Abstract
We observed live fission yeast expressing pairs of functional fluorescent fusion proteins to test the popular model that the cytokinetic contractile ring assembles from a single myosin II progenitor or a Cdc12p-Cdc15p spot. Under our conditions, the anillin-like protein Mid1p establishes a broad band of small dots or nodes in the cortex near the nucleus. These nodes mature by the addition of conventional myosin II (Myo2p, Cdc4p, and Rlc1p), IQGAP (Rng2p), pombe Cdc15 homology protein (Cdc15p), and formin (Cdc12p). The nodes coalesce laterally into a compact ring when Cdc12p and profilin Cdc3p stimulate actin polymerization. We did not observe assembly of contractile rings by extension of a leading cable from a single spot or progenitor. Arp2/3 complex and its activators accumulate in patches near the contractile ring early in anaphase B, but are not concentrated in the contractile ring and are not required for assembly of the contractile ring. Their absence delays late steps in cytokinesis, including septum formation and cell separation.
Introduction
Deciphering cytokinesis is challenging because it involves >50 proteins in both fungi and animal cells (Gönczy et al., 2000; Guertin et al., 2002; Balasubramanian et al., 2004; Eggert et al., 2004). In fission yeast (Wu et al., 2003) and Drosophila melanogaster cells (Dean et al., 2005), a conserved set of core proteins follow similar temporal pathways to assemble a contractile ring of actin filaments and myosin II that pinches the dividing cell in two. Nevertheless, even in fission yeast, 25 yr of genetic analysis (Guertin et al., 2002; Balasubramanian et al., 2004; Wolfe and Gould, 2005) and measurements of the global and local concentrations of 28 of the proteins (Wu and Pollard, 2005) have left many fundamental questions unanswered.
One point of disagreement concerns the initial assembly of the contractile ring. A popular hypothesis is that the anillin-like protein Mid1p recruits a progenitor spot containing myosin II or formin Cdc12p and the pombe Cdc15 homology (PCH) protein Cdc15p to the division site, followed by extension of a leading cable from the spot around the circumference of the equator (Chang et al., 1997; Chang, 1999, 2000; Arai and Mabuchi, 2002; Carnahan and Gould, 2003; Hou and McCollum, 2002; Wong et al., 2002). On the other hand, we (Bezanilla et al., 2000; Wu et al., 2003) and others (Bähler et al., 1998a; Motegi et al., 2000, 2004; Paoletti and Chang, 2000) observed that some contractile ring proteins first form a broad band of small puncta around the middle of the cell and then coalesce to form a contractile ring. The nodes in the broad band contain Mid1p, myosin II Myo2p and associated light chains, and IQGAP Rng2p, but it was not known whether Cdc15p and Cdc12p concentrate in nodes or if localization of all of the node proteins depends on Mid1p.
The role of the actin-related protein 2/3 (Arp2/3) complex in cytokinesis has also been uncertain. The Arp2/3 complex nucleates branched actin filaments (Pollard et al., 2000) and concentrates in actin patches, but not the contractile ring in fixed fission yeast (McCollum et al., 1996; Arai et al., 1998; Morrell et al., 1999). D. melanogaster cells do not require the Arp2/3 complex for cytokinesis (Rogers et al., 2003; Eggert et al., 2004), and fission yeast with the conditional mutations arp2-1 and arp3-c1 have defects in septation, but form normal actin contractile rings under restrictive conditions (McCollum et al., 1996; Morrell et al., 1999). However, Pelham and Chang (Pelham and Chang, 2002) detected GFP-tagged ARPC5 (the smallest subunit, Arc5p/Arc15p, of the Arp2/3 complex) in contractile rings of live fission yeast and showed that the Arp2/3 complex is required for actin polymerization in contractile rings of permeabilized yeast. Furthermore, Carnahan and Gould (2003) presented evidence that the PCH protein Cdc15p recruits both formin Cdc12p (by direct interaction) and the Arp2/3 complex (by interaction with myosin I Myo1p, which is an activator of the Arp2/3 complex) to the contractile ring. Others observed myosin I Myo1p in the contractile ring (Takeda and Chang, 2005).
We addressed these issues by fluorescence microscopy of fission yeast strains expressing functional fusion proteins from their normal chromosomal loci under control of their native promoters. We found that most of the nodes around the equator of G2/M cells contain the seven proteins associated with the assembly of the contractile ring, and that the presence of all of these proteins in nodes depends on Mid1p. We reproduced most of the reported observations on progenitor spots, but found that nodes marked with functional fusion proteins condense laterally into a contractile ring without forming a progenitor spot or extending a leading cable. We also found that Arp2/3 complex contributes to septation, but neither Arp2/3 complex nor its activators concentrate in contractile rings. Instead, they begin to accumulate in endocytic actin patches lateral to the contractile ring after its formation. Remarkably, Cdc15p completely transitions from interphase endocytic actin patches dependent on the Arp2/3 complex to the contractile ring dependent on formin Cdc12p during mitosis.
Results
Participation of progenitor spots containing Myp2p and regulatory light chain Rlc1p in contractile ring assembly
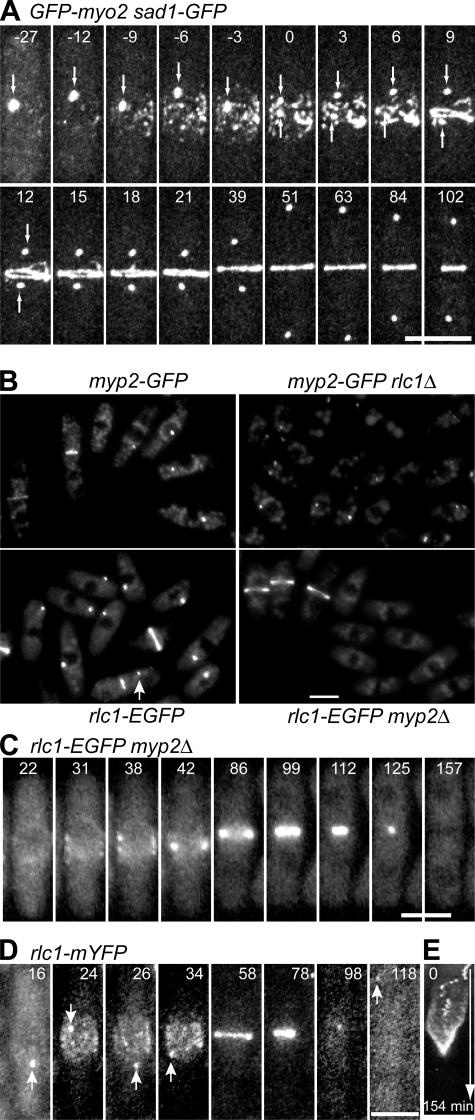
Our first question was if the contractile ring forms by growth from a single progenitor spot containing both myosin II isoforms, Myo2p and Myp2p, and the myosin regulatory light chain, Rlc1p (Kitayama et al., 1997; Bezanilla et al., 2000; Hou and McCollum, 2002; Wong et al., 2002). Time-lapse microscopy of cells expressing both functional GFP-Myo2p and a spindle pole body (SPB) marker, Sad1p-GFP, at their endogenous levels showed that the contractile ring assembles by lateral coalescence from a broad band of nodes before the onset of anaphase B (Fig. 1 A and Video 1, available at http://www.jcb.org/cgi/content/full/jcb.200602032/DC1). The behavior of Rlc1p-GFP is similar to Myo2p, including the number of molecules present in nodes (Wu and Pollard, 2005). Myp2p is not a component of the broad band, but joins the contractile ring ∼10 min after it forms (Bezanilla et al., 2000; Wu et al., 2003).
Figure 1.
The Rlc1p spot is not essential for the assembly of the contractile ring. Elapsed time is given in minutes. (A) A time-lapse series of one cell showing that the contractile ring forms from a broad band of nodes. Cells of strain GFP-myo2 sad1-GFP (JW800) were imaged in EMM5S at 22°C. A stack of 18 fluorescence Z sections spaced at 0.4 μm was taken every 3 min and projected into a 2D image using a maximum intensity projection. The arrows indicate the SPBs. The SPB separation is defined as time zero. The whole series can be viewed in Video 1. (B–E) The assembly of contractile rings independent of the Rlc1p spot. Cells grown exponentially at 25°C were shifted to 36°C for 2–4 h, and then imaged at 25°C. (B) Myp2p and Rlc1p localize to a spot after growth at 36°C and the Rlc1p spot formation depends on Myp2p. Images are maximum intensity projections of eight 0.64-μm Z sections. Arrow indicates a cell with a spot and a contracting ring. (C) A cell of strain rlc1-EGFP myp2Δ (MLP240) forms a contractile ring normally without the Rlc1p spot. The whole series can be viewed in Video 2. (D) The Rlc1p-mYFP spot (strain JW991) incorporates into the contractile ring after the broadband formation. Arrows indicate Rlc1p spots. The micrographs are maximum intensity projections of five 0.9-μm Z sections. (E) Kymograph of the cell in D over 154 min showing the movement of the Rlc1p spot. The kymograph was constructed using the maximum intensity projections, with a 5-μm slit cross the midplane of the cell. Videos 1 and 2 are available at http://www.jcb.org/cgi/content/full/jcb.200602032/DC1. Bars, 5 μm.
What is the nature of the progenitor spot? We confirmed that the partially functional YFP-Myp2p fusion protein (with YFP on the N terminus) formed spots that persist >50 min after ring constriction (Wu et al., 2003). Even the functional fusion proteins Myp2p-YFP and Rlc1p–monomeric CFP (mCFP) expressed from their native promoters colocalized in one or more spots at 36°C, but rarely at 25°C (Fig. S1 A, available at http://www.jcb.org/cgi/content/full/jcb.200602032/DC1). These spots did not interfere with cytokinesis and contained no Myo2p, even at 36°C (Fig. S1 B). Myp2p remained in a spot after Myo2p condensed into a contractile ring (Fig. S1 B, 67 min). Thus, the Myp2p-Rlc1p spot does not contain Myo2p.
The presence of Rlc1p in the progenitor spot depended on Myp2p. Myp2p-GFP formed a spot at 36°C in most cells lacking Rlc1p, but no Rlc1p-GFP spot appeared in a strain lacking Myp2p (Fig. 1 B). Cells lacking a Rlc1p spot formed a normal broad band and a contractile ring, and the ring constricted normally (Fig. 1 C and Video 2, available at http://www.jcb.org/cgi/content/full/jcb.200602032/DC1). Myp2p-Rlc1p spots incorporated into the contractile ring at various times, but these spots could persist after the assembly of a contractile ring (Fig. 1 B, arrow). The movements of Rlc1p spots appeared to be independent of the movements of the nodes in the broad band (Fig. 1, D and E). Although the exact nature of the spot is still unknown, our data provide strong evidence that it is not essential for the formation of the contractile ring.
Composition of the nodes in a broad band around the equator of the cell
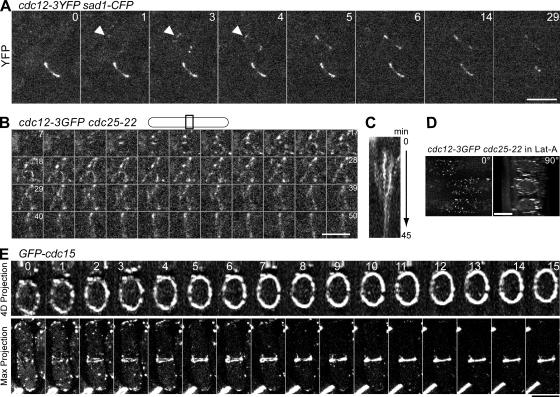
Extensive observations of cells expressing functional fusion proteins show that at least five proteins (anillin Mid1p, myosin II and two associated light chains, and IQGAP Rng2p) form a broad equatorial band of nodes before condensing into a contractile ring (see Introduction). However, it was not clear if other proteins are present in the nodes or if each node contains all of these proteins. In particular, we were uncertain about formin Cdc12p because it is the least abundant of the known cytokinesis proteins in S. pombe, with only ∼600 molecules per cell and 300 molecules in the contractile ring (Wu and Pollard, 2005). Thus, it is very difficult to detect Cdc12p by microscopy as a GFP fusion protein or with fluorescent antibodies without overexpression (Chang et al., 1997; Chang, 1999, 2000). To obtain a stronger signal, we fused three tandem copies of YFP or GFP to the C terminus of Cdc12p. These fusion proteins are functional, and the fluorescent intensities are three times those of Cdc12p fusion proteins with a single YFP or GFP (Wu and Pollard, 2005). These brighter fusion proteins enabled us to observe Cdc12p during the early stages of ring formation.
Within 1 min after SPB separation at the onset of mitosis, Cdc12p concentrated in small nodes located in a broad band around the equator of the cell (Fig. 2 A, arrowheads). Subsequently, these nodes coalesced laterally to form a ring that constricted normally (Video 3, available at http://www.jcb.org/cgi/content/full/jcb.200602032/DC1). We confirmed these observations using synchronized cells expressing Cdc12p-3GFP (Fig. 2, B and C; and Video 4). Some nodes marked with Cdc12p formed linear arrays as they condensed into a ring, but not a single leading cable. In the presence of latrunculin A (Lat-A) to inhibit actin polymerization, or in cells depleted of profilin Cdc3p, Cdc12p nodes formed normally, but did not coalesce into a contractile ring (Fig. 2 D, Fig. S2, and Videos 5 and 6). In the absence of profilin, the actin filaments nucleated by formin Cdc12p cannot elongate from the fast-growing barbed ends, but grow very slowly from their pointed ends (Kovar et al., 2003). Thus, formin Cdc12p localizes to nodes in the broad band independent of actin filaments and profilin. Furthermore, Cdc12p-dependent polymerization of actin filaments is required for nodes to coalesce into a contractile ring.
Figure 2.
Localization of the formin Cdc12p and PCH protein Cdc15p to a broad band of nodes around the equator of cells observed by fluorescence microscopy. (A) Cdc12p localizes to a broad band of nodes (arrowhead) at the onset of mitosis in asynchronous cells expressing Cdc12p-3YFP Sad1p-CFP (strain JW1114). Only YFP images are shown. Three YFP fluorescence Z sections spaced at 0.8 μm of two cells were projected into a 2D image using a maximum intensity projection. Elapsed times are given in minutes. The whole series can be viewed in Video 3. (B and C) Cdc12p localizes to a broad band of nodes in synchronized cdc12-3GFP cdc25-22 cells (JW1118). Cells were grown at 36°C for 4 h and shifted to 24°C for 1 h. (B) The central part of one cell, as indicated, is shown with the long axis of the cell positioned horizontally. Maximum intensity projections of 20 fluorescence Z sections spaced at 0.3 μm. Time points (at 45-s intervals) 7–50 are shown. The 3D projection of the series can be viewed in Video 4. (C) Kymograph of the division site of the cell in B over 45 min showing condensation of the broad band. The kymograph was constructed using maximum intensity projections with a 5.5-μm-long slit parallel to the long axis of cell. (D) Cdc12p accumulates in a broad band of nodes in the absence of actin filaments. Strain cdc12-3GFP cdc25-22 (JW1118) was arrested at G2/M after being grown at 36°C for 4 h, treated with 100 μM Lat-A at 36°C for 30 min, and then released to 23°C for 90 min in Lat-A. 81 Z sections spaced at 0.1 μm were collected, deconvolved, and projected into 3D. The three cells are viewed at 0 and 90° to the y-axis. The whole series can be viewed in Video 5. (E) GFP-Cdc15p (strain JW1038) forms a broad band of nodes around the equator before coalescing laterally into a compact ring. A stack of 36 0.2-μm Z sections was taken every minute. The time series of the same cell is displayed in two ways; (top) sections across the midplane of the cell; (bottom) maximum intensity projections in the X–Y plane of deconvolved images. The cross-section series can be viewed in Video 7. Videos 3–5 and 7 are available at http://www.jcb.org/cgi/content/full/jcb.200602032/DC1. Bars, 5 μm.
The localization of Cdc15p in the broad band of nodes was also in question. GFP-Cdc15p concentrates in patches near the ends of the cell during interphase and in the contractile ring during cell division (Fankhauser et al., 1995; Carnahan and Gould, 2003; Wu et al., 2003). Under our conditions, GFP-Cdc15p first concentrated in many small nodes around the equator, followed by condensation of these nodes into a continuous contractile ring (Fig. 2 E and Video 7, available at http://www.jcb.org/cgi/content/full/jcb.200602032/DC1), rather than forming a spot that initiates a leading cable. The forming ring of GFP-Cdc15p initially had many gaps, but the gaps filled in (Fig. 2 E) as more Cdc15p molecules were recruited (Wu and Pollard, 2005). Cdc15p also localized to a broad band of nodes in cells arrested at G2/M in Lat-A (Fig. 3, H and J; Wu et al., 2003).
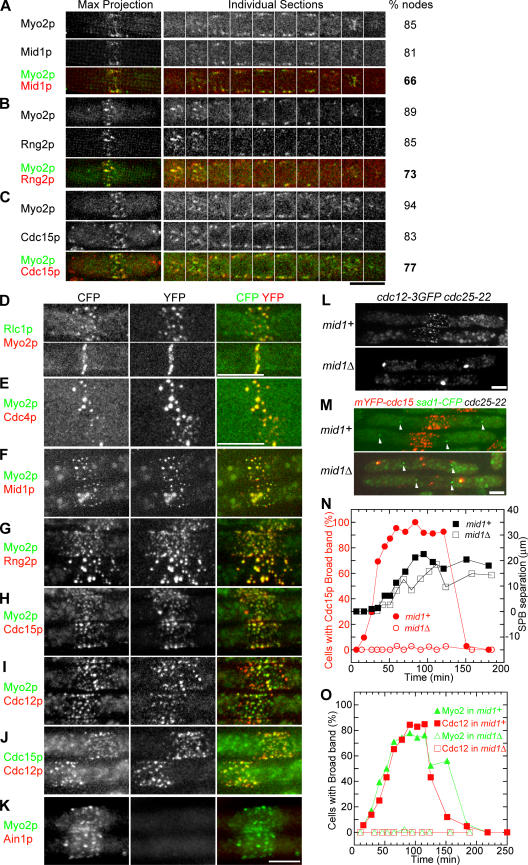
Figure 3.
Localization of pairs of fluorescent fusion proteins in the broad bands of nodes, and the dependence of the node formation on Mid1p. Wild-type cells (A–E) or cdc25-22 cells (F–O) expressing pairs of CFP- and YFP-fusion proteins under the control of their native promoters were observed directly (A–C) or treated with 100 μM Lat-A for at least 30 min before observation to prevent condensation of nodes into a contractile ring (D–O). (D and E) Cells were treated with Lat-A at 23°C. (F–O) Cells with the cdc25-22 temperature-sensitive mutation were arrested at the G2/M transition by incubation at 36°C for 4 h, incubated in Lat-A at 36°C for another 30 min, and then released to 23°C in Lat-A at time zero and observed. The images, except for those on the right in A–C, are maximum intensity projections of 13 0.6- or 36 0.2-μm Z sections (with exposures of 198 ms). (A–C, left) Maximum intensity projections of the whole cells; (middle) consecutive individual sections of the division site of the cells in the leftmost images; and (right) percentage of nodes with the proteins listed before the left images. D–K show the division sites of two cells. (L and M) show two whole cells in each strain background. To reduce background autofluorescence and increase the contrast for CFP, the cells in D and G–I were photobleached briefly at 442 nm before images were taken. (M) Arrowheads indicate the SPBs. (N) Red symbols and lines use the scale on the left. All others use the scale on the right. (N and O) Graphs of the time courses of the fraction of cells with fluorescent proteins in broad bands and the separation of spindle pole bodies. Samples were taken at the indicated time, imaged, and quantified. The following strains were used: (A) mid1-YFP CFP-myo2 (JW851); (B) mYFP-rng2 CFP-myo2 (JW928); (C) mYFP-cdc15 CFP-myo2 (JW1173); (D) JW968; (E) JW929; (F) JW1024; (G) JW1023; (H) JW1181; (I) JW1183; (J) JW1216; (K) JW1026; (L) JW1118 and JW1192; (M and N) JW1220 (n = 423 cells) and JW1218 (n = 450 cells); and (O) cdc12-3YFP mCFP-myo2 (JW1183; n = 641 cells) and mid1Δ cdc12-3YFP mCFP-myo2 (JW1226; n = 343 cells). Bars, 5 μm (same bar for F–K).
To determine if each node in the broad band contains all seven proteins known to accumulate in nodes, we observed cells expressing pairs of contrasting fluorescent fusion proteins in wild-type (Fig. 3, A–E) or cdc25-22 cells (Fig. 3, F–O). To facilitate some observations (Fig. 3, D–O), cells were treated with Lat-A to prevent nodes from condensing into compact rings. We observed fluorescent fusion proteins in cdc25-22 cells arrested at G2/M and then released into Lat-A for two reasons. First, by arresting cells at G2/M and then releasing them, we enriched for cells forming broad bands. Second, Rng2p, Cdc15p, and Cdc12p normally accumulate in nodes over a period of 10 min after myosin II, followed quickly by condensation of the nodes into a contractile ring (Wu et al., 2003). Thus, Myo2 normally colocalizes in nodes with Rng2p, Cdc15p, or Cdc12p for just a few minutes, unless Lat-A prevents condensation of the nodes. The weaker fluorescence of CFP and higher background autofluorescence in the CFP relative to the YFP channel are also challenges for imaging some pairs of proteins.
We observed that most nodes contained anillin-like Mid1p, conventional myosin II heavy chain Myo2p and light chains Cdc4p and Rlc1p, IQGAP Rng2p, PCH protein Cdc15p, and formin Cdc12p (Fig. 3). Approximately 70% of nodes in wild-type cells contained Myo2p, Mid1p, Rng2p, and Cdc15p (Fig. 3, A–C). In cells treated with Lat-A, a similar or even higher fraction of nodes contained all seven proteins (Fig. 3, D–O). Nearly all nodes marked with Myo2p also contained myosin light chains Rlc1p and Cdc4p, anillin-like protein Mid1p, and IQGAP Rng2p (Fig. 3, D–G). A majority of nodes marked with Myo2p also contained the PCH protein Cdc15p and formin Cdc12p. Most nodes marked by Cdc15p also contained Cdc12p (Fig. 3 J), but the Cdc15p signal was stronger in some nodes because of the fivefold excess of Cdc15p over Cdc12p (Wu and Pollard, 2005). The lack of signal from α-actinin Ain1p-YFP (a late arriving component of contractile rings) in the broad band (Fig. 3 K) indicated that the signals from the other proteins do not arise from artifacts of microscopy or physiology in the cdc25-22 background.
The dependence of Cdc12p and Cdc15p on Mid1p for localization in nodes had not been established, so we compared the behavior of these proteins in mid1+ and mid1Δ backgrounds. We synchronized cdc25-22 cells expressing tagged Cdc12p and Cdc15p by incubation at 36°C and release to 23°C into Lat-A to prevent condensation of nodes into a contractile ring. In the presence of Mid1p, Myo2p, Cdc12p, and Cdc15p formed broad bands of nodes (Fig. 3, H–J and L–O). Myo2p persisted in the nodes for ∼100 min during incubation in Lat-A, and then dispersed. Cdc15p and Cdc12p formed aggregates during long incubations in Lat-A. In the absence of Mid1p, none of Myo2p, Cdc12p, or Cdc15p concentrated in a broad band of nodes (Fig. 3, L–O). Myo2p remained diffusely spread in the cytoplasm, but Cdc12p formed aggregates faster in the absence, rather than in the presence, of Mid1p (Fig. S3, available at http://www.jcb.org/cgi/content/full/jcb.200602032/DC1). Thus, Mid1p is required for node formation.
Roles of the Arp2/3 complex and its activators in cytokinesis
Studies in the literature differ regarding the participation of the Arp2/3 complex and its activators in contractile ring function. One study reported the Arp2/3 complex in the contractile ring (Pelham and Chang, 2002), and two studies presented evidence that assembly of contractile ring filaments depends on the Arp2/3 complex (Pelham and Chang, 2002; Carnahan and Gould, 2003). Other genetic and cellular studies presented evidence that the Arp2/3 complex is not required for actin-ring assembly (McCollum et al., 1996; Arai et al., 1998; Morrell et al., 1999). Our new experiments show that the Arp2/3 complex and its activators contribute to septation, but not assembly of the contractile ring.
Experiments with YFP-actin.
YFP-actin expressed at low concentrations from a plasmid incorporates into actin filaments nucleated by the Arp2/3 complex in actin patches (Wu and Pollard, 2005). Wu and Pollard (2005) mentioned, but did not document, the absence of YFP-actin fluorescence in the contractile ring, despite the high local concentration of filaments (Video 8, available at http://www.jcb.org/cgi/content/full/jcb.200602032/DC1). This difference is strong evidence that the Arp2/3 complex nucleates few actin filaments in the contractile ring.
Role of Arp2/3 complex activators in equatorial actin patches.
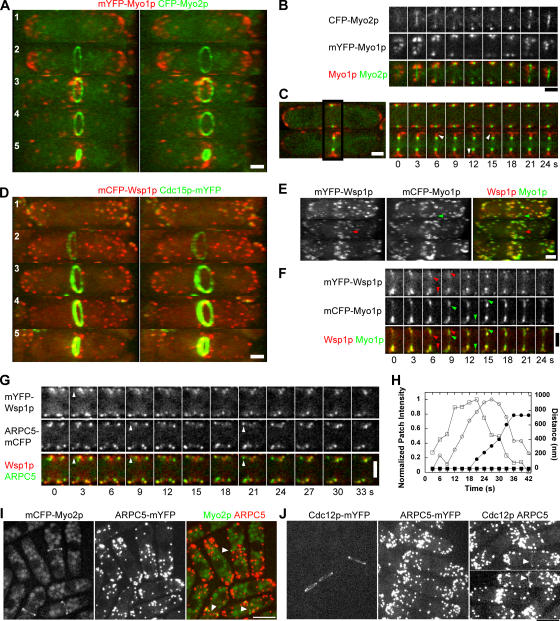
The activators of the Arp2/3 complex, myosin I Myo1p and the Wiskott-Aldrich syndrome protein (WASp) Wsp1p, localized to dynamic patches that were distinguished in time and space from contractile rings marked by Myo2p or Cdc15p. During interphase (Fig. 4 A, cell 1; and Video 9, available at http://www.jcb.org/cgi/content/full/jcb.200602032/DC1), monomeric YFP (mYFP)–Myo1p concentrated in patches at cell tips, whereas CFP-Myo2p was diffuse throughout cytoplasm. While CFP-Myo2p assembled a ring, mYFP-Myo1p remained in patches at cell tips (Fig. 4 A, cell 2). Patches of Myo1p began to accumulate laterally to the ring 15.2 ± 3.1 min (n = 7 cells; strain TP210) after SPB separation and ∼5 min after the compaction of nodes into a contractile ring (Fig. 4, A [cell 3] and B). As the CFP-Myo2p ring constricted (Fig. 4 A, cells 4 and 5), mYFP-Myo1p patches remained adjacent to the ring with little overlap with the CFP-Myo2p ring. Superimposition gave the impression that some of these patches overlap the ring, but careful inspection of Z sections showed that the mYFP-Myo1p patches were separate (Fig. 4 B). Both before and after the onset of CFP-Myo2p ring constriction, mYFP-Myo1p patches were highly dynamic, with lifetimes of 10–20 s (Fig. 4 C).
Figure 4.
Localization and dynamics of Arp2/3 complex and its activators myosin I Myo1p and WASp Wsp1p in actin patches at the cell-division site. (A–C) Cells expressing mYFP-Myo1p (red) and CFP-Myo2p (green) show that endocytic actin patches containing Myo1p are spatially and temporally distinct from contractile rings labeled with Myo2p. (A) Stereo projections of 3D reconstructions made from Z series of confocal images at 0.6-μm intervals and projected at 15 and 24° tilts. The whole series can be viewed in Video 9. (B) Individual confocal sections through the division site of cell 3 from A at 0.6-μm intervals. (C) Time series at 3-s intervals in a single confocal plane showing red mYFP-Myo1p patches surrounding the green CFP-Myo2p ring. Arrowheads mark the appearance of new mYFP-Myo1p patches. (D) Stereo projections of cells expressing mCFP-Wsp1p (red) and Cdc15p-mYFP (green) show that Cdc15p appears in cortical patches at cell tips during interphase and in contractile rings during mitosis, whereas Wsp1p concentrates in actin patches throughout the cell cycle. (E and F) Localization and dynamics of mYFP-Wsp1p (red) and mCFP-Myo1p (green) in patches at the division site. (E) 3D reconstructions made from Z series of confocal images at 0.6-μm intervals. Arrowheads point out examples of patches labeled only with Wsp1p (red) or Myo1p (green). (F) Time course of the activators' assembly into patches at the division site in a single confocal plane at 3-s intervals. Arrowheads point out examples of nascent patches where appearance of Myo1p (green) lags behind Wsp1p (red) by 3–6 s. (G and H) Dynamics of mYFP-Wsp1p and ARPC5-mCFP in patches at the division site. (G) Time course of Wsp1p (red) and ARPC5 (green) patch assembly at the division site in a single confocal plane at 3-s intervals. Arrowheads mark the appearance of Wsp1p at 3 s, appearance of ARPC5 at 9 s, and initiation of patch motility at 21 s in a single patch. The whole series can be viewed in Video 10. (H) Time course of fluorescence intensity of (□) mYFP-Wsp1p and (◯) ARPC5-mCFP and total distance moved by (▪) mYFP-Wsp1p and (•) ARPC5-mCFP for the patch marked in G. (I and J) The images are maximum-intensity projections of 12 0.6-μm Z sections. Arrowheads indicate contractile rings. (I) ARPC5 does not colocalize with Myo2p in the contractile ring in cells expressing mCFP-Myo2p and ARPC5 (Arc5p)-mYFP (JW1221). (J) The concentration of ARPC5 in the contractile ring location is less than that of the formin Cdc12p. Cells expressing Cdc12p-mYFP (KV345), ARPC5-mYFP (JW1223), and Cdc12p-mYFP ARPC5-mYFP (JW1227) were imaged under identical conditions. Videos 9 and 10 are available at http://www.jcb.org/cgi/content/full/jcb.200602032/DC1. Bars: (A–G) 2 μm; (I and J) 5 μm.
Cdc15p was proposed to recruit Arp2/3 complex activators to the contractile ring (Carnahan and Gould, 2003), so we compared the localization of Cdc15p-mYFP with mCFP-Wsp1p (Fig. 4 D) and, with similar results, mCFP-Myo1p (unpublished data). Cdc15p is unique in that it concentrated in patches during interphase and in contractile rings during mitosis, whereas throughout the cell cycle Myo1p and Wsp1p localized exclusively to patches, never to rings. Of 147 interphase patches (16 cells) scored, we detected both Cdc15p-mYFP and mCFP-Wsp1p in 56%, Cdc15p-mYFP alone in 25%, and mCFP-Wsp1p alone in 19% of patches (Fig. 4 D, cell 1). Early in mitosis, Cdc15p-mYFP completely left behind mCFP-Wsp1p in patches at cell tips and concentrated in nodes that condensed into a contractile ring (Fig. 4 D, cells 2 and 3). As the Cdc15p-mYFP ring matured and constricted (Fig. 4 D, cells 4 and 5), mCFP-Wsp1p, but not Cdc15p, concentrated in patches at the division site. Fluorescent patches marked with Arp2/3 complex activators appear to overlap rings marked with Cdc15p-mYFP more often than rings marked with CFP-Myo2p because Cdc15p-mYFP is brighter, owing to its greater abundance (Wu and Pollard, 2005). Careful inspection of Z sections showed that these patches are adjacent to the rings.
The behavior of proteins in patches next to the contractile ring was similar to interphase patches (Sirotkin et al., 2005). The mYFP-Wsp1p signal appeared 3–6 s before mCFP-Myo1p in approximately half of 45 patches scored and at the same time in the remaining patches (Fig. 4, E and F). In dividing cells expressing mCFP-Wsp1p and mYFP-Myo1p, mCFP-Wsp1p arrived before mYFP-Myo1p in 5 of 28 equatorial patches, at the same time in 21 of these patches, and later in 2 patches. ARPC5-mCFP appeared in these patches near the contractile ring 6–9 s after mYFP-Wsp1p and accumulated over 9–12 s (Fig. 4, G and H; and Video 10, available at http://www.jcb.org/cgi/content/full/jcb.200602032/DC1). As ARPC5-mCFP approached its peak intensity, the patch moved with an average velocity of ∼0.1 μm/s away from the activators over 0.5–1 μm during the next ∼12 s, while gradually fading (Fig. 4 H). The mYFP-Wsp1p fluorescence rapidly dissipated after ARPC5-mCFP patches moved. The assembly of Wsp1p ahead of Myo1p in equatorial patches lacking Cdc15p in cells with contractile rings is inconsistent with the proposal that interaction of Cdc15p with Myo1p localizes Arp2/3 complex at the division site (Carnahan and Gould, 2003).
Timing and localization of Arp2/3 complex at the cell cleavage site.
We reexamined the presence of the Arp2/3 complex in the contractile ring. Movies of cells expressing Sad1p-CFP to mark spindle pole bodies and the ARPC5 subunit of the Arp2/3 complex tagged with YFP showed that patches of Arp2/3 complex began to accumulate lateral to the division site 14.6 ± 2.5 min (n = 21 cells) after SPB separation. At this time, the seven proteins present in the nodes (Mid1p, Myo2p, Cdc4p, Rlc1p, Rng2p, Cdc15p, and Cdc12p) have already formed a compact contractile ring (Wu et al., 2003; Fig. 2). ARPC5 was absent from sharp Myo2p rings (Fig. 4 I, arrowheads). Among 250 cells examined, 13 had full-size contractile rings and 14 had partially constricted rings containing mCFP-Myo2p, but none of these rings concentrated ARPC5-mYFP. In a strain (JW1227) simultaneously expressing Cdc12p-mYFP (the least abundant of the known contractile-ring proteins, with only 300 molecules present at a local concentration of 3 μM) and ARPC5-mYFP (Fig. 4 J), the Cdc12p-mYFP fluorescence was clearly stronger in the ring than in the surrounding cytoplasm (arrowheads), despite the high background concentration of ∼1.9 μM ARPC5-mYFP (Wu and Pollard, 2005). However, no ARPC5-mYFP fluorescence was concentrated in the ring region of 396 cells expressing ARPC5-mYFP alone (Fig. 4 J). Thus, ARPC5 was not obviously concentrated in contractile rings.
Effects of mutations of the Arp2/3 complex and its activators on cytokinesis.
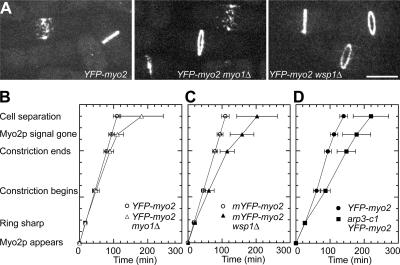
We used time-lapse microscopy to investigate cell division in strains with the cold-sensitive mutant arp3-c1 (McCollum et al., 1996) and the deletion mutants myo1Δ and wsp1Δ (Sirotkin et al., 2005). The fluorescence of mYFP-Myo2p in broad bands and rings was indistinguishable in arp3-c1, myo1Δ, or wsp1Δ strains and wild-type cells (Fig. 5 A). Myo2p rings formed with a normal time course in all of these strains (Fig. 5, B–D). However, the initiation of ring constriction, the rate of ring constriction, the timing of septum formation, the rate of ring disassembly, and the timing of cell separation were all delayed slightly in strains lacking Myo1p or Wsp1p or with the cold-sensitive mutation in Arp3p. We underestimated the mean times for cell separation in mutants because 25–50% of mutant cells failed to separate by the end of movies, whereas all wild-type cells separated. Thus, the Arp2/3 complex and its activators contribute to septum formation and cell separation, but not to the assembly of the contractile ring.
Figure 5.
The effect of the conditional mutation arp3-c1 and activator deletion mutants on the assembly of the contractile ring during cell division. (A–C) Strains expressing mYFP-Myo2p from its native promoter in wild type (JW1110), myo1Δ (JW1248), and wsp1Δ (JW1247) were grown in YE5S medium at 25°C, washed in EMM5S, and imaged in EMM5S plus 25% gelatin at 25°C (23°C for cells in A). (A) The Myo2p ring forms normally in activator deletion mutants. Images are maximum-intensity projections of 13 0.6-μm Z sections. (B–D) Plots of the observed mean times (±1 SD) of cytokinesis events (X-axis) versus the established time of these events in wild-type cells (Wu et al., 2003). Differential interference contrast (DIC) and fluorescence images of mYFP-Myo2p in a single optical section were taken every 2 min. (B) Comparison of wild-type cells (n = 26 cells) and cells lacking myo1 (n = 29). (C) Comparison of the same wild-type sample as in B with cells lacking wsp1 (n = 20). (D) Comparison of wild-type mYFP-myo2 (JW1233; n = 18) and mYFP-myo2 arp3-c1 (JW1235; n = 12) at the restrictive temperature 19°C. Cells were grown exponentially in YE5S at 32°C, shifted to 19°C for 6 h, and then imaged at 19°C. DIC and fluorescence images in a single optical section were taken every 4 min. Bar, 5 μm.
Discussion
How does the assembly of the contractile ring start?
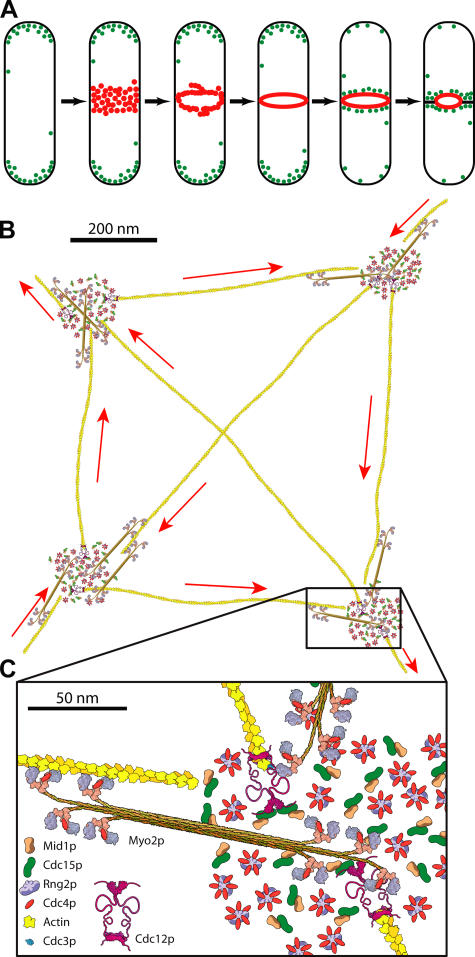
Our time-lapse microscopy and 3D reconstructions using functional fusion proteins expressed from their chromosomal loci, under the control of their native promoters, clarify the early steps in the assembly of the contractile ring (Fig. 6 A). We found that the anillin-like protein Mid1p initiates the assembly as a broad band of nodes. Mid1p exits from the nucleus and specifies a broad band of ∼75 nodes around the cell equator. Mid1p first recruits dephosphorylated type II myosin Myo2p with the light chains Cdc4p and Rlc1p, as well as recruiting IQGAP Rng2p to nodes in this broad band before the onset of mitosis (Wu et al., 2003; Motegi et al., 2004). Myo2p is insoluble at physiological ionic strength (Bezanilla and Pollard, 2000; Lord and Pollard, 2004), so it may assemble into minifilaments like other cytoplasmic type II myosins. The role of Rng2p in the process is unclear. PCH protein Cdc15p and formin Cdc12p are recruited to the nodes at the onset of mitosis. Formin Cdc12p dimers initiate the nucleation of unbranched actin filaments and remain attached to their barbed end (Kovar et al., 2003, 2006; Kovar and Pollard, 2004).
Figure 6.
Lateral contraction model for contractile ring assembly. (A) Diagram of the assembly of a contractile ring (red) from a broad band of nodes (red) followed by relocation of actin patches (green) containing Arp2/3 complex from the ends of the cell to the cleavage site. (B and C) Scale drawings of the proteins known to be present in the nodes that assemble into the contractile ring. The numbers of protein molecules shown in each node are based on the total numbers of each protein in the broad band divided by 75 nodes, resulting in ∼20 molecules of anillin-like Mid1p, myosin II dimer, IQGAP Rng2p, and PCH protein Cdc15p, and 2 formin Cdc12p dimers (Wu and Pollard, 2005). The four nodes are separated by ∼0.7 μm as in live cells. The barbed ends of actin filaments are anchored to formin Cdc12p dimers in one node and project radially to interact with putative myosin II minifilaments in adjacent nodes.
We assume that nodes attach to the inner surface of the plasma membrane and that a unitary node consists of ∼20 anillin Mid1p, 20 dimers of myosin II (Myo2p and light chains Cdc4p and Rlc1p), 20 IQGAP Rng2p, 20 PCH protein Cdc15p, 2 dimers of formin Cdc12p, and ∼250 of the essential light chain Cdc4p in excess of the binding sites on Myo2p (Fig. 6; Wu and Pollard, 2005). Myo2p heads interact with actin filaments (Lord and Pollard, 2004), Myo2p tails interact with each other (Bezanilla and Pollard, 2000) and Mid1p (Motegi et al., 2004), Cdc4p interacts with IQ motifs of Rng2p (D'Souza et al., 2001), and the N termini of Cdc15p and Cdc12p interact with each other (Carnahan and Gould, 2003). Given these ratios and interactions, we expect nodes to have an organized macromolecular structure and orderly assembly (Fig. 6, B and C). However, the physical interactions among the node proteins are not yet established. Mid1p, but not polymerized actin, is required for the localization of Myo2p, Rng2p, Cdc15p, and Cdc12p to the nodes (Fig. 3; Wu et al., 2003). Cdc4p is required for the localization of Myo2p (Naqvi et al., 1999) and Rng2p (Wu et al., 2003) to the nodes. Cdc12p is not required for localization of Myo2p (Motegi et al., 2000) and Rng2p (Wu et al., 2003) to the nodes. Without the nodes in mid1Δ cells, some cells fail to assemble a contractile ring, and some cells make misplaced and defective rings belatedly (Wu et al., 2003). Thus, it seems that nodes contribute to both the proper positioning and efficient assembly of the contractile ring.
How does the broad band of nodes transform into a contractile ring?
Detection of formin Cdc12p in equatorial nodes is crucial for proposing a mechanism to transform the broad band into a contractile ring. Formins nucleate actin filaments at the division site (Pelham and Chang, 2002; Kovar et al., 2003) and remain attached to their barbed ends so that they may also anchor the filaments in nodes. Because actin subunits add to barbed ends associated with Cdc12p, we presume that the pointed ends of these filaments radiate from nodes, which are separated by ∼0.7 ± 0.2 μm. Actin filaments >0.7 μm long might encounter myosin II in adjacent nodes. Movement of myosin II toward the barbed end of an actin filament anchored in an adjacent node might pull nodes together as they coalesce into a compact ring (Fig. 6 B). This hypothesis is consistent with the fact that assembly of actin filaments from nodes depends on Cdc12p and profilin Cdc3p and that actin filaments are required for nodes to coalesce laterally into a sharp, compact ring around the cell equator.
Our lateral contraction model shares some features with models of cytokinesis in animal cells (White and Borisy, 1983; White, 1990), which postulate gradients of cortical tension that move the components to the equator where tension is highest (White and Borisy, 1983), and with the observation that poorly organized actin filaments spread over the medial cortex become aligned into parallel bundles around the equator ( Fishkind and Wang, 1993; Mabuchi, 1994).
Our evidence suggests that the fission yeast contractile ring assembles from a broad band of nodes (Fig. 6) rather than by extension of a leading cable around the circumference of the cell from a single myosin progenitor spot (Hou and McCollum, 2002; Wong et al., 2002) or a Cdc12p-Cdc15p spot (Chang et al., 1997; Chang, 1999, 2000; Carnahan and Gould, 2003). We and others have also observed many proteins in one or more spots in fission yeast, including formin Cdc12p (Chang et al., 1997; Chang, 1999, 2000), PCH protein Cdc15p (Carnahan and Gould, 2003), conventional myosin II Myo2p (Kitayama et al., 1997; Wong et al., 2002), myosin II regulatory light chain Rlc1p (Wong et al., 2002), unconventional myosin II Myp2p (Bezanilla et al., 2000), and α-actinin Ain1p (Wu et al., 2001). These spots may be nonfunctional aggregates because they appear if proteins are overexpressed, nonfunctional, tagged with weakly dimeric GFP, or expressed at a high temperature. Alternatively, spots might be a storage form of inactive protein, as observed for inactive Cdc2-cyclin B in the cytoplasm of starfish oocytes (Slepchenko and Terasaki, 2003; Terasaki et al., 2003). The dissolution or incorporation of spots into the contractile ring during mitosis might result from the depletion of the inactive molecules in the cytoplasm. We favor the first possibility although we cannot rule out the latter because Cdc12p has been detected in a spot using an antibody (Chang, 1999).
Participation of the Arp2/3 complex and activators in cell division
Assembly of actin patches in fungi during endocytosis (Kaksonen et al., 2003, 2005) depends on the Arp2/3 complex to nucleate branched actin filaments (Weaver et al., 2003). On the other hand, assembly of actin filaments for the contractile ring in fission yeast depends on formin Cdc12p and profilin Cdc3p (Balasubramanian et al., 1994; Chang et al., 1997; Pelham and Chang, 2002). Formin Cdc12p nucleates unbranched actin filaments that grow at their barbed ends in the presence of profilin in vitro (Kovar et al., 2003, 2006; Kovar and Pollard, 2004). Contractile ring actin filaments appear to be unbranched in animal cells (Schroeder, 1973; Sanger and Sanger, 1980; Maupin and Pollard, 1986), but less is known about their structure in yeast.
Our observations support previous evidence that the Arp2/3 complex and its activators Myo1p and Wsp1p participate in late steps in cytokinesis. They nucleate actin filaments for patches on either side of the contractile ring, which may contribute to the membrane traffic during endocytosis, accompanying the formation of new membrane and the septum.
Our experiments also add to evidence that the Arp2/3 complex does not participate in the assembly of the contractile ring. First, the strongest evidence is the presence of YFP-actin in actin patches mediated by Arp2/3 complex but not in the contractile ring. The high concentration of YFP-actin in patches (Wu and Pollard, 2005) established that it could add to the free barbed end of filaments nucleated by the Arp2/3 complex. If the Arp2/3 complex nucleated any actin filaments in the contractile ring, we would observe more YFP-actin fluorescence in the ring than in the surrounding cytoplasm. A simple explanation for the lack of YFP-actin fluorescence in the contractile ring is that formin Cdc12p might nucleate all of the filaments and cannot add YFP-actin to growing barbed ends. Formins have a bias against actin with a fluorescent dye conjugated to Cys374 (Kovar et al., 2006) located near the N terminus in the actin structure. Our observations on live cells suggest that Cdc12p does not use YFP-actin to polymerize contractile ring actin filaments. Second, well after the assembly of the contractile ring, actin patches containing the Arp2/3 complex and its activators accumulate lateral to the contractile ring, but not in the contractile ring. Third, strains with a conditional mutation in Arp3p (arp3-c1) or lacking activators of the Arp2/3 complex (myo1Δ and wsp1Δ) assemble Myo2p nodes and compact rings at the same rate as wild-type cells, but then progress through subsequent steps in cytokinesis slightly slower.
The PCH protein Cdc15p crosses over between endocytic actin patches and the contractile ring. During interphase, Cdc15p concentrates with Arp2/3 complex in some, but not all, endocytic patches at the ends of cells (Fig. 4; Carnahan and Gould, 2003). At the onset of mitosis, Cdc15p leaves the endocytic actin patches and concentrates in nodes with myosin II and formin Cdc12p that condense into the contractile ring.
Available evidence is consistent with the proposal that formins, rather than the Arp2/3 complex, nucleate most or all contractile ring actin filaments in other eukaryotes. Arp2/3 complex is not required for the actin-ring assembly in budding yeast (Winter et al., 1999) or C. elegans (Severson et al., 2002), and is not required for cytokinesis in D. melanogaster (Rogers et al., 2003; Eggert et al., 2004). On the other hand, formins are essential for actin-ring assembly and cytokinesis in fungi and animals (Chang et al., 1997; Wasserman, 1998; Tolliday et al., 2002; Rogers et al., 2003).
Materials and methods
Strain construction, growth conditions, and cellular methods
Table S1 (available at http://www.jcb.org/cgi/content/full/jcb.200602032/DC1) lists the S. pombe strains used in this study. We constructed the strains by PCR-based gene targeting (Bähler et al., 1998b; Wu et al., 2003) and standard genetic methods (Moreno et al., 1991). For constructing strains tagged with 3GFP and 3YFP by gene targeting, plasmids pFA6a-3GFP-kanMX6 and pFA6a-3YFP-kanMX6 were used as templates in PCR to amplify DNAs for transformation. The plasmids were obtained by cloning triple GFP or YFP (a gift from W.-L. Lee and J. Cooper, Washington University, St. Louis, MO) into the pFA6a vector (Bähler et al., 1998b). All strains except where noted expressed fusion proteins under the control of their native promoters and from their normal chromosomal locus. We tested the functionality of each fusion protein in previous papers (Wu et al., 2003; Lord and Pollard, 2004; Sirotkin et al., 2005; Wu and Pollard, 2005). We restreaked all cells, except for the strains JW816 and JW1288, from frozen stocks on YE5S plates and grew colonies at appropriate temperatures for 2–4 d. We inoculated cells from these colonies into 5–15 ml YE5S liquid medium in 50-ml baffled flasks and grew exponential cultures at densities of 1–10 × 106 in the dark at 25°C with shaking at 200 rpm for 36–48 h before observations and/or experiments. Strains JW816 (with a YFP-actin plasmid) and JW1288 (cdc3 under the control of repressible 81nmt1 promoter) were grown in EMM medium. Some cultures contained 100 μM Lat-A to inhibit actin polymerization (Wu et al., 2001, 2003). Cells were concentrated by centrifugation at 4,500 rpm for 5–10 s and resuspended in growth medium. 10 μl of cells were mounted directly onto a slide (for some single time-point experiments) or onto a thin layer of 100–150 μl YE5S or EMM5S medium containing 25% gelatin (G-2500; Sigma-Aldrich) and 0.1 mM n-propyl-gallate (for time-lapse experiments), sealed under a coverslip with Valap, and observed at 23°C, except where noted.
Microscopy and data analysis
We observed live cells in growth chambers (Wu et al., 2003) with inverted microscopes (IX-71; Olympus) equipped with a 60×/1.4 NA objective (Plan-Apo; Olympus) and appropriate filters (DIC, CFP, FITC, and YFP) or equipped with a 100×/1.4 NA objective (Plan-Apo) and a spinning-disk confocal system (UltraView RS; Perkin Elmer) with excitation by a 442-nm helium cadmium laser or 488- or 514-nm argon ion lasers. All images were acquired using cooled charge-coupled device cameras (ORCA-ER; Hamamatsu). Digital images and kymographs were processed with Image J software (National Institutes of Health; http://rsb.info.nih.gov/ij/), as previously described (Wu et al., 2003). Some raw images from the confocal system were opened using the UltraView Opener plug-ins (provided by A. Isaacson and B. Mohler, University of Connecticut, Farmington, CT). Node compositions were quantified by stepping through the Z sections. YFP- and CFP-tagged proteins were considered in the same nodes if the fluorescence signals in the nodes were obviously stronger than the cytoplasmic background and the outlines of the nodes overlapped. The images for Video 5 and some images in Fig. 2 (D and the max projection in E) were deconvolved with AutoQuant software (AutoQuant Imaging, Inc.)
Online supplemental material
Fig. S1 shows myosin II spots, Fig. S2 shows that profilin Cdc3p is not required for concentration of formin in nodes, and Fig. S3 depicts formin aggregates. 10 videos are included to show that contractile ring assembly starts from a broad band of nodes (Videos 1 and 2), that the formin Cdc12p and the PCH protein Cdc15p localize to a broad band of nodes (Videos 3–7), and that YFP-actin, Arp2/3 complex, and its activators localize in dynamic actin patches, but do not concentrate in the contractile ring (Videos 8–10). Table S1 lists fission yeast strains. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200602032/DC1.
Supplementary Material
Acknowledgments
We thank Marc Manix, Lindsey Zhao, and Keely Macmillan for making some strains; Wei-Lih Lee and John Cooper for 3GFP and 3YFP plasmids; and Ariel Isaacson and Bill Mohler for UltraView Opener plug-ins.
This work was supported by National Institutes of Health (NIH) grants GM-26132 and GM-261338 (to T.D. Pollard), an Anna Fuller Fund Fellowship (to J.-Q. Wu), an American Heart Association postdoctoral fellowship (to V. Sirotkin), an NIH National Research Service Award fellowship (to D.R. Kovar), and a career award from the Burroughs Wellcome Fund (to J.R. Kuhn).
D.R. Kovar's present address is Depts. of Molecular Genetics and Cell Biology and Biochemistry and Molecular Biology, University of Chicago, Chicago, IL 60637.
M. Lord's present address is Dept. of Molecular Physiology and Biophysics, University of Vermont, Burlington, VT 05405.
Abbreviations used in this paper: Arp2/3, actin-related protein 2/3; DIC, differential interference contrast; Lat-A, latrunculin A; mCFP, monomeric CFP; mYFP, monomeric YFP; PCH, pombe Cdc15 homology; SPB, spindle pole body; WASp, Wiskott-Aldrich syndrome protein.
References
- Arai, R., and I. Mabuchi. 2002. F-actin ring formation and the role of F-actin cables in the fission yeast Schizosaccharomyces pombe. J. Cell Sci. 115:887–898. [DOI] [PubMed] [Google Scholar]
- Arai, R., K. Nakano, and I. Mabuchi. 1998. Subcellular localization and possible function of actin, tropomyosin and actin-related protein 3 (Arp3) in the fission yeast Schizosaccharomyces pombe. Eur. J. Cell Biol. 76:288–295. [DOI] [PubMed] [Google Scholar]
- Bähler, J., A.B. Steever, S. Wheatley, Y.-l. Wang, J.R. Pringle, K.L. Gould, and D. McCollum. 1998. a. Role of polo kinase and Mid1p in determining the site of cell division in fission yeast. J. Cell Biol. 143:1603–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bähler, J., J.-Q. Wu, M.S. Longtine, N.G. Shah, A. McKenzie III, A.B. Steever, A. Wach, P. Philippsen, and J.R. Pringle. 1998. b. Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast. 14:943–951. [DOI] [PubMed] [Google Scholar]
- Balasubramanian, M.K., B.R. Hirani, J.D. Burke, and K.L. Gould. 1994. The Schizosaccharomyces pombe cdc3+ gene encodes a profilin essential for cytokinesis. J. Cell Biol. 125:1289–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian, M.K., E. Bi, and M. Glotzer. 2004. Comparative analysis of cytokinesis in budding yeast, fission yeast and animal cells. Curr. Biol. 14:R806–R818. [DOI] [PubMed] [Google Scholar]
- Bezanilla, M., and T.D. Pollard. 2000. Myosin-II tails confer unique functions in Schizosaccharomyces pombe: characterization of a novel myosin-II tail. Mol. Biol. Cell. 11:79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla, M., J.M. Wilson, and T.D. Pollard. 2000. Fission yeast myosin-II isoforms assemble into contractile rings at distinct times during mitosis. Curr. Biol. 10:397–400. [DOI] [PubMed] [Google Scholar]
- Carnahan, R.H., and K.L. Gould. 2003. The PCH family protein, Cdc15p, recruits two F-actin nucleation pathways to coordinate cytokinetic actin ring formation in Schizosaccharomyces pombe. J. Cell Biol. 162:851–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, F. 1999. Movement of a cytokinesis factor cdc12p to the site of cell division. Curr. Biol. 9:849–852. [DOI] [PubMed] [Google Scholar]
- Chang, F. 2000. Microtubule and actin-dependent movement of the formin cdc12p in fission yeast. Microsc. Res. Tech. 49:161–167. [DOI] [PubMed] [Google Scholar]
- Chang, F., D. Drubin, and P. Nurse. 1997. cdc12p, a protein required for cytokinesis in fission yeast, is a component of the cell division ring and interacts with profilin. J. Cell Biol. 137:169–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean, S.O., S.L. Rogers, N. Stuurman, R.D. Vale, and J.A. Spudich. 2005. Distinct pathways control recruitment and maintenance of myosin II at the cleavage furrow during cytokinesis. Proc. Natl. Acad. Sci. USA. 102:13473–13478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza, V.M., N.I. Naqvi, H. Wang, and M.K. Balasubramanian. 2001. Interactions of Cdc4p, a myosin light chain, with IQ-domain containing proteins in Schizosaccharomyces pombe. Cell Struct. Funct. 26:555–565. [DOI] [PubMed] [Google Scholar]
- Eggert, U.S., A.A. Kiger, C. Richter, Z.E. Perlman, N. Perrimon, T.J. Mitchison, and C.M. Field. 2004. Parallel chemical genetic and genome-wide RNAi screens identify cytokinesis inhibitors and targets. PLoS Biol. 2:e379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fankhauser, C., A. Reymond, L. Cerutti, S. Utzig, K. Hofmann, and V. Simanis. 1995. The S. pombe cdc15 gene is a key element in the reorganization of F-actin at mitosis. Cell. 82:435–444. [DOI] [PubMed] [Google Scholar]
- Fishkind, D.J., and Y.L. Wang. 1993. Orientation and three-dimensional organization of actin filaments in dividing cultured cells. J. Cell Biol. 123:837–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gönczy, P., C. Echeverri, K. Oegema, A. Coulson, S.J. Jones, R.R. Copley, J. Duperon, J. Oegema, M. Brehm, E. Cassin, et al. 2000. Functional genomic analysis of cell division in C. elegans using RNAi of genes on chromosome III. Nature. 408:331–336. [DOI] [PubMed] [Google Scholar]
- Guertin, D.A., S. Trautmann, and D. McCollum. 2002. Cytokinesis in eukaryotes. Microbiol. Mol. Biol. Rev. 66:155–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou, M.-C., and D. McCollum. 2002. Cytokinesis: myosin spots the ring. Curr. Biol. 12:R334–R336. [DOI] [PubMed] [Google Scholar]
- Kaksonen, M., Y. Sun, and D.G. Drubin. 2003. A pathway for association of receptors, adaptors, and actin during endocytic internalization. Cell. 115:475–487. [DOI] [PubMed] [Google Scholar]
- Kaksonen, M., C.P. Toret, and D.G. Drubin. 2005. A modular design for the clathrin- and actin-mediated endocytosis machinery. Cell. 123:305–320. [DOI] [PubMed] [Google Scholar]
- Kitayama, C., A. Sugimoto, and M. Yamamoto. 1997. Type II myosin heavy chain encoded by the myo2 gene composes the contractile ring during cytokinesis in Schizosaccharomyces pombe. J. Cell Biol. 137:1309–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovar, D.R., and T.D. Pollard. 2004. Insertional assembly of actin filament barbed ends in association with formins produces piconewton forces. Proc. Natl. Acad. Sci. USA. 101:14725–14730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovar, D.R., J.R. Kuhn, A.L. Tichy, and T.D. Pollard. 2003. The fission yeast cytokinesis formin Cdc12p is a barbed end actin filament capping protein gated by profilin. J. Cell Biol. 161:875–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovar, D.R., E.S. Harris, R. Mahaffy, H.N. Higgs, and T.D. Pollard. 2006. Control of the assembly of ATP- and ADP-actin by formins and profilin. Cell. 124:423–435. [DOI] [PubMed] [Google Scholar]
- Lord, M., and T.D. Pollard. 2004. UCS protein Rng3p activates actin filament gliding by fission yeast myosin-II. J. Cell Biol. 167:315–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabuchi, I. 1994. Cleavage furrow: timing of emergence of contractile ring actin filaments and establishment of the contractile ring by filament bundling in sea urchin eggs. J. Cell Sci. 107:1853–1862. [DOI] [PubMed] [Google Scholar]
- Maupin, P., and T.D. Pollard. 1986. Arrangement of actin filaments and myosin–like filaments in the contractile ring and of actin–like filaments in the mitotic spindle of dividing HeLa cells. J. Ultrastruct. Mol. Struct. Res. 94:92–103. [DOI] [PubMed] [Google Scholar]
- McCollum, D., A. Feoktistova, M. Morphew, M. Balasubramanian, and K.L. Gould. 1996. The Schizosaccharomyces pombe actin-related protein, Arp3, is a component of the cortical actin cytoskeleton and interacts with profilin. EMBO J. 15:6438–6446. [PMC free article] [PubMed] [Google Scholar]
- Moreno, S., A. Klar, and P. Nurse. 1991. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 194:795–823. [DOI] [PubMed] [Google Scholar]
- Morrell, J.L., M. Morphew, and K.L. Gould. 1999. A mutant of Arp2p causes partial disassembly of the Arp2/3 complex and loss of cortical actin function in fission yeast. Mol. Biol. Cell. 10:4201–4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motegi, F., K. Nakano, and I. Mabuchi. 2000. Molecular mechanism of myosin-II assembly at the division site in Schizosaccharomyces pombe. J. Cell Sci. 113:1813–1825. [DOI] [PubMed] [Google Scholar]
- Motegi, F., M. Mishra, M.K. Balasubramanian, and I. Mabuchi. 2004. Myosin-II reorganization during mitosis is controlled temporally by its dephosphorylation and spatially by Mid1 in fission yeast. J. Cell Biol. 165:685–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi, N.I., K. Eng, K.L. Gould, and M.K. Balasubramanian. 1999. Evidence for F-actin-dependent and -independent mechanisms involved in assembly and stability of the medial actomyosin ring in fission yeast. EMBO J. 18:854–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti, A., and F. Chang. 2000. Analysis of mid1p, a protein required for placement of the cell division site, reveals a link between the nucleus and the cell surface in fission yeast. Mol. Biol. Cell. 11:2757–2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham, R.J., and F. Chang. 2002. Actin dynamics in the contractile ring during cytokinesis in fission yeast. Nature. 419:82–86. [DOI] [PubMed] [Google Scholar]
- Pollard, T.D., L. Blanchoin, and R.D. Mullins. 2000. Molecular mechanisms controlling actin filament dynamics in nonmuscle cells. Annu. Rev. Biophys. Biomol. Struct. 29:545–576. [DOI] [PubMed] [Google Scholar]
- Rogers, S.L., U. Wiedemann, N. Stuurman, and R.D. Vale. 2003. Molecular requirements for actin-based lamella formation in Drosophila S2 cells. J. Cell Biol. 162:1079–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger, J.M., and J.W. Sanger. 1980. Banding and polarity of actin filaments in interphase and cleaving cells. J. Cell Biol. 86:568–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder, T.E. 1973. Actin in dividing cells: contractile ring filaments bind heavy meromyosin. Proc. Natl. Acad. Sci. USA. 70:1688–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severson, A.F., D.L. Baillie, and B. Bowerman. 2002. A formin homology protein and a profilin are required for cytokinesis and Arp2/3-independent assembly of cortical microfilaments in C. elegans. Curr. Biol. 12:2066–2075. [DOI] [PubMed] [Google Scholar]
- Sirotkin, V., C.C. Beltzner, J.-B. Marchand, and T.D. Pollard. 2005. Interactions of WASp, myosin-I, and verprolin with Arp2/3 complex during actin patch assembly in fission yeast. J. Cell Biol. 170:637–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slepchenko, B.M., and M. Terasaki. 2003. Cyclin aggregation and robustness of bio-switching. Mol. Biol. Cell. 14:4695–4706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda, T., and F. Chang. 2005. Role of fission yeast myosin I in organization of sterol-rich membrane domains. Curr. Biol. 15:1331–1336. [DOI] [PubMed] [Google Scholar]
- Terasaki, M., E.-I. Okumura, B. Hinkle, and T. Kishimoto. 2003. Localization and dynamics of Cdc2-cyclin B during meiotic reinitiation in starfish oocytes. Mol. Biol. Cell. 14:4685–4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolliday, N., L. Verplank, and R. Li. 2002. Rho1 directs formin-mediated actin ring assembly during budding yeast cytokinesis. Curr. Biol. 12:1864–1870. [DOI] [PubMed] [Google Scholar]
- Wasserman, S. 1998. FH proteins as cytoskeletal organizers. Trends Cell Biol. 8:111–115. [DOI] [PubMed] [Google Scholar]
- Weaver, A.M., M.E. Young, W.-L. Lee, and J.A. Copper. 2003. Integration of signals to the Arp2/3 complex. Curr. Opin. Cell Biol. 15:23–30. [DOI] [PubMed] [Google Scholar]
- White, J.G. 1990. Laterally mobile, cortical tension elements can self-assemble into a contractile ring. Ann. N. Y. Acad. Sci. 582:50–59. [DOI] [PubMed] [Google Scholar]
- White, J.G., and G.G. Borisy. 1983. On the mechanisms of cytokinesis in animal cells. J. Theor. Biol. 101:289–316. [DOI] [PubMed] [Google Scholar]
- Winter, D.C., E.Y. Choe, and R. Li. 1999. Genetic dissection of the budding yeast Arp2/3 complex: a comparison of the in vivo and structural roles of individual subunits. Proc. Natl. Acad. Sci. USA. 96:7288–7293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe, B.A., and K.L. Gould. 2005. Split decisions: coordinating cytokinesis in yeast. Trends Cell Biol. 15:10–18. [DOI] [PubMed] [Google Scholar]
- Wong, K.C.Y., V.M. D'souza, N.I. Naqvi, F. Motegi, I. Mabuchi, and M.K. Balasubramanian. 2002. Importance of a myosin II-containing progenitor for actomyosin ring assembly in fission yeast. Curr. Biol. 12:724–729. [DOI] [PubMed] [Google Scholar]
- Wu, J.-Q., and T.D. Pollard. 2005. Counting cytokinesis proteins globally and locally in fission yeast. Science. 310:310–314. [DOI] [PubMed] [Google Scholar]
- Wu, J.-Q., J. Bähler, and J.R. Pringle. 2001. Roles of a fimbrin and an α-actinin-like protein in fission yeast cell polarization and cytokinesis. Mol. Biol. Cell. 12:1061–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, J.-Q., J.R. Kuhn, D.R. Kovar, and T.D. Pollard. 2003. Spatial and temporal pathway for assembly and constriction of the contractile ring in fission yeast cytokinesis. Dev. Cell. 5:723–734. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.