Abstract
During peripheral nervous system (PNS) myelination, Schwann cells must interpret extracellular cues to sense their environment and regulate their intrinsic developmental program accordingly. The pathways and mechanisms involved in this process are only partially understood. We use tissue-specific conditional gene targeting to show that members of the Rho GTPases, cdc42 and rac1, have different and essential roles in axon sorting by Schwann cells. Our results indicate that although cdc42 is required for normal Schwann cell proliferation, rac1 regulates Schwann cell process extension and stabilization, allowing efficient radial sorting of axon bundles.
Introduction
Myelination allows the rapid propagation of action potentials along the axon and is an important prerequisite for the normal function of the nervous system. Schwann cells (SCs) are the myelin-forming cells of the peripheral nervous system (PNS). During development, neural crest–derived SC precursors populate outgrowing axon bundles, where they proliferate and differentiate into immature SCs (for review see Jessen and Mirsky, 2005). Possibly as a result of increasing cell density (Martin and Webster, 1973), these cells extend cytoplasmic processes into axon bundles to segregate and establish one-to-one relationships with individual axons in a process referred to as radial sorting (Webster, 1971; Martin and Webster, 1973). During this process, large caliber fibers are sorted and later become myelinated, whereas the remaining small caliber axons are engaged by nonmyelinating SCs (Mirsky and Jessen, 1999).
To sense their environment and regulate their intrinsic developmental program accordingly, SCs need to interpret instructive cues originating within the extracellular environment, among which growth factors and proteins of the ECM are essential components. This process is likely to involve GTPases of the Rho subfamily. Cdc42, rac1, and rhoA are the best-characterized family members. They are well known for their roles in regulating signaling pathways linking extracellular stimuli to the assembly and organization of the actin cytoskeleton (Hall, 1998). In addition, Rho GTPases control microtubule dynamics, cell polarity, membrane trafficking, and gene transcription (Etienne-Manneville and Hall, 2002; Jaffe and Hall, 2005).
Rho GTPases are expressed by SCs (Terashima et al., 2001). In vitro experiments using dominant-negative and constitutively active forms of rac1 and cdc42 suggested that these small GTPases together with FAK may serve to link growth factor activation with SC motility (Cheng et al., 2000). More recently, Yamauchi et al. (2003), again using an in vitro approach, suggested that cdc42 and rac1 regulate the JNK signaling cascade to enhance migration of SCs in response to TrkC tyrosine kinase receptor activation by endogenous neurotrophin-3. Further work by the same investigators proposed that TrkC activation by neurotrophin-3 stimulates SC migration through two parallel signaling pathways involving cdc42 or rac1 (Yamauchi et al., 2005). Precise control of rac1 activity also regulates SC morphology and promotes normal axonal interaction (Nakai et al., 2006). Cells derived from SC tumors (schwannomas) in which rac1 activity is deregulated showed a disorganized cytoskeleton (Pelton et al., 1998) and failed to interact with axons (Nakai et al., 2006). Reestablishing normal rac1 activity levels in these cells restored SC spindle morphology and their capacity to interact with axons (Nakai et al., 2006).
In this study, we examined the role of cdc42 and rac1 signaling in peripheral nerve development using tissue-specific conditional gene ablation specifically in the SC lineage. Ablation of either Cdc42 or Rac1 impairs radial sorting of axons. However, these proteins play different roles during SC development. Our results indicate that although cdc42 is required for SC proliferation, rac1 is necessary for correct SC process extension and stabilization. Furthermore, we show that cdc42 activation can be induced by neuregulin-1 (NRG1) and is critically involved in SC proliferation. Rac1 is activated by β1 integrin signaling and regulates SC process extension and stabilization.
Results
Conditional ablation of Cdc42 or Rac1 in SCs
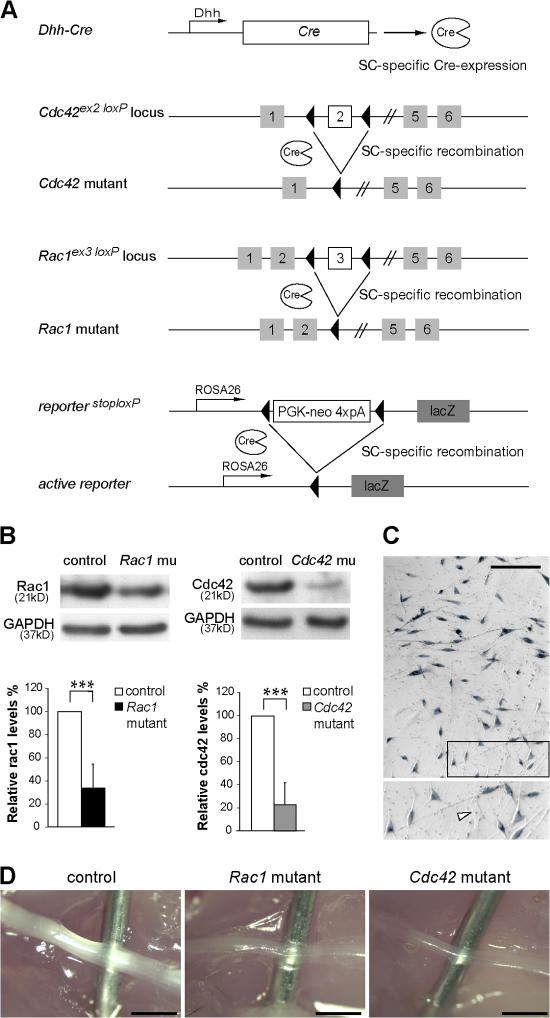
To study the role of cdc42 and rac1 signaling in SCs, we conditionally ablated Cdc42 or Rac1 by expressing Cre recombinase (Cre) under the control of the desert hedgehog (Dhh) gene regulatory sequences (Fig. 1 A). In this setting, Cre is active in SC precursors from embryonic day (E) 12 (Joseph et al., 2004). To identify the recombined cells, we bred also the conditional LacZ reporter allele from the ROSA26 reporter mouse (Soriano, 1999) into control, Cdc42 mutant, and Rac1 mutant mice.
Figure 1.
Recombination of the conditional Cdc42 and Rac1 alleles in SCs of mutant mice. (A) The regulatory sequences of the Dhh gene direct Cre recombinase (Cre) expression in SCs. Depicted are also the conditional Cdc42, Rac1, and the conditional reporter LacZ allele. Upon Dhh-Cre–mediated recombination, the genomic region between the two LoxP sites is excised, inactivating the Cdc42 and Rac1 genes. Recombination of the conditional LacZ reporter triggers the expression of β-galactosidase in SCs. (B) Western blot analysis shows a significant decrease in cdc42 and rac1 protein levels in P1 mutant sciatic nerve lysates (n = 5). ***, P < 0.001. Error bars indicate mean ± SD. (C) Widespread expression of β-galactosidase in SCs obtained from P0 nerves, as assessed by X-gal staining. The magnified box shows X-gal–positive SCs and an X-gal–negative fibroblast (arrowhead). (D) Sciatic nerves from P14 control, Cdc42 mutant, and Rac1 mutant mice. Note that both Cdc42 and Rac1 mutant sciatic nerves are thinner and more transparent than controls. For clarity, a needle is inserted behind the nerves. Bars, 1 mm.
Recombination of the conditional Cdc42 or Rac1 alleles (Fig. 1 A) led to a strong reduction in cdc42 and rac1 protein in lysates obtained from the sciatic nerves of postnatal day (P) 1 Cdc42 and Rac1 mutant mice, respectively (Fig. 1 B). The low residual cdc42 and rac1 protein levels detected in the mutant lysates are likely due to the presence of endoneurial and perineurial fibroblasts as well as some unrecombined SCs. In acute SC cultures obtained from P1 sciatic nerves, Dhh-Cre–mediated recombination of the conditional lacZ allele was found in 87% of the SCs (400 of 460 counted cells) as assessed by X-gal staining (Fig. 1 C). At P14, the sciatic nerves of Cdc42 and Rac1 mutant mice were thinner and more transparent (Fig. 1 D). Both Cdc42 and Rac1 mutants displayed a progressive hind limb accentuated paresis. At around P30, Cdc42 mutants developed hind limb paralysis. Although less affected, Rac1 mutants died of unknown causes at ∼P40. Thus, for both mutants, our analysis was performed at P24 or earlier.
Axon sorting and myelination deficits in Rac1 and Cdc42 mutant mice
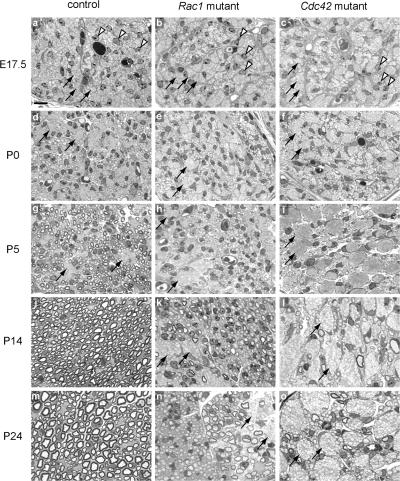
During postnatal development, SCs segregate and myelinate individual large caliber axons from the axon bundles formed during embryogenesis (Fig. 2, d, g, j, and m). We compared control and mutant sciatic nerves at different developmental stages. At E17.5, SCs were present between bundles of tightly apposed axons in the sciatic nerves of control, Rac1 mutant, and Cdc42 mutant mice (Fig. 2, a–c). Although there were still a few unsorted bundles of axons in control mice at P5 (Fig. 2 g), the majority of large caliber axons were already engaged in a one-to-one relationship with SCs. By P24, practically all large caliber axons were sorted and myelinated (Fig. 2 m).
Figure 2.
Axon sorting and myelination are impaired in Rac1 and Cdc42 mutant sciatic nerves. Semithin cross- sections of sciatic nerves from control (a, d, g, j, and m), Rac1 mutant (b, e, h, k, and n), and Cdc42 mutant (c, f, i, l, and o) mice stained with toluidine blue. In the controls, SCs (arrowheads) gradually segregate and myelinate single axons from the axon bundles (arrows). In contrast, in Rac1 and Cdc42 mutant nerves, axon sorting and myelination is impaired and axon bundles (arrows) persist. Although in P24 Rac1 mutant nerves, many axons are progressively sorted and myelinated, only very few axons are myelinated in P24 Cdc42 mutant nerves (o). Bar, 10 μm.
In contrast, in the mutant nerves, axon sorting and myelination was impaired and bundles of axons containing large caliber axons persisted. This feature was more pronounced in Cdc42 mutant nerves (Fig. 2, f, i, l, and o) than in Rac1 mutant nerves, where a considerable number of larger caliber axons progressively became sorted and myelinated over time (Fig. 2, e, h, k, and n). Very few axons were myelinated in Cdc42 mutant nerves (Fig. 2, l and o).
As the mutant SCs also carried the conditional LacZ allele (Fig. 1 A), we could follow the fate of recombined cells by detecting the activity of the β-galactosidase by Bluogal staining. To this end, we performed Bluogal EM analysis on P24 Cdc42 and Rac1 mutant nerves. Bluogal precipitates were detected in promyelinating and myelinating SCs of both P24 Rac1 and Cdc42 mutant nerves (Fig. S1 A, available at http://www.jcb.org/cgi/content/full/jcb.200610108/DC1). This suggests the concomitant recombination of the Cdc42 or Rac1 conditional alleles in these cells.
The Bluogal EM data also indicated that the improvement of the radial sorting phenotype observed in P24 Rac1 mutant nerves is not the result of cellular compensation by unrecombined, rac1-positive SCs. This conclusion was further supported by Western blot analysis of protein lysates obtained from sciatic nerves of P24 Rac1 mutant and control mice. The levels of rac1 in mutant lysates were strongly reduced in relation to control lysates (Fig. S1 B), comparable to the one previously detected in P1 Rac1 mutant nerves (Fig. 1 B).
Defective extension and stabilization of SC processes in Rac1 mutant nerves
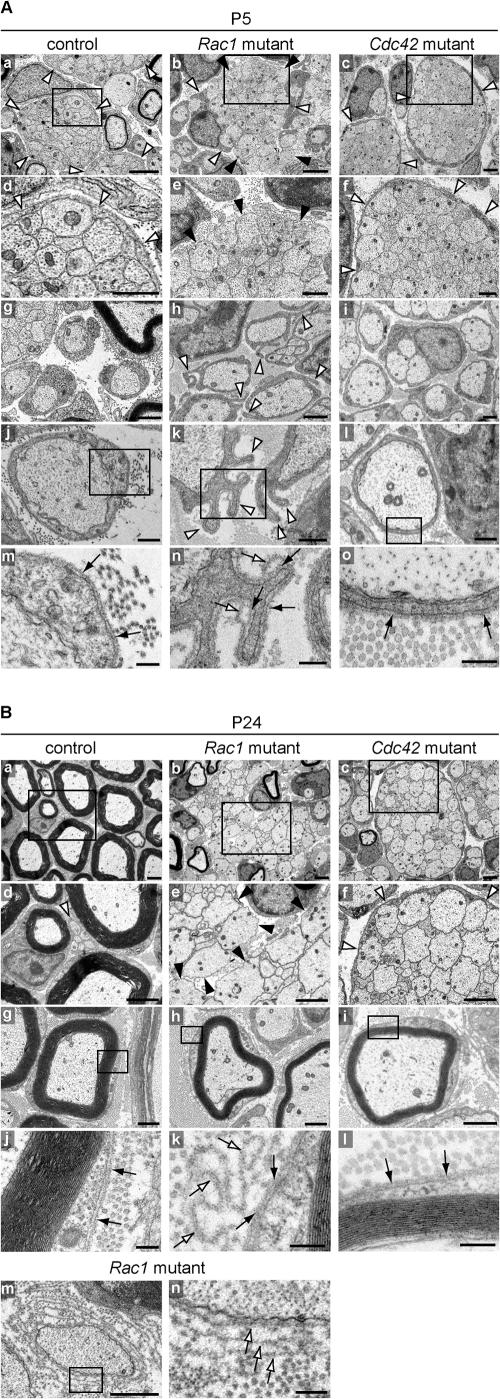
To understand why axon sorting was affected in mutant nerves, we performed EM analysis of P5 and P24 control and mutant nerves. At P5, this analysis revealed SCs at different stages of differentiation, including immature SCs associated with axon bundles, promyelinating SCs in a one-to-one relationship with large caliber axons, and myelinating SCs that had started to form a compact myelin sheath. In control (Fig. 3 A, a and d) and Cdc42 mutant nerves (Fig. 3 A, c and f), immature SCs extended long processes that fully enveloped axon bundles, a normal feature of SCs at this stage of differentiation. This, however, was not the case in Rac1 mutant nerves (Fig. 3 A, b and e), where immature SC processes were shorter and often failed to envelope axon bundles.
Figure 3.
SC process extension and stabilization deficits in Rac1 mutant sciatic nerves. EM analysis of sciatic nerve cross-sections of control, Rac1 mutant, and Cdc42 mutant animals at P5 (A) and P24 (B). (A) At P5, immature SCs in control (a and d) and Cdc42 mutant (c and f) nerves extend long processes that fully envelop axon bundles (white arrowheads), a normal feature of SCs at this stage of differentiation. In contrast, in Rac1 mutant nerves, immature SC processes are shorter (b, white arrowheads) and often fail to envelope axon bundles (b and e, black arrowheads). Control (g and j) and Cdc42 mutant (i and l) promyelinating SCs are surrounded by an apposed BL (black arrows in m and o, respectively). In contrast, Rac1 mutant promyelinating SCs contain abnormal cytoplasmic protrusions that extend in various directions (h and k, white arrowheads). These protrusions are also surrounded by an apposed BL (n, black arrows); however, they often display empty BL loops above the apposed BL (n, white arrows). At the sites where they emerge, these BL loops are continuous with the apposed BL. Bars: (a–c) 2 μm; (d–i) 1 μm; (j–l) 500 nm; (m–o) 200 nm. (B) At P24, radial sorting in control nerves is virtually completed. While large caliber axons are being myelinated (a, d, and g), the small caliber axons are engaged by nonmyelinating SCs (d, white arrowhead). Myelinating SCs are surrounded by an apposed BL (j, black arrows). In P24 mutant nerves, hypomyelination is pronounced and resembles the situation at P5. Axon bundles in Rac1 mutant nerves are still not enveloped by immature SC processes (b and e, black arrowheads). Rac1 mutant myelinating SCs (h) do not show cytoplasmic protrusions. However, several loops of empty BL in the shape of the protrusions (k, white arrows) remain attached to the apposed BL (k, black arrows). In Rac1 mutant nerves, some naked axons are surrounded by several layers of BL, suggesting that processes of adjacent SCs repeatedly engaged and retracted from these axons, leaving behind empty BL layers (m and n, white arrows). P24 Cdc42 mutant nerves still contain many axon bundles (c), which as in P5 nerves (c and d, white arrowhead), are fully enveloped by immature SC processes (f, white arrowheads). P24 Cdc42 mutant nerves also contain myelinating SC-axon profiles (i), which are surrounded by an apposed BL (l, black arrows). Bars: (a–f) 2 μm; (g–i and m) 1 μm; (j–l and n) 200 nm.
At the promyelination stage, SC-axon profiles in control (Fig. 3 A, g and j) and Cdc42 mutant nerves (Fig. 3 A, i and l) were surrounded by an apposed basal lamina (BL; Fig. 3 A, m and o, arrows). In contrast, Rac1 mutant SC-axon profiles contained abnormal cytoplasmic protrusions that extended in various directions (Fig. 3 A, h and k, arrowheads). These protrusions were surrounded by an apposed BL covering their entire surface (Fig. 3 A n, black arrows) and often displayed empty loops of redundant BL (Fig. 3 A n, white arrows). At the sites where they emerged, these loops were continuous with the apposed BL. Retracting protrusions may have detached from their original BL and, while leaving behind these loops of empty BL, produced a new apposed BL layer.
At P24, radial sorting of large caliber fibers in control nerves was virtually completed and mature SCs formed a compact multilayered sheath of myelin around single large caliber axons (Fig. 3 B a). Small caliber axons were engaged by nonmyelinating SCs (Fig. 3 B d, arrowhead). SC-axon profiles at the myelinating stage were surrounded by an apposed BL (Fig. 3 B, g and j, arrows). In P24 mutant nerves, hypomyelination was pronounced, resembling the situation at P5. Axon bundles in Rac1 mutant nerves were still not enveloped by immature SC processes (Fig. 3 B, b and e). SC-axon profiles at the myelinating stage (Fig. 3 B h) did not show any of the protrusions seen at the promyelinating stage (Fig. 3 A, h and k, arrowheads), but several loops of empty BL in the shape of the protrusions remained attached to the apposed BL (Fig. 3 B k, white and black arrows, respectively). In Rac1 mutant nerves, some naked axons were surrounded by several layers of BL, suggesting that SC processes repeatedly engaged and retracted from these axons, leaving behind empty BL layers (Fig. 3 B, m and n, arrows). P24 Cdc42 mutant nerves contained many axon bundles (Fig. 3 B c), which as in P5 nerves were fully enveloped by immature SC processes (Fig. 3 B f, arrowheads). Cdc42 mutant nerves contained also a small number of SC-axon profiles at the myelinating stage (Fig. 3 B i), which were surrounded by an apposed BL (Fig. 3 B l, arrows). Interestingly, and in contrast to what was described by Feltri et al. (2002) in SC-specific conditional β1 integrin mutant nerves, and more recently by Grove et al. (2007) in SC-specific conditional FAK mutant nerves, we have not observed perineurial cells in contact with axon bundles in Rac1 or in Cdc42 mutant nerves.
We conclude that in Rac1 mutant nerves, SC process extension and stabilization is severely affected in a timely and spatially controlled manner. It is likely that such deficits are the reason why radial sorting is delayed. In contrast, and despite their severe radial sorting defects, SC process extension in Cdc42 mutant nerves appears to be normal, suggesting that the mechanisms associated with radial sorting failure in the two mutants are intrinsically different.
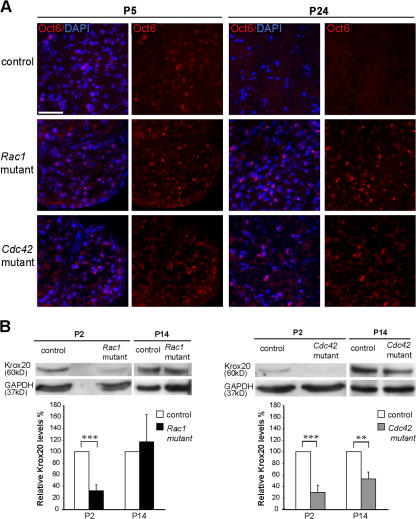
Altered Oct6 and Krox20 expression in Cdc42 and Rac1 mutant nerves
To further confirm that the differentiation of Cdc42 and Rac1 mutant SCs was severely delayed or even blocked, we used immunofluorescence analysis to examine the expression of the transcription factor Oct6 on transverse sections of control and mutant P5 and P24 sciatic nerves and Western blotting to assess the expression levels of Krox20 in mutant and control sciatic nerves at P2 and P14. The expression of Oct6 peaks at the promyelinating and early myelinating stages and is down-regulated at later stages of myelination (Jaegle et al., 1996). At P5, control, Rac1 mutant, and Cdc42 mutant nerves contained similar numbers of Oct6-positive SCs, whereas at P24, Oct6-positive SCs were only present in Rac1 or Cdc42 mutant nerves but not in control nerves (Fig. 4 A). Krox20 expression is required for down-regulation of Oct6 and for the activation of myelin genes, such as peripheral myelin protein 22, myelin protein zero, and myelin basic protein (Topilko et al., 1994). At P2, Krox20 expression was detected by Western blotting in control but only at very low levels in Rac1 and Cdc42 mutant nerves (Fig. 4 B). Later, at P14, Krox20 was expressed at similar levels in control and Rac1 mutant nerves while it was reduced in Cdc42 mutant nerves (Fig. 4 B). These findings corroborate our morphological data showing developmental SC abnormalities (Fig. 2, k and n).
Figure 4.
Altered Oct6 and Krox20 expression in Cdc42 and Rac1 mutant nerves. (A) Transverse sections of P5 and P24 sciatic nerves from control, Rac1 mutant, and Cdc42 mutant mice were stained for Oct6 (red) and DAPI (blue). Oct6-positive nuclei are present in P5 control, Rac1 mutant, and Cdc42 mutant nerves. At P24, Oct6 is still expressed in Rac1 and Cdc42 mutant nerves, whereas no Oct6-positive nuclei are present in control nerves. Bar, 100 μm. (B) Western blot analysis of sciatic nerve lysates of P2 Rac1 and Cdc42 mutant nerves show a significant decrease of Krox20 expression in relation to control. At P14, Krox20 levels were still significantly lower in Cdc42 mutant but not in Rac1 mutant nerves. Error bars indicate mean ± SD. **, P < 0.01; ***, P < 0.001.
Cdc42 is required for normal SC proliferation
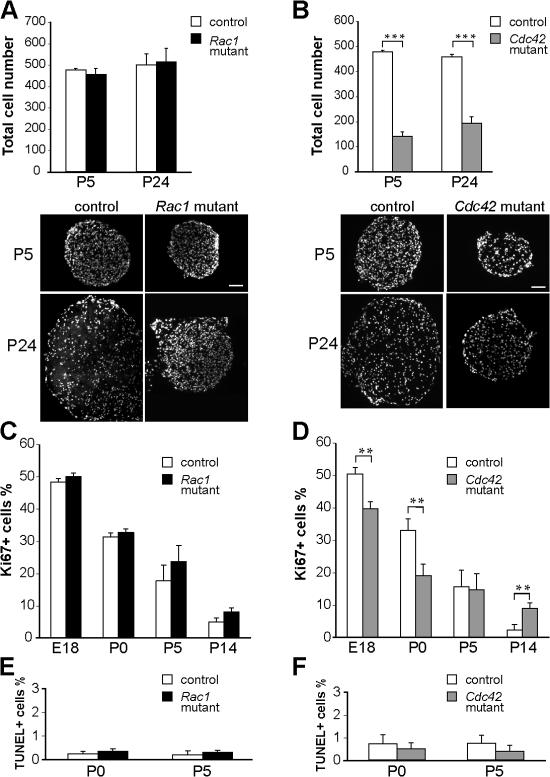
Insufficient numbers of SCs, as a result of reduced proliferation or survival, could potentially explain the persistence of axon bundles, in particular in Cdc42 mutants. Thus, we compared the total numbers of DAPI-stained nuclei present within transverse sections of P5 and P24 sciatic nerves of Rac1 mutant, Cdc42 mutant, and control mice (Fig. 5, A and B). Although at both ages the numbers of DAPI-positive nuclei in Cdc42 mutant nerves were significantly lower compared with control nerves (Fig. 5 B), no differences were found between Rac1 mutant and control nerves (Fig. 5 A).
Figure 5.
Total cell number, proliferation, and apoptosis in Rac1 and Cdc42 mutant sciatic nerves. (A) DAPI-stained P5 and P24 nerve cross-sections show similar numbers of cells in control and Rac1 mutant nerves but a significant decrease (B) of the total cell number in P5 and P24 Cdc42 mutant nerves. Transversal nerve sections obtained from mutant nerves are smaller than those of controls. ***, P < 0.001. (C) At E18, P0, P5, and P14, the numbers of proliferating Ki67-positive cells are not significantly different in sciatic nerves of control and Rac1 mutant mice. (D) In contrast, proliferation is significantly decreased in Cdc42 mutant nerves at E18 and P0 but significantly higher at P14. At P0 and P5, the number of apoptotic, TUNEL-positive cells is not significantly different in Rac1 mutant (E), Cdc42 mutant (F), and control nerves. Error bars indicate mean ± SD. **, P < 0.01. Bars, 100 μm.
Transverse sections obtained from P5 and P24 mutant nerves were smaller in size than those obtained from control nerves (Fig. 5, A and B). This reduction in size is likely the result of the severe hypomyelination in mutant nerves. To analyze potential proliferation defects directly, we compared the percentage of proliferating Ki67-positive cells in control, Cdc42 mutant, and Rac1 mutant nerves at E17.5, P0, P5, and P14. As expected, proliferation was normal in the absence of rac1 (Fig. 5 C) but was significantly diminished in Cdc42 mutant nerves at E18 and P0. At P5, there was no difference between control and Cdc42 mutant cell proliferation, and at P14, cell proliferation in Cdc42 mutant nerves was even higher than in control nerves (Fig. 5 D). Despite this continuing late proliferation, most likely due to delayed SC differentiation, the total number of cells present in P24 Cdc42 mutant nerves was still less than half of those present in control nerves (Fig. 5 B).
To investigate whether the reduction in cell numbers was influenced by increased cell death, we compared the percentage of TUNEL-positive cells in P0 and P5 in control, Cdc42 mutant, and Rac1 mutant nerves. No significant differences were found in all experimental settings examined (Fig. 5, E and F). We conclude that the loss of cdc42 leads specifically to a reduction in SC proliferation but does not affect cell survival.
During PNS development, β1 integrin regulates the activity of rac1 in SCs
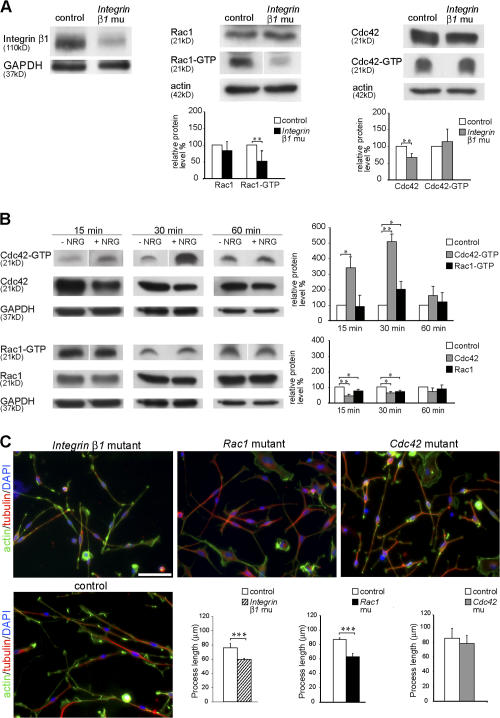
Similar to Cdc42 and Rac1 mutant nerves, conditional ablation of the β1 integrin during SC development also results in the impairment of radial sorting (Feltri et al., 2002). This and the knowledge that in a variety of different cell types, integrin signaling can regulate the activity of Rho GTPases (Schwartz and Shattil, 2000), including rac1 and cdc42, prompted us to investigate whether β1 integrin regulates the activity of these two proteins in SCs. Therefore, we performed Rho GTPase activity assays (see Material and methods) in sciatic nerve lysates obtained from P5 2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNP)–Cre β1 integrin mutant and control mice (Benninger et al., 2006). In these mice, the Cre recombinase is under the control of the CNP gene regulatory sequences and is expressed in SCs from E12 on (Genoud et al., 2002; unpublished data). Western blot analysis of protein lysates obtained from β1 integrin mutant nerves confirmed that the expression of β1 integrin was strongly reduced (Fig 6 A). The following Rho GTPase activity assays showed that in β1 integrin mutant nerve lysates, rac1 activity was significantly reduced compared with controls (Fig. 6 A). In contrast, the cdc42 activity showed a slight tendency to increase but, interestingly, the total expression level of cdc42 was reduced (Fig. 6 A).
Figure 6.
β1 integrin and NRG1 signaling regulate the activity of rac1 and cdc42. (A) Western blot analysis shows that β1 integrin protein expression is strongly reduced in P1 β1 integrin mutant (CNP-Cre + β1 integrinlox/0) sciatic nerve lysates compared with control (CNP-Cre − β1 integrinlox/wt). Levels of active rac1 (rac1-GTP) and cdc42 (cdc42-GTP) were measured in a pull-down assay using GST-PAK-CD constructs. Nerve lysates from P5 β1 integrin mutant mice show significantly decreased levels of active rac1 compared with control lysates (n = 6). Activity of cdc42 is not significantly different between control and mutant lysates (n = 8). Total protein levels of cdc42 are significantly reduced. **, P < 0.01. (B) Exposure to 20 ng/ml NRG1 for 15 and 30 min significantly increases the levels of cdc42 activation in cultures of rat SCs compared with cultures of non–NRG1-treated SCs (control). Rac1 activity is only significantly higher than control after 30 min of exposure to NRG1. Note that the activity levels of cdc42 are increased severalfold in relation to those of rac1 after 15 and 30 min of NRG1 exposure. After 60 min of exposure to NRG1, both cdc42 and rac1 activities returned to control levels. After 15 or 30 min of NRG1 exposure, the total levels of cdc42 and rac1 are significantly reduced in relation to control. No significant differences were observed after 60 min. All experiments were repeated three times. *, P < 0.05; **, P < 0.01. (C) Acute SC cultures obtained from sciatic nerves of P1 β1 integrin, Rac1, and Cdc42 mutant and control mice were cultured for 16 h on PDL/laminin-2–coated dishes. The actin cytoskeleton (green), microtubules (red), and nuclei (blue) were visualized by phalloidin, tubulin antibodies, and DAPI, respectively. The mean length of SC processes is significantly decreased in β1 integrin and Rac1 mutant SC cultures but not in cdc42 mutant SCs (n = 5). Error bars indicate mean ± SD. ***, P < 0.001. Bar, 50 μm.
We conclude that in SCs, rac1 activity is dependent on β1 integrin signaling, whereas cdc42 activity is not, suggesting that activation of cdc42 and rac1 relies, at least partially, on different pathways. This hypothesis is consistent with our findings that cdc42 is required for SC proliferation, a process that is not dependent on β1 integrin (Feltri et al., 2002) or rac1 signaling (this work). SC proliferation is, however, modulated by growth factors such as NRG1 (Garratt et al., 2000). As Rho GTPase activity can also be regulated by growth factors, we hypothesized that in SCs, exposure to NRG1 may preferentially activate cdc42 rather than rac1.
We tested this hypothesis by examining the levels of activated cdc42 and rac1 in cultured rat SCs after 15, 30, and 60 min of exposure to NRG1. As expected, we found that exposure to NRG1 significantly increased the activation of cdc42 in relation to control non–NRG1-treated SCs (Fig. 6 B). Cdc42 activation was elevated after 15 min of NRG1 stimulation, peaked after 30 min, and returned to almost control levels after 60 min. In contrast, the increase in rac1 activation was modest and only significantly higher than control levels after 30 min of NRG1 stimulation (Fig. 6 B). The increase in cdc42 and rac1 activity was accompanied by a reduction in the expression levels of both molecules (Fig. 6 B).
β1 integrin and Rac1 regulate SC process extension in culture
Our morphological data demonstrated that process extension and stabilization in Rac1 mutant SCs is defective. SC process extension is thought to require ECM–integrin interactions and mutations in Laminin genes, and the conditional ablation of β1 integrin in SCs appears to affect the extension and targeting of SC processes, resulting in impaired radial sorting (Feltri et al., 2002; Colognato et al., 2005). Therefore, we hypothesized that when plated on laminin-2, a component of SC BL and a ligand for SC laminin integrin receptors containing the β1 integrin subunit, rac1 but not cdc42 mutant SCs would behave as β1 integrin mutant cells and produce shorter processes. Similar assays and rationale were applied before to study the role of ECM–integrin signaling and downstream molecules in the regulation of oligodendrocyte process extension (Buttery and ffrench-Constant, 1999; Relvas et al., 2001; Colognato et al., 2002; Thurnherr et al., 2006).
Thus, we plated primary SCs obtained from sciatic nerves of neonate control, Rac1 mutant, and Cdc42 mutant mice on laminin-2 substrates and grew the cells in defined media for 16 h, sufficient time for SCs to extend several thin processes (Fig. 6 C). SCs prepared from sciatic nerves of β1 integrin mutant mice were treated in the same way. Visualization of the actin and microtubule cytoskeleton by immunofluorescence allowed us to measure and compare the length of the SC processes formed. β1 integrin and Rac1 mutant SCs produced significantly shorter processes than their control counterparts, whereas the length of the processes produced by Cdc42 mutant SCs was not significantly different from controls (Fig. 6 C). Collectively, these results indicate that rac1 activation is required for normal SC process extension and support the view that the specific radial sorting defects in the nerves of Rac1 mutant mice are a consequence of deficient SC process extension.
Discussion
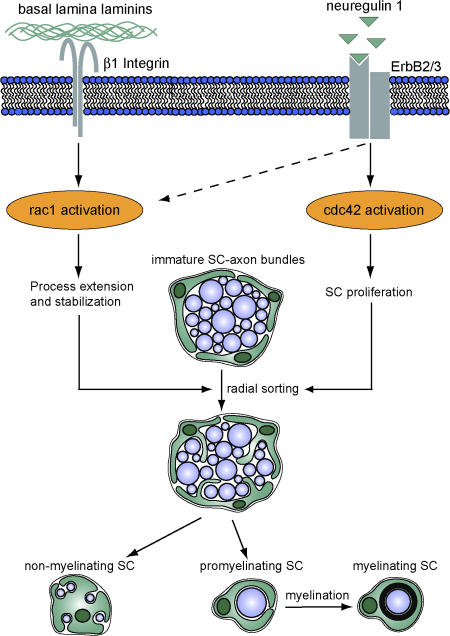
Using tissue-specific conditional gene ablation, we demonstrate essential roles for the small Rho GTPases cdc42 and rac1 during PNS myelination. We show that although both proteins foster radial sorting, they regulate different crucial aspects of SC biology. Cdc42 is required for SC proliferation at the onset of radial sorting, whereas rac1 is critical for SC process extension and stabilization during radial sorting of axon bundles. We also reveal that although rac1 activity is specifically dependent on β1 integrin signaling, cdc42 can be activated by NRG1, suggesting that the activity of these two GTPases in SCs are regulated, at least in part, by different pathways (Fig. 7).
Figure 7.
Proposed mechanism for the regulation of SC development by rac1 and cdc42. Cdc42 and rac1 play different but essential roles during SC development. Although rac1 can be activated by β1 integrin signaling and mediates SC process extension and stabilization, cdc42 is preferentially activated by NRG1 and is required for SC proliferation. Although rac1 and cdc42 play different roles in SC development, both are required for efficient radial sorting of axon bundles, a prerequisite for subsequent axon myelination.
Radial sorting defects in rac1 mutant nerves
Loss of rac1 in SCs led to hypomyelination in postnatal sciatic nerves of mutant mice characterized by the persistence of axon bundles throughout development. Such radial sorting defects have also been described in Laminin mutant mice. Laminin-2 is the major laminin isoform present in the PNS, and laminin-2– deficient mice (dy and dy2J) show bundles of unsorted axons in spinal roots and, to a lesser extent, in sciatic nerves (Bradley and Jenkison, 1975; Stirling, 1975; Weinberg et al., 1975). In these mice, however, brachial nerves myelinated normally, which raises the possibility that other laminin isoforms, such as laminin-8 or -10, could compensate for the loss of laminin-2 function. Indeed, in laminin-2/laminin-8 null mice, all nerves were severely hypomyelinated (Yang et al., 2005). The lack of laminin γ1 chain, present in all laminin isoforms expressed in the PNS, also led to the persistence of unsorted axon bundles (Chen and Strickland, 2003). Furthermore, the conditional ablation of β1 integrin in SCs also resulted in impaired radial sorting (Feltri et al., 2002).
Integrins are the most important receptors for ECM proteins, including laminins. The finding that the loss of β1 integrin subunit, necessary for the formation of SC laminin receptors such as α6β1 and α1β1, caused radial sorting defects highlights the importance of the ECM–integrin signaling in this process. In vitro studies showed that ECM–integrin signaling, among other important cellular functions, control cytoskeleton dynamics via the regulation of Rho GTPase activity (Schwartz and Shattil, 2000). Using a biochemical assay, we demonstrate here that in sciatic nerves, the activity of rac1 is dependent on β1 integrin signaling, suggesting that the radial sorting phenotype observed in β1 integrin mutant nerves (Feltri et al., 2002) could, at least in part, be explained by decreased rac1 activity. In line with this hypothesis, the morphological phenotypes of Rac1 and β1 integrin mutant nerves are similar. Both mutants show radial sorting defects, possibly as a consequence of deficits in SC process extension and stabilization. In Rac1 mutant nerves, evidence for abnormal SC process extension and stabilization includes (1) the inability of immature mutant SC processes to envelope axon bundles; (2) the formation of numerous abnormal cytoplasmic protrusions extending randomly from the surface of SC-axon profiles at the promyelinating stage; (3) the presence of bare axons surrounded by multiple layers of loose BL, suggesting several failed attempts of mutant SCs to engage the same axons. Furthermore, loops of redundant BL are found on the surface of SC-axon profiles at the promyelinating and myelinating stages; and (4) the finding that mutant SCs cultured on laminin-2 produce shorter processes, in line with the same observation made using β1 integrin–deficient SCs. Thus, based on our results and taking into account the similarity of the phenotypes in laminin, β1 integrin, and rac1 mutant nerves, we propose that radial sorting of axons during PNS development is regulated by laminin/β1 integrin signaling in a rac1-dependent way (Fig. 7).
Despite the striking similarities, the radial sorting phenotype of β1 integrin mutants appears to be more severe than in Rac1 mutants. This indicates that β1 integrin signaling in SCs may not exclusively act via rac1 and another pathway may also be involved in mediating β1 signaling. For example, β1 integrin regulates the function and physically interacts with several focal adhesion proteins, such as FAK, which is essential for radial sorting of axons by SCs (Grove et al., 2007).
Loss of cdc42 results in reduced SC proliferation and in radial sorting impairment
Our results suggest that cdc42 activity and function is controlled differently from rac1: (1) cdc42 activity is not dependent on β1 integrin signaling; (2) exposure of rat SCs to NRG1 strongly increases cdc42 activity but has only a moderate effect on the activity of rac1; (3) Cdc42 mutant nerves are not affected by process extension and stabilization defects as present in immature and promyelinating Rac1 mutant SCs; and (4) when plated on laminin-2 substrates, Cdc42 mutant SCs produced processes of normal length. Still, the loss of cdc42 caused a radial sorting phenotype similar to that observed in Rac1 mutant nerves and to the one previously described in mutant β1 integrin nerves (Feltri et al., 2002). It is thought that SC proliferation, which peaks at the onset of radial sorting, is intimately associated with this process (Webster, 1971; Martin and Webster, 1973). As the total number of SCs present in Cdc42 mutant sciatic nerves was lower than in control nerves at P5 and P24, we investigated SC proliferation and survival in Cdc42 mutant nerves. We showed that the loss of cdc42 resulted in a decrease of mutant SC proliferation but did not affect survival. Although we cannot completely exclude a direct role for cdc42 in radial sorting, it is likely that reduced proliferation and insufficient SC numbers are the main cause of the radial sorting defects observed in Cdc42 mutant nerves. Growth factor signaling can activate Rho GTPases in different cell types (Ridley et al., 1992; Nobes et al., 1995), and in line with this, we show that exposure to NRG1, a known SC mitogen (Garratt et al., 2000), induces a strong activation of cdc42 (but not of rac1) in cultured SCs. This suggests that cdc42 activation might be required to promote SC proliferation by growth factors such as NRG1 (NRG1; Garratt et al., 2000). Proliferation is also reduced in SCs lacking laminin γ1 (Yu et al., 2005). In this mutant, it was proposed that proliferation is reduced because laminin γ1–deficient SCs fail to extend cytoplasmic processes and, as a consequence, to interact with axonal mitogens (Yu et al., 2005). Adhesion to ECM substrates, including laminins, is known to increase the sensitivity to mitogenic factors in a variety of cell types (Yamada and Even-Ram, 2002). Although we cannot formally exclude the possibility that the lack of cdc42 directly affects SC cytoskeletal organization and process extension, our data show that the loss of cdc42 did not substantially affect SC process extension on laminin substrates and suggest that the extension and stabilization of SC processes in Cdc42 mutant nerves was normal. Therefore, it appears unlikely that deficient substrate adhesion is the primary cause for the proliferation defects seen in Cdc42 mutant nerves. To test whether proliferation is indeed the primary defect will require further mechanistic studies.
In addition to reduced proliferation, deficient migration or cell survival could also reduce the number of SCs present in the nerves and consequently impair radial sorting. Based on chemotactic Boyden chamber assays, Yamauchi et al. (2003, 2005) postulated that rac1 and cdc42 activation regulates SC migration. Although the onset of Cre expression (E12) in Rac1 and Cdc42 mutant mice prevents us from directly addressing SC migration, morphological analysis of semithin transverse sections of E17.5 nerves suggest that SCs populate the nerves of control, Rac1 mutant, and Cdc42 mutant mice in similar numbers. Furthermore, in Rac1 mutant nerves in which proliferation and cell survival were normal, the numbers of cells in P5 and P14 nerves were similar to those in controls, suggesting that in our in vivo setting, the loss of rac1 did not affect SC migration.
Cdc42 and rac1 play different roles in peripheral and central nervous system myelination
Using tissue-specific conditional gene targeting approaches, we have previously reported that ablation of cdc42 or rac1 in oligodendrocytes does not affect proliferation, migration, or in vitro differentiation, but results in the enlargement of the inner tongue of the oligodendrocyte process and the formation of a particular type of myelin outfoldings (Thurnherr et al., 2006). In view of the roles played by cdc42 and rac1 in other cells of the central nervous system, which include the regulation of proliferation, migration, process extension, and differentiation, among others (Govek et al., 2005), such a stage-specific myelination phenotype was largely unexpected. Here, we show that the functions of cdc42 and rac1 in SCs are also vastly different from our observation in oligodendrocytes. This supports the view that Rho GTPases regulate multiple pathways and cellular functions, and it is becoming increasingly clear that the signaling role of a given small Rho GTPase in a specific cell type cannot predict its function in another cell type (Wang and Zheng, 2007). With regard to the specific case of myelinating cells, it should be kept in mind that although SCs already start to lay down a pronounced BL early in embryonic development, which is maintained and extended into adulthood (Jessen and Mirsky, 2005), oligodendrocytes do not produce a BL. Given the pronounced role of small Rho GTPases in signal transduction of receptors for components of the BL, as emphasized by the present work, such differences may not be completely surprising and deserve further experimental investigation.
Materials and methods
Generation of conditional knockout mice
Conditional Cdc42 mutant (Wu et al., 2006) and Rac1 mutant (Chrostek et al., 2006) mice have been described. Mice homozygous for the Cdc42 floxed allele (Cdc42 lox/lox) were crossed with mice heterozygous for the Cdc42 floxed allele, and additionally expressing the Cre recombinase under the control of the Dhh promoter (Dhh-Cre + Cdc42 lox/wt; Lindeboom et al., 2003), to obtain Dhh-Cre + Cdc42 lox/lox mice (hereafter called Cdc42 mutant mice) and Dhh-Cre + Cdc42 lox/wt (hereafter called Cdc42 control mice). To follow the fate of recombined cells by detection of β-galactosidase expression, we also bred the conditional LacZ allele from the ROSA26 reporter mouse strain (Soriano, 1999) into control and mutant mice (provided by P. Soriano, Fred Hutchinson Cancer Research Center, Seattle, WA). Genotypes were determined by PCR on genomic DNA. Mutant and control Rac1 mice were produced using the same breeding strategy as for Cdc42 mice.
The generation of CNP-Cre β1 integrin mutant and control mice has been described (Lappe-Siefke et al., 2003; Benninger et al., 2006). CNP-Cre mice were also recently used successfully to target the excision of a conditional FAK allele to SCs (Grove et al., 2007).
EM
Mice were deeply anesthetized with Pentobarbital (150 mg/kg, i.p.; Nembutal; Abbott Laboratories) and then perfused with 0.1 M phospate buffer, pH 7.4, followed by buffer containing 3% glutaraldehyde and 4% PFA. Fixed tissues were postfixed in 2% osmium tetroxide, dehydrated through a graded acetone series, and embedded in Spurrs resin (Electron Microscopy Sciences). Semithin sections were stained with toluidine blue for analysis at the light microscope, and ultrathin sections were contrasted with 3% uranyl acetate and 1% lead citrate before observation in a transmission electron microscope (H-600; Hitachi) at 75 kV.
Immunofluorescence, TUNEL staining, and X-gal staining
After fixation with 4% PFA, sciatic nerve sections were blocked for 1 h with 10% goat serum and 0.1% Triton X-100 in PBS. Incubation with primary antibodies was performed overnight at 4°C. Rat monoclonal antibodies against myelin basic protein (1:50; Serotec) and Ki67 (1:50; DakoCytomation), mouse monoclonal antibodies against NF160 (1:150; Sigma-Aldrich), and polyclonal antibodies against Oct6 (Ghazvini et al., 2002) were used. On the following day, tissue sections were washed in PBS and incubated with the appropriate secondary antibodies for 1 h at room temperature. Secondary antibodies conjugated to fluorescein or rhodamine were obtained from Jackson Immunoresearch Laboratories. The sections were mounted in Citifluor (Citifluor Ltd) containing DAPI to stain the nuclei.
Mouse SC cultures were fixed in 4% PFA in MP buffer (65 mM Pipes, 25 mM Hepes, 10 mM EGTA, and 3 mM MgCl2, pH 6.9) for 10 min at room temperature. The cells were permeabilized with 0.2% Triton X-100 in MP buffer for 5 min at room temperature. Primary monoclonal antibody against α-tubulin (1:100; Sigma-Aldrich) and Alexa Fluor 488 phalloidin (1:100; Invitrogen) were incubated in 1 mg/ml BSA in PBS overnight at 4°C, followed by incubation with Cy3-conjugated anti-mouse (1:200; Jackson ImmunoResearch Laboratories) at room temperature.
Apoptotic cell death was analyzed by TUNEL staining using biotin-labeled UTP and FITC-conjugated streptavidin complex according to the manufacturer's instructions (Roche Diagnostics). For X-gal staining, cells or sections were incubated overnight in PBS containing 5 mM potassium ferrocyanide, 5 mM potassium ferricyanide, 2 mM magnesium chloride, 0.1% sodium desoxycholate, 0.02% NP-40, and 2 mM X-gal (AppliChem).
Cells and tissue sections were visualized in a fluorescence microscope (Axioplan 2; Carl Zeiss MicroImaging, Inc.) equipped with 10×/NA 0.30, 20×/NA 0.50, and 40×/NA 0.75 Plan-Neofluar objectives (Carl Zeiss MicroImaging, Inc.). Images were acquired using a charge-coupled device camera (MRm Axiocam; Carl Zeiss MicroImaging, Inc.) connected to a PC running the Axiovision 4 acquisition software (Carl Zeiss MicroImaging, Inc.). Images were further processed (levels adjustment) using Photoshop 7.0 software (Adobe). Fig. 1 C was acquired as a black-and-white image, and artificial color was added using Photoshop.
Primary cell culture
Primary mouse SC cultures were obtained from P0–P2 sciatic nerves. In brief, nerves were digested in enzyme buffer (0.7 mg/ml collagenase type I and 0.25% Trypsin [Invitrogen] in HBSS [Invitrogen]). After trituration, cells were grown on poly-d-lysine/laminin–coated dishes in minimal medium plus 0.5% FCS. Minimal medium is DME/F12 (Invitrogen), containing 100 μg/ml human apo-transferrin, 60 ng/ml progesterone, 5 mg/ml insulin, 16 μg/ml putrescine, 400 ng/ml l-thyroxin, 160 ng/ml selenium, 10 ng/ml triiodothyronine, and 300 mg/ml BSA (Fluka). Supplements were obtained from Sigma-Aldrich unless stated otherwise.
Rat SCs were isolated from Wistar rats and grown in SC medium (DME; Invitrogen), containing 10% FCS, 50 μg/ml gentamicin (Sigma-Aldrich), 100 μg/ml crude GGF (BioReba Biotechnology, Inc.), and 2 μM forskolin (Sigma-Aldrich) as described previously (Atanasoski et al., 2004). Before the Rho GTPase activity assays and exposure to NRG1, freshly plated rat SCs were starved for 6 h in DME without forskolin, growth factors, and serum.
Western blot
Sciatic nerve tissue was homogenized with a chilled mortar and pestle in lysis buffer (0.1% SDS, 10 mM TrisHCl, 150 mM NaCl, 50 mM NaF, 1 mM NaVO4, 1 mM EDTA, 0.5% sodium-deoxycholate, and protease inhibitor cocktail [Sigma-Aldrich]). Extracts were processed using standard SDS-PAGE and Western blotting procedures. The following antibodies were used: Krox20 (1:100; Babco), β-actin (1:1,000; Sigma-Aldrich), α-tubulin (1:1,000; Sigma-Aldrich), GAPDH (1:20,000; HyTest), rac1 (1:1,000; BD Biosciences), and cdc42 (1:1,000; Santa Cruz Biotechnology, Inc.). Secondary antibodies were obtained from Pierce Chemical Co. and Santa Cruz Biotechnology, Inc. Immunoreactive proteins were detected using ECL (GE Healthcare). Densitometry and quantification of the relative levels were performed on scanned images of Western blots using Quantity One software (Bio-Rad Laboratories, Inc.).
Rho GTPase activity assay
The GST-PAK-CD construct was provided by J. Collard (The Netherlands Cancer Institute, Amsterdam, Holland). Rac1 or cdc42 activity was measured essentially as described previously (Sander et al., 1998). In brief, for each independent experiment, sciatic nerves from three P5 mutant mice, three P5 control mice, or rat SCs were pooled, homogenized in FISH buffer (10% glycerol, 50 mM Tris-HCl, pH 7.4, 100 mM NaCl, 1% NP-40, 2 mM MgCl2, and protease inhibitor cocktail), and centrifuged for 5 min at 21,000 g at 4°C. Aliquots were taken from the supernatant to determine the total protein amounts. The supernatant was incubated with bacterially produced GST-PAK-CD fusion protein, bound to glutathione-coupled Sepharose beads (GE Healthcare) at 4°C for 30 min. The beads and proteins bound to the fusion protein were washed three times in an excess of FISH buffer, eluted in Laemmli sample buffer, and analyzed for bound GTP-cdc42 or GTP-rac1 by Western blotting.
Statistical analysis
Data show the mean ± SD. Statistical significance was determined using t test. Significance was set at *, P < 0.05; **, P < 0.01; ***, P < 0.001. n represents the number of independent experiments, which was three unless stated otherwise.
Online supplemental material
Fig. S1 shows Bluogal precipitates in premyelinating and myelinating SCs of P24 Rac1 and Cdc42 mutant nerves and that in P24 Rac1 mutant nerves the levels of rac1 protein were reduced. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200610108/DC1.
Supplementary Material
Acknowledgments
This work was supported by a Technische Hochschule grant from Eidgenössische Technische Hochschule Zurich (Y. Benninger, T. Thurnherr, D. Herzog, U. Suter, and J.B. Relvas), by the Fundação para a Ciência e Tecnologia, Portugal (SFRH/BD/16677/2004; J. Pereira), and by the Swiss National Science Foundation and the National Centre for Competence in Research “Neural Plasticity and Repair” (U. Suter).
We thank Mike Peacock and Sebastian Knüsel for their assistance, Phil Soriano for ROSA26 mice, and Ned Mantei for critically reading the manuscript.
Y. Benninger and T. Thurnherr contributed equally to this paper.
Abbreviations used in this paper: BL, basal lamina; CNP, 2′,3′-cyclic nucleotide 3′-phosphodiesterase; E, embryonic day; NRG1, neuregulin-1; P, postnatal day; PNS, peripheral nervous system; SC, Schwann cell.
References
- Atanasoski, S., L. Notterpek, H.Y. Lee, F. Castagner, P. Young, M.U. Ehrengruber, D. Meijer, L. Sommer, E. Stavnezer, C. Colmenares, and U. Suter. 2004. The protooncogene Ski controls Schwann cell proliferation and myelination. Neuron. 43:499–511. [DOI] [PubMed] [Google Scholar]
- Benninger, Y., H. Colognato, T. Thurnherr, R.J. Franklin, D.P. Leone, S. Atanasoski, K.A. Nave, C. ffrench-Constant, U. Suter, and J.B. Relvas. 2006. Beta1-integrin signaling mediates premyelinating oligodendrocyte survival but is not required for CNS myelination and remyelination. J. Neurosci. 26:7665–7673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley, W.G., and M. Jenkison. 1975. Neural abnormalities in the dystrophic mouse. J. Neurol. Sci. 25:249–255. [DOI] [PubMed] [Google Scholar]
- Buttery, P.C., and C. ffrench-Constant. 1999. Laminin-2/integrin interactions enhance myelin membrane formation by oligodendrocytes. Mol. Cell. Neurosci. 14:199–212. [DOI] [PubMed] [Google Scholar]
- Chen, Z.L., and S. Strickland. 2003. Laminin γ1 is critical for Schwann cell differentiation, axon myelination, and regeneration in the peripheral nerve. J. Cell Biol. 163:889–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, H.L., M.L. Steinway, J.W. Russell, and E.L. Feldman. 2000. GTPases and phosphatidylinositol 3-kinase are critical for insulin-like growth factor-I-mediated Schwann cell motility. J. Biol. Chem. 275:27197–27204. [DOI] [PubMed] [Google Scholar]
- Chrostek, A., X. Wu, F. Quondamatteo, R. Hu, A. Sanecka, C. Niemann, L. Langbein, I. Haase, and C. Brakebusch. 2006. Rac1 is crucial for hair follicle integrity but is not essential for maintenance of the epidermis. Mol. Cell. Biol. 26:6957–6970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colognato, H., W. Baron, V. Avellana-Adalid, J.B. Relvas, A. Baron-Van Evercooren, E. Georges-Labouesse, and C. ffrench-Constant. 2002. CNS integrins switch growth factor signalling to promote target-dependent survival. Nat. Cell Biol. 4:833–841. [DOI] [PubMed] [Google Scholar]
- Colognato, H., C. ffrench-Constant, and M.L. Feltri. 2005. Human diseases reveal novel roles for neural laminins. Trends Neurosci. 28:480–486. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville, S., and A. Hall. 2002. Rho GTPases in cell biology. Nature. 420:629–635. [DOI] [PubMed] [Google Scholar]
- Feltri, M.L., D. Graus Porta, S.C. Previtali, A. Nodari, B. Migliavacca, A. Cassetti, A. Littlewood-Evans, L.F. Reichardt, A. Messing, A. Quattrini, et al. 2002. Conditional disruption of β1 integrin in Schwann cells impedes interactions with axons. J. Cell Biol. 156:199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garratt, A.N., S. Britsch, and C. Birchmeier. 2000. Neuregulin, a factor with many functions in the life of a schwann cell. Bioessays. 22:987–996. [DOI] [PubMed] [Google Scholar]
- Genoud, S., C. Lappe-Siefke, S. Goebbels, F. Radtke, M. Aguet, S.S. Scherer, U. Suter, K.A. Nave, and N. Mantei. 2002. Notch1 control of oligodendrocyte differentiation in the spinal cord. J. Cell Biol. 158:709–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazvini, M., W. Mandemakers, M. Jaegle, M. Piirsoo, S. Driegen, M. Koutsourakis, X. Smit, F. Grosveld, and D. Meijer. 2002. A cell type- specific allele of the POU gene Oct-6 reveals Schwann cell autonomous function in nerve development and regeneration. EMBO J. 21:4612–4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govek, E.E., S.E. Newey, and L. Van Aelst. 2005. The role of the Rho GTPases in neuronal development. Genes Dev. 19:1–49. [DOI] [PubMed] [Google Scholar]
- Grove, M., N.H. Komiyama, K.A. Nave, S.G. Grant, D.L. Sherman, and P.J. Brophy. 2007. FAK is required for axonal sorting by Schwann cells. J. Cell Biol. 176:277–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, A. 1998. Rho GTPases and the actin cytoskeleton. Science. 279:509–514. [DOI] [PubMed] [Google Scholar]
- Jaegle, M., W. Mandemakers, L. Broos, R. Zwart, A. Karis, P. Visser, F. Grosveld, and D. Meijer. 1996. The POU factor Oct-6 and Schwann cell differentiation. Science. 273:507–510. [DOI] [PubMed] [Google Scholar]
- Jaffe, A.B., and A. Hall. 2005. Rho GTPases: biochemistry and biology. Annu. Rev. Cell Dev. Biol. 21:247–269. [DOI] [PubMed] [Google Scholar]
- Jessen, K.R., and R. Mirsky. 2005. The origin and development of glial cells in peripheral nerves. Nat. Rev. Neurosci. 6:671–682. [DOI] [PubMed] [Google Scholar]
- Joseph, N.M., Y.S. Mukouyama, J.T. Mosher, M. Jaegle, S.A. Crone, E.L. Dormand, K.F. Lee, D. Meijer, D.J. Anderson, and S.J. Morrison. 2004. Neural crest stem cells undergo multilineage differentiation in developing peripheral nerves to generate endoneurial fibroblasts in addition to Schwann cells. Development. 131:5599–5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappe-Siefke, C., S. Goebbels, M. Gravel, E. Nicksch, J. Lee, P.E. Braun, I.R. Griffiths, and K.A. Nave. 2003. Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nat. Genet. 33:366–374. [DOI] [PubMed] [Google Scholar]
- Lindeboom, F., N. Gillemans, A. Karis, M. Jaegle, D. Meijer, F. Grosveld, and S. Philipsen. 2003. A tissue-specific knockout reveals that Gata1 is not essential for Sertoli cell function in the mouse. Nucleic Acids Res. 31:5405–5412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, J.R., and H.D. Webster. 1973. Mitotic Schwann cells in developing nerve: their changes in shape, fine structure, and axon relationships. Dev. Biol. 32:417–431. [DOI] [PubMed] [Google Scholar]
- Mirsky, R., and K.R. Jessen. 1999. The neurobiology of Schwann cells. Brain Pathol. 9:293–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai, Y., Y. Zheng, M. MacCollin, and N. Ratner. 2006. Temporal control of Rac in Schwann cell-axon interaction is disrupted in NF2-mutant schwannoma cells. J. Neurosci. 26:3390–3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobes, C.D., P. Hawkins, L. Stephens, and A. Hall. 1995. Activation of the small GTP-binding proteins rho and rac by growth factor receptors. J. Cell Sci. 108:225–233. [DOI] [PubMed] [Google Scholar]
- Pelton, P.D., L.S. Sherman, T.A. Rizvi, M.A. Marchionni, P. Wood, R.A. Friedman, and N. Ratner. 1998. Ruffling membrane, stress fiber, cell spreading and proliferation abnormalities in human Schwannoma cells. Oncogene. 17:2195–2209. [DOI] [PubMed] [Google Scholar]
- Relvas, J.B., A. Setzu, W. Baron, P.C. Buttery, S.E. LaFlamme, R.J. Franklin, and C. ffrench-Constant. 2001. Expression of dominant-negative and chimeric subunits reveals an essential role for beta1 integrin during myelination. Curr. Biol. 11:1039–1043. [DOI] [PubMed] [Google Scholar]
- Ridley, A.J., H.F. Paterson, C.L. Johnston, D. Diekmann, and A. Hall. 1992. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 70:401–410. [DOI] [PubMed] [Google Scholar]
- Sander, E.E., S. van Delft, J.P. ten Klooster, T. Reid, R.A. van der Kammen, F. Michiels, and J.G. Collard. 1998. Matrix-dependent Tiam1/Rac signaling in epithelial cells promotes either cell-cell adhesion or cell migration and is regulated by phosphatidylinositol 3-kinase. J. Cell Biol. 143:1385–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz, M.A., and S.J. Shattil. 2000. Signaling networks linking integrins and rho family GTPases. Trends Biochem. Sci. 25:388–391. [DOI] [PubMed] [Google Scholar]
- Soriano, P. 1999. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 21:70–71. [DOI] [PubMed] [Google Scholar]
- Stirling, C.A. 1975. Abnormalities in Schwann cell sheaths in spinal nerve roots of dystrophic mice. J. Anat. 119:169–180. [PMC free article] [PubMed] [Google Scholar]
- Terashima, T., H. Yasuda, M. Terada, S. Kogawa, K. Maeda, M. Haneda, A. Kashiwagi, and R. Kikkawa. 2001. Expression of Rho-family GTPases (Rac, cdc42, RhoA) and their association with p-21 activated kinase in adult rat peripheral nerve. J. Neurochem. 77:986–993. [DOI] [PubMed] [Google Scholar]
- Thurnherr, T., Y. Benninger, X. Wu, A. Chrostek, S.M. Krause, K.A. Nave, R.J. Franklin, C. Brakebusch, U. Suter, and J.B. Relvas. 2006. Cdc42 and Rac1 signaling are both required for and act synergistically in the correct formation of myelin sheaths in the CNS. J. Neurosci. 26:10110–10119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topilko, P., S. Schneider-Maunoury, G. Levi, A. Baron-Van Evercooren, A.B. Chennoufi, T. Seitanidou, C. Babinet, and P. Charnay. 1994. Krox-20 controls myelination in the peripheral nervous system. Nature. 371:796–799. [DOI] [PubMed] [Google Scholar]
- Wang, L., and Y. Zheng. 2007. Cell type-specific functions of Rho GTPases revealed by gene targeting in mice. Trends Cell Biol. 17:58–64. [DOI] [PubMed] [Google Scholar]
- Webster, H.D. 1971. The geometry of peripheral myelin sheaths during their formation and growth in rat sciatic nerves. J. Cell Biol. 48:348–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg, H.J., P.S. Spencer, and C.S. Raine. 1975. Aberrant PNS development in dystrophic mice. Brain Res. 88:532–537. [DOI] [PubMed] [Google Scholar]
- Wu, X., F. Quondamatteo, T. Lefever, A. Czuchra, H. Meyer, A. Chrostek, R. Paus, L. Langbein, and C. Brakebusch. 2006. Cdc42 controls progenitor cell differentiation and beta-catenin turnover in skin. Genes Dev. 20:571–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada, K.M., and S. Even-Ram. 2002. Integrin regulation of growth factor receptors. Nat. Cell Biol. 4:E75–E76. [DOI] [PubMed] [Google Scholar]
- Yamauchi, J., J.R. Chan, and E.M. Shooter. 2003. Neurotrophin 3 activation of TrkC induces Schwann cell migration through the c-Jun N-terminal kinase pathway. Proc. Natl. Acad. Sci. USA. 100:14421–14426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi, J., Y. Miyamoto, A. Tanoue, E.M. Shooter, and J.R. Chan. 2005. Ras activation of a Rac1 exchange factor, Tiam1, mediates neurotrophin-3-induced Schwann cell migration. Proc. Natl. Acad. Sci. USA. 102:14889–14894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, D., J. Bierman, Y.S. Tarumi, Y.P. Zhong, R. Rangwala, T.M. Proctor, Y. Miyagoe-Suzuki, S. Takeda, J.H. Miner, L.S. Sherman, et al. 2005. Coordinate control of axon defasciculation and myelination by laminin-2 and -8. J. Cell Biol. 168:655–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, W.M., M.L. Feltri, L. Wrabetz, S. Strickland, and Z.L. Chen. 2005. Schwann cell-specific ablation of laminin gamma1 causes apoptosis and prevents proliferation. J. Neurosci. 25:4463–4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.