Abstract
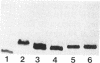
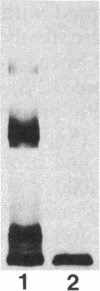
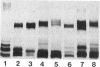
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis was used to analyze the macromolecular heterogeneity of lipopolysaccharides (LPS) from seven fresh clinical isolates and three culture collection strains of the human pathogen Helicobacter pylori. All the clinical isolates produced smooth-form LPS with O side chains of relatively homogeneous chain length, whereas the culture collection strains yielded rough-form LPS. A better yield of the latter LPS was obtained when combined protease pretreatment and hot phenol-water extraction were used than when the conventional phenol-water technique alone was used for extraction. The LPS of the three culture collection strains (S-24, C-5437, and NCTC 11637) were chemically characterized. Constituents common to all the LPS were fucose, D-mannose, D-glucose, D-galactose, D-glycero-D-manno-heptose, L-glycero-D-manno-heptose, and 3-deoxy-D-manno-2-octulosonic acid. The molar ratios of the hexoses differed between different strains, thereby reflecting structural differences. Phosphate, phosphorylethanolamine, and pyrophosphorylethanolamine were present also. Free lipid A contained D-glucosamine and fatty acids, with phosphate and a minor amount of ethanolamine. The major fatty acids were ester- and amide-bound 3-hydroxyoctadecanoic acid and ester-bound octadecanioc and 3-hydroxyhexadecanoic acids, with minor amounts of ester-bound tetradecanoic and hexadecanoic acids. In addition to the uncommonly long 3-hydroxy fatty acids, an unusual phosphorylation pattern was deduced to be present in the lipid A.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Blaser M. J. Epidemiology and pathophysiology of Campylobacter pylori infections. Rev Infect Dis. 1990 Jan-Feb;12 (Suppl 1):S99–106. doi: 10.1093/clinids/12.supplement_1.s99. [DOI] [PubMed] [Google Scholar]
- Brade H., Brade L., Rietschel E. T. Structure-activity relationships of bacterial lipopolysaccharides (endotoxins). Current and future aspects. Zentralbl Bakteriol Mikrobiol Hyg A. 1988 Apr;268(2):151–179. doi: 10.1016/s0176-6724(88)80001-4. [DOI] [PubMed] [Google Scholar]
- Brade H., Galanos C. A method to detect 2-keto-3-deoxyoctanat and related compounds on pherograms and chromatograms. Anal Biochem. 1983 Jul 1;132(1):158–159. doi: 10.1016/0003-2697(83)90440-2. [DOI] [PubMed] [Google Scholar]
- Chester I. R., Murray R. G. Analysis of the cell wall and lipopolysaccharide of Spirillum serpens. J Bacteriol. 1975 Dec;124(3):1168–1176. doi: 10.1128/jb.124.3.1168-1176.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley J. S., Lallier R., Shaw D. H., Trust T. J. Electrophoretic and immunochemical analyses of the lipopolysaccharides from various strains of Aeromonas hydrophila. J Bacteriol. 1985 Oct;164(1):263–269. doi: 10.1128/jb.164.1.263-269.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Geis G., Leying H., Suerbaum S., Opferkuch W. Unusual fatty acid substitution in lipids and lipopolysaccharides of Helicobacter pylori. J Clin Microbiol. 1990 May;28(5):930–932. doi: 10.1128/jcm.28.5.930-932.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANES C. S., ISHERWOOD F. A. Separation of the phosphoric esters on the filter paper chromatogram. Nature. 1949 Dec 31;164(4183):1107-12, illust. doi: 10.1038/1641107a0. [DOI] [PubMed] [Google Scholar]
- Hase S., Rietschel E. T. Isolation and analysis of the lipid A backbone. Lipid A structure of lipopolysaccharides from various bacterial groups. Eur J Biochem. 1976 Mar 16;63(1):101–107. doi: 10.1111/j.1432-1033.1976.tb10212.x. [DOI] [PubMed] [Google Scholar]
- Helander I. M., Kitunen V. Cleavage of the O antigen 4, 5, 12 of Salmonella typhimurium by hydrofluoric acid. FEBS Lett. 1989 Jul 3;250(2):565–569. doi: 10.1016/0014-5793(89)80797-5. [DOI] [PubMed] [Google Scholar]
- Helander I. M., Lindner B., Brade H., Altmann K., Lindberg A. A., Rietschel E. T., Zähringer U. Chemical structure of the lipopolysaccharide of Haemophilus influenzae strain I-69 Rd-/b+. Description of a novel deep-rough chemotype. Eur J Biochem. 1988 Nov 15;177(3):483–492. doi: 10.1111/j.1432-1033.1988.tb14398.x. [DOI] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingsworth R. I., Lill-Elghanian D. A. Isolation and characterization of the unusual lipopolysaccharide component, 2-amino-2-deoxy-2-N-(27-hydroxyoctacosanoyl)-3-O-(3-hydroxy- tetradecanoyl)-gluco-hexuronic acid, and its de-O-acylation product from the free lipid A of Rhizobium trifolii ANU843. J Biol Chem. 1989 Aug 25;264(24):14039–14042. [PubMed] [Google Scholar]
- LOWRY O. H., ROBERTS N. R., LEINER K. Y., WU M. L., FARR A. L. The quantitative histochemistry of brain. I. Chemical methods. J Biol Chem. 1954 Mar;207(1):1–17. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marshall B. J., Warren J. R. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984 Jun 16;1(8390):1311–1315. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- Mayer H., Krauss J. H., Yokota A., Weckesser J. Natural variants of lipid A. Adv Exp Med Biol. 1990;256:45–70. doi: 10.1007/978-1-4757-5140-6_3. [DOI] [PubMed] [Google Scholar]
- Mills S. D., Bradbury W. C., Penner J. L. Basis for serological heterogeneity of thermostable antigens of Campylobacter jejuni. Infect Immun. 1985 Oct;50(1):284–291. doi: 10.1128/iai.50.1.284-291.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran A. P., Rietschel E. T., Kosunen T. U., Zähringer U. Chemical characterization of Campylobacter jejuni lipopolysaccharides containing N-acetylneuraminic acid and 2,3-diamino-2,3-dideoxy-D-glucose. J Bacteriol. 1991 Jan;173(2):618–626. doi: 10.1128/jb.173.2.618-626.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran A. P., Zähringer U., Seydel U., Scholz D., Stütz P., Rietschel E. T. Structural analysis of the lipid A component of Campylobacter jejuni CCUG 10936 (serotype O:2) lipopolysaccharide. Description of a lipid A containing a hybrid backbone of 2-amino-2-deoxy-D-glucose and 2,3-diamino-2,3-dideoxy-D-glucose. Eur J Biochem. 1991 Jun 1;198(2):459–469. doi: 10.1111/j.1432-1033.1991.tb16036.x. [DOI] [PubMed] [Google Scholar]
- Palva E. T., Mäkelä P. H. Lipopolysaccharide heterogeneity in Salmonella typhimurium analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis. Eur J Biochem. 1980;107(1):137–143. doi: 10.1111/j.1432-1033.1980.tb04634.x. [DOI] [PubMed] [Google Scholar]
- Perez-Perez G. I., Blaser M. J. Conservation and diversity of Campylobacter pyloridis major antigens. Infect Immun. 1987 May;55(5):1256–1263. doi: 10.1128/iai.55.5.1256-1263.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston M. A., Penner J. L. Structural and antigenic properties of lipopolysaccharides from serotype reference strains of Campylobacter jejuni. Infect Immun. 1987 Aug;55(8):1806–1812. doi: 10.1128/iai.55.8.1806-1812.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetz C. R. Biochemistry of endotoxins. Annu Rev Biochem. 1990;59:129–170. doi: 10.1146/annurev.bi.59.070190.001021. [DOI] [PubMed] [Google Scholar]
- Rauws E. A., Tytgat G. N. Cure of duodenal ulcer associated with eradication of Helicobacter pylori. Lancet. 1990 May 26;335(8700):1233–1235. doi: 10.1016/0140-6736(90)91301-p. [DOI] [PubMed] [Google Scholar]
- Rietschel E. T. Absolute configuration of 3-hydroxy fatty acids present in lipopolysaccharides from various bacterial groups. Eur J Biochem. 1976 May 1;64(2):423–428. doi: 10.1111/j.1432-1033.1976.tb10318.x. [DOI] [PubMed] [Google Scholar]
- Rietschel E. T., Brade L., Brandenburg K., Flad H. D., de Jong-Leuveninck J., Kawahara K., Lindner B., Loppnow H., Lüderitz T., Schade U. Chemical structure and biologic activity of bacterial and synthetic lipid A. Rev Infect Dis. 1987 Sep-Oct;9 (Suppl 5):S527–S536. doi: 10.1093/clinids/9.supplement_5.s527. [DOI] [PubMed] [Google Scholar]
- Rietschel E. T., Gottert H., Lüderitz O., Westphal O. Nature and linkages of the fatty acids present in the lipid-A component of Salmonella lipopolysaccharides. Eur J Biochem. 1972 Jul 13;28(2):166–173. doi: 10.1111/j.1432-1033.1972.tb01899.x. [DOI] [PubMed] [Google Scholar]
- STROMINGER J. L., PARK J. T., THOMPSON R. E. Composition of the cell wall of Staphylococcus aureus: its relation to the mechanism of action of penicillin. J Biol Chem. 1959 Dec;234:3263–3268. [PubMed] [Google Scholar]
- Sleytr U. B., Messner P. Crystalline surface layers in procaryotes. J Bacteriol. 1988 Jul;170(7):2891–2897. doi: 10.1128/jb.170.7.2891-2897.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonesson A., Jantzen E., Bryn K., Larsson L., Eng J. Chemical composition of a lipopolysaccharide from Legionella pneumophila. Arch Microbiol. 1989;153(1):72–78. doi: 10.1007/BF00277544. [DOI] [PubMed] [Google Scholar]
- Sukupolvi S., Vaara M., Helander I. M., Viljanen P., Mäkelä P. H. New Salmonella typhimurium mutants with altered outer membrane permeability. J Bacteriol. 1984 Aug;159(2):704–712. doi: 10.1128/jb.159.2.704-712.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surcel H. M., Sarvas M., Helander I. M., Herva E. Membrane proteins of Francisella tularensis LVS differ in ability to induce proliferation of lymphocytes from tularemia-vaccinated individuals. Microb Pathog. 1989 Dec;7(6):411–419. doi: 10.1016/0882-4010(89)90021-1. [DOI] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- WARAVDEKAR V. S., SASLAW L. D. A sensitive colorimetric method for the estimation of 2-deoxy sugars with the use of the malonaldehyde-thiobarbituric acid reaction. J Biol Chem. 1959 Aug;234(8):1945–1950. [PubMed] [Google Scholar]
- Weckesser J., Mayer H. Different lipid A types in lipopolysaccharides of phototrophic and related non-phototrophic bacteria. FEMS Microbiol Rev. 1988 Apr-Jun;4(2):143–153. doi: 10.1111/j.1574-6968.1988.tb02740.x. [DOI] [PubMed] [Google Scholar]
- Weintraub A., Zähringer U., Wollenweber H. W., Seydel U., Rietschel E. T. Structural characterization of the lipid A component of Bacteroides fragilis strain NCTC 9343 lipopolysaccharide. Eur J Biochem. 1989 Aug 1;183(2):425–431. doi: 10.1111/j.1432-1033.1989.tb14945.x. [DOI] [PubMed] [Google Scholar]
- Wollenweber H. W., Schramek S., Moll H., Rietschel E. T. Nature and linkage type of fatty acids present in lipopolysaccharides of phase I and II Coxiella burnetii. Arch Microbiol. 1985 Jun;142(1):6–11. doi: 10.1007/BF00409228. [DOI] [PubMed] [Google Scholar]