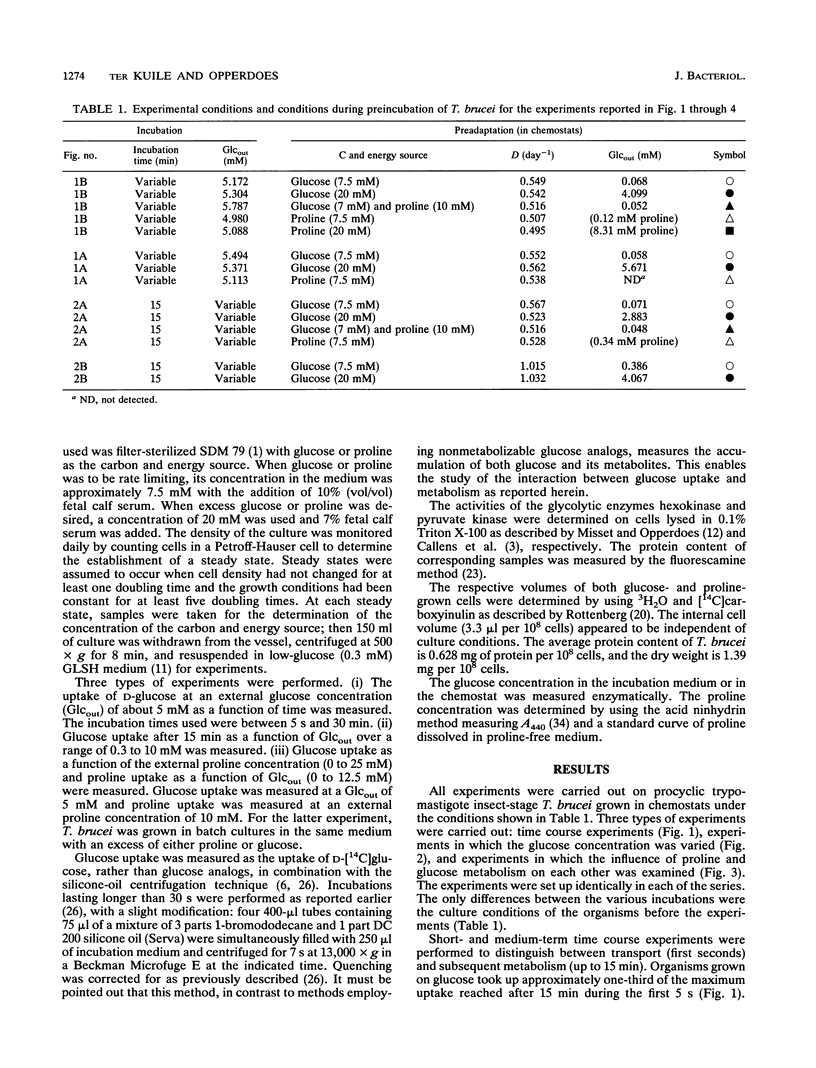
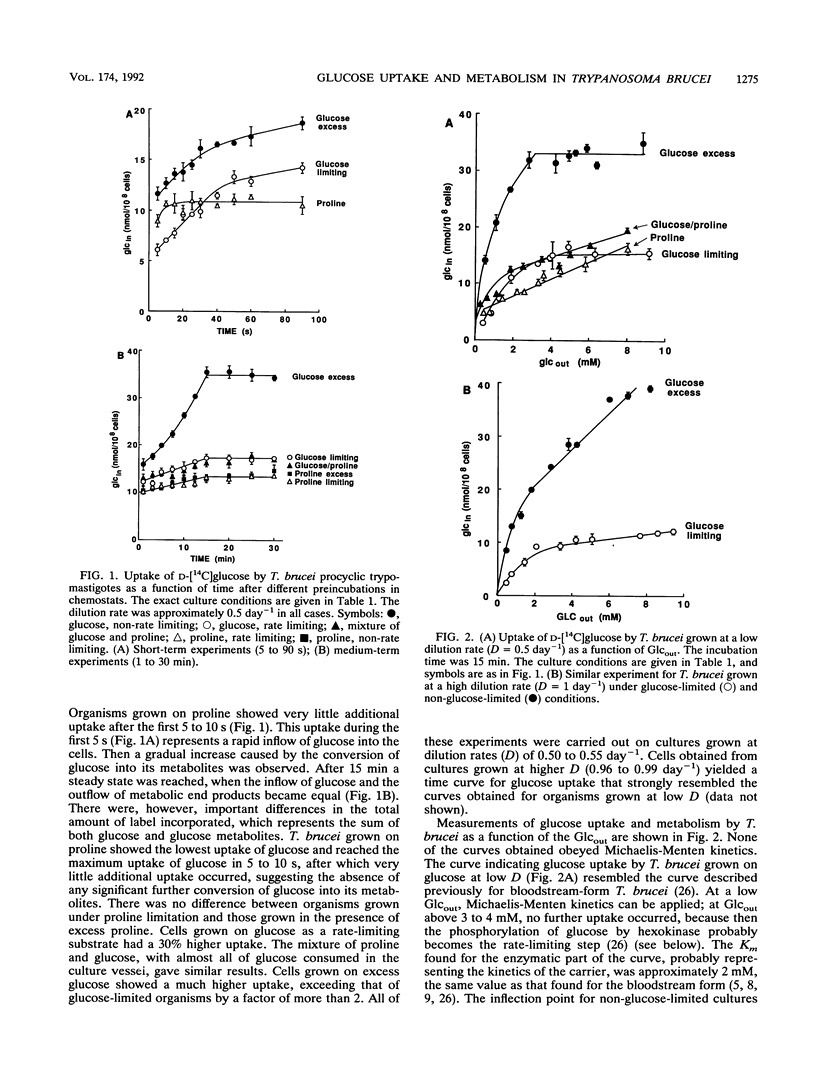
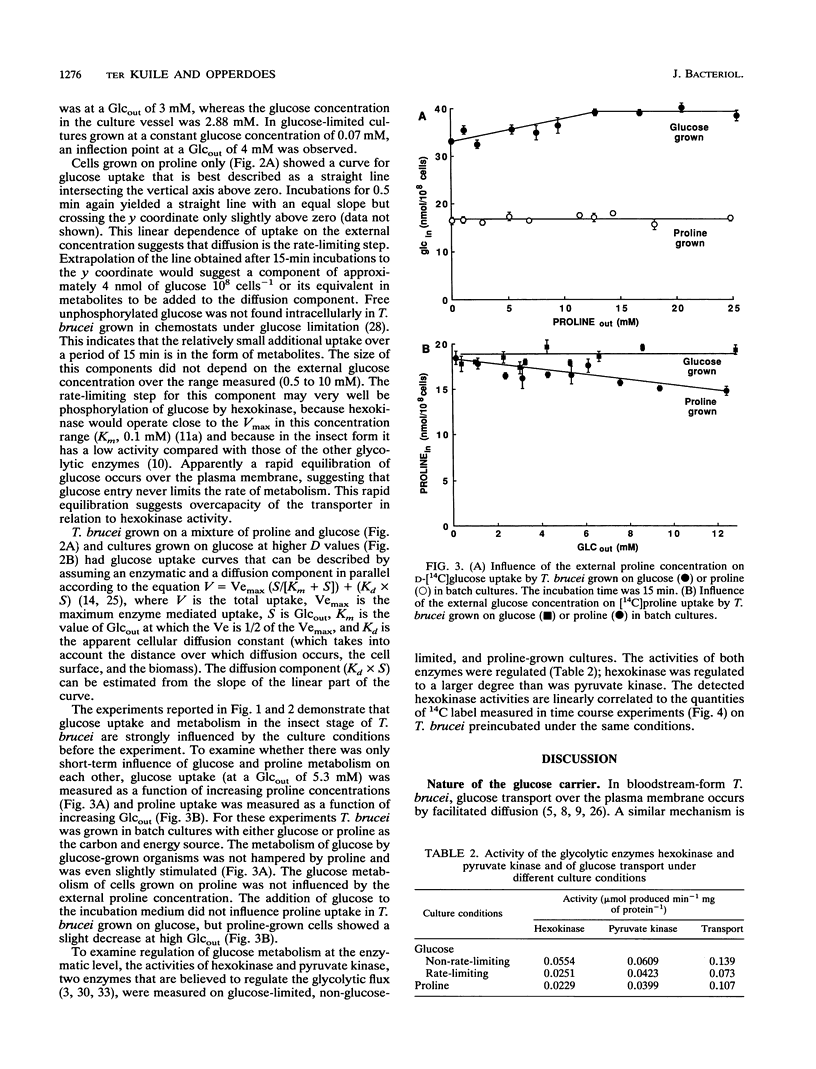
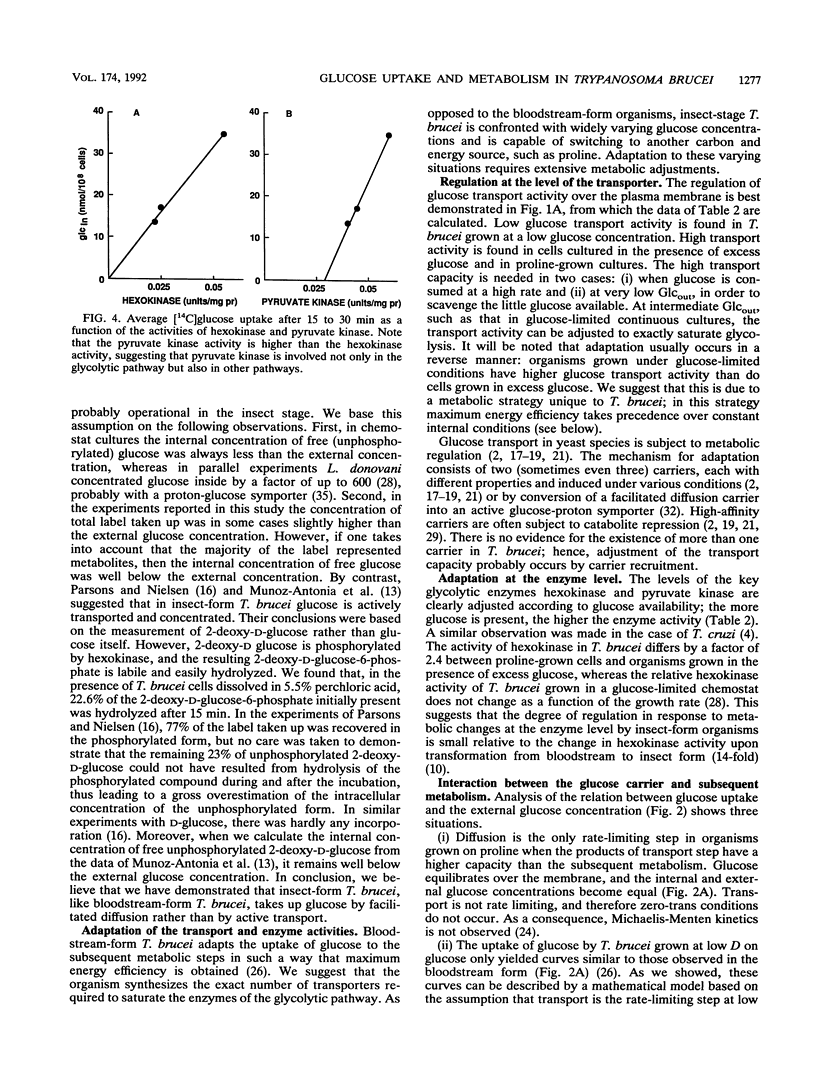
The mutual adjustment of glucose uptake and metabolism in the insect stage of the protozoan parasite Trypanosoma brucei was studied. T. brucei was preadapted in the chemostat to conditions in which either glucose or proline served as the major carbon and energy source. Cells were grown and adapted to either energy or non-energy limitation at a low dilution rate (0.5 day-1) or a high dilution rate (1 day-1). The cells were then used in short- to medium-term uptake experiments with D-[14C]glucose as a tracer. In time course experiments a steady state was reached after 15 min regardless of the preadaptation conditions. This steady-state level increased with increasing glucose availability during preadaptation. The rate of glucose uptake and the hexokinase activity were linearly correlated. In short-term 5- to 90-s) uptake experiments a high transport rate was measured with cultures grown in excess glucose, an intermediate rate was measured with proline-grown cultures, and a low rate was measured in organisms grown under glucose limitation. Glucose metabolism and proline metabolism did not affect each other during the 15-min incubations. Glucose uptake, as a function of the external glucose concentration, did not obey simple Michaelis-Menten kinetics but could be described by a two-step mechanism: (i) transport of glucose by facilitated diffusion and (ii) subsequent metabolism of glucose. The respective rates of the two steps were adjusted to each other. It is concluded that T. brucei is capable of adjusting the different metabolic processes in a way that gives maximum energy efficiency at the cost of short-term flexibility.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brun R., Schönenberger Cultivation and in vitro cloning or procyclic culture forms of Trypanosoma brucei in a semi-defined medium. Short communication. Acta Trop. 1979 Sep;36(3):289–292. [PubMed] [Google Scholar]
- Busturia A., Lagunas R. Catabolite inactivation of the glucose transport system in Saccharomyces cerevisiae. J Gen Microbiol. 1986 Feb;132(2):379–385. doi: 10.1099/00221287-132-2-379. [DOI] [PubMed] [Google Scholar]
- Callens M., Kuntz D. A., Opperdoes F. R. Characterization of pyruvate kinase of Trypanosoma brucei and its role in the regulation of carbohydrate metabolism. Mol Biochem Parasitol. 1991 Jul;47(1):19–29. doi: 10.1016/0166-6851(91)90144-u. [DOI] [PubMed] [Google Scholar]
- Cazzulo J. J., Franke de Cazzulo B. M., Engel J. C., Cannata J. J. End products and enzyme levels of aerobic glucose fermentation in trypanosomatids. Mol Biochem Parasitol. 1985 Sep;16(3):329–343. doi: 10.1016/0166-6851(85)90074-x. [DOI] [PubMed] [Google Scholar]
- Eisenthal R., Game S., Holman G. D. Specificity and kinetics of hexose transport in Trypanosoma brucei. Biochim Biophys Acta. 1989 Oct 2;985(1):81–89. doi: 10.1016/0005-2736(89)90107-7. [DOI] [PubMed] [Google Scholar]
- Ellenberger T. E., Beverley S. M. Biochemistry and regulation of folate and methotrexate transport in Leishmania major. J Biol Chem. 1987 Jul 25;262(21):10053–10058. [PubMed] [Google Scholar]
- Evans D. A., Brown R. C. The utilization of glucose and proline by culture forms of Trypanosoma brucei. J Protozool. 1972 Nov;19(4):686–690. doi: 10.1111/j.1550-7408.1972.tb03561.x. [DOI] [PubMed] [Google Scholar]
- Game S., Holman G., Eisenthal R. Sugar transport in Trypanosoma brucei: a suitable kinetic probe. FEBS Lett. 1986 Jan 1;194(1):126–130. doi: 10.1016/0014-5793(86)80063-1. [DOI] [PubMed] [Google Scholar]
- Gruenberg J., Sharma P. R., Deshusses J. D-Glucose transport in Trypanosoma brucei. D-Glucose transport is the rate-limiting step of its metabolism. Eur J Biochem. 1978 Sep 1;89(2):461–469. doi: 10.1111/j.1432-1033.1978.tb12549.x. [DOI] [PubMed] [Google Scholar]
- Hart D. T., Misset O., Edwards S. W., Opperdoes F. R. A comparison of the glycosomes (microbodies) isolated from Trypanosoma brucei bloodstream form and cultured procyclic trypomastigotes. Mol Biochem Parasitol. 1984 May;12(1):25–35. doi: 10.1016/0166-6851(84)90041-0. [DOI] [PubMed] [Google Scholar]
- Jadin J., Le Ray D. Acquisitions récentes dans les techniques de culture deTrypanosomes africains. Ann Soc Belges Med Trop Parasitol Mycol. 1969;49(4):331–339. [PubMed] [Google Scholar]
- Misset O., Opperdoes F. R. Simultaneous purification of hexokinase, class-I fructose-bisphosphate aldolase, triosephosphate isomerase and phosphoglycerate kinase from Trypanosoma brucei. Eur J Biochem. 1984 Nov 2;144(3):475–483. doi: 10.1111/j.1432-1033.1984.tb08490.x. [DOI] [PubMed] [Google Scholar]
- Munoz-Antonia T., Richards F. F., Ullu E. Differences in glucose transport between blood stream and procyclic forms of Trypanosoma brucei rhodesiense. Mol Biochem Parasitol. 1991 Jul;47(1):73–81. doi: 10.1016/0166-6851(91)90149-z. [DOI] [PubMed] [Google Scholar]
- Opperdoes F. R. Compartmentation of carbohydrate metabolism in trypanosomes. Annu Rev Microbiol. 1987;41:127–151. doi: 10.1146/annurev.mi.41.100187.001015. [DOI] [PubMed] [Google Scholar]
- Parsons M., Nielsen B. Active transport of 2-deoxy-D-glucose in Trypanosoma brucei procyclic forms. Mol Biochem Parasitol. 1990 Sep-Oct;42(2):197–203. doi: 10.1016/0166-6851(90)90162-f. [DOI] [PubMed] [Google Scholar]
- Postma E., Scheffers W. A., van Dijken J. P. Kinetics of growth and glucose transport in glucose-limited chemostat cultures of Saccharomyces cerevisiae CBS 8066. Yeast. 1989 May-Jun;5(3):159–165. doi: 10.1002/yea.320050305. [DOI] [PubMed] [Google Scholar]
- Postma E., Van den Broek P. J. Continuous-culture study of the regulation of glucose and fructose transport in Kluyveromyces marxianus CBS 6556. J Bacteriol. 1990 Jun;172(6):2871–2876. doi: 10.1128/jb.172.6.2871-2876.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottenberg H. The measurement of membrane potential and deltapH in cells, organelles, and vesicles. Methods Enzymol. 1979;55:547–569. doi: 10.1016/0076-6879(79)55066-6. [DOI] [PubMed] [Google Scholar]
- Srivastava H. K., Bowman I. B. Adaptation in oxidative metabolism of Trypanosoma rhodesiense during transformation in culture. Comp Biochem Physiol B. 1971 Dec 15;40(4):973–981. doi: 10.1016/0305-0491(71)90042-3. [DOI] [PubMed] [Google Scholar]
- Ter Kuile B. H., Opperdoes F. R. Chemostat cultures of Leishmania donovani promastigotes and Trypanosoma brucei procyclic trypomastigotes. Mol Biochem Parasitol. 1991 Mar;45(1):171–173. doi: 10.1016/0166-6851(91)90039-9. [DOI] [PubMed] [Google Scholar]
- Ter Kuile B. H., Opperdoes F. R. Glucose uptake by Trypanosoma brucei. Rate-limiting steps in glycolysis and regulation of the glycolytic flux. J Biol Chem. 1991 Jan 15;266(2):857–862. [PubMed] [Google Scholar]
- Van Schaftingen E., Opperdoes F. R., Hers H. G. Effects of various metabolic conditions and of the trivalent arsenical melarsen oxide on the intracellular levels of fructose 2,6-bisphosphate and of glycolytic intermediates in Trypanosoma brucei. Eur J Biochem. 1987 Aug 3;166(3):653–661. doi: 10.1111/j.1432-1033.1987.tb13563.x. [DOI] [PubMed] [Google Scholar]
- Verma R. S., Spencer-Martins I., Van Uden N. Role of de novo protein synthesis in the interconversion of glucose transport systems in the yeast Pichia ohmeri. Biochim Biophys Acta. 1987 Jun 12;900(1):139–144. doi: 10.1016/0005-2736(87)90285-9. [DOI] [PubMed] [Google Scholar]
- Visser N., Opperdoes F. R. Glycolysis in Trypanosoma brucei. Eur J Biochem. 1980 Feb;103(3):623–632. doi: 10.1111/j.1432-1033.1980.tb05988.x. [DOI] [PubMed] [Google Scholar]
- Wu G. Y., Seifter S. Periodate oxidation products of hydroxylysine in the synthesis of 5-substituted prolines. Anal Biochem. 1985 May 15;147(1):103–107. doi: 10.1016/0003-2697(85)90014-4. [DOI] [PubMed] [Google Scholar]
- Zilberstein D., Dwyer D. M. Protonmotive force-driven active transport of D-glucose and L-proline in the protozoan parasite Leishmania donovani. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1716–1720. doi: 10.1073/pnas.82.6.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]


