Abstract
Protein kinase D (PKD) has been identified as a crucial regulator of secretory transport at the trans-Golgi network (TGN). Recruitment and activation of PKD at the TGN is mediated by the lipid diacylglycerol, a pool of which is generated by sphingomyelin synthase from ceramide and phosphatidylcholine. The nonvesicular transfer of ceramide from the endoplasmic reticulum to the Golgi complex is mediated by the lipid transfer protein CERT (ceramide transport). In this study, we identify CERT as a novel in vivo PKD substrate. Phosphorylation on serine 132 by PKD decreases the affinity of CERT toward its lipid target phosphatidylinositol 4-phosphate at Golgi membranes and reduces ceramide transfer activity, identifying PKD as a regulator of lipid homeostasis. We also show that CERT, in turn, is critical for PKD activation and PKD-dependent protein cargo transport to the plasma membrane. Thus, the interdependence of PKD and CERT is key to the maintenance of Golgi membrane integrity and secretory transport.
Introduction
PKD is a family of serine/threonine-specific protein kinases comprising three structurally related members: PKD1/PKCμ, PKD2, and PKD3/PKCν. PKD contains two zinc finger–like cysteine-rich motifs that bind DAG, a pleckstrin homology (PH), and a kinase domain. PKD localizes to the cytosol, nucleus, Golgi complex, and plasma membrane, where it regulates diverse cellular processes, including vesicle trafficking (Rykx et al., 2003; Wang, 2006). Thus far, only a few physiological PKD substrates are known (e.g., the neuronal protein Kidins220, the Ras effector RIN1, HDAC5, and PI4KIIIβ; Iglesias et al., 2000; Wang et al., 2002; Vega et al., 2004; Hausser et al., 2005). At the TGN, PKD is critically involved in the fission of transport carriers en route to the cell surface (Liljedahl et al., 2001; Yeaman et al., 2004). PKD is recruited to the TGN by its cysteine-rich regions (Maeda et al., 2001; Baron and Malhotra, 2002; Hausser et al., 2002), where it is activated by PKCη-mediated phosphorylation (Diaz Anel and Malhotra, 2005). PKD-mediated phosphorylation of PI4KIIIβ stimulates its lipid kinase activity, resulting in enhanced phosphatidylinositol 4-phosphate (PI(4)P) production and cargo transport to the plasma membrane (Hausser et al., 2005).
In this study, we demonstrate that PKD also phosphorylates and regulates the activity of the Golgi-localized ceramide transfer protein (CERT; also known as Goodpasture antigen-binding protein), a cytosolic protein essential for the nonvesicular delivery of ceramide from its site of production at the ER to Golgi membranes, where conversion to sphingomyelin (SM) takes place (Hanada et al., 2003). Two CERT isoforms exist: the more abundantly expressed, alternatively spliced form missing a 26–amino acid serine-rich region and the full-length 624–amino acid protein, which is designated CERTL (Raya et al., 2000). Both CERT isoforms possess a steroidogenic acute regulatory lipid transfer (START) domain that is necessary and sufficient for ceramide binding and transport (Hanada et al., 2003). START domains are ∼210 amino acids in length and form a hydrophobic tunnel that accommodates a monomeric lipid (Soccio and Breslow, 2003; Alpy and Tomasetto, 2005). They are found in 15 mammalian proteins, with CERT being most closely related to Pctp, which binds and shuttles phosphatidylcholine (PC) between membranes, and StarD10, a lipid transfer protein specific for PC and phosphatidylethanolamine (Soccio and Breslow, 2003; Olayioye et al., 2005; Wirtz, 2006). CERT proteins further contain an N-terminal PH domain with specificity for PI(4)P that contributes to Golgi localization (Levine and Munro, 2002; Hanada et al., 2003) and an FFAT motif (two phenylalanines in an acidic tract) that targets the protein to the ER via interaction with the ER resident transmembrane proteins VAP-A and VAP-B (vesicle-associated membrane protein–associated protein; Loewen et al., 2003; Kawano et al., 2006). Nonvesicular lipid transfer is thought to occur at membrane contact sites, at which the ER comes into close apposition with other organelles (Levine and Loewen, 2006). CERT may thus shuttle a very short distance between ER and Golgi membranes or perhaps contact both compartments simultaneously. When overexpressed, the START domain of CERT is sufficient for ceramide transfer to the Golgi complex (Kawano et al., 2006). However, under physiological conditions, both Golgi and ER targeting motifs are essential for CERT function. In the CHO cell line LY-A, CERT was identified to contain a mutation within its PH domain (G67E), rendering the protein defective in PI(4)P binding, which resulted in reduced cellular SM levels (Hanada et al., 2003). The PI(4)P requirement for CERT function is further supported by a recent study showing that PI4KIIIβ activity is necessary for efficient ceramide trafficking to the Golgi (Toth et al., 2006). We now provide evidence that PKD phosphorylates CERT on serine 132 adjacent to the PH domain, whereby PI(4)P binding, Golgi targeting, and ceramide transfer activity are negatively regulated. Furthermore, by transferring ceramide that is required for DAG production to Golgi membranes, CERT stimulates PKD activity and ensures the maintenance of constitutive secretory transport.
Results and discussion
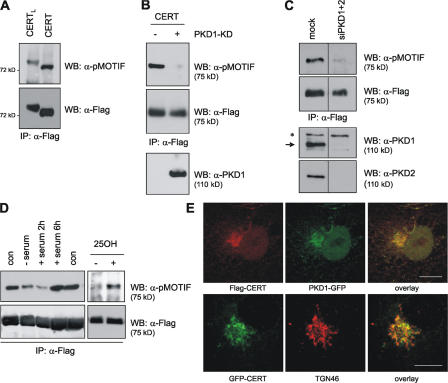
PKD is a key regulator at the Golgi complex, with PI4KIIIβ being the only local substrate identified thus far (Hausser et al., 2005). To test whether the Golgi complex–localized CERT protein may serve as a substrate for PKD, we made use of a phosphospecific substrate antibody, termed pMOTIF, that was raised against consensus motifs phosphorylated by PKD (Doppler et al., 2005). HEK293T cells were transfected with expression vectors encoding Flag-tagged CERT and CERTL. Immunoprecipitated CERT isoforms were analyzed by Western blotting with the pMOTIF antibody (Fig. 1 A). A pMOTIF signal corresponding to the molecular weight of CERT and, more weakly, to that of CERTL was detected (Fig. 1 A). The weaker detection of the CERTL isoform by ∼25% compared with CERT may be related to its known behavior to form aggregates, which may impact phosphosite accessibility to kinases (Raya et al., 2000). To investigate whether recognition of CERT by the pMOTIF antibody was dependent on PKD, we expressed CERT together with a kinase-dead (KD) dominant-negative PKD1 variant (PKD1-KD) in HEK293T cells. Coexpression of inactive PKD1 abolished CERT detection by the pMOTIF antibody, suggesting that the signal was indeed the result of PKD-mediated CERT phosphorylation (Fig. 1 B). To address the question of which PKD isoform was responsible for CERT phosphorylation, we used an RNAi approach to down-regulate PKD. Silencing of only one isoform did not influence the level of CERT phosphorylation as judged by immunoblotting with the pMOTIF antibody (unpublished data). However, simultaneous knockdown of PKD1 and PKD2 greatly reduced CERT phosphorylation (Fig. 1 C), suggesting that these two isoforms were primarily responsible for phosphorylating CERT, whereas PKD3 appeared to play a minor role. This is in accordance with previously reported overlapping substrate specificities of PKD1 and PKD2, which both phosphorylate PI4KIIIβ, whereas PKD3 fails to do so (Hausser et al., 2005).
Figure 1.
CERT is detected by a PKD substrate antibody. (A) HEK293T cells were transfected with expression plasmids encoding Flag-tagged CERTL and CERT. Cells were lysed, and CERT isoforms were immunoprecipitated with anti-Flag antibody. Immunoprecipitated proteins were subjected to SDS-PAGE followed by immunoblotting with PKD substrate antibody (pMOTIF; top) and, after stripping, with anti-Flag antibody (bottom). (B) HEK293T cells were transfected with Flag-CERT expression plasmid along with GFP-PKD1-KD or empty vector. CERT was analyzed by Western blotting as described in A. The expression of PKD1-KD was verified by immunoblotting with a PKD1-specific antibody (bottom). (C) HEK293T cells were either mock transfected or transfected with PKD1- and PKD2-specific siRNAs followed by transfection with Flag-CERT expression plasmid 48 h later. After 24 h, CERT phosphorylation was analyzed as described in A (top). Silencing of PKD1 and PKD2 was verified by immunoblotting of lysates with specific antibodies (bottom). The band marked with an asterisk is the result of nonspecific binding. PKD1 is marked with an arrow. (D) HEK293T cells were transfected with Flag-CERT expression plasmid. Cells were left untreated (con) or were serum starved overnight followed by stimulation with either 10% serum for 2 and 6 h or 2.5 μg/ml 25-hydroxycholesterol for 1 h. CERT phosphorylation was analyzed as described in A. (E) COS7 cells expressing Flag-CERT and PKD1-GFP (top) or GFP-CERT (bottom) were fixed and stained with Flag- and TGN46-specific antibodies (red), respectively. Bars, 10 μm.
The phosphorylation status of CERT was strongly reduced in serum-deprived cells and could be restored by the readdition of serum (Fig. 1 D), indicating that CERT phosphorylation is dependent on extracellular stimuli. It was recently reported that OSBP (oxysterol-binding protein) promotes CERT translocation to the Golgi complex in response to stimulation with its ligand, 25-hydroxycholesterol, thereby integrating sterol signaling and SM synthesis (Perry and Ridgway, 2006). In line with these studies, 25-hydroxycholesterol treatment was found to augment CERT phosphorylation (Fig. 1 D), possibly by bringing CERT to the Golgi in the vicinity of PKD. CERT has been demonstrated to colocalize with the cis/medial-Golgi marker GS28 (Hanada et al., 2003). Immunofluorescence analysis of GFP-tagged CERT expressed in COS7 cells showed that the protein localized to GS28-positive Golgi regions (Fig. S1, available at http://www.jcb.org/cgi/content/full/jcb.200612017/DC1). However, lipid transfer proteins are thought to act at membrane contact sites, which are formed between the ER and TGN (Levine and Loewen, 2006), where PKD is localized. Immunofluorescence staining of Flag-tagged CERT coexpressed with GFP-tagged PKD in COS7 cells revealed that the two proteins colocalize at the Golgi complex. Furthermore, staining of the TGN-specific marker protein TGN46 verified that CERT partially localizes to this compartment (Fig. 1 E).
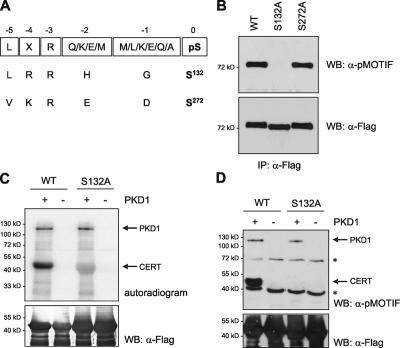
To identify pMOTIF recognition sites in CERT, we searched for potential PKD consensus motifs characterized by a leucine, isoleucine, or valine residue in the −5 position and arginine in the −3 position relative to a serine or threonine. Two serines at positions 132 and 272 matching the PKD consensus motif (Fig. 2 A) were exchanged for alanines by site-directed mutagenesis. Mutants were expressed in HEK293T cells and tested for recognition by the pMOTIF antibody. Interestingly, mutation of serine 132 to alanine abrogated the detection of CERT with the pMOTIF antibody and caused an increase in electrophoretic mobility, which is indicative of the loss of phosphorylation, whereas the S272A mutation did not affect the pMOTIF signal (Fig. 2 B). On low percentage gels, the wild-type (WT) protein migrated as two distinct bands, indicating the presence of a phosphorylated and a nonphosphorylated CERT pool (unpublished data). To confirm that PKD was capable of directly phosphorylating serine 132, we performed in vitro kinase assays with purified PKD1 and recombinant CERT GST fusion proteins comprising the first 138 amino acids of the protein. WT CERT was efficiently phosphorylated by PKD1, whereas the CERT-S132A protein showed a strongly reduced incorporation of radioactivity in this assay (Fig. 2 C). Furthermore, in vitro PKD phosphorylation of WT but not CERT-S132A generated a recognition site for the pMOTIF antibody (Fig. 2 D). Collectively, these results prove that CERT is a genuine PKD substrate in vitro and in vivo and identify serine 132 as a specific PKD phosphorylation site in CERT that can be monitored with the pMOTIF antibody.
Figure 2.
PKD phosphorylates CERT on serine 132. (A) Alignment of the peptide sequences used to raise the pMOTIF antibody and two potential PKD motifs in CERT. (B) HEK293T cells transiently expressing Flag-tagged CERT-WT, -S132A, and -S272A were lysed, and CERT phosphorylation was analyzed as described in Fig. 1 A. (C and D) Recombinant GST-Flag-CERT-WT and -S132A proteins were incubated in kinase buffer containing γ-[32P]ATP (C) or cold ATP (D) in the absence (−) and presence (+) of purified PKD1. Proteins were separated by SDS-PAGE and transferred to membrane. (C) Incorporation of radioactive phosphate was analyzed using a phosphorimager (top) followed by immunoblotting with Flag-specific antibody to verify equal loading of the CERT proteins. (D) Immunoblotting was performed with the pMOTIF antibody and, after stripping, with Flag-specific antibody to verify equal loading of the CERT proteins. PKD1 and CERT proteins are marked with arrows; the bands with asterisks are the results of nonspecific binding.
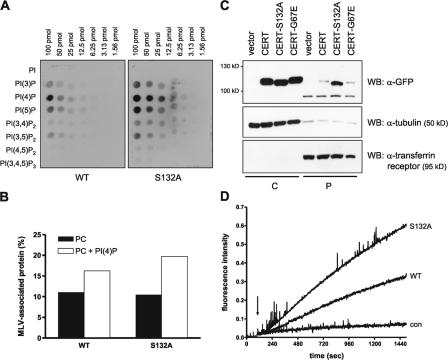
Serine 132 is in close proximity to the CERT PH domain (aa 23–117), making it possible that phosphorylation on this site affects PI(4)P binding by increasing the local negative charge. Therefore, we quantified PI(4)P binding of CERT-WT and -S132A by performing protein–lipid overlay assays. Cytosol from cells transiently expressing the CERT variants was incubated with membranes spotted with a concentration gradient of the different phosphoinositides, and bound CERT proteins were detected via their GFP tag. As reported previously, the WT protein demonstrated weak binding to several phospholipid species but displayed strong interaction with PI(4)P (Levine and Munro, 2002; Hanada et al., 2003). CERT-S132A binding to PI(4)P was detectable at two- to fourfold lower concentrations as compared with that of the WT protein (Fig. 3 A). To corroborate these results, the association of CERT with multilamellar vesicles (MLVs) consisting of PC alone or PC plus 5% PI(4)P was measured. Although the addition of PI(4)P to PC vesicles increased the membrane binding of CERT-WT 1.5-fold, the binding of CERT-S132A was enhanced 1.9-fold, suggesting an increased affinity of the CERT-S132A mutant to PI(4)P (Fig. 3 B). To investigate whether this affected the association with Golgi membranes in intact cells, we performed fractionation studies with cells expressing CERT-WT and -S132A. To estimate the level of ER binding, we included a CERT mutant (G67E) defective in PI(4)P binding. Only a small proportion of the WT and G67E protein were recovered in the pellet fraction, suggesting that under the experimental conditions used, ER binding was negligible, and Golgi association of the WT protein was not maintained (Fig. 3 C). The CERT-S132A mutant protein was highly enriched in the pellet fraction, confirming that the enhanced affinity for PI(4)P stabilizes membrane association in vivo. Together, these data imply that CERT, once bound to the Golgi complex, is phosphorylated by PKD. This then decreases the affinity of CERT to PI(4)P and, thereby, regulates the interaction of CERT with the Golgi complex. Because PI(4)P is also present at the plasma membrane, additional factors must specify CERT targeting to the Golgi complex. One candidate is Arf1, which has been shown to interact with the structurally related proteins OSBP and FAPP1 (Levine and Munro, 2002). Whether CERT phosphorylation influences binding to such additional factors remains to be tested in the future.
Figure 3.
CERT phosphorylation on serine 132 modulates PI(4)P binding and ceramide transfer activity. HEK293T cells transiently expressing the indicated GFP-tagged CERT variants were harvested by hypotonic lysis, and the cytosol fraction was recovered after 100,000 g centrifugation. Samples containing equal amounts of GFP fluorescence were used for protein–lipid overlay (A), flotation (B), and in vitro ceramide transfer assays (D). (A) Phosphatidylinositol phosphate arrays were incubated with cytosol from cells transiently expressing GFP-tagged CERT-WT and -S132A, and bound proteins were detected with GFP-specific primary followed by HRP-labeled secondary antibody. (B) MLVs consisting of PC or PC + 5% PI(4)P were incubated with cytosol and separated by centrifugation. The amount of CERT protein in the top (MLV) and bottom fractions was quantified by measuring GFP fluorescence and set as 100%. Results are plotted as percentages of protein recovered in the MLV fraction. (C) Cytosol (C) and the 100,000 g pellet (P) containing cellular membranes were analyzed by immunoblotting with GFP-specific antibody. The purity of the individual fractions was confirmed by detection of the transferrin receptor in the membrane and tubulin in the cytosolic fraction. (D) Donor liposomes containing TNP-PE and pyrene-ceramide were mixed with unlabeled acceptor liposomes. After 60 s, cytosol from cells transiently expressing GFP-tagged CERT-WT, -S132A, or GFP alone (con) was added, and pyrene fluorescence at 395 nm was recorded.
The CERT protein has been shown to function as a lipid transfer protein (Hanada et al., 2003). Thus, we investigated whether CERT phosphorylation on serine 132 influenced its ability to bind and transfer ceramide between membranes. To this end, GFP-tagged versions of CERT-WT and -S132A were transiently expressed in HEK239T cells, and the cytosol fraction was analyzed for ceramide-specific lipid transfer activity using a fluorescence resonance energy transfer–based assay. In this assay, vesicles containing pyrene-labeled ceramide as a fluorescent donor and quenching amounts of 2,4,6-trinitrophenyl-phosphatidylethanolamine (TNP-PE) were used (Somerharju, 2002; Olayioye et al., 2005). The lipid preparation used was total extract from porcine brain, which is likely to contain PI(4)P. Upon the addition of cytosol-containing CERT-WT, a steady increase in fluorescence was noted, which was not observed when control cytosol of vector-transfected cells was used (Fig. 3 D). Compared with the WT protein, CERT-S132A displayed a higher rate of lipid transfer, which was evident from a more rapid increase in pyrene fluorescence (Fig. 3 D). Similar results were obtained when 0.5% PI(4)P was added to donor liposomes (unpublished data). This suggests that CERT phosphorylation on serine 132 down-regulates ceramide transfer activity, most likely by decreasing association of the protein with membranes. Previous data have already shown that PKD regulates the level of PI(4)P at the Golgi complex by the phosphorylation-mediated activation of PI4KIIIβ (Hausser et al., 2005). Interestingly, PI4KIIIβ is critical for the transport of ceramide between the ER and the Golgi complex (Toth et al., 2006). Accordingly, together with the data presented in this study, a dual role for PKD in maintaining lipid homeostasis of Golgi membranes becomes apparent by controlling the on rate (via PI(4)P levels) and off rate (via direct phosphorylation) of CERT.
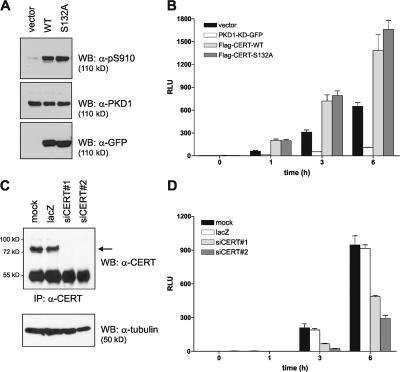
The transfer of ceramide from the ER to the TGN is essential for SM synthesis at this compartment (Hanada et al., 2003). Golgi-localized SM synthase 1 utilizes ceramide and PC to generate SM and DAG (Perry and Ridgway, 2005), the latter being a prerequisite for PKD recruitment and activation. Compounds that block DAG production at the TGN inhibit the binding of PKD to TGN membranes and interfere with secretory transport (Baron and Malhotra, 2002). Therefore, increased ceramide transfer from the ER to the TGN by the overexpression of CERT should result in an elevated local DAG pool and may consequently stimulate PKD activity and secretory transport. To test this hypothesis, we transiently expressed CERT-WT and -S132A in HEK293T cells and analyzed the autophosphorylation of endogenous PKD. Compared with the control, the expression of both CERT-WT and -S132A increased PKD activity, as revealed by analyses with a phosphospecific PKD antibody (Fig. 4 A). CERT has been reported to possess kinase activity (Raya et al., 2000), making it possible that it activates PKD by direct phosphorylation. However, kinase assays clearly demonstrated that PKD is not phosphorylated by CERT. Moreover, a kinase activity was associated with the CERT protein only under mild detergent conditions (Fig. S1). Thus, our results show that PKD activation is regulated by CERT proteins, most likely as a result of increased ceramide delivery and enforced SM/DAG synthesis. A similar function has recently been described for the lipid transfer protein Nir2 in the maintenance of DAG levels at the Golgi apparatus via regulation of the cytidine-5′-diphosphate–choline pathway (Litvak et al., 2005). RNAi-mediated knockdown of Nir2 decreased DAG and PKD levels at the Golgi complex and blocked secretory transport. Interestingly, this effect could be rescued by the addition of exogenous C6-ceramide (Litvak et al., 2005), indicating a critical role for ceramide in DAG synthesis and PKD recruitment to the Golgi complex.
Figure 4.
CERT regulates PKD activation and secretory transport. (A) HEK293T cells transiently expressing CERT-WT and -S132A were lysed, and PKD activation was analyzed by immunoblotting with pS910 PKD antibody (top). Equal loading was verified by reprobing with PKD1-specific antibody (middle). The expression of CERT proteins was verified by immunoblotting with GFP-specific antibody (bottom). (B and D) HEK293T cells were transfected with the indicated expression plasmids (B), and COS7 cells were transfected with the indicated siRNAs (D) together with ssHRP-Flag plasmid as described in Materials and methods. The medium was analyzed for HRP activity after 0, 1, 3, and 6 h by chemiluminescence. Values correspond to the mean of triplicate samples, and error bars represent SEM. RLU, relative light units. (C) COS7 cells were transfected with the siRNAs indicated, and CERT expression was analyzed after 72 h by immunoprecipitation and Western blotting using a CERT-specific antibody (top). Tubulin levels were not affected (bottom). CERT is marked with an arrow.
To address the question of whether CERT-mediated PKD activation indeed translated into enhanced secretory transport, we made use of a plasmid encoding HRP fused to a signal sequence (ss). The fusion protein ssHRP can be used as a reporter for constitutive protein secretion (Bard et al., 2006). In control cells, secretion of ssHRP could be detected within 1 h and increased over time (Fig. 4 B). Coexpression of PKD1-KD, which inhibits the secretory transport of cargo protein (Liljedahl et al., 2001; Hausser et al., 2005), almost entirely abrogated ssHRP secretion. This confirmed that HRP was secreted in a PKD-dependent manner in our assay. Coexpression of CERT-WT and -S132A strongly augmented the amount of secreted HRP (Fig. 4 B). Conversely, knockdown of CERT by RNAi in COS7 cells inhibited the secretion of HRP (Fig. 4, C and D), confirming the essential role for CERT in the constitutive exocytosis of cargo proteins. We could only detect a slight increase in secretion with the S132A mutant compared with the one observed with the WT protein. This is in accordance with the comparable activation of PKD by CERT-WT and -S132A (Fig. 4 A) but was unexpected in light of the substantially enhanced in vitro lipid transfer activity of the CERT mutant (Fig. 3 C). However, increased levels of ceramide may not necessarily translate into equivalent increases in DAG because DAG synthesis might be limited by the availability of PC and the activity of SM synthase.
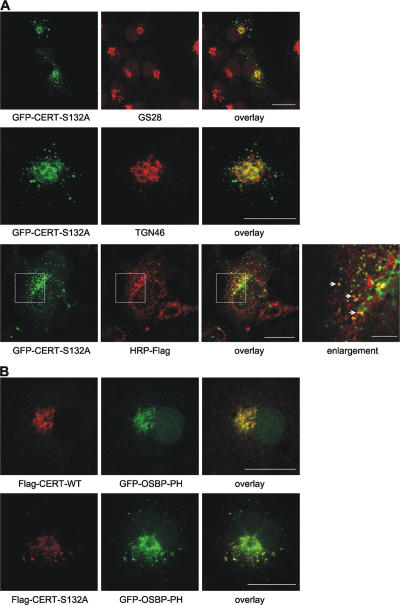
The accumulation of ceramide is known to affect Golgi membrane stability and induces vesicle fission (Weigert et al., 1999; Fukunaga et al., 2000). Therefore, we investigated whether overexpression of the CERT-S132A mutant affected its localization and/or caused morphological changes of the Golgi apparatus. In addition to concentrating in GS28-positive regions of the Golgi complex, the CERT-S132A mutant displayed a dispersed punctate staining (Fig. 5 A). However, the distribution of GS28 itself and that of TGN46 was not affected by the expression of CERT-S132A, nor were these proteins present in the vesicular structures observed with the mutant CERT protein (Fig. 5 A). This rules out fragmentation of the Golgi apparatus as a consequence of CERT-S132A overexpression. Some of the vesicular structures were found to contain the cargo protein ssHRP, providing evidence that these structures represent Golgi-derived transport carriers (Fig. 5 A). It thus appears that the increased membrane affinity of CERT-S132A prevents its dissociation from budding vesicles. Interestingly, when coexpressed with CERT-S132A, the PH domain of OSBP also localized to these vesicles, indicating that these structures are PI(4)P positive (Fig. 5 B). The CERT-S132A mutant may therefore inhibit PI(4)P turnover, thus stabilizing the lipid on transport carriers. Of note, a CERT-S132E protein was indistinguishable from the alanine mutant in terms of cellular localization and, thus, could not be used to mimic the phosphorylated state (unpublished data).
Figure 5.
CERT-S132A localizes to PI(4)P-positive secretory vesicles. (A and B) COS7 cells were transiently transfected with the indicated expression plasmids. Cells were fixed and stained with GS28- (red; A, top), TGN46- (red; A, middle), and Flag-specific antibodies (red; A, bottom; and B). The boxed areas are shown in the enlargement. Double-positive vesicles are marked with arrows. Bars, 20 μm; (enlargement) 5 μm.
Collectively, our data support the following working model: PKD is recruited to the TGN by a local DAG pool that can be generated via different metabolic pathways. PKD then activates PI4KIIIβ, increasing PI(4)P levels at the TGN. This, in turn, recruits the CERT protein to the Golgi complex, where it contributes to PKD activation and vesicular transport processes by providing ceramide as a precursor for further DAG production. The system is tightly regulated by a negative feedback loop: active PKD phosphorylates CERT at serine 132, thus decreasing the affinity of CERT toward its lipid target PI(4)P to ensure continuous rounds of lipid transfer from the ER to the Golgi compartment. In conclusion, we have identified CERT as a PKD substrate and provide evidence for a novel relationship between membrane lipid biogenesis and protein secretion.
Materials and methods
Immunofluorescence microscopy
Cells were fixed in 4% PFA for 10 min, washed, and incubated with PBS containing 0.1 M glycine for 15 min. Cells were permeabilized with PBS containing 0.1% Triton X-100 for 5 min and blocked with 5% goat serum in PBS containing 0.1% Tween 20 for 30 min. Cells were then incubated with primary antibody diluted in blocking buffer for 2 h followed by incubation with secondary antibodies diluted in blocking buffer for 1 h. Coverslips were mounted in Fluoromount G (Southern Biotechnology Associates, Inc.) and analyzed on a confocal laser-scanning microscope (TCS SL; Leica) using 488- and 543-nm excitation and a 40.0/1.25 HCX PL APO objective lens. Images were processed with Photoshop (Adobe). All images shown are stacks of several confocal sections.
Protein extraction, immunoprecipitation, and Western blotting
Whole cell extracts were obtained by solubilizing cells in NP-40 extraction buffer (50 mM Tris, pH 7.5, 150 mM NaCl, 1% NP-40, 1 mM sodium orthovanadate, 10 mM sodium fluoride, and 20 mM β-glycerophosphate plus Complete protease inhibitors [Roche]). Lysates were clarified by centrifugation at 16,000 g for 10 min. For immunoprecipitations, equal amounts of protein were incubated with specific antibodies for 2 h on ice. Immune complexes were collected with protein G–Sepharose beads (GE Healthcare) and washed three times with NP-40 extraction buffer. Whole cell extracts or immunoprecipitated proteins were subjected to SDS-PAGE, and proteins were blotted onto polyvinylidene difluoride membranes (Roth). After blocking with 0.5% blocking reagent (Roche) in PBS containing 0.1% Tween 20, filters were probed with specific antibodies. Proteins were visualized with HRP-coupled secondary antibody using the ECL system (Pierce Chemical Co.). Stripping of membranes was performed in 62.5 mM Tris, pH 6.8, 2% SDS, and 100 mM β-mercaptoethanol for 30 min at 60°C. Membranes were then reprobed with the indicated antibodies.
Recombinant protein purification and in vitro kinase assays
BL21 bacteria were transformed with pGEX6P-Flag-CERT-WT(1–138) and -S132A(1–138) vectors. Expression was induced with 0.5 mM IPTG for 4 h at 30°C. Bacteria were harvested and resuspended in PBS containing 50 μg/ml lysozyme, Complete protease inhibitors (Roche), 10 mM sodium fluoride, and 20 mM β-glycerophosphate. Triton X-100 was added to a final concentration of 1% before sonication. GST-CERT fusions were purified from clarified lysate with glutathione resin (GE Healthcare). Recombinant proteins were incubated with purified PKD1 from insect cells in kinase buffer (50 mM Tris, pH 7.5, 10 mM MgCl2, and 1 mM DTT) in the presence of either 2 μCi γ-[32P]ATP or 75 μM of cold ATP for 30 min. Samples were resolved by SDS-PAGE, blotted onto membrane, analyzed on a phosphorimager (Storm 860; Molecular Dynamics), and detected with the indicated antibodies.
Cellular fractionation
Cells were harvested in hypotonic buffer (50 mM Tris, pH 7.4, containing Complete protease inhibitors, 1 mM PMSF, 5 mM β-glycerophosphate, and 5 mM sodium fluoride) and sheared by passage through a 25-G/16-mm needle. Nuclei were removed by centrifugation at 500 g, and cytosol and membrane fractions were obtained by centrifugation at 100,000 g.
Phosphatidylinositol phosphate arrays, flotation, and ceramide transfer assays
The amount of expressed CERT protein in the cytosolic fraction was quantified by GFP peak emission at 480–550 nm (excitation of 466 nm). Phosphatidylinositol phosphate arrays (Echelon) were blocked in TBS-T (10 mM Tris, pH 8, 150 mM NaCl, and 0.1% Tween 20) containing 3% fatty acid–free BSA (Roth) followed by incubation with 500 μg cytosol containing equal amounts of GFP proteins in 5 ml of blocking buffer for 1 h. Bound proteins were detected with anti-GFP antibody followed by HRP-conjugated secondary antibody. Flotation assays were performed by incubating 50 μl cytosol containing equal amounts of GFP-tagged CERT proteins with 100 μl MLVs in 50 mM Tris, pH 7.5, and 50 mM NaCl buffer for 10 min at RT. The suspension was adjusted to 30% sucrose by the addition of 100 μl of 75% sucrose and overlayed with 200 μl of 25% sucrose in buffer and 50 μl sucrose-free buffer. Samples were centrifuged at 240,000 g for 1 h. The bottom (250 μl) and top (100 μl) fractions were collected and analyzed by fluorescence spectrometry. Protein-mediated transfer of ceramide between small unilamellar vesicles was measured as described previously (Olayioye et al., 2005). The transfer assay mixture contained donor vesicles (2 nmol of lipid/ml) composed of brain lipids, pyrene-labeled C16-ceramide, TNP-PE (provided by P. Somerharju, University of Helsinki, Helsinki, Finland; 88.6:0.4:11 mol percent), and a 10-fold excess of acceptor vesicles composed of brain lipids. Fluorescence intensity was recorded at 395 nm (excitation of 345 nm and slit widths of 4 nm) before and after the addition of 75 μg cytosol from HEK293T cells transiently expressing GFP-tagged CERT-WT and -S132A. Fluorescence intensities were normalized to the maximum intensity obtained after the addition of 0.5% Triton X-100 and the maximum GFP fluorescence to account for different protein expression levels.
Secretion assay
HEK293T cells were cotransfected with ssHRP-Flag plasmid together with empty vector, pEGFPN1-PKD1-KD, pcDNA3-Flag-CERT-WT, and -S132A at a ratio of 1:6.5, respectively. For CERT RNAi, COS7 cells were transfected with ssHRP-Flag plasmid, harvested after 8 h, replated, and transfected with siRNAs. HEK293T and COS7 cells were washed with serum-free medium 24 and 48 h after transfection, respectively, and HRP secretion was quantified by incubation of clarified cell supernatant with ECL reagent. Measurements were performed with a luminometer (Lucy2; Anthos) at 450 nm.
Online supplemental material
Fig. S1 shows that CERT does not phosphorylate PKD directly. Fig. S2 shows the colocalization of CERT-WT and GS28. Supplemental materials and methods provides information about the antibodies and reagents used, DNA constructs, and cell culture and transfection. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200612017/DC1.
Supplementary Material
Acknowledgments
We wish to thank Juan Saus for providing CERT expression plasmids, Vivek Malhotra for the ssHRP-Flag plasmid, Tim Levine for plasmid encoding the GFP-tagged PH domain of OSBP, Pentti Somerharju for fluorescent lipid analogues, and Ruth Jähne for technical assistance.
The laboratory of Monilola A. Olayioye is funded by grants from the Deutsche Forschungsgemeinschaft (SFB 495-Junior Research Group) and the Deutsche Krebshilfe (OM-106708).
T. Fugmann and A. Hausser contributed equally to this paper.
Abbreviations used in this paper: KD, kinase dead; MLV, multilamellar vesicle; PC, phosphatidylcholine; PH, pleckstrin homology; PI(4)P, phosphatidylinositol 4-phosphate; SM, sphingomyelin; START, steroidogenic acute regulatory lipid transfer; TNP-PE, 2,4,6-trinitrophenyl-phosphatidylethanolamine; WT, wild type.
References
- Alpy, F., and C. Tomasetto. 2005. Give lipids a START: the StAR-related lipid transfer (START) domain in mammals. J. Cell Sci. 118:2791–2801. [DOI] [PubMed] [Google Scholar]
- Bard, F., L. Casano, A. Mallabiabarrena, E. Wallace, K. Saito, H. Kitayama, G. Guizzunti, Y. Hu, F. Wendler, R. Dasgupta, et al. 2006. Functional genomics reveals genes involved in protein secretion and Golgi organization. Nature. 439:604–607. [DOI] [PubMed] [Google Scholar]
- Baron, C.L., and V. Malhotra. 2002. Role of diacylglycerol in PKD recruitment to the TGN and protein transport to the plasma membrane. Science. 295:325–328. [DOI] [PubMed] [Google Scholar]
- Diaz Anel, A.M., and V. Malhotra. 2005. PKCη is required for β1γ2/β3γ2- and PKD-mediated transport to the cell surface and the organization of the Golgi apparatus. J. Cell Biol. 169:83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doppler, H., P. Storz, J. Li, M.J. Comb, and A. Toker. 2005. A phosphorylation state-specific antibody recognizes Hsp27, a novel substrate of protein kinase D. J. Biol. Chem. 280:15013–15019. [DOI] [PubMed] [Google Scholar]
- Fukunaga, T., M. Nagahama, K. Hatsuzawa, K. Tani, A. Yamamoto, and M. Tagaya. 2000. Implication of sphingolipid metabolism in the stability of the Golgi apparatus. J. Cell Sci. 113:3299–3307. [DOI] [PubMed] [Google Scholar]
- Hanada, K., K. Kumagai, S. Yasuda, Y. Miura, M. Kawano, M. Fukasawa, and M. Nishijima. 2003. Molecular machinery for non-vesicular trafficking of ceramide. Nature. 426:803–809. [DOI] [PubMed] [Google Scholar]
- Hausser, A., G. Link, L. Bamberg, A. Burzlaff, S. Lutz, K. Pfizenmaier, and F.J. Johannes. 2002. Structural requirements for localization and activation of protein kinase Cμ (PKCμ) at the Golgi compartment. J. Cell Biol. 156:65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausser, A., P. Storz, S. Martens, G. Link, A. Toker, and K. Pfizenmaier. 2005. Protein kinase D regulates vesicular transport by phosphorylating and activating phosphatidylinositol-4 kinase IIIbeta at the Golgi complex. Nat. Cell Biol. 7:880–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias, T., N. Cabrera-Poch, M.P. Mitchell, T.J. Naven, E. Rozengurt, and G. Schiavo. 2000. Identification and cloning of Kidins220, a novel neuronal substrate of protein kinase D. J. Biol. Chem. 275:40048–40056. [DOI] [PubMed] [Google Scholar]
- Kawano, M., K. Kumagai, M. Nishijima, and K. Hanada. 2006. Efficient trafficking of ceramide from the endoplasmic reticulum to the Golgi apparatus requires a VAMP-associated protein-interacting FFAT motif of CERT. J. Biol. Chem. 281:30279–30288. [DOI] [PubMed] [Google Scholar]
- Levine, T., and C. Loewen. 2006. Inter-organelle membrane contact sites: through a glass, darkly. Curr. Opin. Cell Biol. 18:371–378. [DOI] [PubMed] [Google Scholar]
- Levine, T.P., and S. Munro. 2002. Targeting of Golgi-specific pleckstrin homology domains involves both PtdIns 4-kinase-dependent and -independent components. Curr. Biol. 12:695–704. [DOI] [PubMed] [Google Scholar]
- Liljedahl, M., Y. Maeda, A. Colanzi, I. Ayala, J. Van Lint, and V. Malhotra. 2001. Protein kinase D regulates the fission of cell surface destined transport carriers from the trans-Golgi network. Cell. 104:409–420. [DOI] [PubMed] [Google Scholar]
- Litvak, V., N. Dahan, S. Ramachandran, H. Sabanay, and S. Lev. 2005. Maintenance of the diacylglycerol level in the Golgi apparatus by the Nir2 protein is critical for Golgi secretory function. Nat. Cell Biol. 7:225–234. [DOI] [PubMed] [Google Scholar]
- Loewen, C.J., A. Roy, and T.P. Levine. 2003. A conserved ER targeting motif in three families of lipid binding proteins and in Opi1p binds VAP. EMBO J. 22:2025–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda, Y., G.V. Beznoussenko, J. Van Lint, A.A. Mironov, and V. Malhotra. 2001. Recruitment of protein kinase D to the trans-Golgi network via the first cysteine-rich domain. EMBO J. 20:5982–5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olayioye, M.A., S. Vehring, P. Muller, A. Herrmann, J. Schiller, C. Thiele, G.J. Lindeman, J.E. Visvader, and T. Pomorski. 2005. StarD10, a START domain protein overexpressed in breast cancer, functions as a phospholipid transfer protein. J. Biol. Chem. 280:27436–27442. [DOI] [PubMed] [Google Scholar]
- Perry, R.J., and N.D. Ridgway. 2005. Molecular mechanisms and regulation of ceramide transport. Biochim. Biophys. Acta. 1734:220–234. [DOI] [PubMed] [Google Scholar]
- Perry, R.J., and N.D. Ridgway. 2006. Oxysterol-binding protein and vesicle-associated membrane protein-associated protein are required for sterol-dependent activation of the ceramide transport protein. Mol. Biol. Cell. 17:2604–2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raya, A., F. Revert-Ros, P. Martinez-Martinez, S. Navarro, E. Rosello, B. Vieites, F. Granero, J. Forteza, and J. Saus. 2000. Goodpasture antigen-binding protein, the kinase that phosphorylates the goodpasture antigen, is an alternatively spliced variant implicated in autoimmune pathogenesis. J. Biol. Chem. 275:40392–40399. [DOI] [PubMed] [Google Scholar]
- Rykx, A., L. De Kimpe, S. Mikhalap, T. Vantus, T. Seufferlein, J.R. Vandenheede, and J. Van Lint. 2003. Protein kinase D: a family affair. FEBS Lett. 546:81–86. [DOI] [PubMed] [Google Scholar]
- Soccio, R.E., and J.L. Breslow. 2003. StAR-related lipid transfer (START) proteins: mediators of intracellular lipid metabolism. J. Biol. Chem. 278:22183–22186. [DOI] [PubMed] [Google Scholar]
- Somerharju, P. 2002. Pyrene-labeled lipids as tools in membrane biophysics and cell biology. Chem. Phys. Lipids. 116:57–74. [DOI] [PubMed] [Google Scholar]
- Toth, B., A. Balla, H. Ma, Z.A. Knight, K.M. Shokat, and T. Balla. 2006. Phosphatidylinositol 4-kinase IIIbeta regulates the transport of ceramide between the endoplasmic reticulum and Golgi. J. Biol. Chem. 281:36369–36377. [DOI] [PubMed] [Google Scholar]
- Vega, R.B., B.C. Harrison, E. Meadows, C.R. Roberts, P.J. Papst, E.N. Olson, and T.A. McKinsey. 2004. Protein kinases C and D mediate agonist-dependent cardiac hypertrophy through nuclear export of histone deacetylase 5. Mol. Cell. Biol. 24:8374–8385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Q.J. 2006. PKD at the crossroads of DAG and PKC signaling. Trends Pharmacol. Sci. 27:317–323. [DOI] [PubMed] [Google Scholar]
- Wang, Y., R.T. Waldron, A. Dhaka, A. Patel, M.M. Riley, E. Rozengurt, and J. Colicelli. 2002. The RAS effector RIN1 directly competes with RAF and is regulated by 14-3-3 proteins. Mol. Cell. Biol. 22:916–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigert, R., M.G. Silletta, S. Spano, G. Turacchio, C. Cericola, A. Colanzi, S. Senatore, R. Mancini, E.V. Polishchuk, M. Salmona, et al. 1999. CtBP/BARS induces fission of Golgi membranes by acylating lysophosphatidic acid. Nature. 402:429–433. [DOI] [PubMed] [Google Scholar]
- Wirtz, K.W. 2006. Phospholipid transfer proteins in perspective. FEBS Lett. 580:5436–5441. [DOI] [PubMed] [Google Scholar]
- Yeaman, C., M.I. Ayala, J.R. Wright, F. Bard, C. Bossard, A. Ang, Y. Maeda, T. Seufferlein, I. Mellman, W.J. Nelson, and V. Malhotra. 2004. Protein kinase D regulates basolateral membrane protein exit from trans-Golgi network. Nat. Cell Biol. 6:106–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.