Abstract
During epithelial tumor progression, the loss of E-cadherin expression and inappropriate expression of mesenchymal cadherins coincide with increased invasiveness. Reexpression experiments have established E-cadherin as an invasion suppressor. However, the mechanism by which E-cadherin suppresses invasiveness and the role of mesenchymal cadherins are poorly understood. We show that both p120 catenin and mesenchymal cadherins are required for the invasiveness of E-cadherin–deficient cells. p120 binding promotes the up-regulation of mesenchymal cadherins and the activation of Rac1, which are essential for cell migration and invasiveness. p120 also promotes invasiveness by inhibiting RhoA activity, independently of cadherin association. Furthermore, association of endogenous p120 with E-cadherin is required for E-cadherin–mediated suppression of invasiveness and is accompanied by a reduction in mesenchymal cadherin levels. The data indicate that p120 acts as a rheostat, promoting a sessile cellular phenotype when associated with E-cadherin or a motile phenotype when associated with mesenchymal cadherins.
Introduction
E-cadherin is the main epithelial cell–cell adhesion molecule, and either it is functionally disrupted or its expression is lost altogether during tumor progression (Berx and Van Roy, 2001; Conacci-Sorrell et al., 2002). The effect is likely to be direct, because reestablishing E-cadherin function in cadherin-deficient cell lines can reverse the invasive phenotype (Vleminckx et al., 1991). Moreover, experiments in transgenic mice strongly suggest that loss of E-cadherin directly promotes the transition of a benign adenoma into a carcinoma (Perl et al., 1998).
The mechanism by which E-cadherin suppresses invasiveness is still unclear. The intracellular domain of E-cadherin interacts directly with β-catenin and p120 catenin (p120) via separate conserved interaction domains. β-Catenin binding was recently shown to be important for the anti-invasive properties of E-cadherin (Wong and Gumbiner, 2003), although neither increased cell adhesion nor reduced nuclear β-catenin signaling was required for this effect. Unlike β-catenin, p120 has not been implicated in E-cadherin–mediated suppression of invasiveness, although it mislocalizes to the cytoplasm of E-cadherin–deficient cells. This altered localization of p120 in breast or colon carcinomas is prognostic for aggressive disease (Sarrio et al., 2004; Bellovin et al., 2005).
Epithelial-to-mesenchymal transition is a process associated with normal development and wound healing, but its aberrant regulation contributes to cancer progression and metastasis (Thiery, 2002). Epithelial-to-mesenchymal transition is associated with loss of E-cadherin expression and increased expression of mesenchymal cadherins. Indeed, overexpression studies have suggested that increased expression of mesenchymal cadherins (N-cadherin, R-cadherin, and cadherin 11) increases the motility and invasiveness of epithelial cells (Nieman et al., 1999; Hazan et al., 2000; Feltes et al., 2002; Suyama et al., 2002). It is currently unclear whether endogenous mesenchymal cadherins are required for the increased motility/invasiveness of E-cadherin–deficient cells.
The Rho family of GTPases (e.g., RhoA, Rac1, and Cdc42) mediate cytoskeletal dynamics (Nobes and Hall, 1995) and are crucial regulators of both cell motility (Titus et al., 2005) and cadherin-dependent cell adhesion (Braga, 2002). As such, Rho GTPases are thought either to promote intercellular adhesion or to induce cell migration, depending on signals received from the microenvironment. Signaling from the cadherin complexes to Rho GTPases is thought to depend on p120 (Anastasiadis and Reynolds, 2001).
Recent data indicate that p120 binding promotes the stabilization of cadherin complexes on the plasma membrane and thus strengthens cell–cell adhesion (Davis et al., 2003; Xiao et al., 2003). In some cases, p120 can also negatively affect cell adhesion, although the mechanism of this effect remains unclear. p120 overexpression induces dramatic changes in cell morphology and increases cell motility (for review see Anastasiadis and Reynolds, 2001). These effects are apparently mediated by the ability of p120 to suppress RhoA activity (Anastasiadis et al., 2000; Noren et al., 2000) and induce the activities of the related Rho GTPases Rac1 and Cdc42 (Noren et al., 2000; Grosheva et al., 2001). E-cadherin overexpression blocks the effects of p120 on cell morphology, suggesting that the recruitment of p120 to E-cadherin complexes reduces its effects toward Rho GTPases and possibly affects the balance between sessile and motile states.
Using E-cadherin–deficient cells, we show that endogenous p120 mediates both the invasion-promoting effects of mesenchymal cadherins and the invasion-suppressing action of ectopically expressed E-cadherin. Endogenously expressed mesenchymal cadherins are essential for the invasiveness of E-cadherin–deficient cells, and their levels depend on p120 association. Furthermore, p120-induced Rac activation requires binding of p120 to mesenchymal cadherins and promotes invasiveness. p120 also promotes invasiveness by inhibiting RhoA in a cadherin-independent manner. The data indicate that endogenous p120 is an important contributor to both the invasive phenotype of E-cadherin–deficient carcinomas and the sessile phenotype of E-cadherin–expressing epithelial cells.
Results
Endogenous p120 promotes invasiveness in E-cadherin–deficient cells
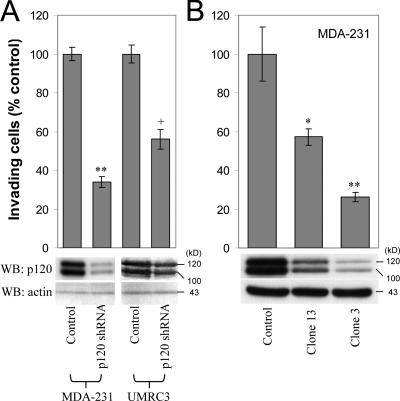
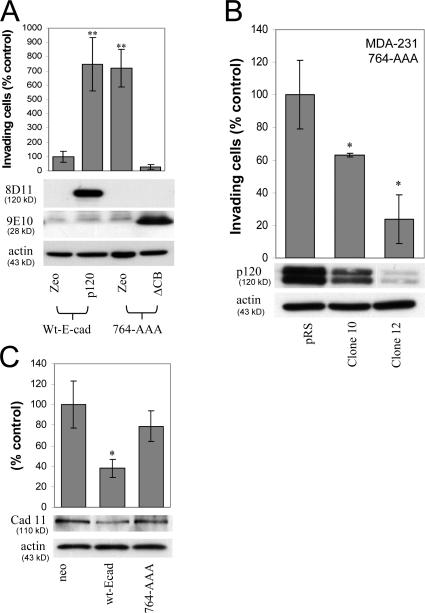
To test whether endogenous p120 promotes the invasiveness of E-cadherin–deficient tumor cells, we measured the invasiveness of cells with diminished p120 expression. MDA-MB-231 (MDA-231) and UMRC3 cells were infected with a retroviral vector (pRS) expressing short hairpin RNA (shRNA) targeted against human p120, as described previously (Davis et al., 2003). UMRC3 cells are highly metastatic renal carcinoma cells that lack E-cadherin expression. Polyclonal cell populations with reduced p120 expression were tested for invasiveness in Matrigel-coated transwells toward a gradient of hepatocyte growth factor (HGF; MDA-231 cells) or 5% FBS (UMRC3 cells). HGF was used as a chemoattractant because blocking HGF signaling prevents MDA-231 metastasis in nude mice (Jiang et al., 2003). As shown in Fig. 1 A, reduction of endogenous p120 levels resulted in significantly reduced invasiveness in both cell lines. Next, we selected individual clones of shRNA-expressing MDA-231 cells with varying expression of endogenous p120 and tested their invasiveness toward HGF. Fig. 1 B demonstrates that the invasiveness of MDA-231 cells in vitro is proportional to the levels of endogenous p120. Control experiments verified that cell line growth was not significantly affected by p120 depletion under our experimental conditions. Identical results were obtained using anti-p120–specific RNAi compared with control RNAi (unpublished data). Combined, the data strongly suggest that endogenous p120 is required for the invasiveness of E-cadherin–deficient cells.
Figure 1.
Endogenous p120 promotes invasiveness. (A) p120 depletion blocks invasiveness. E-cadherin–deficient MDA-231 and UMRC3 cells were infected with control pRS retrovirus or with a retrovirus expressing anti-human p120-specific shRNA. Polyclonal cell populations were generated, and invasion assays were performed in vitro using 20 ng/ml HGF as a chemoattractant for MDA-231 cells or 5% FBS for the UMRC3 cells. After a 20-h incubation, cells on the underside of a Matrigel-coated transwell membrane were counted under a 20× objective (n = 6). (B) Endogenous p120 levels are directly correlated to cell invasion. MDA-231 cells were infected with control retrovirus or retrovirus expressing p120-specific shRNA. Individual clones of shRNA-expressing cells were selected and tested for levels of endogenous p120 and invasiveness in vitro, toward HGF (n = 6). (bottom) Lysates from all cell lines were subjected to SDS-PAGE and Western blotted for expression of endogenous p120 (using mAb pp120) and actin. The two p120 bands correspond to p120 isoforms 1 (top) and 3 (bottom). Error bars indicate SEM. *, P < 0.05; **, P < 0.01; +, P < 0.05 as compared to pRS-infected (control) UMRC3 cells.
Ectopic reexpression of p120, but not of a cadherin-uncoupled p120 mutant, rescues motility and invasiveness
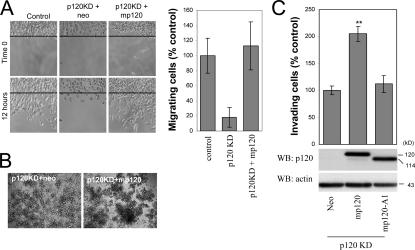
To further validate the in vitro invasion data, we measured cell migration using a scratch-wound assay. Within 12 h, control pRS/Neo-expressing cells moved to fill in the wound much more efficiently than cells expressing human-specific p120 shRNA (p120KD; Fig. 2 A). Expression of full-length murine p120 (mp120) in p120KD cells rescued cell migration in response to HGF. The data argue that endogenous p120 mediates HGF-induced cell migration.
Figure 2.
A cadherin-uncoupled p120 mutant does not promote motility and invasiveness. (A) Ectopic expression of murine p120 rescues the migration of p120-depleted cells. p120-depleted MDA-231 cells (clone 3; Fig. 1 B) were infected with control neo retrovirus or retrovirus expressing murine p120. After G418 selection, stable polyclonal cell lines were used in a scratch-wound assay to test their migration in response to HGF. Images shown are 0 and 12 h after HGF addition. The line denotes the cell front at time 0. The number of individual cells that crossed the line after 12 h was counted, and results were expressed as a percentage of migrating cells compared with control (MDA-231-pRS). n = 9. (B) The increased migration of p120-expressing cells is not due to reduced cell–cell adhesion. Cells were suspended as hanging drops and allowed to aggregate overnight. The strength of cell adhesion was assessed after passing the cell aggregates 10 times through a standard 200-μl pipette tip. Note that p120-expressing cells are more resistant to dissaggregation than cells depleted of endogenous p120. (C) MDA-231 cells with knocked down expression of endogenous p120 (clone 3) were infected with retroviruses expressing neo resistance alone or together with full-length murine p120 or a murine p120 mutant (mp120-A1), which is unable to bind E-cadherin. The invasiveness of p120-reexpressing cells was tested in vitro toward HGF (n = 6). (bottom) Lysates from all cell lines were subjected to SDS-PAGE and Western blotted for expression of p120 (using mAb pp120) and actin. Error bars indicate SEM. **, P < 0.01.
To address the possibility that p120 promotes motility by decreasing cell–cell adhesion, we performed cell aggregation–disaggregation assays. Cells were allowed to form multicellular spheroid aggregates after overnight incubation in a hanging drop of media. Cell aggregates were then pipetted 10 times with a small bore tip, and the extent of cell disaggregation was assessed as a measure of adhesion strength. As can be seen in Fig. 2 B, ectopic expression of mp120 in p120KD cells moderately increased cell–cell adhesion, suggesting that increased motility and invasiveness in response to p120 is not due to reduced adhesion.
Finally, we examined the invasiveness of p120KD cells expressing murine p120 or a p120 mutant that lacks the first Armadillo domain (A1) and is unable to associate with E-cadherin (Ireton et al., 2002). As shown in Fig. 2 C, expression of full-length p120 reversed the p120KD effect and increased cell invasiveness. In contrast, ectopic expression of the p120 A1 mutant (mp120-A1) failed to rescue invasiveness. The data indicate that the effects of p120 depletion on cell invasion and motility are indeed p120 dependent. They also suggest that cadherin binding is required for the effects of p120 on invasion.
Endogenous mesenchymal cadherins promote the invasiveness of E-cadherin–deficient epithelial cells
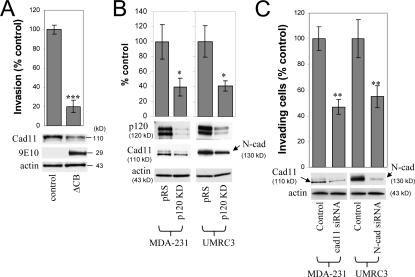
The inability of a cadherin-uncoupled p120 mutant to promote invasiveness suggested the possibility that in these E-cadherin–deficient cells, p120 induces invasiveness via its association with mesenchymal cadherins. MDA-231 cells lack E-cadherin expression but express mesenchymal cadherin 11, whereas UMRC3 cells lack E-cadherin expression but express mesenchymal N-cadherin (unpublished data). Both of these cadherins have been shown to promote motility and invasiveness when overexpressed in tumor cells (Nieman et al., 1999; Hazan et al., 2000). To test the possibility that endogenous p120 acts via mesenchymal cadherins to promote invasiveness, we initially expressed a short myc-tagged fragment of the E-cadherin cytoplasmic tail in MDA-231 cells. This small E-cadherin fragment (ΔCB) binds avidly to p120, but not β-catenin, and recruits it to the cytosol away from any endogenous cadherins (Anastasiadis et al., 2000). As can be seen in Fig. 3 A, expression of ΔCB significantly reduced MDA-231 cell invasiveness. Furthermore, ΔCB expression caused a marked reduction in endogenous cadherin 11 levels, consistent with the hypothesis that p120 binding regulates the levels of endogenous cadherins (Ireton et al., 2002; Davis et al., 2003; Xiao et al., 2003). To directly test whether endogenous p120 regulates the levels of mesenchymal cadherins in E-cadherin–deficient cells, we determined the expression of mesenchymal cadherins after p120 depletion. Fig. 3 B shows that depletion of endogenous p120 reduced mesenchymal cadherin expression in both MDA-231 and UMRC3 cells.
Figure 3.
Endogenous mesenchymal cadherins promote cell invasiveness. (A) Expression of the E-cadherin juxtamembrane domain inhibits cadherin 11 levels and blocks invasiveness. MDA-231 cells were infected with control retrovirus (zeo) or retrovirus expressing a small, p120-binding, myc-tagged fragment of the E-cadherin cytoplasmic tail (ΔCB) that cannot associate with β-catenin. Polyclonal stable cell lines were subjected to invasion assays, toward a gradient of HGF (n = 6). (bottom) Lysates from both cell lines were subjected to SDS-PAGE and Western blotted for expression of cadherin 11, ΔCB (using the myc tag–specific mAb 9E10), and actin. (B) p120 regulates the levels of endogenous mesenchymal cadherins. Polyclonal populations of p120-depleted MDA-231 or UMRC3 cells were examined for their expression of cadherin 11 or N-cadherin, respectively. Note that upon p120 depletion (top), levels of endogenous mesenchymal cadherins (middle and graphs) are reduced (n = 3). (C) Endogenous mesenchymal cadherins promote cell invasiveness. MDA-231 and UMRC3 cells were transiently transfected by electroporation with either control siRNA or siRNA specific for human cadherin 11 or N-cadherin, respectively. Control experiments verified maximal knock down of cadherin expression 3 d after transfection. 2 d after transfection, cells were serum starved overnight and plated in Matrigel-coated transwells. Invasiveness was determined 24 h later in response to a gradient of HGF (MDA-231) or serum (UMRC3). n = 6. Error bars indicate SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Finally, to determine whether endogenously expressed mesenchymal cadherins promote invasion, we treated MDA-231 and UMRC3 cells with siRNAs against cadherin 11 or N-cadherin, respectively, and measured cell invasiveness. In both cases, reduction of the endogenous mesenchymal cadherin levels resulted in significant inhibition of cell invasiveness, indicating that endogenous mesenchymal cadherins mediate motility and invasiveness in E-cadherin–deficient cells (Fig. 3 C).
p120 association promotes endogenous mesenchymal cadherin expression and invasiveness
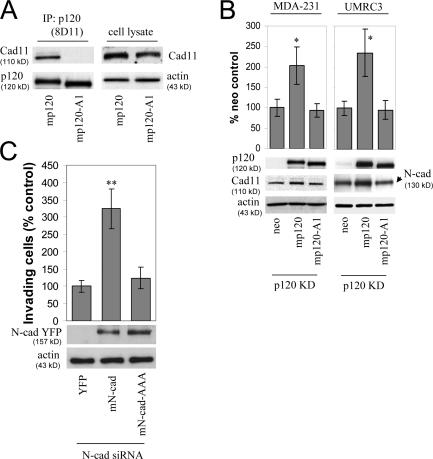
The data in Fig. 3 suggested that one way by which p120 promotes invasiveness is by binding to and regulating the levels of mesenchymal cadherins. Consistent with this hypothesis, the E-cadherin–uncoupled p120 A1 mutant was unable to promote invasiveness when expressed in p120KD cells (Fig. 2 C). However, it is not known whether this p120 mutant (p120-A1) is also unable to associate with mesenchymal cadherins and whether its expression affects mesenchymal cadherin levels. To answer the first question, we performed coimmunoprecipitation assays using full-length p120 as a control. As shown in Fig. 4 A, ectopically expressed murine p120 coprecipitated with endogenous cadherin 11 from MDA-231 cells, whereas the p120-A1 mutant did not. Similar results were also obtained for N-cadherin (unpublished data), indicating that the p120-A1 mutant is uncoupled from both epithelial and mesenchymal cadherins.
Figure 4.
p120 binding regulates the levels of endogenous mesenchymal cadherins. (A) The p120-A1–deletion mutant does not bind mesenchymal cadherins. Lysates of p120-depleted MDA-231 cells (clone 3) reexpressing murine p120 (mp120) or a murine p120 mutant lacking the first armadillo domain (mp120-A1) were immunoprecipitated with the murine p120-specific mAb 8D11. Precipitates were then subjected to SDS-PAGE and Western blotted for the presence of cadherin 11 or p120. As shown, cadherin 11 coimmunoprecipitates with wt p120 but not with mp120-A1. (right) Expression of cadherin 11 in total cell lysates of both cell lines. (B) The p120-A1–deletion mutant does not regulate the levels of endogenous mesenchymal cadherins. p120-depleted MDA-231 cells (clone 3) stably expressing control neo, mp120, or mp120-A1 were lysed and subjected to SDS-PAGE and Western blotted for expression of p120, cadherin 11, or actin as a loading control (left). (right) Similar Western blots of p120-depleted UMRC3 cells blotted for expression of N-cadherin. In both cases, reexpression of mp120, but not mp120-A1, increases mesenchymal cadherin levels. (C) p120 binding is required for the invasion-promoting effects of N-cadherin. UMRC3 cells were transiently transfected with human N-cadherin–specific siRNA together with control YFP, murine N-cadherin–YFP, or N-cad-AAA-YFP, a murine N-cadherin mutant unable to bind p120 (N-cad-AAA). In contrast to wt N-cadherin, N-cad-AAA failed to promote invasiveness. Error bars indicate SEM. *, P < 0.05; **, P < 0.01.
As shown earlier, p120 depletion caused a reduction in mesenchymal cadherin levels (Fig. 3 C). Fig. 4 B shows that p120 reexpression increased the levels of both cadherin 11 and N-cadherin in MDA-231 and UMRC3 cells, respectively. In contrast, expression of the cadherin-uncoupled p120-A1 mutant had no effect on mesenchymal cadherin expression, in agreement with a requirement for p120 association.
We next tested the ability of a p120-uncoupled N-cadherin mutant to promote invasiveness. Fig. 4 C shows that ectopic expression of murine N-cadherin in UMRC3 cells depleted of endogenous N-cadherin rescued cell invasiveness. However, expression of a p120-uncoupled N-cadherin mutant (N-cad-AAA; Chen et al., 2003) failed to promote invasiveness. The data argue that p120 promotes motility and invasiveness by binding to mesenchymal cadherins. The association promotes cadherin stability and possibly signaling events that induce cell migration.
p120-mediated Rac activation requires mesenchymal cadherin binding
Having established that p120 and mesenchymal cadherins are required for cell motility and invasiveness, we next examined possible mechanisms for this action. Given the ability of p120 to affect the activity of Rho-family GTPases, we first asked whether p120 and mesenchymal cadherins cooperatively regulate the activity of Rho GTPases in E-cadherin–deficient cells.
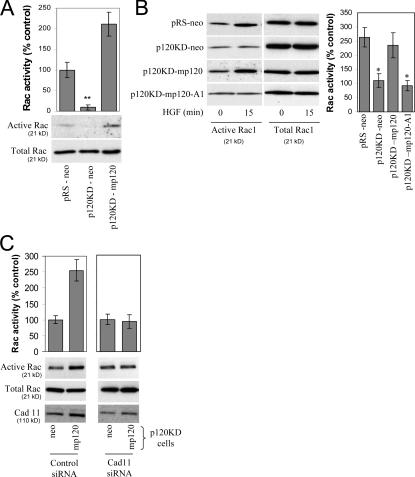
Initially, we examined the activity of Rac1 in control cells (pRS-neo), p120-depleted cells (p120KD-neo), and cells reexpressing p120 (p120KD-mp120), under basal, serum-starved conditions. As shown in Fig. 5 A, under basal conditions, p120 depletion resulted in significant reduction of Rac1 activity, which was reversed by expression of murine p120. p120 depletion also affected Rac1 activity of HGF-treated cells. Fig. 5 B shows that Rac1 activity was increased in control pRS-neo cells treated with HGF but not in p120-depleted cells (p120KD-neo). Expression of murine p120 in p120-depleted cells (p120KD-mp120) restored HGF-mediated Rac1 activation. In contrast, expression of the cadherin-uncoupled p120-A1 mutant failed to restore Rac1 activation, suggesting that p120-mediated activation of Rac1 requires mesenchymal cadherin binding.
Figure 5.
p120-mediated Rac activation requires mesenchymal cadherin binding. (A) Endogenous p120 regulates basal Rac1 activity. Levels of total and GTP-bound Rac1 were determined in serum-starved control MDA-231 cells (pRS-neo), p120-depleted cells (p120KD-neo), or cells reexpressing p120 (p120KD-mp120). Data are also expressed as a percentage of Rac1 activity, determined by densitometric analysis of immunoblots compared with the pRS-neo controls (n = 6). (B) p120 regulates HGF-induced Rac1 activity. Levels of total and GTP-bound Rac1 were determined in the same serum-starved cell lines as above, as well as in p120KD cells expressing mp120-A1, after incubation for 15 min with HGF. Both the p120-depleted cells and cells expressing mp120-A1 failed to induce Rac1 in response to HGF. Data are also expressed as a percentage of Rac activity at time 0 (control). n = 3. (C) Cadherin 11 is required for p120-dependent Rac1 induction. p120KD MDA-231 cells expressing either neo control or mp120 were transiently transfected using electroporation with control or cadherin 11 siRNA. Cells were then serum starved overnight, and the activity of Rac1 was determined using pull-down assays after a 15-min incubation with HGF. Data was also expressed as a percentage of Rac1 activity compared with the neo controls (n = 6). Error bars indicate SEM. *, P < 0.05; **, P < 0.01.
Finally, if the ability of p120 to induce Rac1 activation requires cadherin association, depletion of endogenous cadherins should block p120-mediated Rac1 activation. To test this, we used either control p120-depleted cells (p120KD-neo) or p120-depleted cells reexpressing p120 (p120KD-mp120). Rac1 activation in response to HGF (Fig. 5 B) is p120 dependent under these conditions. Fig. 5 C shows that the ability of p120 to mediate HGF-induced Rac1 activation depends on the levels of cadherin 11, as a cadherin 11–specific siRNA blocks p120-mediated Rac1 activation, whereas a control siRNA has no effect. As expected, cadherin 11 depletion also inhibited HGF-induced Rac1 activation in parental MDA-231 cells (unpublished data). Collectively, these data strongly argue that the p120-mediated activation of Rac1 requires association of p120 with mesenchymal cadherins.
The p120-mediated inhibition of RhoA in E-cadherin–deficient cells is independent of mesenchymal cadherin expression
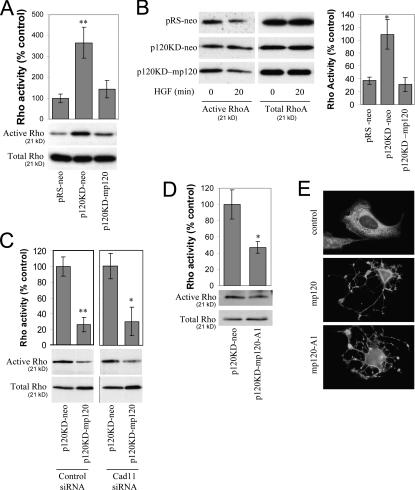
Previous studies have shown that p120 overexpression decreases RhoA activity (Anastasiadis et al., 2000; Noren et al., 2000; Grosheva et al., 2001). Thus, we examined the effect of p120 on RhoA activity under either basal (serum-starved) conditions or after HGF treatment. Fig. 6 A shows that under basal conditions, RhoA activity was significantly increased in p120-depleted cells (p120KD-neo) when compared with control cells (pRS-neo). Consistent with the increased RhoA activity, p120-depleted cells were flatter and contained more stress fibers than control cells (unpublished data). Expression of murine p120 reduced basal RhoA activity levels and restored normal cell morphology, indicating that p120 regulates RhoA activity. Similar results were obtained when cells were treated for 20 min with HGF; control cells and p120-reexpressing cells exhibited reduced Rho activity compared with p120-depleted cells (Fig. 6 B).
Figure 6.
p120 inhibits RhoA independently of mesenchymal cadherin expression. (A) Endogenous p120 regulates basal RhoA activity. Levels of total and GTP-bound RhoA were determined in serum-starved control MDA-231 cells (pRS-neo), p120-depleted cells (p120KD-neo), or cells reexpressing p120 (p120KD-mp120). Densitometric analysis of immunoblots was used to express the data as a percentage of RhoA activity, as compared with the pRS-neo controls (n = 6). (B) p120 regulates HGF-induced effects on RhoA activity. RhoA activity was determined in the same cell lines as explained above after incubation for 20 min with HGF. Data are also expressed as a percentage of Rho activity at time 0 (n = 3). (C) Cadherin 11 is dispensable for p120-dependent RhoA inhibition. p120KD MDA-231 cells expressing neo control or mp120 were transiently transfected with control or cadherin 11 siRNA. Cells were then serum starved overnight, and the activity of RhoA was determined after a 20-min incubation with HGF. Treatment with cadherin 11 siRNA failed to block the p120-mediated inhibition of RhoA under these conditions (n = 3). (D) The cadherin-uncoupled p120-A1 mutant can inhibit RhoA activity. p120-depleted MDA-231 cells expressing neo control or mp120-A1 were subjected to Rho pull-down assays to determine levels of active RhoA after 20 min of HGF treatment. Data are also expressed as a percentage of RhoA activity (n = 6). (E) mp120-A1 can induce “branching” in NIH3T3 cells. NIH3T3 cells were transiently transfected with mp120, mp120-A1, or pcDNA control. 24 h later, cells were fixed in methanol and stained for p120 with mAb 12F4. Photographs show the characteristic branching phenotype of p120-overexpressing cells. Error bars indicate SEM. *, P < 0.05; **, P < 0.01.
We then asked whether the p120-mediated reduction of RhoA activity requires binding to endogenous cadherins. Again, we used p120-depleted (p120KD-neo) and p120-reexpressing cells (p120KD-mp120) and examined RhoA activity in response to HGF treatment in the presence of cadherin 11 or control siRNA. Fig. 6 C shows that p120 can suppress RhoA activity even in cadherin 11–depleted cells, suggesting that cadherin 11 is not required for p120-induced RhoA inhibition. This conclusion was corroborated by the observation that the cadherin-uncoupled p120 A1 mutant, like wild-type (wt) p120, inhibits RhoA activity in MDA-231 cells (Fig. 6 D). Finally, like wt p120, which when overexpressed induces a branching morphology by inhibiting RhoA activity (Anastasiadis et al., 2000), mp120-A1 was able to induce a branching morphology in NIH3T3 cells (Fig. 6 E). The data suggest that, unlike p120-mediated Rac1 activation, the p120-mediated inhibition of RhoA is independent of mesenchymal cadherin expression in these cells.
Rho GTPases regulate the invasiveness of E-cadherin–deficient tumor cells
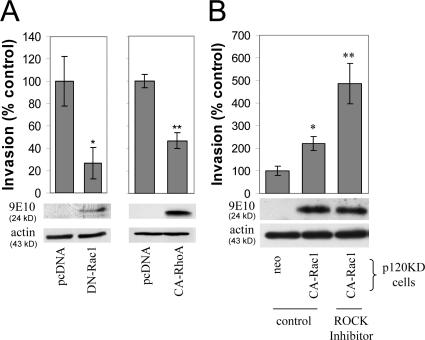
We next sought to establish that the p120-mediated changes in Rho GTPase activities were related to the increased cell migration and invasiveness of E-cadherin–deficient cells. First, we overexpressed constitutively active or dominant-negative Rac1 in MDA-231 cells and measured the in vitro invasiveness of serum-starved cells toward a gradient of HGF. Expression of dominant-negative Rac1 (DN-Rac1) significantly reduced cell invasiveness (Fig. 7 A), suggesting that Rac1 activation is required for HGF-induced invasiveness. Ectopic expression of constitutively active Rac1 did not increase cell invasiveness over cells expressing a vehicle control (pcDNA), suggesting that parental MDA-231 cells maximally activate Rac1 in response to HGF (unpublished data). To test the potential involvement of Rho activation in the invasiveness of MDA-231 cells, we expressed constitutively active RhoA and measured cell invasiveness in response to HGF. As shown in Fig. 7 A, increased levels of active RhoA significantly reduced the invasiveness of MDA-231 cells.
Figure 7.
Restoration of Rho GTPase signaling rescues the invasiveness of p120-depleted cells. (A) Inhibition of Rac1 and activation of RhoA activities block MDA-231 cell invasiveness. MDA-231 cells were transiently transfected by electroporation with a control pcDNA construct or with a myc-tagged pcDNA construct expressing either a dominant-negative N17-Rac1 mutant (DN-Rac1) or a constitutively active V14-RhoA mutant (CA-RhoA). 2 d after transfection, cells were plated in transwell filters, and invasion assays were performed a day later using HGF as a chemoattractant (n = 6). The expression of DN-Rac1 or CA-RhoA was monitored in cell lysates using the myc tag–specific mAb 9E10. (B) Activation of Rac1 and inhibition of Rho signaling promote invasiveness in p120-depleted cells. p120-depleted MDA-231 cells were infected either with retrovirus expressing control neo or with constitutively active Rac1. Stable cell lines were subjected to invasion assays in either control media or media containing a Rho kinase inhibitor (H-1152; 1.6 nM). n = 3. Error bars indicate SEM. *, P < 0.05; **, P < 0.01.
To directly correlate changes in Rho GTPase activities with p120-induced invasiveness, we tested whether activation of Rac1 and inhibition of RhoA signaling can restore the invasiveness of p120-depleted cells. As shown in Fig. 7 B, stable expression of constitutively active Rac1 (CA-Rac1) in p120-depleted MDA-231 cells increased invasiveness toward HGF in vitro. Inhibition of Rho signaling by incubating p120-depleted cells expressing CA-Rac1 with a Rho kinase inhibitor (H1152; 1.6 nM; Ikenoya et al., 2002) further promoted cell invasiveness. Together, the data argue that changes in Rac and Rho signaling are causally involved in p120-mediated effects on cell motility and invasiveness.
The suppression of cell migration and invasiveness by E-cadherin requires p120 binding and reduced expression of mesenchymal cadherins
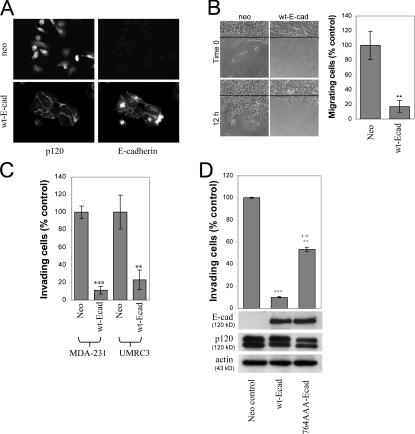
Having established that endogenous p120 promotes the invasive behavior of E-cadherin–deficient cells, we tested the hypothesis that recruitment of p120 to E-cadherin can suppress invasiveness. Ectopic expression of wt human E-cadherin in MDA-231 or UMRC3 cells was accomplished by retroviral infection, followed by G418 selection. More than 95% of infected cells expressed E-cadherin under these conditions. As shown in Fig. 8 A, MDA-231 cells infected with a control neo virus express no E-cadherin, have primarily cytoplasmic p120 staining, and exhibit scattered cell morphology. In contrast, cells expressing wt E-cadherin form epithelial colonies in which p120 is recruited to the E-cadherin–mediated cell–cell junctions. Identical results were also obtained with UMRC3 cells. The ectopic expression of E-cadherin significantly inhibited cell migration (Fig. 8 B) and blocked the ability of either MDA-231 or UMRC3 cells to invade in vitro (Fig. 8 C). Together, these experiments establish that both cell lines represent excellent model systems for studying the mechanism by which E-cadherin suppresses invasiveness and the possible involvement of p120.
Figure 8.
p120-uncoupled E-cadherin is less able to suppress invasiveness. (A) Ectopic E-cadherin recruits p120 to cell junctions. MDA-231 cells were infected with retroviruses expressing neomycin resistance alone or in combination with wt E-cadherin. More than 95% of G418-selected cells expressed wt E-cadherin. Wt E-cadherin accumulated at areas of cell–cell contact (mAb HECD-1). Endogenous p120 was primarily cytoplasmic in control cells and was recruited to cell–cell junctions in cells expressing wt E-cadherin (detected with polyclonal antibody F1αSH). (B) E-cadherin expression suppresses cell migration. After overnight serum starvation, confluent monolayers of control neo cells or MDA-231 cells expressing wt E-cadherin were scratched with a pipette tip, and cell migration into the wound was monitored over time in the presence of HGF. The number of individual cells that crossed the original cell front after 12 h was counted, and results were expressed as a percentage of migrating cells compared with neo control (n = 6). (C) E-cadherin suppresses cell invasiveness. The invasiveness of E-cadherin–expressing MDA-231 or UMRC3 cells was tested in vitro using HGF (MDA-231) or serum (UMRC3) as the chemoattractant (n = 6). (D) p120-uncoupled E-cadherin is less able to suppress invasiveness. Polyclonal populations of MDA-231 cells expressing neo control, wt E-cadherin, or a p120-uncoupled E-cadherin mutant (764-AAA) were subjected to invasion assays in response to HGF (n = 6). Error bars indicate SEM. **, P < 0.01; ***, P < 0.001; ++, P < 0.01, as compared with cells expressing wt E-cadherin (ANOVA).
Next, we compared the invasiveness of MDA-231 cells expressing a p120-uncoupled E-cadherin mutant (764-AAA; Thoreson et al., 2000) to that of control cells or cells expressing wt E-cadherin. As shown in Fig. 8 D, cells expressing the p120-uncoupled 764-AAA E-cadherin mutant were significantly more invasive than cells expressing wt E-cadherin but not as invasive as the neo controls. Control experiments verified that both cadherins were expressed at comparable levels and that cells expressing the p120-uncoupled E-cadherin mutant do form colonies and recruit β-catenin to the membrane (unpublished data; Thoreson et al., 2000).
To directly implicate endogenous p120 in E-cadherin–mediated suppression of invasiveness, we used two complimentary approaches. In the first, we overexpressed p120 in E-cadherin–expressing cells. In the second, we inhibited p120 function in cells expressing the p120-uncoupled E-cadherin mutant. Fig. 9 A shows that p120 overexpression increased the invasiveness of wt E-cadherin–expressing cells in vitro, suggesting that recruitment of endogenous p120 by E-cadherin suppresses invasion. Furthermore, the invasiveness of cells expressing the 764-AAA E-cadherin mutant was potently blocked by coexpression of ΔCB, the small cytoplasmic fragment of E-cadherin that binds selectively to p120 but not β-catenin. The data suggest that the increased invasiveness of cells expressing p120-uncoupled E-cadherin is due to its inability to recruit endogenous p120 to the E-cadherin complex. To confirm this hypothesis, we examined the effect of p120 depletion on the invasiveness of cells expressing the p120-uncoupled E-cadherin mutant. Retroviral expression of p120-specific shRNA, but not control shRNA, significantly reduced the invasiveness of these cells (Fig. 9 B). Examination of individual p120 shRNA clonal lines with varied endogenous p120 expression indicated that cell invasion is directly correlated with endogenous p120 levels (Fig. 9 B). Collectively, the data indicate that p120 binding is required for E-cadherin–mediated suppression of invasiveness.
Figure 9.
p120 binding to E-cadherin suppresses invasiveness and reduces mesenchymal cadherin levels. (A) E-cadherin–free p120 promotes invasiveness. MDA-MB-231 cells stably expressing wt E-cadherin or a p120-uncoupled E-cadherin mutant (764-AAA) were infected with retroviruses expressing zeomycin resistance alone (zeo) or together with murine p120 or ΔCB. ΔCB is a myc-tagged truncation mutant of the E-cadherin cytoplasmic domain, which binds p120 but not β-catenin. Invasion assays using HGF as chemoattractant were performed as described earlier (n = 6). (bottom) Lysates from all four cell lines were subjected to SDS-PAGE and Western blotted for expression of exogenous p120 (mAb 8D11 recognizes murine but not endogenous human p120), ΔCB (9E10), and actin as a loading control. (B) Endogenous p120 promotes the invasiveness of p120-uncoupled E-cadherin in a dose-dependent manner. MDA-231 cells expressing the p120-uncoupled 764-AAA E-cadherin mutant were infected with control pRS retrovirus or viruses expressing p120-specific shRNA. Individual clones of shRNA-expressing cells were selected and examined for levels of endogenous p120 and in vitro invasiveness toward HGF (n = 6). Note that levels of endogenous p120 in the clonal cell lines correlate directly to their invasiveness in vitro. (C) p120 recruitment to E-cadherin inhibits cadherin 11 levels. Lysates of MDA-231 cells stably expressing control neo, wt E-cadherin, or the p120-uncoupled 764-AAA E-cadherin mutant were subjected to SDS-PAGE and Western blotted for cadherin 11 levels or actin as a loading control. (top) Cadherin 11 levels are also expressed as a percentage of neo control (n = 3). Error bars indicate SEM. *, P < 0.05; **, P < 0.01.
Finally, we also examined the effects of E-cadherin reexpression on the levels of endogenous mesenchymal cadherins. As can be seen in Fig. 9 C, E-cadherin expression in MDA-231 cells resulted in a marked reduction of cadherin 11 levels. The data are consistent with the hypothesis that p120 recruitment to E-cadherin causes the loss of p120 binding to mesenchymal cadherins and subsequent reduction of their protein levels. Indeed, expression of p120-uncoupled E-cadherin largely reverses the loss of mesenchymal cadherin expression observed in cells expressing wt E-cadherin and, as shown earlier (Fig. 9 A), restores their invasiveness. Similar results were observed in UMRC3 cells (which express N-cadherin), demonstrating that this effect is not restricted to MDA-231 cells (unpublished data).
Discussion
Several previous studies have suggested that overexpression of p120 catenin (Noren et al., 2000; Grosheva et al., 2001) or overexpression of mesenchymal cadherins (Nieman et al., 1999; Hazan et al., 2000; Johnson et al., 2004) promotes cell migration. We have used E-cadherin–deficient cancer cells to test the hypothesis that p120 is required for the increased migration and invasiveness of these cells in vitro. The results reveal an essential role for p120 in both the migration and invasiveness of these cells and an unexpected role in mediating the proinvasive function of endogenous mesenchymal cadherins. Remarkably, association of endogenous p120 with E-cadherin is required for E-cadherin–mediated suppression of invasiveness and is accompanied by a concomitant reduction in mesenchymal cadherin levels. Mechanistically, p120 seems to regulate migration and invasiveness via three seemingly independent pathways: (1) the p120 association–dependent regulation of mesenchymal cadherin levels, (2) the induction of Rac1 activity after mesenchymal cadherin binding, and (3) the cadherin-independent inhibition of RhoA.
Our data demonstrate that mesenchymal cadherins are essential for the migration and invasiveness of E-cadherin–deficient tumor cells and that both the physical and the functional interaction with p120 are required for the proinvasive function of mesenchymal cadherins. The data are also consistent with a recent study suggesting that the p120-binding juxtamembrane domain of cadherin 11 is responsible for cadherin 11–mediated cell motility (Kiener et al., 2006).
It has been proposed that p120 mediates its effects on cell migration through regulation of Rho GTPases (for review see Anastasiadis and Reynolds, 2001). Interestingly, we show that both the basal and HGF-induced Rac1 activities are inhibited by p120 depletion. Furthermore, experiments using cadherin 11–depleted cells or cadherin-uncoupled p120 mutants indicate that the p120-induced Rac1 activation requires mesenchymal cadherin association and are in agreement with recent data suggesting that the p120-binding juxtamembrane domain is required for E-cadherin– or N-cadherin–induced Rac1 activation upon cell–cell adhesion (Goodwin et al., 2003; Gavard et al., 2004).
p120 depletion increased RhoA activity and decreased the activity of Rac1, providing a potential mechanistic explanation for the ability of dominant-active Rac mutants and dominant-negative RhoA mutants to rescue the defects induced by p120 depletion on Xenopus laevis gastrulation (Fang et al., 2004). However, unlike Rac activation, the data suggest that p120 inhibits RhoA in a cadherin-independent manner in these cells, in agreement with several previous investigations (Anastasiadis et al., 2000; Noren et al., 2000; Magie et al., 2002; Bellovin et al., 2005; Perez-Moreno et al., 2006).
To address the involvement of Rho GTPases in the invasiveness of our E-cadherin–deficient cells, we initially demonstrated that either reduced Rac1 or increased RhoA activities result in decreased cell invasiveness in vitro. Reduced Rac1 and increased RhoA activities mimic the effects of p120 depletion in these cells. Interestingly, activation of Rac1 and inhibition of RhoA signaling cooperatively restored the invasiveness of p120-depleted cells, arguing that changes in Rho GTPase signaling are causally involved in p120-mediated effects on cell motility and invasiveness.
The observation that endogenous p120 promotes the expression of mesenchymal cadherins and increases the invasiveness of E-cadherin–deficient cells suggested the possibility that p120 is also involved in the invasion-suppressive function of E-cadherin. In the simplest scenario, E-cadherin association would reduce the amount of p120 available to bind mesenchymal cadherins and promote invasiveness. Indeed, we show that E-cadherin suppresses invasion, at least in part, by binding endogenous p120. Furthermore, as predicted, mesenchymal cadherin levels were significantly reduced upon the expression of wt E-cadherin but not the p120-uncoupled E-cadherin mutant (764-AAA). The data reveal an important role for p120 binding in E-cadherin–mediated suppression of invasiveness and regulation of the motile or sessile phenotype of epithelial cells. It should be noted that the invasiveness of 764-AAA E-cadherin–expressing cells was lower than that of neo controls (Fig. 8 D), suggesting that a portion of the invasion-suppressive function of E-cadherin may not be related to p120 binding. It is likely that other factors, including recruitment of β-catenin (Wong and Gumbiner, 2003), play important roles in this process.
Our data indicate that p120 binds to and cooperates with mesenchymal cadherins to activate Rac1 and promote motility and invasiveness. However, it is unclear why E-cadherin suppresses migration, despite its ability to activate Rac1 in a p120-dependent manner after its homophilic interaction. One possibility is that the activation of Rac1 in the context of cadherin ligation is effectively different from activation of Rac1 in response to certain growth factors. In agreement with this, lamellipodium extension in response to cadherin activation is reportedly dependent on a PI-3-kinase–Rac1 pathway, whereas cadherin-mediated adhesion proceeds via a PI-3-kinase independent, Rac1-dependent pathway; both responses require the membrane association of p120 with the cadherin complex (Gavard et al., 2004). In addition, E-cadherin may be less effective than mesenchymal cadherins in promoting Rac1 activation in response to promigratory signals, or it may be more capable of suppressing growth factor signaling by sequestering and preventing the ligand-dependent activation of their receptors (Qian et al., 2004). Another possibility is that the differential ability of cadherins to recruit p120 to cell junctions may result in differential regulation of Rho activities. In support of these possibilities, the increased migration of R-cadherin overexpressing BT-20 cells, which normally express E-cadherin, correlates with increased Rac1 and reduced RhoA activities (Johnson et al., 2004), suggesting that E-cadherin and mesenchymal cadherins differentially affect Rho GTPases.
Finally, it is important to note that collagen-mediated integrin signaling can switch the effect of increased Rac1 activation from promoting E-cadherin–mediated adhesion to promoting cell migration (Sander et al., 1998). The data indicate that contextual signals can misdirect Rac signaling to promote cell migration, even in the presence of E-cadherin. Clearly, understanding the functional differences between E-cadherin and mesenchymal cadherins in regulating cell adhesion versus migration will be critical for understanding tumor progression to metastasis and events involved in tissue morphogenesis.
The data presented here imply that E-cadherin competes p120 away from mesenchymal cadherins, which then become destabilized. Further studies will be needed to address the relative affinities of p120 for different cadherins and how these affinities are affected by posttranslational modifications (e.g., p120 phosphorylation). It is possible that the functional disruption of the cadherin–catenin complex, which is often the result of Ras mutations or constitutive receptor tyrosine kinase signaling, promotes a more invasive phenotype by reducing the affinity of p120 for E-cadherin. As invasiveness was tested here using cell culture models, future studies are needed to show whether these results reflect invasive behavior in vivo. In any case, our data indicate that endogenous p120 acts as a rheostat, promoting a sessile cellular phenotype when associated with E-cadherin or a motile phenotype when associated with mesenchymal cadherins.
Materials and methods
Cell culture, infections, and transfections
Culture conditions have been described previously (Ireton et al., 2002). For shRNA expression, cells were infected with pRS and selected with 5 μg/ml puromycin. As indicated, some cells were infected again with LZRS-neo or -zeo and selected with 1 mg/ml G418 or 350 μg/ml zeocin. pRS and LZRS amphotropic retroviruses were produced as described previously (Davis et al., 2003). Clonal MDA-MB-231 cell lines were generated by limiting dilution. Amaxa electroporations were performed according to the company's protocol. In brief, 106 cells were resuspended in 100 μl solution T (Amaxa) containing 2 μg of plasmid DNA. Electroporation was performed using program A-23. Electroporated cells were plated in 60-mm dishes and incubated for 24 h in normal culture media. Cells were then washed in PBS and incubated for another 12 h in serum-free Dulbecco's minimal essential medium.
Immunofluorescence
Immunofluorescence localization procedures have been described in detail (Thoreson et al., 2000). The following primary antibodies were used: 0.5 μg/ml F1αSH p120 polyclonal antibody (Wu et al., 1998) and 1 μg/ml HECD-1 (Zymed Laboratories). The secondary antibodies used were goat anti-mouse Alexa 488 (Invitrogen) and goat anti-rabbit Alexa 596 at 1:600. Cells were visualized under a fluorescent microscope (DM5000B; Leica) using a 63×/1.4 HCX planApo oil objective (Leica). Photos were acquired with the FX4000 program (Leica) using a charge-coupled device camera (DFC350FX; Leica) and compiled in Photoshop (Adobe) and PowerPoint (Microsoft).
Constructs
LZRS-mp120 isoform 1A-neo, LZRS-mp120 A1-neo, LZRS-wt-E-cadherin-neo, and LZRS-764-AAA-neo were described previously (Ireton et al., 2002). The pRS vector was a gift from R. Agami (The Netherlands Cancer Institute, Amsterdam, Netherlands). pRS human p120 shRNA was also described previously (Davis et al., 2003). LZRS-MS-zeocin was provided by A. Reynolds (Vanderbilt University, Nashville, TN) and encodes for zeocin instead of neomycin resistance. Initially, LZRS-ΔCB-GFP was generated by subcloning an EcoRI fragment of pCAN-ΔCB (Anastasiadis et al., 2000) into the EcoRI site of LZRS-MS-GFP (LZRS-ΔCB-GFP). LZRS-ΔCB-zeocin was generated by subcloning a SgfI–SfiI fragment of LZRS-ΔCB-GFP (containing ΔCB) into the respective sites of the LZRS-MS-zeocin vector. The RhoA-V14-myc (CA-RhoA), Rac1-V12-myc (CA-Rac1), and Rac1-N17-myc (DN-Rac1) pcDNA3 constructs were all described previously (Anastasiadis et al., 2000). Murine N-cadherin-YFP and N-cadherin-AAA-YFP were provided by K.J. Green (Northwestern University, Chicago, IL; Chen et al., 2003). All constructs were verified by sequencing. Smartpool siRNAs against human cadherin 11 and N-cadherin were obtained from Dharmacon. Silencing specificity was confirmed using ON-Targetplus nontargeting siRNAs (Dharmacon).
Western blotting
Western blotting procedures were conducted as described previously (Ireton et al., 2002). Primary antibodies were used as follows: 0.25 μg/ml anti-p120 mAbs pp120 and 1 μg/ml 8D11 (does not recognize human p120), anti–E-cadherin mAbs (1/2,500; C-20820; BD Biosciences) and 1 μg/ml HECD-1 (Zymed Laboratories), 1 μg/ml anti–c-Met (C-28; Santa Cruz Biotechnology, Inc.), anti–β-catenin polyclonal antibody (1/1000; C2206; Sigma-Aldrich), 5 μg/ml anti-Flag tag mAb (M2; Sigma-Aldrich), 1 μg/ml anti-myc tag (9E10; Sigma-Aldrich), anti–cadherin 11 antibodies (WTID1 [polyclonal antibody] and 5B2H5 [mAb]; Zymed Laboratories), and 0.6 μg/ml anti-actin goat polyclonal antibody (I-19; Santa Cruz Biotechnology, Inc.). Secondary antibodies were peroxidase-conjugated donkey anti–mouse IgG and mouse anti–rabbit IgG (Jackson ImmunoResearch Laboratories) and donkey anti-goat IgG (Santa Cruz Biotechnology, Inc.) used at 1:10,000.
Invasion assay
Cell invasion was measured in vitro using BioCoat Matrigel-coated invasion chambers (8 μm pore size; Becton Dickinson). Culture medium was changed to Dulbecco's minimal essential medium supplemented with 250 μg/ml BSA, and cells were incubated overnight at 37°C. Cells were then harvested using Cell Stripper (Mediatech, Inc.), to prevent the proteolytic degradation of cadherins, and resuspended in Dulbecco's minimal essential medium/BSA at a density of 5 × 105 cells/ml. 100 μl (5 × 104 cells) of cell suspension was added to the top chamber, whereas Dulbecco's minimal essential medium/BSA containing either 20 ng/ml HGF (Reprotech, Inc.) or 5% FBS was added to the lower chamber as a chemoattractant. Cells were allowed to invade the Matrigel and migrate to the underside of the invasion chamber for 20 h at 37°C in 5% CO2. Cells on the top surface of the chamber were removed by gentle scrubbing with a cotton swab, and cells on the underside were stained with crystal violet and counted. Control experiments established that no growth differences existed between all cell lines tested under the conditions of this assay. Data from several experiments were expressed as percentage of control and represent the mean ± SEM of at least three independent determinations performed in duplicate. One, two, and three asterisks represent P < 0.05, P < 0.01, and P < 0.001, respectively (t test, or one way ANOVA followed by post-hoc comparisons using the Newman-Keuls test). The H-1152 Rho kinase inhibitor (Calbiochem) was used in some experiments at 1.6 nM, which is the reported Ki for this compound.
Scratch-wound assay
Cells were harvested using Cell Stripper, washed twice in PBS, and resuspended at 1 × 106 cells/ml in Dulbecco's minimal essential medium. 3 × 105 cells in 300 μl of media were then cultured in 4-well chamber slides (Nunc). 24 h later, cells were washed again with PBS and supplemented with serum-free media for 12 h. Confluent cell monolayers were scratched using a 200-μl Finnpipette tip, and serum-free medium containing 20 ng/ml HGF was added to the cells. Migration of cells into the wound was monitored in multiple wells using a live cell imaging workstation (AS-MDW; Leica) with a 20×/04 N Plan objective. Images were captured every 60 min, and images shown represent 0 and 12 h after HGF addition.
Aggregation assay
Cells were tested for their ability to aggregate in hanging drop suspension cultures, as previously described (Thoreson et al., 2000). In brief, cells were suspended using Cell Stripper, washed in PBS, and resuspended in Dulbecco's minimal essential medium. 1.5 × 105 cells in 30 μl of media were suspended as hanging drops from the lid of a 24-well culture dish and allowed to aggregate overnight in a humid 5% CO2 incubator at 37°C. Aggregation was assessed 18 h after plating. To assay for tightness of cell–cell adhesion, cells were subjected to shear force by passing them 10 times through a standard 200-μl Finnpipette tip. Cells were photographed within 10 min through the AS-MDW live cell imaging workstation using a 10× phase-contrast objective.
Rho/Rac activity assays
MDA-231 cells were plated in 100-mm dishes. 18 h later, the cells were washed and incubated for an additional 12 h in serum-free media, and RhoA or Rac1 activities were determined as described previously (Anastasiadis et al., 2000). In some cases, serum-deprived cells were treated with 20 ng/ml HGF for the indicated times before cell lysis. Cells were lysed for 5 min at 0°C in 500 μl of lysis buffer (20 mM Hepes, pH 7.5, 0.5% NP-40, 100 mM NaCl, 0.2% deoxycholic acid, 10% glycerol, and 10 mM MgCl2) supplemented with protease and phosphatase inhibitors. Lysates were clarified with a 5-min microcentrifugation, and supernatants were transferred to new tubes containing 30 μg of either Rhotekin RBD or PAK-1 PBD (Upstate Biotechnology) bound to glutathione beads. A 20-μl aliquot of supernatant was also saved for the determination of total RhoA/Rac1 and p120 levels in each sample. After a 45-min incubation at 4°C, beads were washed in wash buffer (20 mM Hepes, pH 7.5, 0.5% NP-40, 100 mM NaCl, 10% glycerol, and 10 mM MgCl2), and bound RhoA- or Rac1-GTP, as well as total RhoA/Rac1, were visualized after SDS-PAGE and Western blotting using either a RhoA-specific mAb (26C4; Santa Cruz Biotechnology, Inc.) or a Rac-1–specific mAb (BD Biosciences). GTPγS- or GDP-labeled cell lysates were used as positive and negative controls, respectively.
Acknowledgments
We thank Drs. Reuven Agami, Albert B. Reynolds, and Kathleen J. Greene for providing constructs. We also thank Drs. E. Aubrey Thompson, Alan P. Fields, and Derek Radisky for their useful comments.
The work was supported by National Institutes of Health grant R01 CA100467 (to P. Anastasiadis) and by Team Science Program grant 05-TSP-01 from the Florida Department of Health.
Abbreviations used in this paper: HGF, hepatocyte growth factor; shRNA, short hairpin RNA; wt, wild-type.
References
- Anastasiadis, P.Z., and A.B. Reynolds. 2001. Regulation of Rho GTPases by p120-catenin. Curr. Opin. Cell Biol. 13:604–610. [DOI] [PubMed] [Google Scholar]
- Anastasiadis, P.Z., S.Y. Moon, M.A. Thoreson, D.J. Mariner, H.C. Crawford, Y. Zheng, and A.B. Reynolds. 2000. Inhibition of RhoA by p120 catenin. Nat. Cell Biol. 2:637–644. [DOI] [PubMed] [Google Scholar]
- Bellovin, D.I., R.C. Bates, A. Muzikansky, D.L. Rimm, and A.M. Mercurio. 2005. Altered localization of p120 catenin during epithelial to mesenchymal transition of colon carcinoma is prognostic for aggressive disease. Cancer Res. 65:10938–10945. [DOI] [PubMed] [Google Scholar]
- Berx, G., and F. Van Roy. 2001. The E-cadherin/catenin complex: an important gatekeeper in breast cancer tumorigenesis and malignant progression. Breast Cancer Res. 3:289–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga, V.M. 2002. Cell-cell adhesion and signalling. Curr. Opin. Cell Biol. 14:546–556. [DOI] [PubMed] [Google Scholar]
- Chen, X., S. Kojima, G.G. Borisy, and K.J. Green. 2003. p120 catenin associates with kinesin and facilitates the transport of cadherin-catenin complexes to intercellular junctions. J. Cell Biol. 163:547–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conacci-Sorrell, M., J. Zhurinsky, and A. Ben-Ze'ev. 2002. The cadherin-catenin adhesion system in signaling and cancer. J. Clin. Invest. 109:987–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, M.A., R.C. Ireton, and A.B. Reynolds. 2003. A core function for p120-catenin in cadherin turnover. J. Cell Biol. 163:525–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang, X., H. Ji, S.W. Kim, J.I. Park, T.G. Vaught, P.Z. Anastasiadis, M. Ciesiolka, and P.D. McCrea. 2004. Vertebrate development requires ARVCF and p120 catenins and their interplay with RhoA and Rac. J. Cell Biol. 165:87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltes, C.M., A. Kudo, O. Blaschuk, and S.W. Byers. 2002. An alternatively spliced cadherin-11 enhances human breast cancer cell invasion. Cancer Res. 62:6688–6697. [PubMed] [Google Scholar]
- Gavard, J., M. Lambert, I. Grosheva, V. Marthiens, T. Irinopoulou, J.F. Riou, A. Bershadsky, and R.M. Mege. 2004. Lamellipodium extension and cadherin adhesion: two cell responses to cadherin activation relying on distinct signalling pathways. J. Cell Sci. 117:257–270. [DOI] [PubMed] [Google Scholar]
- Goodwin, M., E.M. Kovacs, M.A. Thoreson, A.B. Reynolds, and A.S. Yap. 2003. Minimal mutation of the cytoplasmic tail inhibits the ability of E-cadherin to activate Rac but not phosphatidylinositol 3-kinase: direct evidence of a role for cadherin-activated Rac signaling in adhesion and contact formation. J. Biol. Chem. 278:20533–20539. [DOI] [PubMed] [Google Scholar]
- Grosheva, I., M. Shtutman, M. Elbaum, and A.D. Bershadsky. 2001. p120 catenin affects cell motility via modulation of activity of Rho-family GTPases: a link between cell-cell contact formation and regulation of cell locomotion. J. Cell Sci. 114:695–707. [DOI] [PubMed] [Google Scholar]
- Hazan, R.B., G.R. Phillips, R.F. Qiao, L. Norton, and S.A. Aaronson. 2000. Exogenous expression of N-cadherin in breast cancer cells induces cell migration, invasion, and metastasis. J. Cell Biol. 148:779–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikenoya, M., H. Hidaka, T. Hosoya, M. Suzuki, N. Yamamoto, and Y. Sasaki. 2002. Inhibition of rho-kinase-induced myristoylated alanine-rich C kinase substrate (MARCKS) phosphorylation in human neuronal cells by H-1152, a novel and specific Rho-kinase inhibitor. J. Neurochem. 81:9–16. [DOI] [PubMed] [Google Scholar]
- Ireton, R.C., M.A. Davis, J. van Hengel, D.J. Mariner, K. Barnes, M.A. Thoreson, P.Z. Anastasiadis, L. Matrisian, L.M. Bundy, L. Sealy, et al. 2002. A novel role for p120 catenin in E-cadherin function. J. Cell Biol. 159:465–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, W.G., D. Grimshaw, T.A. Martin, G. Davies, C. Parr, G. Watkins, J. Lane, R. Abounader, J. Laterra, and R.E. Mansel. 2003. Reduction of stromal fibroblast-induced mammary tumor growth, by retroviral ribozyme transgenes to hepatocyte growth factor/scatter factor and its receptor, c-MET. Clin. Cancer Res. 9:4274–4281. [PubMed] [Google Scholar]
- Johnson, E., C.S. Theisen, K.R. Johnson, and M.J. Wheelock. 2004. R-cadherin influences cell motility via Rho family GTPases. J. Biol. Chem. 279:31041–31049. [DOI] [PubMed] [Google Scholar]
- Kiener, H.P., C.S. Stipp, P.G. Allen, J.M. Higgins, and M.B. Brenner. 2006. The Cadherin-11 cytoplasmic juxtamembrane domain promotes α-catenin turnover at adherens junctions and intercellular motility. Mol. Biol. Cell. 17:2366–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magie, C.R., D. Pinto-Santini, and S.M. Parkhurst. 2002. Rho1 interacts with p120ctn and alpha-catenin, and regulates cadherin-based adherens junction components in Drosophila. Development. 129:3771–3782. [DOI] [PubMed] [Google Scholar]
- Nieman, M.T., R.S. Prudoff, K.R. Johnson, and M.J. Wheelock. 1999. N-cadherin promotes motility in human breast cancer cells regardless of their E-cadherin expression. J. Cell Biol. 147:631–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobes, C.D., and A. Hall. 1995. Rho, Rac, and Cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 81:53–62. [DOI] [PubMed] [Google Scholar]
- Noren, N.K., B.P. Liu, K. Burridge, and B. Kreft. 2000. p120 catenin regulates the actin cytoskeleton via Rho family GTPases. J. Cell Biol. 150:567–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Moreno, M., M.A. Davis, E. Wong, H.A. Pasolli, A.B. Reynolds, and E. Fuchs. 2006. p120-catenin mediates inflammatory responses in the skin. Cell. 124:631–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perl, A.K., P. Wilgenbus, U. Dahl, H. Semb, and G. Christofori. 1998. A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature. 392:190–193. [DOI] [PubMed] [Google Scholar]
- Qian, X., T. Karpova, A.M. Sheppard, J. McNally, and D.R. Lowy. 2004. E-cadherin-mediated adhesion inhibits ligand-dependent activation of diverse receptor tyrosine kinases. EMBO J. 23:1739–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander, E.E., S. van Delft, J.P. ten Klooster, T. Reid, R.A. van der Kammen, F. Michiels, and J.G. Collard. 1998. Matrix-dependent Tiam1/Rac signaling in epithelial cells promotes either cell-cell adhesion or cell migration and is regulated by phosphatidylinositol 3-kinase. J. Cell Biol. 143:1385–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarrio, D., B. Perez-Mies, D. Hardisson, G. Moreno-Bueno, A. Suarez, A. Cano, J. Martin-Perez, C. Gamallo, and J. Palacios. 2004. Cytoplasmic localization of p120ctn and E-cadherin loss characterize lobular breast carcinoma from preinvasive to metastatic lesions. Oncogene. 23:3272–3283. [DOI] [PubMed] [Google Scholar]
- Suyama, K., I. Shapiro, M. Guttman, and R.B. Hazan. 2002. A signaling pathway leading to metastasis is controlled by N-cadherin and the FGF receptor. Cancer Cell. 2:301–314. [DOI] [PubMed] [Google Scholar]
- Thiery, J.P. 2002. Epithelial-mesenchymal transitions in tumour progression. Nat. Rev. Cancer. 2:442–454. [DOI] [PubMed] [Google Scholar]
- Thoreson, M.A., P.Z. Anastasiadis, J.M. Daniel, R.C. Ireton, M.J. Wheelock, K.R. Johnson, D.K. Hummingbird, and A.B. Reynolds. 2000. Selective uncoupling of p120(ctn) from E-cadherin disrupts strong adhesion. J. Cell Biol. 148:189–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titus, B., M.A. Schwartz, and D. Theodorescu. 2005. Rho proteins in cell migration and metastasis. Crit. Rev. Eukaryot. Gene Expr. 15:103–114. [DOI] [PubMed] [Google Scholar]
- Vleminckx, K., L. Vakaet Jr., M. Mareel, W. Fiers, and F. van Roy. 1991. Genetic manipulation of E-cadherin expression by epithelial tumor cells reveals an invasion suppressor role. Cell. 66:107–119. [DOI] [PubMed] [Google Scholar]
- Wong, A.S., and B.M. Gumbiner. 2003. Adhesion-independent mechanism for suppression of tumor cell invasion by E-cadherin. J. Cell Biol. 161:1191–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, J., D.J. Mariner, M.A. Thoreson, and A.B. Reynolds. 1998. Production and characterization of monoclonal antibodies to the catenin p120ctn. Hybridoma. 17:175–183. [DOI] [PubMed] [Google Scholar]
- Xiao, K., D.F. Allison, K.M. Buckley, M.D. Kottke, P.A. Vincent, V. Faundez, and A.P. Kowalczyk. 2003. Cellular levels of p120 catenin function as a set point for cadherin expression levels in microvascular endothelial cells. J. Cell Biol. 163:535–545. [DOI] [PMC free article] [PubMed] [Google Scholar]