Abstract
Spheroids of differentiating embryonic stem cells, denoted embryoid bodies, constitute a high-quality model for vascular development, particularly well suited for loss-of-function analysis of genes required for early embryogenesis. This review examines vasculogenesis and angiogenesis in murine embryoid bodies and discusses the promise of stem cell–based models for the study of human vascular development.
Introduction
In the early 1980s, methodology was established to allow for culture of pluripotent embryonic stem cells (ESCs) isolated from the inner cell mass of mouse blastocysts (for review see Glaser et al., 2005). Gene targeting in ESCs was soon thereafter achieved through homologous recombination, followed by generation of mouse strains from such manipulated ESCs. Occasionally, gene targeting results in early embryonic lethality, which precludes understanding of the contribution of genes to subsequent developmental processes including de novo blood vessel formation (vasculogenesis) and formation of new blood vessels from preexisting vessels (angiogenesis).
Blood vessels are essential for the delivery of nutrients and oxygen to tissues, as well as for removal of waste products. All blood vessels share a number of basic features, although the detailed gene expression pattern, morphology, and function vary between different vascular beds (e.g., arteries, veins, and capillaries). The inside of blood vessels is lined with endothelium, a thin layer of endothelial cells (ECs), which separates the blood from tissues. The outside of the endothelium is covered with a specialized layer of connective tissue (the basement membrane) followed by a layer of mural cells (pericytes and vascular smooth muscle cells). Angiogenesis is a tightly controlled process where EC proliferation and migration is regulated by secreted factors as well as by surrounding cells and matrix. There are currently considerable efforts invested into the development of drugs aimed to control blood vessel growth in conditions such as ischemia and cancer, characterized by deficient or excessive vessel growth, respectively. To this end, it is essential to create easily accessible models by which vessel development can be both manipulated and studied at high resolution.
Vascular development and sprouting angiogenesis in embryoid bodies
The isolation of EC lines and the establishment of conditions required for their maintenance in cell culture represents a milestone in the vascular biology field (Gimbrone et al., 1973). However, such cultures do not provide a proper microenvironment, involving three-dimensional (3D) interactions between ECs and adjacent supporting cells and matrix that are known to be absolutely vital in regulation of vascular processes. In contrast, cultures of human and murine ESCs possess the capacity to differentiate into most if not all major cell lineages (Thomson et al., 1998), creating an environment with parallel development of several cell types. Thus, in differentiating ESCs assembled into embryoid bodies (EBs), vascular development occurs in a context of continuous interactions with adjacent non-ECs. The first indication that EC development and subsequent vascular morphogenesis in differentiating ESC cultures proceed in an in vivo–like fashion was provided by Doetschman et al. (1985).
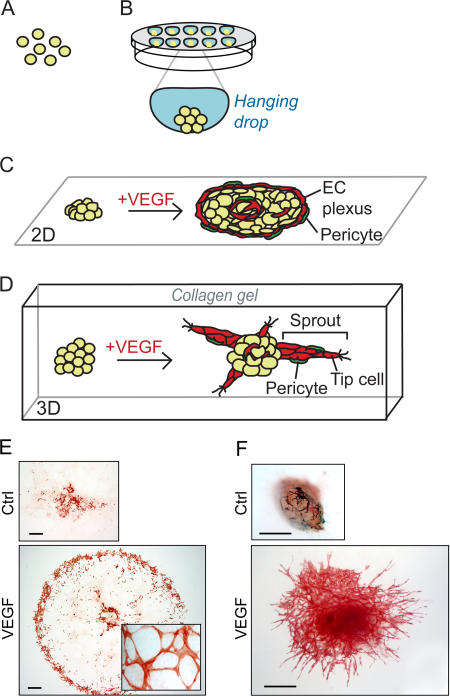
Formation of EBs can be controlled through aggregation of ESCs in hanging drops (Fig. 1, A and B), after the removal of feeder cells and leukemia inhibitory factor that otherwise are used to keep the ESCs pluripotent. The hanging drop culture proceeds for a few days to allow EB growth and differentiation, followed by seeding into a two-dimensional (2D) culture (Fig. 1, C and E), or into a 3D collagen gel (Fig. 1, D and F). For more information on EB culture procedures, see Jakobsson et al. (2006) (detailed protocols will be made available upon request to the authors). At d 3 of differentiation, the onset of vasculogenesis is demonstrated by the presence of a precursor common for endothelial and hematopoietic cells, the hemangioblast. The hemangioblast, which expresses T cell acute leukemia 1/stem cell leukemia (TAL/SCL), vascular endothelial growth factor receptor (VEGFR)-2, and brachyury, has also been detected in human EBs (Choi et al., 1998; Kennedy et al., 2007). Subsequently, hemangioblasts will be committed to either the hematopoietic or the EC lineage. The EC precursors, the angioblasts, undergo sequential maturation to eventually express a set of markers characteristic for mature ECs such as VEGFR-2, CD31, vascular endothelial (VE)– cadherin, Tie-1, and Tie-2. Angioblast development in EBs thus closely mimics the in vivo maturation process (Vittet et al., 1996).
Figure 1.
Outline of 2D and 3D EB models for vasculogenesis and angiogenesis. Stem cells are trypsinized (d 0), (A) and aggregated to create EBs, in drops hanging from the lid of a Petri dish (B). Aggregation can also occur spontaneously by seeding ESCs in suspension in a nonadhesive Petri dish, resulting in EBs of variable size. After 4 d, EBs are seeded on a tissue culture slide (2D), (C) or alternatively embedded in a 3D collagen gel (D). Addition of VEGF induces formation of a peripheral vascular plexus in 2D (C and E) and endothelial cell sprouts (“angiogenesis”) in 3D (D and F) (bottom panel in F adopted from Magnusson et al., [2005]). Whole-mount stainings for CD31 of 2D (E) or 3D (F) EBs at d 10 of differentiation, untreated (Ctrl, top) or induced with VEGF (bottom). Bars, 500 μm.
The primary vascular plexus in the EB is remodeled from d 6 and onwards, by sprouting angiogenesis. This process is regulated by growth factors, which may be produced endogenously or added in as exogenous factors. Also without growth factor treatment but in the presence of 15% serum, vascular development is evident by the presence of blood islands that differentiate to form small networks of ECs located in the center of the EB. Addition of growth factors stimulates further expansion of the endothelium. There is a distinct morphology of the vascular plexus formed in 2D EB cultures dependent on the growth factor present in the culture; VEGF isoforms VEGF-A121 and VEGF-A165, fibroblast growth factor-2, and platelet-derived growth factor (PDGF)-BB each enhance vessel formation in a distinct pattern. Typically, VEGF-A165 stimulates the formation of a peripheral capillary plexus in 2D EB cultures (Fig. 1, C and E) (Jakobsson et al., 2006).
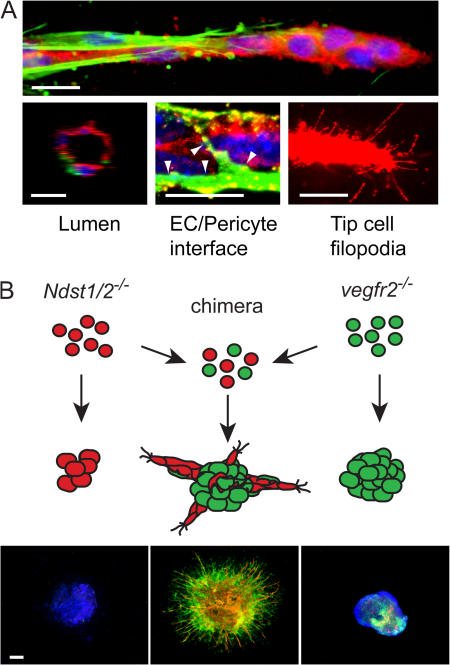
Invasive angiogenesis in 3D collagen gels is preferentially induced by VEGF-A165 and manifested around d 8 by the formation of EC sprouts protruding from the central core of the EB. The stalk cells are guided by tip cells with numerous filopodia, a process with striking similarities to vascular development in zebra fish and the retina (Fig. 1, D and F; and Fig. 2 A) (Lawson and Weinstein, 2002; Gerhardt et al., 2003). Subsequently, the sprouts branch and occasional tip cells fuse with adjacent vessels to form networks. The EC sprouts are surrounded by perivascular cells that share features such as morphology (i.e., close apposition to the endothelial cells) and protein expression pattern (expressing nerve-glia2 [NG2] and/or α-smooth muscle actin [αSMA]) with pericytes seen in vivo (Fig. 2 A). Furthermore, the vessels are enclosed by a vascular basement membrane whose detailed composition, dynamics, and function in the EBs remain to be described. Lumen formation is detectable at about d 10 of EB differentiation, and occasionally a mature lumen is evident at d 12 (Fig. 2 A). Subsequently, large lumenized vascular networks become established. It is an interesting possibility that the EB endothelium has the capacity to undergo arterial/venous specification, as endothelial cells formed from ESCs in vitro specifically express either ephrin B2 or EphB4, which are markers for arterial and venous endothelium, respectively (Muller-Ehmsen et al., 2006).
Figure 2.
Angiogenic sprouts invade the surrounding matrix. (A) Features of blood vessel sprouts formed in 3D collagen matrix in response to VEGF. Expression is shown of the endothelial cell marker CD31/platelet-endothelial cell adhesion molecule (PECAM; red), the pericyte markers αSMA (green, top), and NG2 (green, bottom middle). Hoechst 33342 was used to indicate nuclei (blue). Lumen formation is evident in larger vessels (left, cross section [z-stack] of a sprout [d 18] generated by confocal microscopy). The tip cell at the front of growing sprouts send out filopodia to sense growth factor gradients. Occasional filopodia are also detected on stalk cells that lack pericyte coverage. Bars, 10 μm. (B) Knockout EBs and the assembly of chimeric EBs. Cells deficient in production of HS (Ndst1/2 −/−) or lacking VEGFR-2 (vegfr2 −/−) do not form vascular sprouts in the EB model. However, chimeric EBs generated by mixing of the two ESC lines before EB formation respond to VEGF and form sprouts. In the chimeras, the endothelial cells (CD31; red) are derived from Ndst1/2 −/− cells expressing VEGFR-2, whereas functional HS is provided by pericytes (αSMA; green) lacking VEGFR-2 (Jakobsson et al., 2006). Bar, 300 μm.
Embryoid bodies; beyond early lethality of transgenic animals
Inactivation of genes with a vascular function (vegfr2, vegfa, VE-cadherin, or pdgfrβ) results in similar phenotypes in the EB model as in vivo, with regard to temporal effects on development and consequences for EC morphology (Table I) (Olsson et al., 2006). For example, deletion of VEGF-A, one of the main VEGFR-2 ligands, results in an arrest in vascular development and remodeling in vivo as well as in vitro (Ng et al., 2004). The EB model is particularly suitable in this context because it allows rapid and easy testing of the unique contribution of different VEGF-A isoforms to vascular development. Accordingly, treatment of VEGF-A–deficient EBs with purified VEGF-A165 rescued EC morphogenesis (Bautch et al., 2000), in agreement with the fact that mice expressing only VEGF-A165 display normal vascular development. In certain cases, data generated using in vivo models have been extended by studies performed in different ESC-based culture models. For example, VEGFR-2 was shown by differentiation of vegfr2 −/− ESCs to be required for vascular morphogenesis but not essential for early endothelial and hematopoietic cell commitment (Schuh et al., 1999). Furthermore, a number of gene deletions have resulted in developmental arrest before the onset of vasculogenesis, for example due to defects in implantation. However, by generation of ESCs from recombinant blastocysts, critical stages incompatible with in vivo growth may be studied in the EB model (see Table I for a comparison of vascular phenotypes in gene-targeted embryos and EBs).
Table I.
Vascular phenotypes in mouse embryos and EBs, as a consequence of specific gene targeting
| Phenotype
|
||
|---|---|---|
| Genotype | Embryo | EB |
| vegfr2 −/− |
aE8.5–9.5. Defective blood-island formation and vasculogenesis (Shalaby et al., 1995) |
Defective EC development and vascular remodeling (Jakobsson et al., 2006) |
| vegfa +/− | E11–12. Defective vascular development (Carmeliet et al., 1996; Ferrara et al., 1996) |
Reduced EC development and vascular remodeling; partial rescue by exogenous VEGF (Bautch et al., 2000) |
| vegfa −/− | E9.5–10.5. Generated by aggregation of ESCs with tetraploid embryos. More severely affected than vegfa +/− embryos (Carmeliet et al., 1996) |
Attenuated EC development and vascular remodeling; partial rescue by exogenous VEGF (Bautch et al., 2000; Ng et al., 2004) |
| VE-cadherin −/− | E9.5. Defective vascular development and angiogenesis (Carmeliet et al., 1999) |
Defective vascular formation and morphogenesis (Vittet et al., 1997) |
| N-cadherin −/− | E10. Impaired angiogenesis, defective yolk sac vasculature (Radice et al., 1997) |
Reduced pericyte coating, otherwise intact vascular sprouting and remodeling (Tillet et al., 2005) |
| pdgfrβ−/− | Lethal shortly before birth, hemorrhagic (Soriano, 1994). Loss of vSMC recruitment to small arteries in limb, heart and skin (Hellström et al., 1999) |
Loss of vSMC/pericyte recruitment to angiogenic sprouts (Rolny et al., 2006) |
| Ndst1/2 −/− | Lethal before gastrulation (Holmborn et al., 2004) | Attenuated vascular development; rescue by HS presented in trans by non-EC (Jakobsson et al., 2006) |
| fgfr1 −/− | E9.5. Reduced blood-island formation (Deng et al., 1994) | Exaggerated vascularization and angiogenic sprouting in the absence of exogenous growth factors (Magnusson et al., 2005) |
| β1integrin −/− | E5.5 (Fässler and Meyer, 1995) | Poor vessel branching, disturbed VEGF-induced morphogenesis (Bloch et al., 1997) |
Indicates time point of embryonic lethality.
A severe developmental phenotype (lethal before gastrulation) is caused by simultaneous deletion of the enzymes N-deacetylase/N-sulfotransferase 1 and 2 (NDST1/2), central to the synthesis of heparan sulfate (HS) (Holmborn et al., 2004). Proteins modified by attachment of HS, so-called proteoglycans, are essential co-receptors for many tyrosine kinase receptors, including VEGFR-2. Deletion of the NDST1/2 enzymes severely hampers ESC differentiation with a close to complete loss of vascular development (Fig. 2). However, rescue of vascular development was achieved in chimeric EB cultures composed of a mix of Ndst1/2 −/− and vegfr2 −/− ESCs (Jakobsson et al., 2006). Here, endothelial cells derived from the HS-deficient Ndst1/2 −/− stem cells (expressing VEGFR-2) were complemented by normal HS produced by pericytes derived form the vegfr2 −/− ESCs (Fig. 2). This exemplifies the versatility of the EB model, which allows combinations of knock-out ESCs to study the requirement for genes in subpopulations of cells during cell specification and development, to unravel new mechanisms in cell communication.
Restrictions of the EB model
Although ESCs have the capacity to produce cells of essentially any type, it is likely that complex processes that require progression through several developmental stages may be underrepresented. For example, although blood EC development is faithfully reproduced, the subsequent differentiation of lymphatic ECs from blood vessels is difficult to control (Kreuger et al., 2006). Furthermore, EBs lack blood flow, and dependent on the process under study, this aspect may be vital as flow- induced shear stress influences remodeling of the vascular system (Nguyen et al., 2006). Another drawback is that the detailed organization of vascular structures in individual EBs is much more variable compared with the precise vascular patterning in embryos. In the authors' experience, the serum chosen for the embryoid body cultures influences the properties of the cultures with regard to spontaneous vascularization, or alternatively, the ability to respond to exogenously added growth factors. The ongoing development of serum-free culture conditions might therefore contribute to an increased stability of the system. On the other hand, the model as such appears very stable as ESC lines of different genetic background show remarkably similar properties with regard to vascular development and sprouting angiogenesis.
Manipulated ESCs in vascular biology research
As described above, the flexibility of the EB model is remarkable due to the fact that ESCs can be derived from all types of genetically engineered mice. Thus, ESC-based models may in the future be used widely to complement and occasionally even replace animal experimentation, especially when very early embryonic lethality becomes a severe limitation (see Table I). ESCs from transgenic mice and gene-targeted mutant mice can be evaluated independently, or in combination, by generating chimeric cultures of two or more different ESC types (Jakobsson et al., 2006). Inducible gene expression and deletion systems, such as the Tetracyclin-On and -Off expression systems, and the site-specific DNA recombinase system Cre/loxP, in combination with labeling of cells by expression of reporter genes (e.g., β-galactosidase or fluorescent proteins) will allow clonal analysis and lineage tracing similar to what can be done in animal models (Glaser et al., 2005; Ueno and Weissman, 2006). Several ESCs with fluorescent reporters under the control of EC-specific promoters have already been created, constituting powerful tools for live imaging at single-cell or even subcellular resolution by confocal or two-photon excitation microscopy (Fraser et al., 2005).
Generation of homozygous gene deletions and recombinant animals is expensive, time consuming, and laborious. Gene silencing can instead be achieved by RNA-interference, where RNAi can be delivered to both murine and human ESCs through lentiviral transduction (Zaehres et al., 2005). Because RNAi delivery can be traced and enriched through selection for reporter gene expression, a very high degree of silencing can be attained. Moreover, previously reported problems with clonal selection and loss of expression during differentiation of ES cells appear to be circumvented when using lentivirus as a strategy for introduction of RNAi (see Zaehres et al., 2005 for further discussion).
Future perspectives
The fact that stem cells under proper conditions have the capacity to differentiate into both blood and lymphatic ECs may be explored for therapeutic purposes (Nishikawa et al., 1998). Importantly, both mouse and human ESCs can be used to generate functional ECs contributing to formation of stable vessels that connect to the host circulation (Yurugi-Kobayashi et al., 2003; Wang et al., 2007). The use of human ESCs for therapeutic purposes obviously presents a moral dilemma. Possibly, retrieval of ESCs from other locations than the fetus, such as umbilical cord blood or amniotic sources may present a feasible alternative in the future (Zhang et al., 2006). Furthermore, it is an interesting possibility that pathological conditions characterized by impaired blood and lymph vessel function may be treated by administration of adult stem cells or progenitors isolated from the patient's own bone marrow. For a recent review on the contribution of circulating stem cells to angiogenesis, see Kopp et al. (2006).
Despite striking similarities between mouse and human development, numerous therapies developed in mice (e.g., to treat diseases such as cancer) have failed when tested in humans. A contributing factor to such failures may be genetic differences between the species. Because most experimentation on humans is prohibited for ethical reasons, preclinical testing has relied solely on animal experimentation. In the future, application of human ESCs may constitute an additional step in the development and testing of drugs, with regard to toxicity and teratogenic effects. Furthermore, experimentation with human ESCs offers means to study human embryonic development. Already, homologous recombination and introduction of RNAi have been demonstrated in human ESCs, paving the way for new insights in human biology (Zwaka and Thomson, 2003).
Acknowledgments
Owing to space limitations, we have not been able to cite all relevant work; we apologize to those whose work has been omitted. We thank Drs. Vittet and Bautch for useful comments.
The authors wish to acknowledge funding from the Swedish Research Council, the Swedish Cancer Foundation, the EU 6th framework projects Lymphangiogenomics and Angiotargeting, the Swedish Foundation for Strategic Research, and the Wenner-Gren Foundations.
Abbreviations used in this paper: 2D, two-dimensional; 3D, three-dimensional; EB, embryoid body; EC, endothelial cell; ESC, embryonic stem cell; HS, heparan sulfate; NDST, N-deacetylase N-sulfotransferase; VEGFR, VEGF receptor.
References
- Bautch, V.L., S.D. Redick, A. Scalia, M. Harmaty, P. Carmeliet, and R. Rapoport. 2000. Characterization of the vasculogenic block in the absence of vascular endothelial growth factor-A. Blood. 95:1979–1987. [PubMed] [Google Scholar]
- Bloch, W., E. Forsberg, S. Lentini, C. Brakebusch, K. Martin, H.W. Krell, U.H. Weidle, K. Addicks, and R. Fässler. 1997. Beta 1 integrin is essential for teratoma growth and angiogenesis. J. Cell Biol. 139:265–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet, P., V. Ferreira, G. Breier, S. Pollefeyt, L. Kieckens, M. Gertsenstein, M. Fahrig, A. Vandenhoeck, K. Harpal, C. Eberhardt, et al. 1996. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 380:435–439. [DOI] [PubMed] [Google Scholar]
- Carmeliet, P., M.G. Lampugnani, L. Moons, F. Breviario, V. Compernolle, F. Bono, G. Balconi, R. Spagnuolo, B. Oostuyse, M. Dewerchin, et al. 1999. Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell. 98:147–157. [DOI] [PubMed] [Google Scholar]
- Choi, K., M. Kennedy, A. Kazarov, J.C. Papadimitriou, and G. Keller. 1998. A common precursor for hematopoietic and endothelial cells. Development. 125:725–732. [DOI] [PubMed] [Google Scholar]
- Deng, C.X., A. Wynshaw-Boris, M.M. Shen, C. Daugherty, D.M. Ornitz, and P. Leder. 1994. Murine FGFR-1 is required for early postimplantation growth and axial organization. Genes Dev. 8:3045–3057. [DOI] [PubMed] [Google Scholar]
- Doetschman, T.C., H. Eistetter, M. Katz, W. Schmidt, and R. Kemler. 1985. The in vitro development of blastocyst-derived embryonic stem cell lines: formation of visceral yolk sac, blood islands and myocardium. J. Embryol. Exp. Morphol. 87:27–45. [PubMed] [Google Scholar]
- Fässler, R., and M. Meyer. 1995. Consequences of lack of beta 1 integrin gene expression in mice. Genes Dev. 9:1896–1908. [DOI] [PubMed] [Google Scholar]
- Ferrara, N., K. Carver-Moore, H. Chen, M. Dowd, L. Lu, K.S. O'Shea, L. Powell-Braxton, K.J. Hillan, and M.W. Moore. 1996. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 380:439–442. [DOI] [PubMed] [Google Scholar]
- Fraser, S.T., A.K. Hadjantonakis, K.E. Sahr, S. Willey, O.G. Kelly, E.A. Jones, M.E. Dickinson, and M.H. Baron. 2005. Using a histone yellow fluorescent protein fusion for tagging and tracking endothelial cells in ES cells and mice. Genesis. 42:162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt, H., M. Golding, M. Fruttiger, C. Ruhrberg, A. Lundkvist, A. Abramsson, M. Jeltsch, C. Mitchell, K. Alitalo, D. Shima, and C. Betsholtz. 2003. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J. Cell Biol. 161:1163–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimbrone, M.A., Jr., R.S. Cotran, and J. Folkman. 1973. Endothelial regeneration: studies with human endothelial cells in culture. Ser. Haematol. 6:453–455. [PubMed] [Google Scholar]
- Glaser, S., K. Anastassiadis, and A.F. Stewart. 2005. Current issues in mouse genome engineering. Nat. Genet. 37:1187–1193. [DOI] [PubMed] [Google Scholar]
- Hellström, M., M. Kalen, P. Lindahl, A. Abramsson, and C. Betsholtz. 1999. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development. 126:3047–3055. [DOI] [PubMed] [Google Scholar]
- Holmborn, K., J. Ledin, E. Smeds, I. Eriksson, M. Kusche-Gullberg, and L. Kjellen. 2004. Heparan sulfate synthesized by mouse embryonic stem cells deficient in NDST1 and NDST2 is 6-O-sulfated but contains no N-sulfate groups. J. Biol. Chem. 279:42355–42358. [DOI] [PubMed] [Google Scholar]
- Jakobsson, L., J. Kreuger, K. Holmborn, L. Lundin, I. Eriksson, L. Kjellen, and L. Claesson-Welsh. 2006. Heparan sulfate in trans potentiates VEGFR-mediated angiogenesis. Dev. Cell. 10:625–634. [DOI] [PubMed] [Google Scholar]
- Kennedy, M., S.L. D'Souza, M. Lynch-Kattman, S. Schwantz, and G. Keller. 2007. Development of the hemangioblast defines the onset of hematopoiesis in human ES cell differentiation cultures. Blood. 109:2679–2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp, H.G., C.A. Ramos, and S. Rafii. 2006. Contribution of endothelial progenitors and proangiogenic hematopoietic cells to vascularization of tumor and ischemic tissue. Curr. Opin. Hematol. 13:175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuger, J., I. Nilsson, D. Kerjaschki, T. Petrova, K. Alitalo, and L. Claesson-Welsh. 2006. Early lymph vessel development from embryonic stem cells. Arterioscler. Thromb. Vasc. Biol. 26:1073–1078. [DOI] [PubMed] [Google Scholar]
- Lawson, N.D., and B.M. Weinstein. 2002. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev. Biol. 248:307–318. [DOI] [PubMed] [Google Scholar]
- Magnusson, P.U., R. Ronca, P. Dell'Era, P. Carlstedt, L. Jakobsson, J. Partanen, A. Dimberg, and L. Claesson-Welsh. 2005. Fibroblast growth factor receptor-1 expression is required for hematopoietic but not endothelial cell development. Arterioscler. Thromb. Vasc. Biol. 25:944–949. [DOI] [PubMed] [Google Scholar]
- Muller-Ehmsen, J., A. Schmidt, B. Krausgrill, R.H. Schwinger, and W. Bloch. 2006. Role of erythropoietin for angiogenesis and vasculogenesis: from embryonic development through adulthood. Am. J. Physiol. Heart Circ. Physiol. 290:H331–H340. [DOI] [PubMed] [Google Scholar]
- Ng, Y.S., M. Ramsauer, R.M. Loureiro, and P.A. D'Amore. 2004. Identification of genes involved in VEGF-mediated vascular morphogenesis using embryonic stem cell-derived cystic embryoid bodies. Lab. Invest. 84:1209–1218. [DOI] [PubMed] [Google Scholar]
- Nguyen, T.H., A. Eichmann, F. Le Noble, and V. Fleury. 2006. Dynamics of vascular branching morphogenesis: the effect of blood and tissue flow. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 73:061907. [DOI] [PubMed] [Google Scholar]
- Nishikawa, S.I., S. Nishikawa, M. Hirashima, N. Matsuyoshi, and H. Kodama. 1998. Progressive lineage analysis by cell sorting and culture identifies FLK1+VE-cadherin+ cells at a diverging point of endothelial and hemopoietic lineages. Development. 125:1747–1757. [DOI] [PubMed] [Google Scholar]
- Olsson, A.K., A. Dimberg, J. Kreuger, and L. Claesson-Welsh. 2006. VEGF receptor signalling - in control of vascular function. Nat. Rev. Mol. Cell Biol. 7:359–371. [DOI] [PubMed] [Google Scholar]
- Radice, G.L., H. Rayburn, H. Matsunami, K.A. Knudsen, M. Takeichi, and R.O. Hynes. 1997. Developmental defects in mouse embryos lacking N-cadherin. Dev. Biol. 181:64–78. [DOI] [PubMed] [Google Scholar]
- Rolny, C., I. Nilsson, P. Magnusson, A. Armulik, L. Jakobsson, P. Wentzel, P. Lindblom, J. Norlin, C. Betsholtz, R. Heuchel, et al. 2006. Platelet-derived growth factor receptor-beta promotes early endothelial cell differentiation. Blood. 108:1877–1886. [DOI] [PubMed] [Google Scholar]
- Schuh, A.C., P. Faloon, Q.L. Hu, M. Bhimani, and K. Choi. 1999. In vitro hematopoietic and endothelial potential of flk-1(−/−) embryonic stem cells and embryos. Proc. Natl. Acad. Sci. USA. 96:2159–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalaby, F., J. Rossant, T.P. Yamaguchi, M. Gertsenstein, X.F. Wu, M.L. Breitman, and A.C. Schuh. 1995. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 376:62–66. [DOI] [PubMed] [Google Scholar]
- Soriano, P. 1994. Abnormal kidney development and hematological disorders in PDGF beta-receptor mutant mice. Genes Dev. 8:1888–1896. [DOI] [PubMed] [Google Scholar]
- Thomson, J.A., J. Itskovitz-Eldor, S.S. Shapiro, M.A. Waknitz, J.J. Swiergiel, V.S. Marshall, and J.M. Jones. 1998. Embryonic stem cell lines derived from human blastocysts. Science. 282:1145–1147. [DOI] [PubMed] [Google Scholar]
- Tillet, E., D. Vittet, O. Feraud, R. Moore, R. Kemler, and P. Huber. 2005. N-cadherin deficiency impairs pericyte recruitment, and not endothelial differentiation or sprouting, in embryonic stem cell-derived angiogenesis. Exp. Cell Res. 310:392–400. [DOI] [PubMed] [Google Scholar]
- Ueno, H., and I.L. Weissman. 2006. Clonal analysis of mouse development reveals a polyclonal origin for yolk sac blood islands. Dev. Cell. 11:519–533. [DOI] [PubMed] [Google Scholar]
- Wang, Z.Z., P. Au, T. Chen, Y. Shao, L.M. Daheron, H. Bai, M. Arzigian, D. Fukumura, R.K. Jain, and D.T. Scadden. 2007. Endothelial cells derived from human embryonic stem cells form durable blood vessels in vivo. Nat. Biotechnol. 25:317–318. [DOI] [PubMed] [Google Scholar]
- Vittet, D., M.H. Prandini, R. Berthier, A. Schweitzer, H. Martin-Sisteron, G. Uzan, and E. Dejana. 1996. Embryonic stem cells differentiate in vitro to endothelial cells through successive maturation steps. Blood. 88:3424–3431. [PubMed] [Google Scholar]
- Vittet, D., T. Buchou, A. Schweitzer, E. Dejana, and P. Huber. 1997. Targeted null-mutation in the vascular endothelial-cadherin gene impairs the organization of vascular-like structures in embryoid bodies. Proc. Natl. Acad. Sci. USA. 94:6273–6278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurugi-Kobayashi, T., H. Itoh, J. Yamashita, K. Yamahara, H. Hirai, T. Kobayashi, M. Ogawa, S. Nishikawa, and K. Nakao. 2003. Effective contribution of transplanted vascular progenitor cells derived from embryonic stem cells to adult neovascularization in proper differentiation stage. Blood. 101:2675–2678. [DOI] [PubMed] [Google Scholar]
- Zaehres, H., M.W. Lensch, L. Daheron, S.A. Stewart, J. Itskovitz-Eldor, and G.Q. Daley. 2005. High-efficiency RNA interference in human embryonic stem cells. Stem Cells. 23:299–305. [DOI] [PubMed] [Google Scholar]
- Zhang, L., R. Yang, and Z.C. Han. 2006. Transplantation of umbilical cord blood-derived endothelial progenitor cells: a promising method of therapeutic revascularisation. Eur. J. Haematol. 76:1–8. [DOI] [PubMed] [Google Scholar]
- Zwaka, T.P., and J.A. Thomson. 2003. Homologous recombination in human embryonic stem cells. Nat. Biotechnol. 21:319–321. [DOI] [PubMed] [Google Scholar]