Abstract
A recent meeting entitled Frontiers in Live Cell Imaging was attended by more than 400 cell biologists, physicists, chemists, mathematicians, and engineers. Unlike typical special topics meetings, which bring together investigators in a defined field primarily to review recent progress, the purpose of this meeting was to promote cross-disciplinary interactions by introducing emerging methods on the one hand and important biological applications on the other. The goal was to turn live cell imaging from a “technique” used in cell biology into a new exploratory science that combines a number of research fields.
Over the past decades, the advances in live cell imaging have dramatically transformed the biological sciences. Critical developments include fluorescence, confocal, and two-photon optics, green fluorescent protein, photoelectronic detectors, and image deconvolution. Although these developments have often involved the serendipitous convergence of multiple disciplines, there have been minimal direct interactions between researchers from different disciplines. The pace of development should be greatly accelerated by promoting cross-disciplinary interactions and by targeting research toward important and appropriate biological questions. To achieve this goal, speakers and session chairs in this meeting were requested to present their research in a broad perspective, and biosketches of speakers who may be unfamiliar to some of the audience were provided in the program book. These steps allowed the meeting to be conducted in a highly cohesive “language” despite the diversity of the participants. To expand the horizon of the participants, one of the keynote speeches featured an astrophysicist, Roberto Ragazzoni (University of Padova). He inspired the audience with vivid descriptions of his pioneering work in the application of adaptive optics (in which the characteristics of optical components are adjusted automatically and at high speed in response to observation conditions) to address highly challenging problems in astronomy. The meeting covered technical advances in probes, optics, and image informatics, as well as imaging applications at the single-molecule, cellular, and tissue level. The program book and streaming video of the presentations are available at http://www.cellmigration.org/consortium/2006imaging.workshop.
Probes and sensors
On the development of new probes, Atsushi Miyawaki (RIKEN) described new generations of fluorescent proteins that allow the use of light to switch them reversibly between bright and dim fluorescent states (“protein highlighting;” Ando et al., 2004; Dedecker et al., 2006), whereas Gerard Marriott (University of Wisconsin) prepared organic probes that undergo rapid and reversible optical switching between states of widely different chemical and photophysical properties (Sakata et al., 2005a,b). When conjugated to target proteins, these probes could be used to measure the dynamics or manipulate the chemical state of the protein repeatedly. For example, fluorescent protein highlighting was used to demonstrate the stimulation of bidirectional nuclear transport of MAP kinase upon growth factor activation (Ando et al., 2004), and optical switching has allowed researchers to reversibly and rapidly control protein dipolar interactions inside cells. These techniques also can be used in combination with innovative strategies of FRAP, FRET, and super-resolution imaging (described later). Equally significant are new inorganic probes that are small, bright, and resistant to photodegradation (Robert Dickson, Georgia Tech; Zheng et al., 2004). Once these probes are conjugated to specific targets, they should allow the detection of single molecules in living cells. Other biosensors can report the activation states (e.g., conformation and phosphorylation) of endogenous proteins with minimal perturbation (Klaus Hahn, University of North Carolina; Nalbant et al., 2004; Pertz et al., 2006). These biosensors consist of an affinity element that binds only to the activated state of the target protein and a bright dye whose fluorescence emission responds to the binding. The development of the affinity element was further facilitated by using a phage display library combined with high-throughput screening of sequences capable of reporting a wide range of protein activities.
A long-standing challenge with biosensors has been delivering them into living cells and targeting them to specific proteins or organelles of interest. Newly designed peptide sequences have now allowed the import of probes into living cells via nonendocytic pathways, thereby alleviating the problem of generating a constellation of brightly fluorescent vesicles (Klaus Hahn). In addition, Alice Ting (Massachusetts Institute of Technology) described how to exploit natural enzymes for protein-specific labeling (Chen et al., 2005; Howarth et al., 2005). For example, Escherichia coli biotin ligase is known to ligate biotin to a lysine side chain within a specific 15–amino acid substrate sequence. By genetically fusing the 15–amino acid acceptor peptide to proteins of interest, coexpressing the biotin ligase, and providing a ketone analogue of biotin, her group showed that proteins in living cells could be specifically labeled with a variety of small-molecule probes, including fluorophores and photoaffinity labels.
Image informatics
The session on image informatics delivered the important message that a wealth of information, much beyond human vision, can be obtained automatically through the use of computational analyses. The development is being driven both by the large volume of images collected via automated microscopy of live cells, and by the complexity and subtlety of patterns in three-dimensional (3D), time-series images. Even the analysis of conventional fluorescence images can benefit greatly from automated interpretation of subcellular patterns. For example, Gaudenz Danuser (Scripps Research Institute) described computational methods for the quantification of the dynamics of hundreds of thousands of fluorescent speckles, at a high spatial and temporal resolution, to probe with great detail the nonsteady state behavior of actin filament structures in migrating cells (Ponti et al., 2004; Ji and Danuser, 2005; Danuser and Waterman-Storer, 2006). Bob Murphy (Carnegie-Mellon University) described computer programs that can recognize the patterns of major subcellular structures better than trained human observers. The information allowed the classification of proteins by their subcellular location and will facilitate the discovery of new distribution patterns as well as realistic simulations of protein distribution for modeling cell behavior (Chen and Murphy, 2005; Chen et al., 2006). Roland Eils (German Cancer Research Center) described a fast, automated method for analyzing a large collection of time-lapse image sequences for the purpose of screening the effects of siRNA. The process involves “training” a classifier to recognize various phenotypes, followed by applying the classifier to images in a time series. This approach has permitted fully automated identification of genes involved in such important functions as cell cycle regulation.
Imaging techniques and optical strategies
The integration of multiple disciplines is evident in the recent advancement of several imaging techniques. For example, fluorescence correlation spectroscopy (FCS) is being used with laser scanning microscopes, high-speed electron multiplying charge coupled device (EMCCD) cameras (Enrico Gratton, University of California, Irvine; Petra Schwille, Technische Universität, Dresden), total internal reflection fluorescence (TIRF) optics, and increasingly sophisticated mathematical analysis tools (Nancy Thompson, University of North Carolina, Chapel Hill; Enrico Gratton) to measure molecular concentrations, diffusion, binding, and aggregation densities both on the surface and inside living cells with high spatial and temporal resolution (Thompson et al., 2002; Lieto et al., 2003; Digman et al., 2005a,b; Bacia et al., 2006; Ries and Schwille, 2006). Cross-correlation methods further allow direct comparison of the mobility of multiple components labeled with different color fluorophores and reveal their interactions within complexes (Petra Schwille and Enrico Gratton). These studies will benefit from new fluorescence dyes that can be excited at a common wavelength while emitting at easily separable wavelengths (Atsushi Miyawaki). Another elegant example is the application of switchable fluorescent probes, as described above, to build images by switching on (and bleaching off) a few diffraction-limited spots (Airy disks) at a time. Referred to as photoactivated localization microscopy (PALM), this approach provides superb spatial resolution (although at the expense of temporal resolution, since it takes time to cover the entire image with hundreds of thousands of Airy disks), as centroids of these individual Airy disks are well separated and may be determined at nanometer precision (Eric Betzig, Howard Hughes Medical Institute; Betzig et al., 2006).
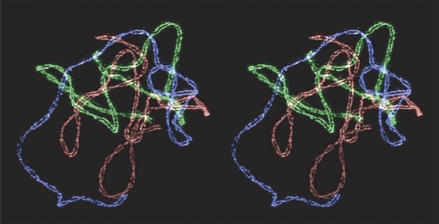
A number of clever optical strategies have increased both the resolution and versatility of microscopy. For example, Tony Wilson (Oxford University) showed that the quality of images with conventional optics can approach that of confocal images, by illuminating thick samples with a laser through a one-dimensional grid, followed by removal of the pattern with computer processing (Neil et al., 2000). A second application of patterned illumination, combining stripe illumination at multiple orientations with computer image processing, was developed by the University of California, San Francisco, cooperative (John Sedat, David Agard, and Mats Gustafsson) to overcome the diffraction limit and obtain unprecedented details from images of chromosomes (Fig. 1). Here, periodic illumination is used to extend the optical response of the microscope to high-frequency signals along a particular direction, by generating a moiré interference effect with greater (and therefore detectable) periodicity (Gustafsson, 2000; Wells, 2004). A technically more demanding technique of patterned illumination was described by Betzig, who used complex optical interference phenomena to generate a 3D lattice of diffraction-limited illumination spots for high-speed, high-resolution imaging of thick samples (Betzig, 2005). These spots illuminate a large number of regions simultaneously, with a photon efficiency far exceeding that provided by a confocal microscope.
Figure 1.
Stereo pair of maize meiotic chromosomes imaged with structured illumination, stained using immunofluorescence against ZmAFD1, a Rec8 homologue, which localizes to the axis of each chromosome. The maize nucleus has ten pairs of chromosomes; for ease of viewing, three have been false-colored and computationally excised from the rest. The 200-nm spacing of the chromosome axes is visible, as is the tendency of the axes to twist around each other. Courtesy of Rachel Wang (University of California, Berkeley) and Pete Carlton (University of California, San Francisco).
By combining optical manipulations with computation or innovative probes, many new powerful approaches may be invented with surprisingly low cost and great simplicity. In addition to patterned illumination and PALM described above, this includes combining oblique focus scanning (where optical sections are collected while the sample is shifted horizontally at the same time) and deconvolution to generate stereo pairs (Wilson and Sedat), and using flash illumination to limit how far single molecules diffuse during the detection phase as in strobe photography of high speed objects (Sunney Xie, Harvard University; Xie et al., 2006). Future developments may further incorporate new mechanisms to generate image contrast and to extend the usable range of electromagnetic waves, in order to take advantage of special chemical and physical characteristics of the materials. For example, Xie developed 3D coherent anti-stoke Raman scattering (CARS) microscopy (Evans et al., 2005), which combines two laser beams at different wavelengths to generate a beating frequency that matches the vibration frequency of target molecules. These beams produce much stronger signals of Raman light scattering than conventional Raman scattering techniques. The technique has been used to image the dynamics of lipid droplets and to distinguish different lipids in unstained living cells, based on their different vibration frequencies. Finally, Carolyn Larabell's group (Lawrence Berkeley Lab) is developing soft X-ray microscopy as a new tool for whole-cell imaging (Le Gros et al., 2005). The approach uses zone plate (diffractive) optics to focus X-rays onto the sample, and then onto a detector. At the wavelengths used, X-rays are absorbed by carbon and nitrogen, but not by water. The technique should be capable of producing high-contrast, 3D images of unstained cells at a resolution as high as 10 nm.
Applications to biology—single molecules to single cells
Half of the meeting was dedicated to the applications of cutting edge imaging techniques to various important biological problems. A significant trend is the emphasis on single-molecule or single-organelle techniques, which remove the ensemble average in conventional fluorescence imaging to obtain behavior distributions, local dynamics, and kinetics without the need to synchronize individual molecules or cells. Using single-molecule FRET or single-particle tracking, investigators can observe the kinetics of molecular interactions, the appearance of conformational intermediates, and the molecular responses to force applied by an optical trap (Xiaowei Zhuang, Harvard University; Taekjip Ha, University of Illinois, Urbana-Champaign; McKinney et al., 2005). Single-molecule observations are also being used in cells to determine distances at nanometer accuracy, by accurately measuring the center of the diffraction-limited spot emanating from single molecules. This approach has been used to reveal the mechanisms by which molecular motors such as dynein, kinesin, and myosin V move (Paul Selvin, University of Illinois, Urbana-Champaign; Kural et al., 2005; Park et al., 2006; Toprak et al., 2006). In addition, single-molecule tracking in cells is revealing distributions of diffusion coefficients for membrane proteins and lipids, as well as linear, directed motion arising from treadmilling of bacterial actin before and after cell division (W.E. Moerner, Stanford University).
Single-organelle techniques are being used to understand the mechanisms that underlie endocytosis, exocytosis, and viral entry into cells (Zhuang; Rebecca Heald, University of California, Berkeley; Sandy Simon, Rockefeller University; Jennifer Lippincott-Schwartz, National Institutes of Health), while correlation spectroscopy, speckle microscopy, and molecule counting regimes are being used to understand dynamics of the cytoskeleton and substrate adhesions (Clare Waterman-Storer, Scripps Research Institute; Edward Salmon, University of North Carolina, Chapel Hill; Enrico Gratton).
Applications to biology—whole tissues
Although imaging cells in tissues is not as well developed as studying cells in culture, it is a critical frontier, and progress is promising. If excitation light enters the specimen orthogonal to the objective lens (using single plane illumination), fluorescence can be measured from all molecules that are located in the very narrow volume defined by the plane of excitation light. This greatly reduces photodamage and photobleaching and enhances the axial resolution (Ernst Stelzer, EMBL, Heidelberg; Huisken et al., 2004). Although such new instrumentation (as well as lattice illumination described earlier) is likely to make a strong impact, significant advances have already been made using commercially available confocal optics. Peter Friedl (University of Würzburg) and John Condeelis (Albert Einstein College of Medicine) showed impressive imaging of cellular movements in skin and breast tissue, respectively, in live animals. Tumor cells in skin reside in a variety of states including single cells and collectives, and they migrate by either mesenchymal or amoeboid type motility (Wolf et al., 2003). Migration through collagen requires strategic clipping of collagen at constriction points as the cell squeezes through the matrix network, leaving a localized trail of oriented collagen fibers. In addition, breast tumor cells undergo a chemotactic migration along collagen fibers, guided by epidermal growth factor secreted by perivascular macrophages (Goswami et al., 2005).
Equally impressive is the mapping of neural responses in the visual cortex by applying calcium indicators and two-photon optics to the brain of live animals (Clay Reid, Harvard Medical School). The responses of neurons, monitored within cubes that have sides ∼300 μm in length, show sharp changes as the animal receives the visual stimulation of a bar moving in different directions (Ohki et al., 2005). Finally, Scott Fraser and his colleagues (California Institute of Technology) have developed comprehensive imaging and informatic systems for high-throughput, parallel analysis of the cellular trajectories of hundreds or thousands of cells in live zebrafish embryos, with the ultimate goal of understanding the exact pathways of cellular movements during embryonic development (Forouhar et al., 2006).
The synergy and enthusiasm generated during the meeting reflect the importance of live cell imaging, the incisive applications to date, and the prospects for significant developments in the near future. The localized nature of biological phenomena serves as the driving force for the development of technologies to observe, assay, and parse their mechanisms. The meeting pointed to a very bright future for live cell imaging, by showing that higher resolution, better reagents, and more powerful methods of revealing interactions and dynamics are well within reach. In addition, as suggested by Roger Tsien (University of California, San Diego), one of the keynote speakers, basic research in this area may soon lead to revolutionary advances in clinical diagnosis and treatments, both by direct applications of “optical physiology”—the probing and manipulation of physiological events with optical techniques—and by spinning off techniques involved in probe development and delivery.
Acknowledgments
The authors wish to thank the many speakers from the meeting for commenting on this article.
Frontiers in Live Cell Imaging was sponsored by the National Institutes of General Medical Sciences and the Cell Migration Consortium.
References
- Ando, R., H. Mizuno, and A. Miyawaki. 2004. Regulated fast nucleocytoplasmic shuttling observed by reversible protein highlighting. Science. 306:1370–1373. [DOI] [PubMed] [Google Scholar]
- Bacia, K., S.A. Kim, and P. Schwille. 2006. Fluorescence cross-correlation spectroscopy in living cells. Nat. Meth. 3:83–89. [DOI] [PubMed] [Google Scholar]
- Betzig, E. 2005. Multifocal three-dimensional imaging with optical lattice excitation. Microsc. Microanal. 11:80–81. [Google Scholar]
- Betzig, E., G.H. Patterson, R. Sougrat, O.W. Lindwasser, S. Olenych, J.S. Bonifacino, M.W. Davidson, J. Lippincott-Schwartz, and H.F. Hess. 2006. Imaging intracellular fluorescent proteins at nanometer resolution. Science. 10.1126/science.1127344. [DOI] [PubMed]
- Chen, I., M. Howarth, W. Lin, and A.Y. Ting. 2005. Site-specific labeling of cell surface proteins with biophysical probes using biotin ligase. Nat. Meth. 2:99–104. [DOI] [PubMed] [Google Scholar]
- Chen, S.C., and R.F. Murphy. 2006. A graphical model approach to 2005. Objective clustering of proteins based on subcellular location patterns. J. Biomed. Biotech. 2005:87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, X., M. Velliste, and R.F. Murphy. 2006. Automated interpretation of subcellular patterns in fluorescence microscope images for location proteomics. Cytometry. 69A:631–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danuser, G., and C.M. Waterman-Storer. 2006. Quantitative fluorescent speckle microscopy of cytoskeleton dynamics. Annu. Rev. Biophys. Biomol. Struct. 35:361–387. [DOI] [PubMed] [Google Scholar]
- Dedecker, P., J.I. Hotta, R. Ando, A. Miyawaki, Y. Engelborghs, J. Hofkens. Fast and reversible photoswitching of the fluorescent protein Dronpa as evidenced by fluorescence correlation spectroscopy. Biophys J. 2006. 10.1529/biophysj.106.089789. [DOI] [PMC free article] [PubMed]
- Digman, M.A., C.M. Brown, P. Sengupta, P.W. Wiseman, A.R. Horwitz, and E. Gratton. 2005. a. Measuring fast dynamics in solutions and cells with a laser scanning microscope. Biophys. J. 89:1317–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Digman, M.A., P. Sengupta, P.W. Wiseman, C.M. Brown, A.R. Horwitz, and E. Gratton. 2005. b. Fluctuation correlation spectroscopy with a laser-scanning microscope: exploiting the hidden time structure. Biophys. J. 88:L33–L36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans, C.L., E.O. Potma, M. Puoris'haag, D. Cote, C.P. Lin, and X.S. Xie. 2005. Chemical imaging of tissue in vivo with video-rate coherent anti-Stokes Raman scattering microscopy. Proc. Natl. Acad. Sci. USA. 102:16807–16812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forouhar, A.S., M. Liebling, A. Hickerson, A. Nasiraei-Moghaddam, H.J. Tsai, J.R. Hove, S.E. Fraser, M.E. Dickinson, and M. Gharib. 2006. The embryonic vertebrate heart tube is a dynamic suction pump. Science. 312:751–753. [DOI] [PubMed] [Google Scholar]
- Goswami, S., E. Sahai, J.B. Wyckoff, M. Cammer, D. Cox, F.J. Pixley, E.R. Stanley, J.E. Segall, and J.S. Condeelis. 2005. Macrophages promote the invasion of breast carcinoma cells via a colony-stimulating factor-1/epidermal growth factor paracrine loop. Cancer Res. 65:5278–5283. Erratum published in 65:7031. [DOI] [PubMed] [Google Scholar]
- Gustafsson, M.G.L. 2000. Surpassing the lateral resolution limit by a factor of two using structured illumination microscopy. J. Microsc. 198:82–87. [DOI] [PubMed] [Google Scholar]
- Howarth, M., K. Takao, Y. Hayashi, and A.Y. Ting. 2005. Targeting quantum dots to surface proteins in living cells with biotin ligase. Proc. Natl. Acad. Sci. USA. 102:7583–7588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisken, J., J. Swoger, F. Del Bene, J. Wittbrodt, and E.H. Stelzer. 2004. Optical sectioning deep inside live embryos by selective plane illumination microscopy. Science. 305:1007–1009. [DOI] [PubMed] [Google Scholar]
- Ji, L., and G. Danuser. 2005. Tracking quasi-stationary flow of weak fluorescent signals by adaptive multi-frame correlation. J. Microsc. 220:150–167. [DOI] [PubMed] [Google Scholar]
- Kural, C., H. Kim, S. Syed, G. Goshima, V.I. Gelfand, and P.R. Selvin. 2005. Kinesin and dynein move a peroxisome in vivo: a tug-of-war or coordinated movement? Science. 308:1469–1472. [DOI] [PubMed] [Google Scholar]
- Le Gros, M.A., G. McDermott, and C.A. Larabell. 2005. X-ray tomography of whole cells. Curr. Opin. Struct. Biol. 15:593–600. [DOI] [PubMed] [Google Scholar]
- Lieto, A.M., R.C. Cush, and N.L. Thompson. 2003. Ligand-receptor kinetics measured by total internal reflection with fluorescence correlation spectroscopy. Biophys. J. 85:3294–3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney, S.A., A.D. Freeman, D.M. Lilley, and T. Ha. 2005. Observing spontaneous branch migration of Holliday junctions one step at a time. Proc. Natl. Acad. Sci. USA. 102:5715–5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalbant, P., L. Hodgson, V. Kraynov, A. Toutchkine, and K.M. Hahn. 2004. Activation of endogenous Cdc42 visualized in living cells. Science. 305:1615–1619. [DOI] [PubMed] [Google Scholar]
- Neil, M.A.A., A. Squire, R. Juskaitis, P.I.H. Bastiaens, and T. Wilson. 2000. Wide-field optically sectioning fluorescence microscopy with laser illumination. J. Microsc. 197:1–4. [DOI] [PubMed] [Google Scholar]
- Ohki, K., S. Chung, Y.H. Ch'ng, P. Kara, and R.C. Reid. 2005. Functional imaging with cellular resolution reveals precise micro-architecture in visual cortex. Nature. 433:597–603. [DOI] [PubMed] [Google Scholar]
- Park, H., B. Ramamurthy, M. Travaglia, D. Safer, L.Q. Chen, C. Franzini-Armstrong, P.R. Selvin, and H.L. Sweeney. 2006. Full-length myosin VI dimerizes and moves processively along actin filaments upon monomer clustering. Mol. Cell. 21:331–336. [DOI] [PubMed] [Google Scholar]
- Pertz, O., L. Hodgson, R.L. Klemke, and K.M. Hahn. 2006. Spatiotemporal dynamics of RhoA activity in migrating cells. Nature. 440:1069–1072. [DOI] [PubMed] [Google Scholar]
- Ponti, A., M. Machacek, S.L. Gupton, C.M. Waterman-Storer, and G. Danuser. 2004. Two distinct actin networks drive the protrusion of migrating cells. Science. 305:1782–1786. [DOI] [PubMed] [Google Scholar]
- Ries, J., and P. Schwille. 2006. Studying Slow Membrane Dynamics with Continuous Wave Scanning Fluorescence Correlation Spectroscopy. Biophys. J. 10.1529/biophysj.106.082297. [DOI] [PMC free article] [PubMed]
- Sakata, T., Y. Yan, and G. Marriott. 2005. a. Family of site-selective molecular optical switches. J. Org. Chem. 70:2009–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata, T., Y. Yan, and G. Marriott. 2005. b. Optical switching of dipolar interactions on proteins. Proc. Natl. Acad. Sci. USA. 102:4759–4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, N.L., A.M. Lieto, and N.W. Allen. 2002. Recent advances in fluorescence correlation spectroscopy. Curr. Opin. Struct. Biol. 12:634–641. [DOI] [PubMed] [Google Scholar]
- Toprak, E., J. Enderlein, S. Syed, S.A. McKinney, R.G. Petschek, T. Ha, Y.E. Goldman, and P.R. Selvin. 2006. Defocused orientation and position imaging (DOPI) of myosin V. Proc. Natl. Acad. Sci. USA. 103:6495–6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells, W.A. 2004. Man the nanoscopes. J. Cell Biol. 164:337–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf, K., I. Mazo, H. Leung, K. Engelke, U.H. von Andrian, E.I. Deryugina, A.Y. Strongin, E.B. Brocker, and P. Friedl. 2003. Compensation mechanism in tumor cell migration: mesenchymal-amoeboid transition after blocking of pericellular proteolysis. J. Cell Biol. 160:267–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, X.S., J. Yu, and W.Y. Yang. 2006. Living cells as test tubes. Science. 312:228–230. [DOI] [PubMed] [Google Scholar]
- Zheng, J., C. Zhang, and R.M. Dickson. 2004. Highly fluorescent, water-soluble, size-tunable gold quantum dots. Phys. Rev Lett. 93:077402. [DOI] [PubMed] [Google Scholar]