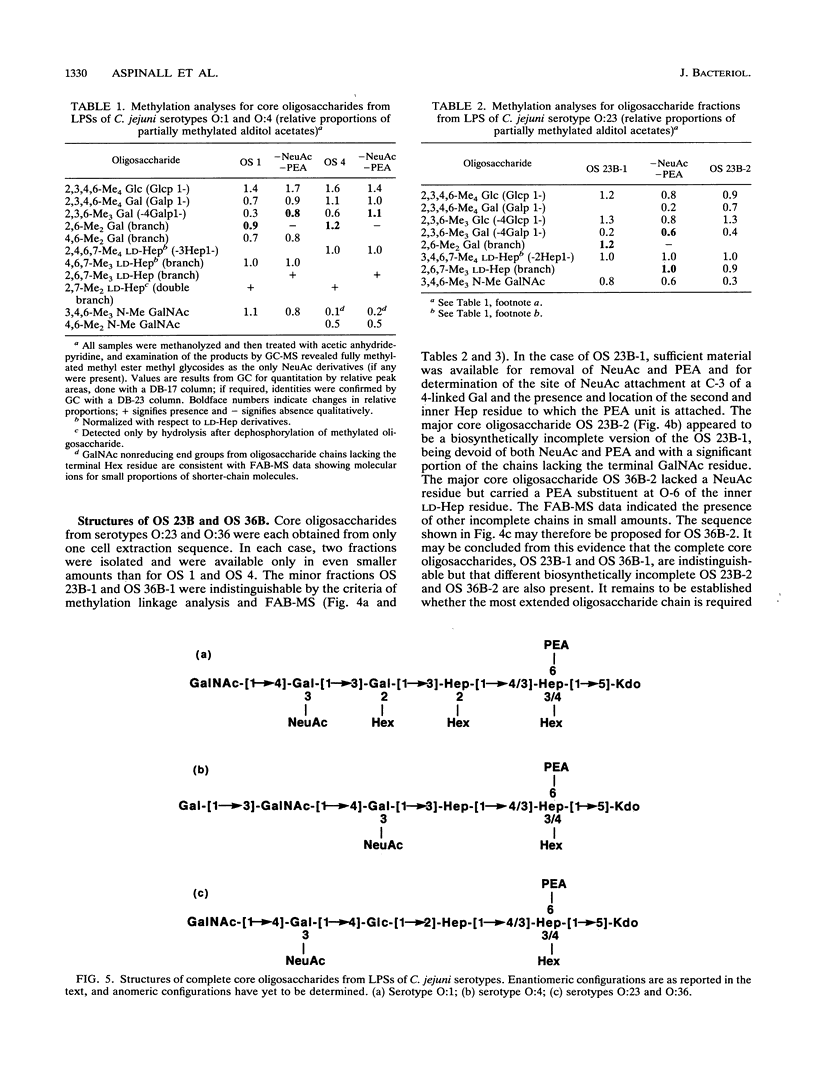
Abstract
Low-Mr lipopolysaccharides (LPS) of Campylobacter jejuni reference strains for serotypes O:1, O:4, O:23, and O:36 were examined through the liberation of core oligosaccharides by mild acid cleavage of the ketosidic linkage of 3-deoxy-D-manno-2-octulosonic acid residues to the lipid A moiety. The liberated oligosaccharides were examined for chemical structure by compositional analysis and methylated linkage analysis in conjunction with fast atom bombardment-mass spectrometry of permethylated oligosaccharide derivatives. The results showed (i) that the LPS contained short oligosaccharide chains of branched nonrepetitive structure, to many of which N-acetylneuraminic acid residues remained attached by 2----3 linkages to 4-linked D-galactose residues in the core structure; (ii) that serotypical differences, which are not readily defined through qualitatively similar compositions, are clearly reflected in variations in linkage types and sequences of sugar residues in the outer core attached to an inner region of invariable structure; but (iii) that the presence or absence of NeuAc residues does not appear to be a basis for serotypical differences. The results also showed that oligosaccharide chains from LPS of serotypes O:1 and O:4 are distinctly different and are distinct again from those of the cross-reacting serotypes O:23 and O:36, between whose core oligosaccharide chains no differences were found. It is concluded that the structurally variable low-Mr LPS from C. jejuni show greater similarities to the lipooligosaccharides from Neisseria spp. than to the highly conserved core regions of Salmonella species. Those strains (serotypes O:23 and O:36) which also furnish high-Mr LPS are unique among gram-negative bacteria in possessing both low-Mr molecules of the Neisseria lipooligosaccharide type and high-Mr LPS of the Salmonella smooth type.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beer W., Adam M., Seltmann G. Monosaccharide composition of lipopolysaccharides from Campylobacter jejuni and Campylobacter coli. J Basic Microbiol. 1986;26(4):201–204. doi: 10.1002/jobm.3620260405. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee A. K., Jennings H. J. Determination of the linkages in some methylated, sialic acid-containing, meningococcal polysaccharides by mass spectrometry. Carbohydr Res. 1976 Nov;51(2):253–261. doi: 10.1016/s0008-6215(00)83333-9. [DOI] [PubMed] [Google Scholar]
- Dawson C. R., Jones D. B., Kaufman H. E., Barron B. A., Hauck W. W., Wilhelmus K. R. Design and organization of the herpetic eye disease study (HEDS). Curr Eye Res. 1991;10 (Suppl):105–110. doi: 10.3109/02713689109020365. [DOI] [PubMed] [Google Scholar]
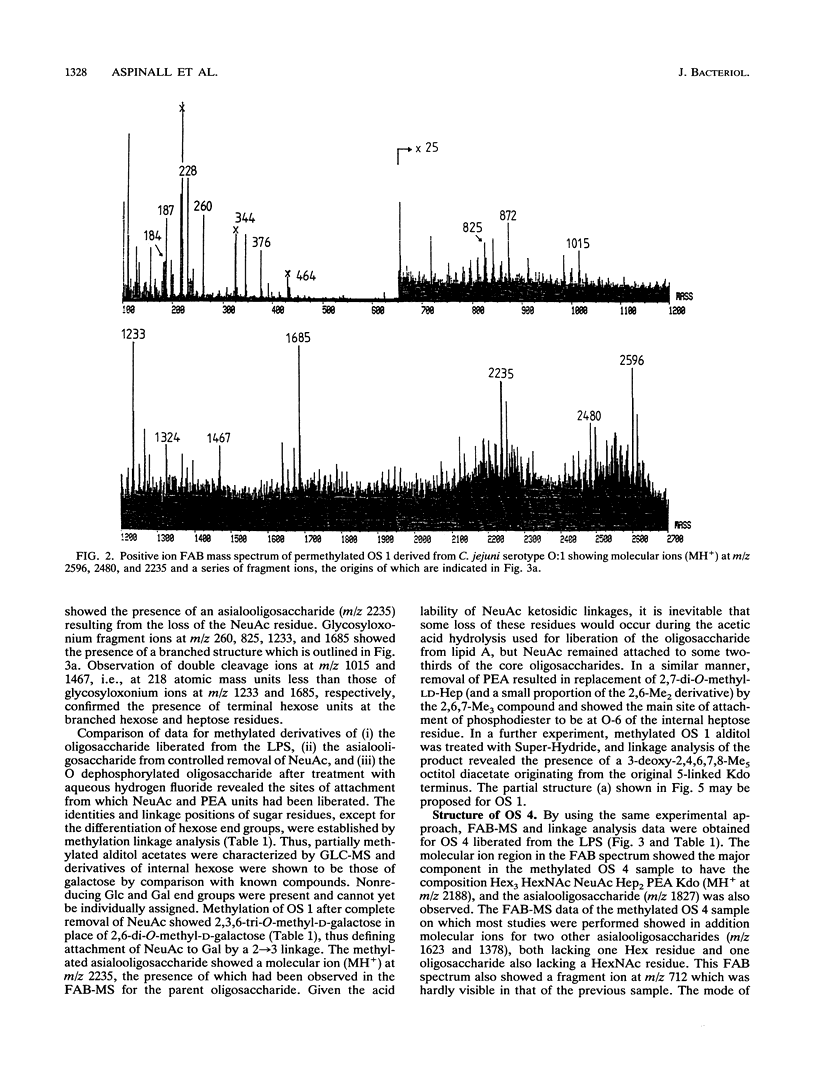
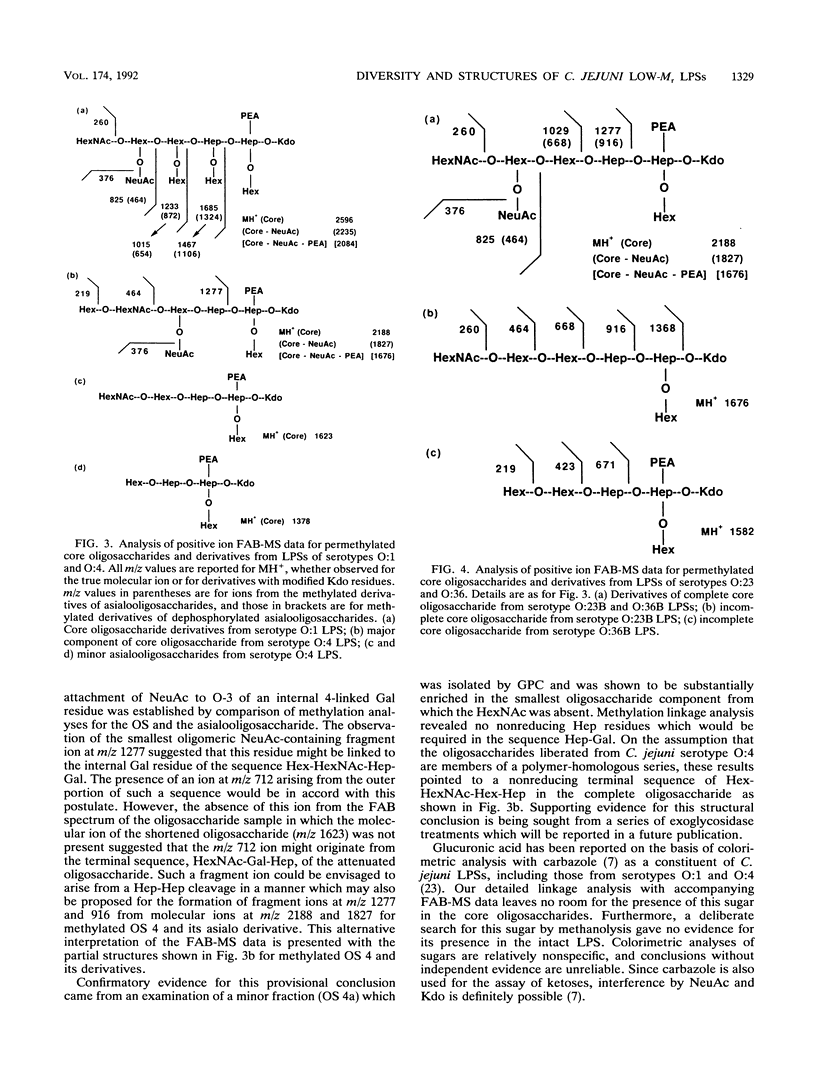
- Dell A., Azadi P., Tiller P., Thomas-Oates J., Jennings H. J., Beurret M., Michon F. Analysis of oligosaccharide epitopes of meningococcal lipopolysaccharides by fast-atom-bombardment mass spectrometry. Carbohydr Res. 1990 Apr 25;200:59–76. doi: 10.1016/0008-6215(90)84182-t. [DOI] [PubMed] [Google Scholar]
- Griffiss J. M., O'Brien J. P., Yamasaki R., Williams G. D., Rice P. A., Schneider H. Physical heterogeneity of neisserial lipooligosaccharides reflects oligosaccharides that differ in apparent molecular weight, chemical composition, and antigenic expression. Infect Immun. 1987 Aug;55(8):1792–1800. doi: 10.1128/iai.55.8.1792-1800.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
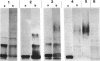
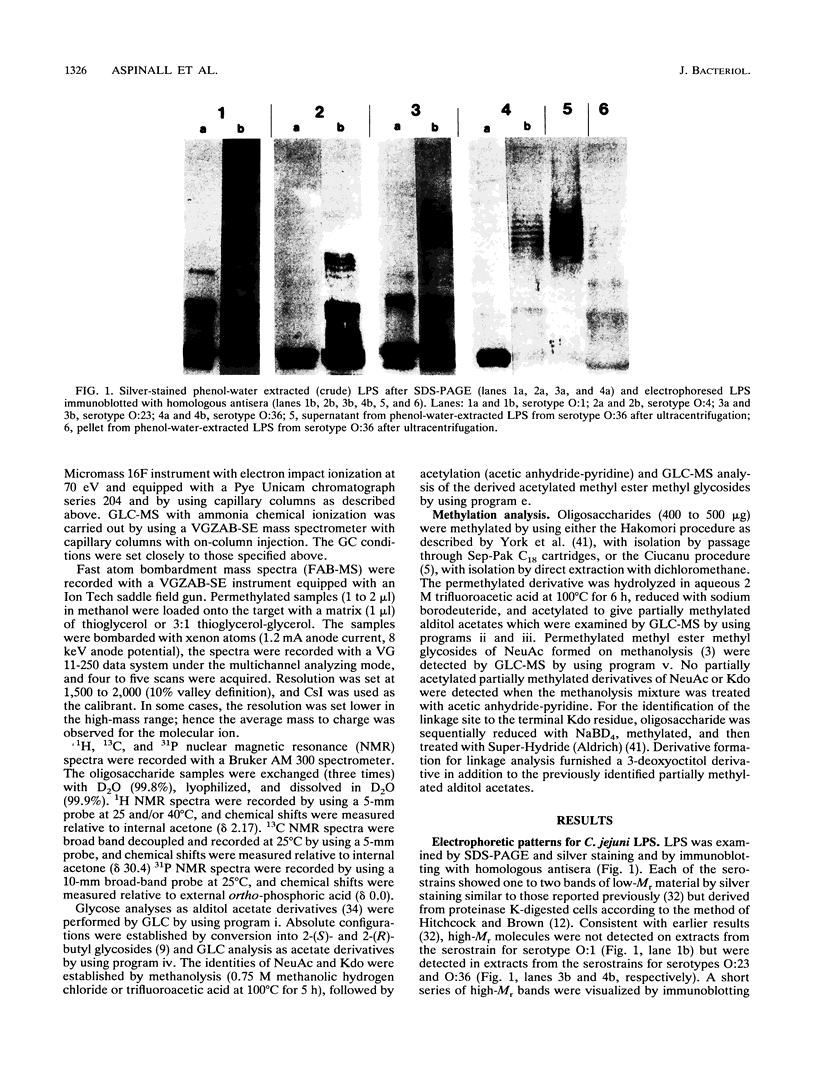
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inzana T. J., Seifert W. E., Jr, Williams R. P. Composition and antigenic activity of the oligosaccharide moiety of Haemophilus influenzae type b lipooligosaccharide. Infect Immun. 1985 May;48(2):324–330. doi: 10.1128/iai.48.2.324-330.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janda J., Work E. A colorimetric estimation of lipopolysaccharides. FEBS Lett. 1971 Sep 1;16(4):343–345. doi: 10.1016/0014-5793(71)80386-1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Logan S. M., Trust T. J. Structural and antigenic heterogeneity of lipopolysaccharides of Campylobacter jejuni and Campylobacter coli. Infect Immun. 1984 Jul;45(1):210–216. doi: 10.1128/iai.45.1.210-216.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrell R. E., Kim J. J., John C. M., Gibson B. W., Sugai J. V., Apicella M. A., Griffiss J. M., Yamasaki R. Endogenous sialylation of the lipooligosaccharides of Neisseria meningitidis. J Bacteriol. 1991 May;173(9):2823–2832. doi: 10.1128/jb.173.9.2823-2832.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrell R., Schneider H., Apicella M., Zollinger W., Rice P. A., Griffiss J. M. Antigenic and physical diversity of Neisseria gonorrhoeae lipooligosaccharides. Infect Immun. 1986 Oct;54(1):63–69. doi: 10.1128/iai.54.1.63-69.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills S. D., Bradbury W. C., Penner J. L. Basis for serological heterogeneity of thermostable antigens of Campylobacter jejuni. Infect Immun. 1985 Oct;50(1):284–291. doi: 10.1128/iai.50.1.284-291.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran A. P., Rietschel E. T., Kosunen T. U., Zähringer U. Chemical characterization of Campylobacter jejuni lipopolysaccharides containing N-acetylneuraminic acid and 2,3-diamino-2,3-dideoxy-D-glucose. J Bacteriol. 1991 Jan;173(2):618–626. doi: 10.1128/jb.173.2.618-626.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naess V., Hofstad T. Chemical composition and biological activity of lipopolysaccharides prepared from type strains of Campylobacter jejuni and Campolybacter coli. Acta Pathol Microbiol Immunol Scand B. 1984 Aug;92(4):217–222. doi: 10.1111/j.1699-0463.1984.tb02824.x. [DOI] [PubMed] [Google Scholar]
- Naess V., Hofstad T. Chemical studies of partially hydrolysed lipopolysaccharides from four strains of Campylobacter jejuni and two strains of Campylobacter coli. J Gen Microbiol. 1984 Nov;130(11):2783–2789. doi: 10.1099/00221287-130-11-2783. [DOI] [PubMed] [Google Scholar]
- Penner J. L., Hennessy J. N., Congi R. V. Serotyping of Campylobacter jejuni and Campylobacter coli on the basis of thermostable antigens. Eur J Clin Microbiol. 1983 Aug;2(4):378–383. doi: 10.1007/BF02019474. [DOI] [PubMed] [Google Scholar]
- Penner J. L., Hennessy J. N. Passive hemagglutination technique for serotyping Campylobacter fetus subsp. jejuni on the basis of soluble heat-stable antigens. J Clin Microbiol. 1980 Dec;12(6):732–737. doi: 10.1128/jcm.12.6.732-737.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penner J. L. The genus Campylobacter: a decade of progress. Clin Microbiol Rev. 1988 Apr;1(2):157–172. doi: 10.1128/cmr.1.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez Perez G. I., Blaser M. J. Lipopolysaccharide characteristics of pathogenic campylobacters. Infect Immun. 1985 Feb;47(2):353–359. doi: 10.1128/iai.47.2.353-359.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez G. I., Hopkins J. A., Blaser M. J. Antigenic heterogeneity of lipopolysaccharides from Campylobacter jejuni and Campylobacter fetus. Infect Immun. 1985 May;48(2):528–533. doi: 10.1128/iai.48.2.528-533.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips N. J., John C. M., Reinders L. G., Gibson B. W., Apicella M. A., Griffiss J. M. Structural models for the cell surface lipooligosaccharides of Neisseria gonorrhoeae and Haemophilus influenzae. Biomed Environ Mass Spectrom. 1990 Nov;19(11):731–745. doi: 10.1002/bms.1200191112. [DOI] [PubMed] [Google Scholar]
- Preston M. A., Penner J. L. Characterization of cross-reacting serotypes of Campylobacter jejuni. Can J Microbiol. 1989 Feb;35(2):265–273. doi: 10.1139/m89-040. [DOI] [PubMed] [Google Scholar]
- Preston M. A., Penner J. L. Structural and antigenic properties of lipopolysaccharides from serotype reference strains of Campylobacter jejuni. Infect Immun. 1987 Aug;55(8):1806–1812. doi: 10.1128/iai.55.8.1806-1812.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skirrow M. B. Campylobacter enteritis: a "new" disease. Br Med J. 1977 Jul 2;2(6078):9–11. doi: 10.1136/bmj.2.6078.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. Pathogenicity and the microbe in vivo. The 1989 Fred Griffith Review Lecture. J Gen Microbiol. 1990 Mar;136(3):377–393. doi: 10.1099/00221287-136-3-377. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Yao K., Ubuka T., Masuoka N., Kinuta M., Ikeda T. Direct determination of bound sialic acids in sialoglycoproteins by acidic ninhydrin reaction. Anal Biochem. 1989 Jun;179(2):332–335. doi: 10.1016/0003-2697(89)90138-3. [DOI] [PubMed] [Google Scholar]