Abstract
To perform the vital functions of motility and division, cells must undergo dramatic shifts in cell polarity. Recent evidence suggests that polarized distributions of phosphatidylinositol 4,5-bisphosphate and phosphatidylinositol 3,4,5-trisphosphate, which are clearly important for regulating cell morphology during migration, also play an important role during the final event in cell division, which is cytokinesis. Thus, there is a critical interplay between the membrane phosphoinositides and the cytoskeletal cortex that regulates the complex series of cell shape changes that accompany these two processes.
Cell migration, cytokinesis, and phosphoinositide signaling
Many of the current questions facing cell biologists are related to the mechanisms that allow cells to move and divide. Cell migration and division are profoundly important in shaping the embryo and wiring the developing nervous system. In the adult, these two processes are involved in tissue maintenance, regeneration, and the immune response, as well as in the pathology of numerous diseases. The morphological changes required for cell migration and during cytokinesis are similar in many respects. Both processes involve elaborate control of the cytoskeleton to produce and dynamically modulate cell polarity. A moving cell typically maintains a single front and back, whereas a dividing cell is bipolar, with two poles flanking the future site of cleavage. In many migratory cells, there is a seamless transition between these morphologies (Fig. 1). As a migrating cell prepares for cytokinesis, it rounds up and appears to “erase” its polarity. It then elongates and begins to constrict at the midline. Membranes from opposite sides of the cell invaginate, defining the fission furrow, and then fuse, pinching off the newly formed daughter cells, which typically move apart. The backs of the new cells correspond to the point of cleavage, and the fronts derive from the former poles of the dividing cell. Some cells lack a significant migratory phase, yet still cycle through the unipolar, bipolar, and nonpolar stages.
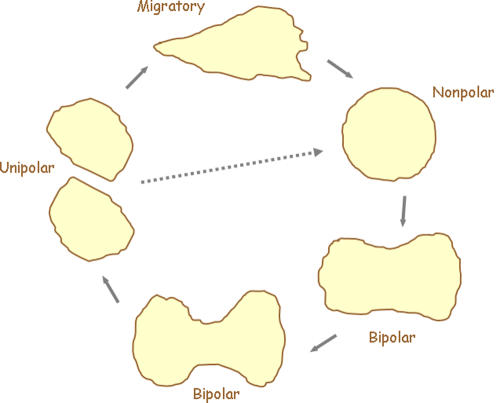
Figure 1.
Diagram depicting the life cycle of a migratory cell. Migratory cells generally have a distinct front and back and display a polarized morphology. As cells prepare to enter cytokinesis, they normally round up and suppress actin-filled projections. Control of the cytoskeleton is then handed over to the forming spindle. As the spindle apparatus elongates, the cell follows suit, taking on a bipolar morphology, with a furrow forming at its equator. Constriction at the midline will ultimately lead to abscission at the cell bridge and the formation of two unipolar cells. Migratory behavior will then resume and the cycle can start again. If migratory cues are not initiated, cells will bypass this stage and continue through the cell cycle (dotted arrow).
The morphological symmetry seen between migration and cytokinesis carries through on a molecular level. A series of proteins that localize to the fronts of migrating cells, including RacE, dynacortin, coronin, and scar, are also found at the poles of dividing cells, whereas others, such as myosin II, Rho GTPases, and cortexillin, which associate with the back of moving cells, are concentrated at the furrow during cytokinesis (De Lozanne and Spudich, 1987; Kishi et al., 1993; Drechsel et al., 1997; Kitayama et al., 1997; Bi et al., 1998; Lippincott and Li, 1998; Weber et al., 1999). These localizations ensure that newly polymerized actin creates leading edge projections, or polar ruffles, whereas actomyosin creates contraction at the rear and within the ring that pinches apart the daughter cells (Gerisch et al., 1999; Fukui, 2000).
The polarization of the cytoskeleton requires signaling from the plasma membrane, and this communication involves the phosphoinositides (PIPs), chiefly phosphatidylinositol 4,5-bisphosphate (PI[4,5]P2) and phosphatidylinositol 3,4,5-trisphosphate (PI[3,4,5]P3). PI(4,5)P2, which is relatively more abundant in the plasma membrane, interacts permissively with a variety of cytoskeletal proteins in different spatial localizations. PI(4,5)P2 influences or activates several regulators of actin polymerization, including WASP, profilin, cofilin, and capping protein (Yin and Janmey, 2003). In vitro PI(4,5)P2 also binds to septins and ezrin-radixin-moesin (ERM) family members like α-actinin (Hirao et al., 1996; Matsui et al., 1999; Nakamura et al., 1999; Zhang et al., 1999; Barret et al., 2000). Studies on migrating cells show that PI(3,4,5)P3 accumulates in the membrane at the cell's leading edge and aligns the cell along the chemoattractant gradient (Haugh et al., 2000; Wang et al., 2002). The locally elevated PI(3,4,5)P3 levels instruct new actin polymerization and pseudopodia extension at the front. The lower levels at the rear inhibit projections in this region and help specify the back (Chen et al., 2003). Positive feedback between the actin cytoskeleton and plasma membrane PI(3,4,5)P3 reinforces the asymmetric response and can spontaneously bring about polarity, even in the absence of a gradient (Niggli, 2000; Weiner et al., 2002; Devreotes and Janetopoulos, 2003).
The participation of PIPs, which typically serve as secondary messengers, seems natural for migration, but might seem unusual for cytokinesis, where there is no known receptor-mediated signaling. Polarity during cytokinesis depends instead on internal cues. It is thought that astral microtubules interact with and alter the cell cortex to set polarity, and that feedback from the polarizing cortex controls the progress and orientation of the spindle (Rappaport, 1986; Cao and Wang, 1996; Wheatley and Wang, 1996; Rieder et al., 1997; Robinson and Spudich, 2004; Rosenblatt et al., 2004). New evidence now indicates that lipid-signaling pathways appear to coordinate membrane and cortical events during cytokinesis. Several investigators have recently found that regulation of PI(4,5)P2 is necessary for proper furrow ingression during cytokinesis. Similarly, we have found that a “polarity circuit,” involving the temporal and spatial regulation of PI(3,4,5)P3 metabolism, plays a key role in establishing polarity for cell division.
PI(4,5)P2 and regulation of the contractile ring
In one of the first experiments to directly suggest a role for PI(4,5)P2 in cytokinesis, injection of anti-PI(4,5)P2 antibodies into Xenopus laevis embryos was shown to lengthen the cell cycle and inhibit cleavage furrow progression (Han et al., 1992). Earlier experiments on sea urchin zygotes had shown that Li+ blocks cytokinesis (Forer and Sillers, 1987). Li+ inhibits inositol monophosphatase and inositol polyphosphate-1-phosphatase, which can lead to depletion of PIPs (Parthasarathy and Eisenberg, 1986; Rana and Hokin, 1990). This inhibitory effect was reversed by the precursor myoinositol (Forer and Sillers, 1987; Becchetti and Whitaker, 1997).
Genetic manipulations and drugs have assessed the role of PI(4,5)P2 metabolism in cytokinesis. Mutations in the Drosophila melanogaster gene four wheel drive (fwd), which encodes PI4Kβ, resulted in a cytokinesis defect during male meiosis, suggesting a vital role for PI(4)P synthesis during this process (Brill et al., 2000). In Schizosaccharomyces pombe, both PI(4)P 5-kinase (its3) and its product PI(4,5)P2 were concentrated in the medial ring during cytokinesis. Also in S. pombe, the homologue of both fwd and its3 were found to be required for proper cell division (Zhang et al., 2000; Desautels et al., 2001). PI(4,5)P2 was also found to be important in mammalian cells, as its depletion or sequestration with neomycin blocked cytokinesis in cultured cells (Zhang et al., 1999). Furthermore, in crane fly spermatocytes, Li+, the PI-kinase inhibitors wortmannin and LY294002, and the PLC inhibitor U73122 all stopped or slowed furrowing after initiation (Saul et al., 2004). These initial studies have raised further questions. In general, are PI(4,5)P2 and the enzymes that regulate it localized to the furrow? What are the targets of PI(4,5)P2 and/or its products?
Brill et al. (2000) reported a series of experiments indicating that hydrolysis of PI(4,5)P2 to IP3 and DAG could be a key step for completion of cytokinesis. They depleted PI(4,5)P2 by various methods, such as dephosphorylation by the Salmonella enterica phosphoinositide phosphatase SigD (Marcus et al., 2001; Terebiznik et al., 2002) and sequestration by PLCδ-PH-GFP and by the cell-permeable PI(4,5)P2-binding peptide PBP10 (Cunningham et al., 2001). All three treatments caused cytokinesis defects (Wong et al., 2005). With SigD treatment, the spermatids contained multiple nuclei; the majority of spermatids contained three or four nuclei, suggesting failures at both meiosis I and II. Expression of PLCδ-PH-GFP caused a statistically significant, but minor, defect in <2% of transformed cells. PBP10, which was administered to cells after furrow ingression had initiated, caused regression followed by cytokinesis failure in the majority of cells treated. When cells were treated with the PLC inhibitor U73122, furrow regression occurred in a rapid and dose-dependent manner (Fig. 2 A). When cells were treated with the IP3R antagonist 2-APB, cleavage furrows regressed, suggesting that Ca2+ is required for completion of cytokinesis (Wong et al., 2005). Further evidence for Ca2+ was obtained when cells maintained in Ca2+-free buffer were treated with the membrane permeable Ca2+chelator, BAPTA-AM. Cytokinesis failure rates as high as 40% (10 μM) and 90% (300 μM) were recorded. Based on these observations, the authors suggest that the hydrolysis of PI(4,5)P2 and the release of Ca2+ may be involved in contractility by activating myosin or by stimulating vesicle fusion events by activating SNAREs (Chen et al., 1999; Littleton et al., 2001). Interestingly, PLCδ-PH-GFP, which specifically binds PI(4,5)P2, was found localized to the plasma membranes, but was not highly enriched in the furrow.
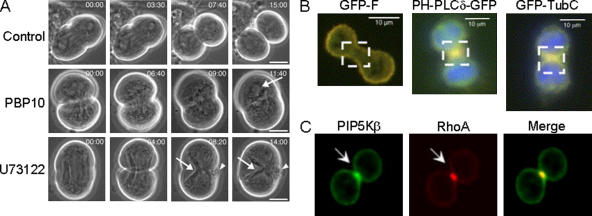
Figure 2.
A series of experiments show evidence for a role of PI(4,5)P2 during cytokinesis. (A) Spermatocyte meiosis I cytokinesis requires both PI(4,5)P2 and PI(4,5)P2 hydrolysis. Control cells progressed through cytokinesis normally, whereas cells treated with 1 μM PBP10 or 10 μM U73122 failed to undergo normal cleavage (Wong et al., 2005). Arrows, a reformed nucleus and parafusorial membranes that continued to constrict in the PBP10 and U73122 treated cells, respectively; Arrowheads, blebs of plasma membrane at equator. (B) PI(4,5)P2 appears to be enriched in the furrow in HeLa cells. Two markers for PI(4,5)P2, PLCδ-PH-GFP, and GFP-TubbyC both localized to the furrow, whereas the expression of farnesylated EGFP was uniformly distributed around the periphery of the cell (Field et al., 2005). Note the localization of these three markers in the boxed areas. (C) PIP5Kβ colocalizes with RhoA in the cleavage furrow. CHO cells were fixed and stained with anti-PIP5Kβ antibody and anti-RhoA antibody (Emoto et al., 2005). Arrow, cleavage furrow.
Although PI(4,5)P2 was uniformly distributed in D. melanogaster spermatocytes, two other groups found that PI(4,5)P2 was localized in the furrow in mammalian tissue cells during ingression (Emoto et al., 2005; Field et al., 2005). They also reported that interference with PI(4,5)P2 impaired cytokinesis. Field et al. (2005) expressed a series of PH domains specific for PI(4,5)P2 in HeLa, CHO, RAW, and 3T3 cells. PLCδ-PH-GFP and GFP-TubC were associated with the plasma membrane and enriched in the cleavage furrow of dividing cells, suggesting that PI(4,5)P2 was elevated in these regions (Fig. 2 B). Low expression levels of these constructs did not interfere with cytokinesis. However, a 10-fold overexpression resulted in a separation of cytoskeletal actin from the plasma membrane. Similarly, there was detachment of actin from the plasma membrane at the furrow in cells expressing the PI(4,5)P2 phosphatase synaptojanin. They also found that high levels of PLCδ-PH-GFP and GFP-TubC both independently doubled the duration of cytokinesis and increased the frequency of multinucleate cells. This is consistent with the PLCδ-PH-GFP data from Wong et al. (2005). Furthermore, expression of synaptojanin, as well as a dominant-negative, kinase-inactive PI(4)P-5 kinase, both reduced the plasma membrane PI(4,5)P2 and increased the amount of multinucleate cells. Based on these observations, the authors suggest that plasma membrane PI(4,5)P2 levels in the constricting furrow must be sufficient to sustain adhesion between the plasma membrane and the underlying actin cytoskeleton for proper maintenance of the furrow.
Emoto et al. similarly found that PI(4,5)P2 localized to the furrow and were able to cause cytokinesis defects when they interfered with PI(4,5)P2 accumulation (Emoto et al., 2005). In both normal cells and in cells specifically arrested during late cytokinesis, PI(4)P-5 kinase, RhoA, and PI(4,5)P2 were highly localized in the furrow of CHO cells (Fig. 2 C). Both the overexpression of catalytically inactive PI(4)P-5 kinase and the microinjection of specific anti-PI(4,5)P2 antibodies impaired cytokinesis. The antibody treatment increased the amount of multinucleate cells from ∼6 to ∼32%. These authors suggest that localized production of PI(4,5)P2 is needed for proper cytokinesis and that there is likely a unique lipid domain in the cleavage furrow. This is also consistent with a recent paper showing a requirement for PLCγ in sea urchin cytokinesis (Ng et al., 2005).
PI(3,4,5)P3 polarity circuits in cytokinesis
Recent studies of Dictyostelium discoideum have revealed a regulatory circuit involving PI(3,4,5)P3 that controls polarity in cytokinesis, as well as in migration (Janetopoulos et al., 2005). The two enzymes controlling PI(3,4,5)P3, PI3K, and PTEN, were reciprocally regulated during cell division in a strikingly similar manner, as has been described previously for migrating cells (Funamoto et al., 2002; Huang et al., 2003). As cells rounded up at the onset of cytokinesis, PTEN-GFP moved uniformly to the membrane, closely resembling the localization of myosin II. At the same time, membrane PI3K-GFP localization was inhibited. Then, as the cell elongated, PI3K-GFP and PTEN-GFP associated with the cell membrane at the poles and furrow, respectively (Fig. 3 A). This spatial modulation of PI3K and PTEN controls the local distribution of PI(3,4,5)P3, and perturbation of the polarity circuit including these enzymes adversely affects migration.
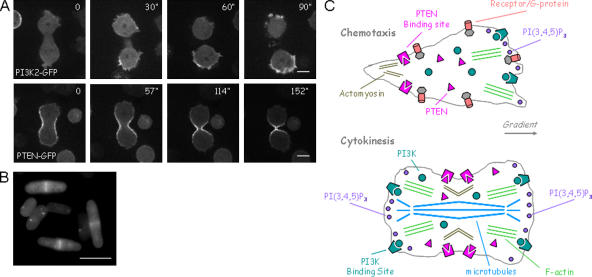
Figure 3.
The enzymes regulating PI(3,4,5)P3 levels are reciprocally regulated during cytokinesis. (A) PI3K and PTEN are localized to the poles and furrow, respectively, of a D. discoideum cell undergoing cytokinesis (Janetopoulos et al., 2005). (B) PTEN localizes to the septa of S. pombe during cell division (Mitra et al., 2004). (C) Diagram depicting the reciprocal regulation of PI3K and PTEN in both migrating and dividing cells. The localization of the enzymes is controlled through the activation of heterotrimeric G proteins in migrating cells, whereas an unknown factor, possibly the spindle or microtubule apparatus, regulates the distribution of the enzymes during cytokinesis. PI(3,4,5)P3 synthesis results in actin polymerization and membrane protrusion. Its inhibition, on the other hand, allows for the contraction of either the back of the cell or the constriction of the furrow and is associated with the actomyosin complex.
This new study demonstrated that inhibiting these enzymes had deleterious effects on the process of cell division as well. PTEN was disrupted in a cell line already lacking PI3K1 and PI3K2. These two PI3Ks account for ∼90% of the PI3K activity in wild-type cells; there are an additional six PI3K enzymes in D. discoideum cells (Zhou et al., 1995; Meili et al., 1999; Janetopoulos et al., 2005). These mutant cells, which contained basal PI(3,4,5)P3 levels higher than normal, but lacked temporal or spatial regulation by external or internal cues, failed to undergo cytokinesis in shaking suspension, resulting in large multinucleate cells. Mutant cell lines missing PI3K1 and PI3K2 or PTEN alone also had modest cytokinesis defects. Furthermore, we found that cells grown on surfaces and treated with the PI3K inhibitors wortmannin or LY294002 were dramatically delayed or failed to undergo cytokinesis nearly 25% of the time.
Based on studies of migrating cells, we have speculated on the role of PI(3,4,5)P3 during cytokinesis. Because local increases in PI(3,4,5)P3 promote actin polymerization and pseudopod extension, cells must carefully regulate PI(3,4,5)P3 accumulation during cell division. At the onset of cytokinesis PI(3,4,5)P3 signaling is globally suppressed, which leads to a loss of actin polymerization and membrane ruffling. This allows the cell to round up and reset its polarity. Then, as the cell elongates, the poles gradually become active zones of PI(3,4,5)P3-labeled, actin-filled projections, whereas the midzone and forming furrow are devoid of PI(3,4,5)P3 and remain quiescent. This polarization of the membrane and cytoskeleton is critical in positioning the initial furrow and in driving the progression of cytokinesis. When we imaged the cells lacking PI(3,4,5)P3-modulation, actin-based projections were apparent all around the cell perimeter. This unregulated activity of the cytoskeleton likely contributes to failure at multiple stages of cytokinesis. First, it would be more difficult for these cells to reset polarity at the onset of cytokinesis. Without appropriate PI(3,4,5)P3 regulation, the elongating spindle may be unable to gain control of the membrane and cytoskeleton. Second, the hyperactivity would destabilize localized activity of the cytoskeleton during furrow formation. The regulation of PI(3,4,5)P3 is likely involved in many types of cell shape changes in numerous systems. A similar association of PTEN with the septa of S. pombe during cell division is likely related to the mechanism described here (Fig. 3 B; Mitra et al., 2004). A recent report found PTEN localized to cell–cell junctions in D. melanogaster photoreceptors, where the enzyme regulates PI(3,4,5)P3 levels and is essential for epithelial cell morphogenesis and apical basal polarity (Pinal et al., 2006).
PI(3,4,5)P3 and PI(4,5)P2 define the “front” and “back” of polarized cells
Evidence that has emerged in the last few years and been brought together here suggests that PIPs play a key role in regulating polarity in cytokinesis, as well as in migration, although the precise mechanisms remain to be defined. The involvement of these intracellular signaling molecules in cytokinesis is consistent with the close parallel of the front and back of migrating cells with the poles and furrow of dividing cells. Similar cytoskeletal components localize at each corresponding zone. There appears to be a seamless transition in cell morphology as cells shift from cell migration to cell division. As D. discoideum cells round up before cytokinesis, proteins normally located at the back, such as myosin, cortexillin, and PTEN, are recruited along the entire cell periphery. Then as the spindle elongates, they localize to the furrow. Likewise, stitching together data from various cell types, there seems to be a general consensus that PI(3,4,5)P3 is found at the front or at poles, whereas PI(4,5)P2 is localized to the back or the furrow. PI(4,5)P2 may play a permissive role in regulating cytoskeletal proteins, several of which contain PI(4,5)P2-binding motifs, whereas in other instances it is clearly the products of PI(4,5)P2 hydrolysis that are important.
Interestingly, PTEN contains a PI(4,5)P2-binding motif (Galvin et al., 1984; Iijima et al., 2004). Is it possible that PI(4,5)P2 helps direct PTEN to the furrow? Considering that the phosphatase activity of PTEN regulates the conversion of PI(3,4,5)P3 to PI(4,5)P2, it is easy to imagine that local levels of PI(4,5)P2 might rise, and PTEN associations would form a powerful feedback loop for polarity in cytokinesis, as well as migration. Although D. discoideum is the only organism in which the localization of PTEN has been observed at the midline of a dividing cell, observations of PI(4,5)P2 at the furrow have been made in other organisms. Also, although PI(3,4,5)P3 has been imaged at the front of many migrating cells, it has only been imaged during cytokinesis in D. discoideum. Nevertheless, the studies discussed in this paper clearly illustrate a key role for phosphoinositide signaling in cytokinesis and provide a missing link between the plasma membrane and cytoskeleton. Future studies will address the generality of these findings and the precise role of the signaling in this fascinating process.
Abbreviations used in this paper: ERM, ezrin-radixin-moesin; PIP, phosphoinositide.
References
- Barret, C., C. Roy, P. Montcourrier, P. Mangeat, and V. Niggli. 2000. Mutagenesis of the phosphatidylinositol 4,5-bisphosphate (PIP2) binding site in the NH2- terminal domain of ezrin correlates with its altered cellular distribution. J. Cell Biol. 151:1067–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becchetti, A., and M. Whitaker. 1997. Lithium blocks cell cycle transitions in the first cell cycles of sea urchin embryos, an effect rescued by myo-inositol. Development. 124:1099–1107. [DOI] [PubMed] [Google Scholar]
- Bi, E., P. Maddox, D.J. Lew, E.D. Salmon, J.N. McMillan, E. Yeh, and J.R. Pringle. 1998. Involvement of an actomyosin contractile ring in Saccharomyces cerevisiae cytokinesis. J. Cell Biol. 142:1301–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brill, J.A., G.R. Hime, M. Scharer-Schuksz, and M.T. Fuller. 2000. A phospholipid kinase regulates actin organization and intercellular bridge formation during germline cytokinesis. Development. 127:3855–3864. [DOI] [PubMed] [Google Scholar]
- Cao, L.G., and Y.L. Wang. 1996. Signals from the spindle midzone are required for the stimulation of cytokinesis in cultured epithelial cells. Mol. Biol. Cell. 7:225–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, L., C. Janetopoulos, Y.E. Huang, M. Iijima, J. Borleis, and P.N. Devreotes. 2003. Two phases of actin polymerization display different dependencies on PI(3,4,5)P3 accumulation and have unique roles during chemotaxis. Mol. Biol. Cell. 14:5028–5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y.A., S.J. Scales, S.M. Patel, Y.C. Doung, and R.H. Scheller. 1999. SNARE complex formation is triggered by Ca2+ and drives membrane fusion. Cell. 97:165–174. [DOI] [PubMed] [Google Scholar]
- Cunningham, C.C., R. Vegners, R. Bucki, M. Funaki, N. Korde, J.H. Hartwig, T.P. Stossel, and P.A. Janmey. 2001. Cell permeant polyphosphoinositide-binding peptides that block cell motility and actin assembly. J. Biol. Chem. 276:43390–43399. [DOI] [PubMed] [Google Scholar]
- De Lozanne, A., and J.A. Spudich. 1987. Disruption of the Dictyostelium myosin heavy chain gene by homologous recombination. Science. 236:1086–1091. [DOI] [PubMed] [Google Scholar]
- Desautels, M., J.P. Den Haese, C.M. Slupsky, L.P. McIntosh, and S.M. Hemmingsen. 2001. Cdc4p, a contractile ring protein essential for cytokinesis in Schizosaccharomyces pombe, interacts with a phosphatidylinositol 4-kinase. J. Biol. Chem. 276:5932–5942. [DOI] [PubMed] [Google Scholar]
- Devreotes, P., and C. Janetopoulos. 2003. Eukaryotic chemotaxis: distinctions between directional sensing and polarization. J. Biol. Chem. 278:20445–20448. [DOI] [PubMed] [Google Scholar]
- Drechsel, D.N., A.A. Hyman, A. Hall, and M. Glotzer. 1997. A requirement for Rho and Cdc42 during cytokinesis in Xenopus embryos. Curr. Biol. 7:12–23. [DOI] [PubMed] [Google Scholar]
- Emoto, K., H. Inadome, Y. Kanaho, S. Narumiya, and M. Umeda. 2005. Local change in phospholipid composition at the cleavage furrow is essential for completion of cytokinesis. J. Biol. Chem. 280:37901–37907. [DOI] [PubMed] [Google Scholar]
- Field, S.J., N. Madson, M.L. Kerr, K.A. Galbraith, C.E. Kennedy, M. Tahiliani, A. Wilkins, and L.C. Cantley. 2005. PtdIns(4,5)P2 functions at the cleavage furrow during cytokinesis. Curr. Biol. 15:1407–1412. [DOI] [PubMed] [Google Scholar]
- Forer, A., and P.J. Sillers. 1987. The role of the phosphatidylinositol cycle in mitosis in sea urchin zygotes. Lithium inhibition is overcome by myo-inositol but not by other cyclitols or sugars. Exp. Cell Res. 170:42–55. [DOI] [PubMed] [Google Scholar]
- Fukui, Y. 2000. Real-time high-resolution optical sectioning suggests biphasic cytokinetic mechanism in Dictyostelium discoideum. Microsc. Res. Tech. 49:183–189. [DOI] [PubMed] [Google Scholar]
- Funamoto, S., R. Meili, S. Lee, L. Parry, and R.A. Firtel. 2002. Spatial and temporal regulation of 3-phosphoinositides by PI 3-kinase and PTEN mediates chemotaxis. Cell. 109:611–623. [DOI] [PubMed] [Google Scholar]
- Galvin, N.J., D. Stockhausen, B.L. Meyers-Hutchins, and W.A. Frazier. 1984. Association of the cyclic AMP chemotaxis receptor with the detergent-insoluble cytoskeleton of Dictyostelium discoideum. J. Cell Biol. 98:584–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerisch, G., M. Maniak, and R. Neujahr. 1999. Patterns of cellular activities based on protein sorting in cell motility, endocytosis and cytokinesis. Biochem. Soc. Symp. 65:1–14. [PubMed] [Google Scholar]
- Han, J.K., K. Fukami, and R. Nuccitelli. 1992. Reducing inositol lipid hydrolysis, Ins(1,4,5)P3 receptor availability, or Ca2+ gradients lengthens the duration of the cell cycle in Xenopus laevis blastomeres. J. Cell Biol. 116:147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugh, J.M., F. Codazzi, M. Teruel, and T. Meyer. 2000. Spatial sensing in fibroblasts mediated by 3′ phosphoinositides. J. Cell Biol. 151:1269–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirao, M., N. Sato, T. Kondo, S. Yonemura, M. Monden, T. Sasaki, Y. Takai, S. Tsukita, and S. Tsukita. 1996. Regulation mechanism of ERM (ezrin/radixin/moesin) protein/plasma membrane association: possible involvement of phosphatidylinositol turnover and Rho-dependent signaling pathway. J. Cell Biol. 135:37–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, Y.E., M. Iijima, C.A. Parent, S. Funamoto, R.A. Firtel, and P. Devreotes. 2003. Receptor-mediated regulation of PI3Ks confines PI(3,4,5)P3 to the leading edge of chemotaxing cells. Mol. Biol. Cell. 14:1913–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima, M., Y.E. Huang, H.R. Luo, F. Vazquez, and P.N. Devreotes. 2004. Novel mechanism of PTEN regulation by its phosphatidylinositol 4,5-bisphosphate binding motif is critical for chemotaxis. J. Biol. Chem. 279:16606–16613. [DOI] [PubMed] [Google Scholar]
- Janetopoulos, C., J. Borleis, F. Vazquez, M. Iijima, and P. Devreotes. 2005. Temporal and spatial regulation of phosphoinositide signaling mediates cytokinesis. Dev. Cell. 8:467–477. [DOI] [PubMed] [Google Scholar]
- Kishi, K., T. Sasaki, S. Kuroda, T. Itoh, and Y. Takai. 1993. Regulation of cytoplasmic division of Xenopus embryo by rho p21 and its inhibitory GDP/GTP exchange protein (rho GDI). J. Cell Biol. 120:1187–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitayama, C., A. Sugimoto, and M. Yamamoto. 1997. Type II myosin heavy chain encoded by the myo2 gene composes the contractile ring during cytokinesis in Schizosaccharomyces pombe. J. Cell Biol. 137:1309–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott, J., and R. Li. 1998. Sequential assembly of myosin II, an IQGAP-like protein, and filamentous actin to a ring structure involved in budding yeast cytokinesis. J. Cell Biol. 140:355–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littleton, J.T., J. Bai, B. Vyas, R. Desai, A.E. Baltus, M.B. Garment, S.D. Carlson, B. Ganetzky, and E.R. Chapman. 2001. Synaptotagmin mutants reveal essential functions for the C2B domain in Ca2+-triggered fusion and recycling of synaptic vesicles in vivo. J. Neurosci. 21:1421–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus, S.L., M.R. Wenk, O. Steele-Mortimer, and B.B. Finlay. 2001. A synaptojanin-homologous region of Salmonella typhimurium SigD is essential for inositol phosphatase activity and Akt activation. FEBS Lett. 494:201–207. [DOI] [PubMed] [Google Scholar]
- Matsui, T., S. Yonemura, S. Tsukita, and S. Tsukita. 1999. Activation of ERM proteins in vivo by Rho involves phosphatidyl-inositol 4-phosphate 5-kinase and not ROCK kinases. Curr. Biol. 9:1259–1262. [DOI] [PubMed] [Google Scholar]
- Meili, R., C. Ellsworth, S. Lee, T.B. Reddy, H. Ma, and R.A. Firtel. 1999. Chemoattractant-mediated transient activation and membrane localization of Akt/PKB is required for efficient chemotaxis to cAMP in Dictyostelium. EMBO J. 18:2092–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra, P., Y. Zhang, L.E. Rameh, M.P. Ivshina, D. McCollum, J.J. Nunnari, G.M. Hendricks, M.L. Kerr, S.J. Field, L.C. Cantley, and A.H. Ross. 2004. A novel phosphatidylinositol(3,4,5)P3 pathway in fission yeast. J. Cell Biol. 166:205–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura, F., L. Huang, K. Pestonjamasp, E.J. Luna, and H. Furthmayr. 1999. Regulation of F-actin binding to platelet moesin in vitro by both phosphorylation of threonine 558 and polyphosphatidylinositides. Mol. Biol. Cell. 10:2669–2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng, M.M., F. Chang, and D.R. Burgess. 2005. Movement of membrane domains and requirement of membrane signaling molecules for cytokinesis. Dev. Cell. 9:781–790. [DOI] [PubMed] [Google Scholar]
- Niggli, V. 2000. A membrane-permeant ester of phosphatidylinositol 3,4,5-trisphosphate (PIP(3)) is an activator of human neutrophil migration. FEBS Lett. 473:217–221. [DOI] [PubMed] [Google Scholar]
- Parthasarathy, R., and F. Eisenberg Jr. 1986. The inositol phospholipids: a stereochemical view of biological activity. Biochem. J. 235:313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinal, N., D.C. Goberdhan, L. Collinson, Y. Fujita, I.M. Cox, C. Wilson, and F. Pichaud. 2006. Regulated and polarized PtdIns(3,4,5)P3 accumulation is essential for apical membrane morphogenesis in photoreceptor epithelial cells. Curr. Biol. 16:140–149. [DOI] [PubMed] [Google Scholar]
- Rana, R.S., and L.E. Hokin. 1990. Role of phosphoinositides in transmembrane signaling. Physiol. Rev. 70:115–164. [DOI] [PubMed] [Google Scholar]
- Rappaport, R. 1986. Establishment of the mechanism of cytokinesis in animal cells. Int. Rev. Cytol. 105:245–281. [DOI] [PubMed] [Google Scholar]
- Rieder, C.L., A. Khodjakov, L.V. Paliulis, T.M. Fortier, R.W. Cole, and G. Sluder. 1997. Mitosis in vertebrate somatic cells with two spindles: implications for the metaphase/anaphase transition checkpoint and cleavage. Proc. Natl. Acad. Sci. USA. 94:5107–5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson, D.N., and J.A. Spudich. 2004. Mechanics and regulation of cytokinesis. Curr. Opin. Cell Biol. 16:182–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblatt, J., L.P. Cramer, B. Baum, and K.M. McGee. 2004. Myosin II-dependent cortical movement is required for centrosome separation and positioning during mitotic spindle assembly. Cell. 117:361–372. [DOI] [PubMed] [Google Scholar]
- Saul, D., L. Fabian, A. Forer, and J.A. Brill. 2004. Continuous phosphatidylinositol metabolism is required for cleavage of crane fly spermatocytes. J. Cell Sci. 117:3887–3896. [DOI] [PubMed] [Google Scholar]
- Terebiznik, M.R., O.V. Vieira, S.L. Marcus, A. Slade, C.M. Yip, W.S. Trimble, T. Meyer, B.B. Finlay, and S. Grinstein. 2002. Elimination of host cell PtdIns(4,5)P(2) by bacterial SigD promotes membrane fission during invasion by Salmonella. Nat. Cell Biol. 4:766–773. [DOI] [PubMed] [Google Scholar]
- Wang, F., P. Herzmark, O.D. Weiner, S. Srinivasan, G. Servant, and H.R. Bourne. 2002. Lipid products of PI(3)Ks maintain persistent cell polarity and directed motility in neutrophils. Nat. Cell Biol. 4:513–518. [DOI] [PubMed] [Google Scholar]
- Weber, I., J. Niewohner, and J. Faix. 1999. Cytoskeletal protein mutations and cell motility in Dictyostelium. Biochem. Soc. Symp. 65:245–265. [PubMed] [Google Scholar]
- Weiner, O.D., P.O. Neilsen, G.D. Prestwich, M.W. Kirschner, L.C. Cantley, and H.R. Bourne. 2002. A PtdInsP(3)- and Rho GTPase-mediated positive feedback loop regulates neutrophil polarity. Nat. Cell Biol. 4:509–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheatley, S.P., and Y. Wang. 1996. Midzone microtubule bundles are continuously required for cytokinesis in cultured epithelial cells. J. Cell Biol. 135:981–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, R., I. Hadjiyanni, H.C. Wei, G. Polevoy, R. McBride, K.P. Sem, and J.A. Brill. 2005. PIP2 hydrolysis and calcium release are required for cytokinesis in Drosophila spermatocytes. Curr. Biol. 15:1401–1406. [DOI] [PubMed] [Google Scholar]
- Yin, H.L., and P.A. Janmey. 2003. Phosphoinositide regulation of the actin cytoskeleton. Annu. Rev. Physiol. 65:761–789. [DOI] [PubMed] [Google Scholar]
- Zhang, J., C. Kong, H. Xie, P.S. McPherson, S. Grinstein, and W.S. Trimble. 1999. Phosphatidylinositol polyphosphate binding to the mammalian septin H5 is modulated by GTP. Curr. Biol. 9:1458–1467. [DOI] [PubMed] [Google Scholar]
- Zhang, Y., R. Sugiura, Y. Lu, M. Asami, T. Maeda, T. Itoh, T. Takenawa, H. Shuntoh, and T. Kuno. 2000. Phosphatidylinositol 4-phosphate 5-kinase Its3 and calcineurin Ppb1 coordinately regulate cytokinesis in fission yeast. J. Biol. Chem. 275:35600–35606. [DOI] [PubMed] [Google Scholar]
- Zhou, K., K. Takegawa, S.D. Emr, and R.A. Firtel. 1995. A phosphatidylinositol (PI) kinase gene family in Dictyostelium discoideum: biological roles of putative mammalian p110 and yeast Vps34p PI 3-kinase homologs during growth and development. Mol. Cell. Biol. 15:5645–5656. [DOI] [PMC free article] [PubMed] [Google Scholar]