Abstract
Serratia marcescens W225 expresses an unconventional iron(III) transport system. Uptake of Fe3+ occurs in the absence of an iron(III)-solubilizing siderophore, of an outer membrane receptor protein, and of the TonB and ExbBD proteins involved in outer membrane transport. The three SfuABC proteins found to catalyze iron(III) transport exhibit the typical features of periplasmic binding-protein-dependent systems for transport across the cytoplasmic membrane. In support of these conclusions, the periplasmic SfuA protein bound iron chloride and iron citrate but not ferrichrome, as shown by protection experiments against degradation by added V8 protease. The cloned sfuABC genes conferred upon an Escherichia coli aroB mutant unable to synthesize its own enterochelin siderophore the ability to grow under iron-limiting conditions (in the presence of 0.2 mM 2.2'-dipyridyl). Under extreme iron deficiency (0.4 mM 2.2'-dipyridyl), however, the entry rate of iron across the outer membrane was no longer sufficient for growth. Citrate had to be added in order for iron(III) to be translocated as an iron citrate complex in a FecA- and TonB-dependent manner through the outer membrane and via SfuABC across the cytoplasmic membrane. FecA- and TonB-dependent iron transport across the outer membrane could be clearly correlated with a very low concentration of iron in the medium. Expression of the sfuABC genes in E. coli was controlled by the Fur iron repressor gene. S. marcescens W225 was able to synthesize enterochelin and take up iron(III) enterochelin. It contained an iron(III) aerobactin transport system but lacked aerobactin synthesis. This strain was able to utilize the hydroxamate siderophores ferrichrome, coprogen, ferrioxamine B, rhodotorulic acid, and schizokinen as sole iron sources and grew on iron citrate as well. In contrast to E. coli K-12, S. marcescens could utilize heme. DNA fragments of the E. coli fhuA, iut, exbB, and fur genes hybridized with chromosomal S. marcescens DNA fragments, whereas no hybridization was obtained between S. marcescens chromosomal DNA and E. coli fecA, fhuE, and tonB gene fragments. The presence of multiple iron transport systems was also indicated by the increased synthesis of at least five outer membrane proteins (in the molecular weight range of 72,000 to 87,000) after growth in low-iron media. Serratia liquefaciens and Serratia ficaria produced aerobactin, showing that this siderophore also occurs in the genus Serratia.
Full text
PDF

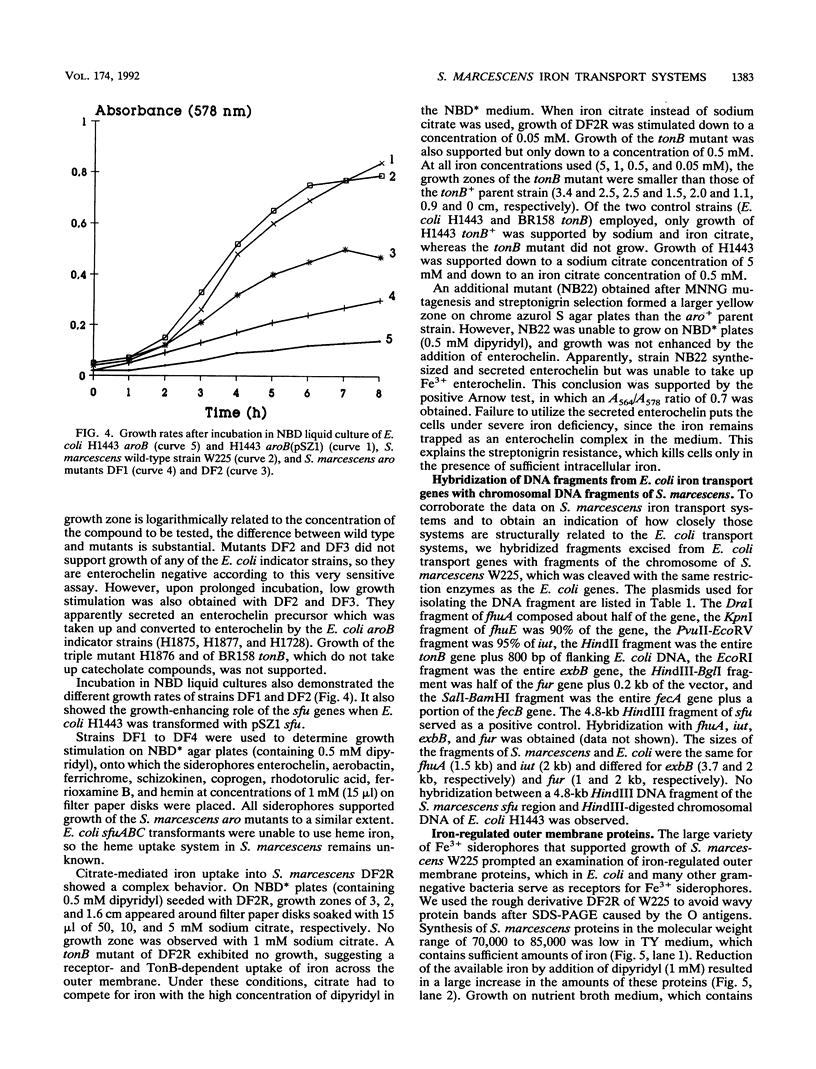




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F., Joshi A. K. Energy coupling in bacterial periplasmic permeases. J Bacteriol. 1990 Aug;172(8):4133–4137. doi: 10.1128/jb.172.8.4133-4137.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames G. F., Mimura C. S., Shyamala V. Bacterial periplasmic permeases belong to a family of transport proteins operating from Escherichia coli to human: Traffic ATPases. FEMS Microbiol Rev. 1990 Aug;6(4):429–446. doi: 10.1111/j.1574-6968.1990.tb04110.x. [DOI] [PubMed] [Google Scholar]
- Angerer A., Gaisser S., Braun V. Nucleotide sequences of the sfuA, sfuB, and sfuC genes of Serratia marcescens suggest a periplasmic-binding-protein-dependent iron transport mechanism. J Bacteriol. 1990 Feb;172(2):572–578. doi: 10.1128/jb.172.2.572-578.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop L., Agbayani R., Jr, Ambudkar S. V., Maloney P. C., Ames G. F. Reconstitution of a bacterial periplasmic permease in proteoliposomes and demonstration of ATP hydrolysis concomitant with transport. Proc Natl Acad Sci U S A. 1989 Sep;86(18):6953–6957. doi: 10.1073/pnas.86.18.6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitter W., Marugg J. D., de Weger L. A., Tommassen J., Weisbeek P. J. The ferric-pseudobactin receptor PupA of Pseudomonas putida WCS358: homology to TonB-dependent Escherichia coli receptors and specificity of the protein. Mol Microbiol. 1991 Mar;5(3):647–655. doi: 10.1111/j.1365-2958.1991.tb00736.x. [DOI] [PubMed] [Google Scholar]
- Braun V., Gross R., Köster W., Zimmermann L. Plasmid and chromosomal mutants in the iron(III)-aerobactin transport system of Escherichia coli. Use of streptonigrin for selection. Mol Gen Genet. 1983;192(1-2):131–139. doi: 10.1007/BF00327658. [DOI] [PubMed] [Google Scholar]
- Braun V., Günter K., Hantke K. Transport of iron across the outer membrane. Biol Met. 1991;4(1):14–22. doi: 10.1007/BF01135552. [DOI] [PubMed] [Google Scholar]
- Burkhardt R., Braun V. Nucleotide sequence of the fhuC and fhuD genes involved in iron (III) hydroxamate transport: domains in FhuC homologous to ATP-binding proteins. Mol Gen Genet. 1987 Aug;209(1):49–55. doi: 10.1007/BF00329835. [DOI] [PubMed] [Google Scholar]
- Coulton J. W., Mason P., Allatt D. D. fhuC and fhuD genes for iron (III)-ferrichrome transport into Escherichia coli K-12. J Bacteriol. 1987 Aug;169(8):3844–3849. doi: 10.1128/jb.169.8.3844-3849.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosa J. H. Genetics and molecular biology of siderophore-mediated iron transport in bacteria. Microbiol Rev. 1989 Dec;53(4):517–530. doi: 10.1128/mr.53.4.517-530.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis N. A., Eisenstadt R. L., East S. J., Cornford R. J., Walker L. A., White A. J. Iron-regulated outer membrane proteins of Escherichia coli K-12 and mechanism of action of catechol-substituted cephalosporins. Antimicrob Agents Chemother. 1988 Dec;32(12):1879–1886. doi: 10.1128/aac.32.12.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean D. A., Davidson A. L., Nikaido H. Maltose transport in membrane vesicles of Escherichia coli is linked to ATP hydrolysis. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9134–9138. doi: 10.1073/pnas.86.23.9134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eick-Helmerich K., Braun V. Import of biopolymers into Escherichia coli: nucleotide sequences of the exbB and exbD genes are homologous to those of the tolQ and tolR genes, respectively. J Bacteriol. 1989 Sep;171(9):5117–5126. doi: 10.1128/jb.171.9.5117-5126.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eick-Helmerich K., Hantke K., Braun V. Cloning and expression of the exbB gene of Escherichia coli K-12. Mol Gen Genet. 1987 Feb;206(2):246–251. doi: 10.1007/BF00333580. [DOI] [PubMed] [Google Scholar]
- Fecker L., Braun V. Cloning and expression of the fhu genes involved in iron(III)-hydroxamate uptake by Escherichia coli. J Bacteriol. 1983 Dec;156(3):1301–1314. doi: 10.1128/jb.156.3.1301-1314.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer E., Günter K., Braun V. Involvement of ExbB and TonB in transport across the outer membrane of Escherichia coli: phenotypic complementation of exb mutants by overexpressed tonB and physical stabilization of TonB by ExbB. J Bacteriol. 1989 Sep;171(9):5127–5134. doi: 10.1128/jb.171.9.5127-5134.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaisser S., Braun V. The tonB gene of Serratia marcescens: sequence, activity and partial complementation of Escherichia coli tonB mutants. Mol Microbiol. 1991 Nov;5(11):2777–2787. doi: 10.1111/j.1365-2958.1991.tb01986.x. [DOI] [PubMed] [Google Scholar]
- Gross R., Engelbrecht F., Braun V. Genetic and biochemical characterization of the aerobactin synthesis operon on pColV. Mol Gen Genet. 1984;196(1):74–80. doi: 10.1007/BF00334095. [DOI] [PubMed] [Google Scholar]
- Gross R., Engelbrecht F., Braun V. Identification of the genes and their polypeptide products responsible for aerobactin synthesis by pColV plasmids. Mol Gen Genet. 1985;201(2):204–212. doi: 10.1007/BF00425661. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Hantke K., Braun V. Iron transport in Escherichia coli K-12. 2,3-Dihydroxybenzoate-promoted iron uptake. Arch Microbiol. 1977 Sep 28;114(3):231–239. doi: 10.1007/BF00446867. [DOI] [PubMed] [Google Scholar]
- Hannavy K., Barr G. C., Dorman C. J., Adamson J., Mazengera L. R., Gallagher M. P., Evans J. S., Levine B. A., Trayer I. P., Higgins C. F. TonB protein of Salmonella typhimurium. A model for signal transduction between membranes. J Mol Biol. 1990 Dec 20;216(4):897–910. doi: 10.1016/S0022-2836(99)80009-6. [DOI] [PubMed] [Google Scholar]
- Hantke K. Dihydroxybenzoylserine--a siderophore for E. coli. FEMS Microbiol Lett. 1990 Jan 15;55(1-2):5–8. doi: 10.1016/0378-1097(90)90158-m. [DOI] [PubMed] [Google Scholar]
- Heidinger S., Braun V., Pecoraro V. L., Raymond K. N. Iron supply to Escherichia coli by synthetic analogs of enterochelin. J Bacteriol. 1983 Jan;153(1):109–115. doi: 10.1128/jb.153.1.109-115.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller K. J., Kadner R. J., Günther K. Suppression of the btuB451 mutation by mutations in the tonB gene suggests a direct interaction between TonB and TonB-dependent receptor proteins in the outer membrane of Escherichia coli. Gene. 1988 Apr 15;64(1):147–153. doi: 10.1016/0378-1119(88)90488-x. [DOI] [PubMed] [Google Scholar]
- Hussein S., Hantke K., Braun V. Citrate-dependent iron transport system in Escherichia coli K-12. Eur J Biochem. 1981 Jul;117(2):431–437. doi: 10.1111/j.1432-1033.1981.tb06357.x. [DOI] [PubMed] [Google Scholar]
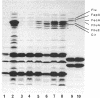
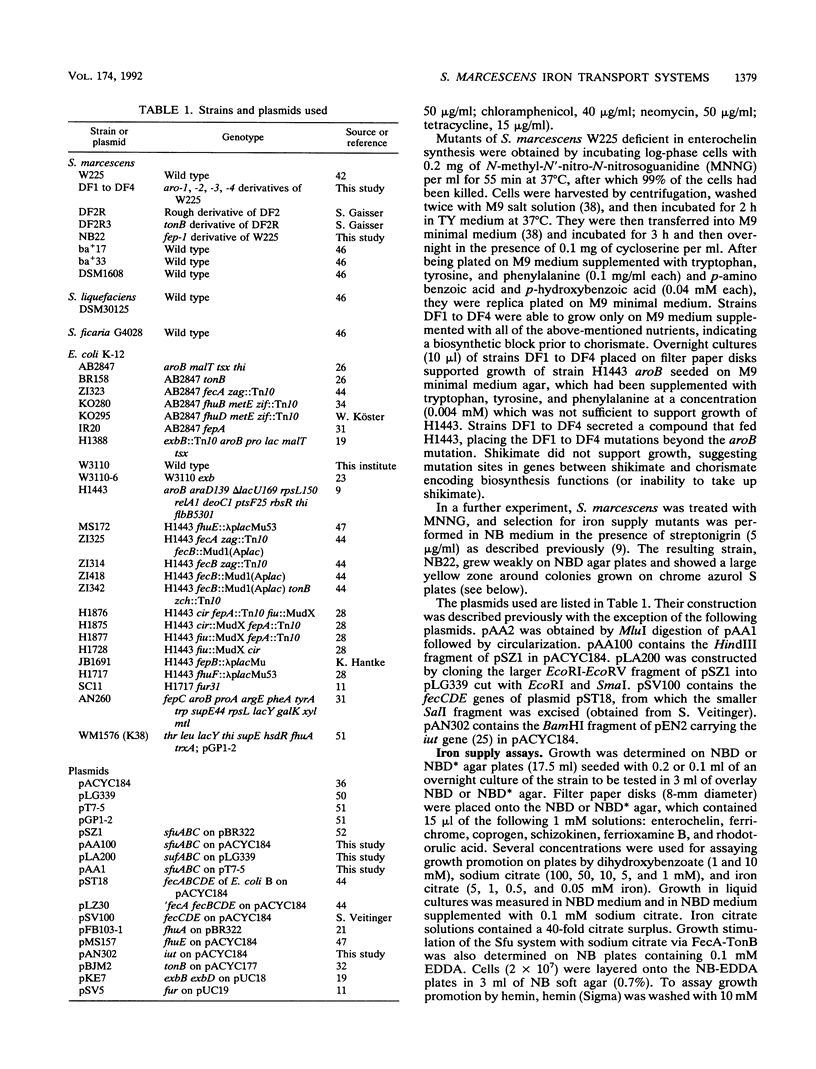
- Köster W., Braun V. Iron (III) hydroxamate transport into Escherichia coli. Substrate binding to the periplasmic FhuD protein. J Biol Chem. 1990 Dec 15;265(35):21407–21410. [PubMed] [Google Scholar]
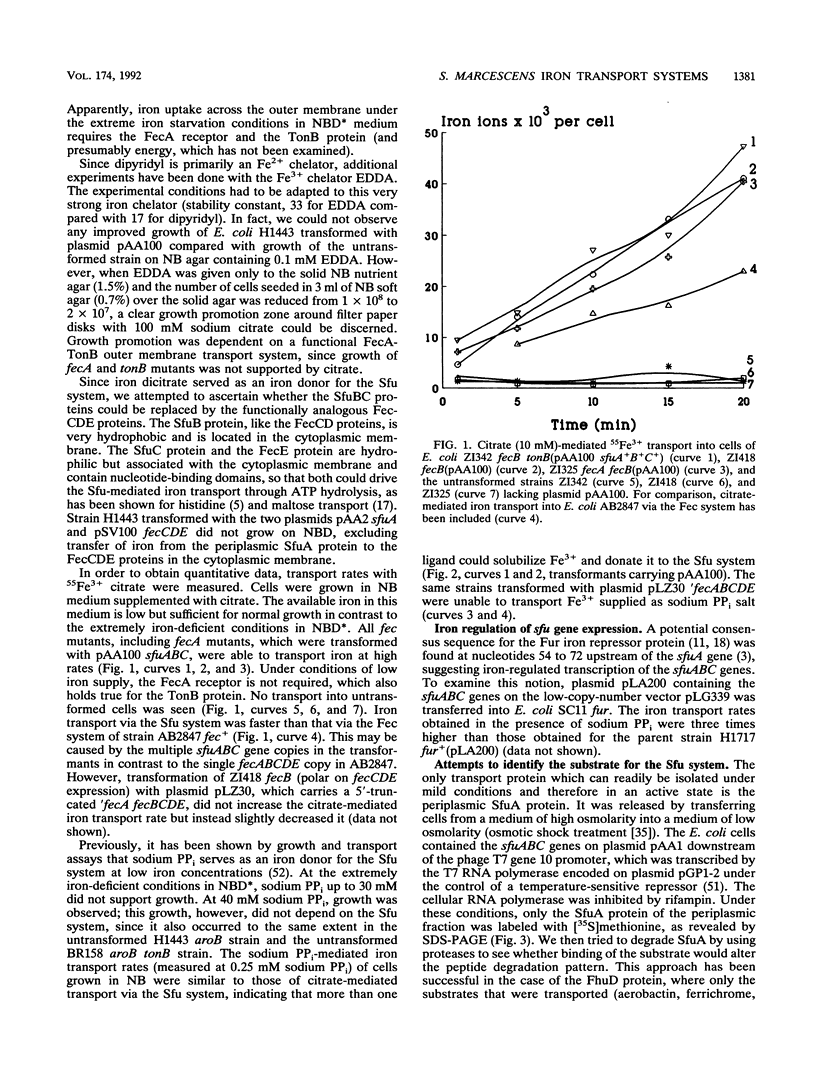
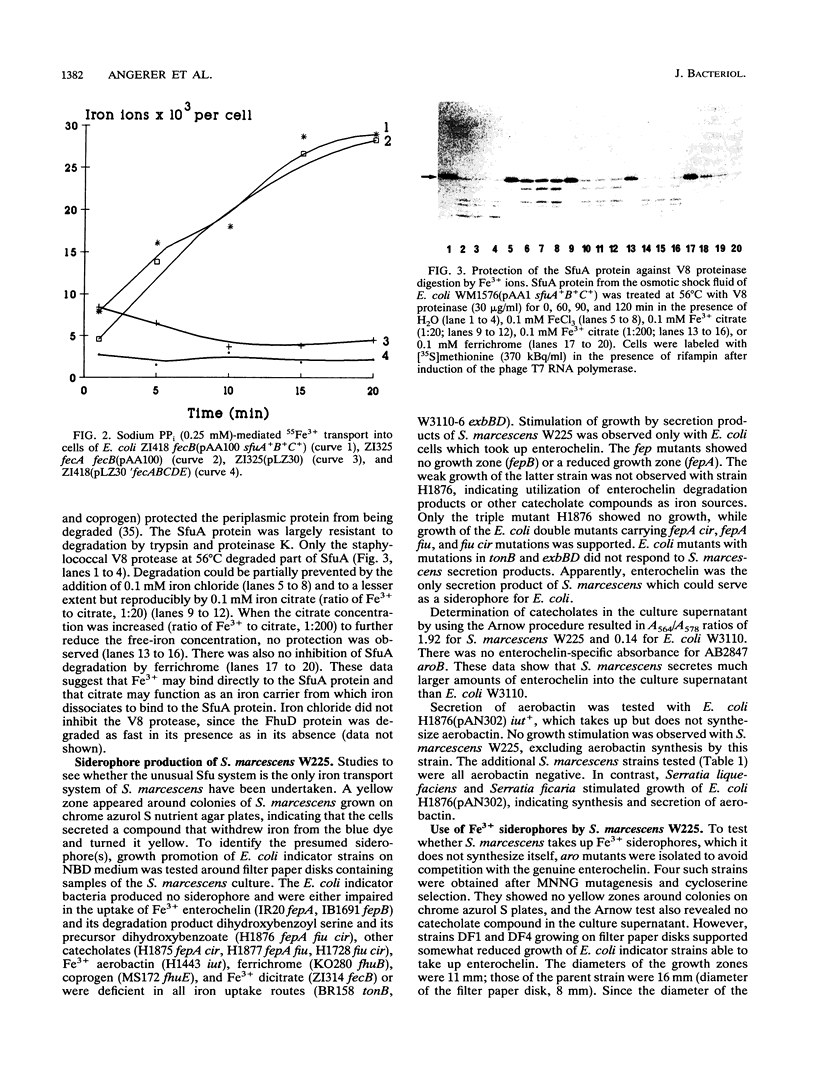
- Köster W., Braun V. Iron hydroxamate transport of Escherichia coli: nucleotide sequence of the fhuB gene and identification of the protein. Mol Gen Genet. 1986 Sep;204(3):435–442. doi: 10.1007/BF00331021. [DOI] [PubMed] [Google Scholar]
- Köster W. Iron(III) hydroxamate transport across the cytoplasmic membrane of Escherichia coli. Biol Met. 1991;4(1):23–32. doi: 10.1007/BF01135553. [DOI] [PubMed] [Google Scholar]
- Martin R. B. Citrate binding of Al3+ and Fe3+. J Inorg Biochem. 1986 Oct-Nov;28(2-3):181–187. doi: 10.1016/0162-0134(86)80081-2. [DOI] [PubMed] [Google Scholar]
- Neilands J. B. Microbial iron compounds. Annu Rev Biochem. 1981;50:715–731. doi: 10.1146/annurev.bi.50.070181.003435. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Rosenberg E. Y. Cir and Fiu proteins in the outer membrane of Escherichia coli catalyze transport of monomeric catechols: study with beta-lactam antibiotics containing catechol and analogous groups. J Bacteriol. 1990 Mar;172(3):1361–1367. doi: 10.1128/jb.172.3.1361-1367.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne S. M. Iron and virulence in the family Enterobacteriaceae. Crit Rev Microbiol. 1988;16(2):81–111. doi: 10.3109/10408418809104468. [DOI] [PubMed] [Google Scholar]
- Poole K., Braun V. Iron regulation of Serratia marcescens hemolysin gene expression. Infect Immun. 1988 Nov;56(11):2967–2971. doi: 10.1128/iai.56.11.2967-2971.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle K. TonB and the gram-negative dilemma. Mol Microbiol. 1990 Dec;4(12):2019–2025. doi: 10.1111/j.1365-2958.1990.tb00561.x. [DOI] [PubMed] [Google Scholar]
- Pressler U., Staudenmaier H., Zimmermann L., Braun V. Genetics of the iron dicitrate transport system of Escherichia coli. J Bacteriol. 1988 Jun;170(6):2716–2724. doi: 10.1128/jb.170.6.2716-2724.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugsley A. P., Zimmerman W., Wehrli W. Highly efficient uptake of a rifamycin derivative via the FhuA-TonB-dependent uptake route in Escherichia coli. J Gen Microbiol. 1987 Dec;133(12):3505–3511. doi: 10.1099/00221287-133-12-3505. [DOI] [PubMed] [Google Scholar]
- Ruan Y., Braun V. Hemolysin as a marker for Serratia. Arch Microbiol. 1990;154(3):221–225. doi: 10.1007/BF00248958. [DOI] [PubMed] [Google Scholar]
- Sauer M., Hantke K., Braun V. Sequence of the fhuE outer-membrane receptor gene of Escherichia coli K12 and properties of mutants. Mol Microbiol. 1990 Mar;4(3):427–437. doi: 10.1111/j.1365-2958.1990.tb00609.x. [DOI] [PubMed] [Google Scholar]
- Staudenmaier H., Van Hove B., Yaraghi Z., Braun V. Nucleotide sequences of the fecBCDE genes and locations of the proteins suggest a periplasmic-binding-protein-dependent transport mechanism for iron(III) dicitrate in Escherichia coli. J Bacteriol. 1989 May;171(5):2626–2633. doi: 10.1128/jb.171.5.2626-2633.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker N. G., Fairweather N. F., Spratt B. G. Versatile low-copy-number plasmid vectors for cloning in Escherichia coli. Gene. 1982 Jun;18(3):335–341. doi: 10.1016/0378-1119(82)90172-x. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann L., Angerer A., Braun V. Mechanistically novel iron(III) transport system in Serratia marcescens. J Bacteriol. 1989 Jan;171(1):238–243. doi: 10.1128/jb.171.1.238-243.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann L., Hantke K., Braun V. Exogenous induction of the iron dicitrate transport system of Escherichia coli K-12. J Bacteriol. 1984 Jul;159(1):271–277. doi: 10.1128/jb.159.1.271-277.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lorenzo V., Giovannini F., Herrero M., Neilands J. B. Metal ion regulation of gene expression. Fur repressor-operator interaction at the promoter region of the aerobactin system of pColV-K30. J Mol Biol. 1988 Oct 20;203(4):875–884. doi: 10.1016/0022-2836(88)90113-1. [DOI] [PubMed] [Google Scholar]