Abstract
Lgl (lethal giant larvae) plays an important role in cell polarity. Atypical protein kinase C (aPKC) binds to and phosphorylates Lgl, and the phosphorylation negatively regulates Lgl activity. In this study, we identify p32 as a novel Lgl binding protein that directly binds to a domain on mammalian Lgl2 (mLgl2), which contains the aPKC phosphorylation site. p32 also binds to PKCζ, and the three proteins form a transient ternary complex. When p32 is bound, PKCζ is stimulated to phosphorylate mLgl2 more efficiently. p32 overexpression in Madin–Darby canine kidney cells cultured in a 3D matrix induces an expansion of the actin-enriched apical membrane domain and disrupts cell polarity. Addition of PKCζ inhibitor blocks apical actin accumulation, which is rescued by p32 overexpression. p32 knockdown by short hairpin RNA also induces cell polarity defects. Collectively, our data indicate that p32 is a novel regulator of cell polarity that forms a complex with mLgl2 and aPKC and enhances aPKC activity.
Introduction
The establishment and maintenance of cell polarity are crucial for a diverse range of biological processes, including cell migration, asymmetric cell division, and epithelial morphogenesis. In contrast, loss of cell polarity correlates with a more invasive phenotype in cancer cells (Bilder, 2004; Igaki et al., 2006). Lgl (lethal giant larvae) was originally identified as a tumor suppressor protein (Mechler et al., 1985) and has recently been shown to play an important role in cell polarity (Bilder et al., 2000; Ohshiro et al., 2000; Peng et al., 2000). Although the molecular mechanism of how Lgl regulates cell polarity is not fully understood, Lgl has been shown to localize at the basal membrane domain, and its activity is required for the specification of basal membrane identity. Lgl bears the characteristics of a molecular scaffold, with WD40 protein–protein interaction motifs at the N terminus, and has no known enzymatic activity. Its roles in cell polarity and tumor suppression are therefore likely to be mediated by protein–protein interactions. In fact, Lgl forms a complex with other cell polarity proteins, including aPKC and Par-6 (Betschinger et al., 2003; Plant et al., 2003; Yamanaka et al., 2003; Dollar et al., 2005; Yasumi et al., 2005). In the Lgl–atypical PKC (aPKC)–Par-6 complex, aPKC phosphorylates Lgl, which alters its conformation (Betschinger et al., 2005), inhibiting its activity and causing it to dissociate from the apical membrane cortex. aPKC also phosphorylates other cell polarity proteins, and the aPKC-catalyzed phosphorylations play a crucial role in the regulation of cell polarity in various cell types (Etienne-Manneville and Hall, 2003; Hurov et al., 2004; Sotillos et al., 2004; Suzuki et al., 2004). How the activity of aPKC itself is regulated, however, is not fully understood. In this study, we identify p32 as a novel Lgl binding protein that enhances the activity of aPKC.
Results and discussion
p32 is a novel mammalian Lgl2 (mLgl2) binding protein
To further understand the functional role of Lgl proteins, we used biochemical affinity purification to identify novel interacting proteins of mLgl. As bait, we used the C terminus of mLgl2 tagged with maltose binding protein (MBP). By applying rat kidney lysate to the fusion protein coupled to amylose resin beads, we purified a protein with a size of 30 kD (Fig. S1 A, left, available at http://www.jcb.org/cgi/content/full/jcb.200612022/DC1). No other mLgl2 binding proteins were identified. Mass spectrometric analysis identified the 30-kD protein as p32 (gC1QR, gC1q-BP, and HABP1), which was first characterized as a splicing factor 2–associated protein (Honore et al., 1993) but later described as a multifunctional chaperone protein (Storz et al., 2000). Western blotting with anti-p32 antibody confirmed the identity of the protein (Fig. S1 A, right).
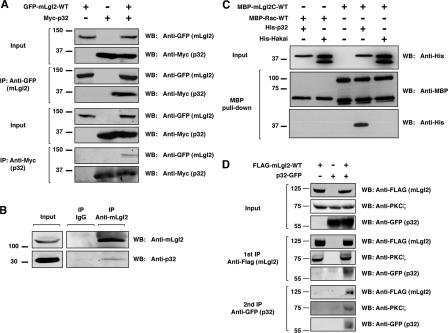
To validate the interaction, we examined whether mLgl2 and p32 were coimmunoprecipitated. GFP-mLgl2-WT (wild type) and Myc-p32 were coexpressed in human embryonic kidney (HEK) 293 cells and immunoprecipitated with either anti-GFP or anti-Myc antibody. Reciprocal coimmunoprecipitation of mLgl2 and p32 was observed, confirming the interaction between the two proteins (Fig. 1 A). Myc-p32 was not coimmunoprecipitated with GFP alone (Fig. S1 B). mLgl1, another mammalian homologue of Lgl, was also coimmunoprecipitated with p32 (Fig. S1 C). We also demonstrated that endogenous p32 and mLgl2 proteins were coimmunoprecipitated from HEK293 cell lysate (Fig. 1 B). We further showed that the interaction between mLgl2 and p32 was direct, as recombinant His-p32 protein bound specifically to MBP-mLgl2C-WT in an in vitro binding assay (Fig. 1 C).
Figure 1.
p32 is a novel mLgl2 binding protein. (A) Reciprocal coimmunoprecipitation of mLgl2 and p32. GFP-mLgl2-WT and Myc-p32 were expressed in HEK293 cells, and immunoprecipitation was performed by using either anti-GFP or anti-Myc antibody, followed by Western blotting with anti-GFP and anti-Myc antibodies. (B) Coimmunoprecipitation of endogenous mLgl2 and p32 proteins. Immunoprecipitation was performed with anti-mLgl2 antibody from HEK293 cell lysate, followed by Western blotting using anti-mLgl2 and anti-p32 antibodies. (C) Direct interaction between p32 and mLgl2. Recombinant MBP-mLgl2C-WT and His-p32 proteins were incubated and pulled down using amylose resin beads, followed by Western blotting using anti-MBP and anti-His antibodies. As controls, MBP-Rac-WT and His-Hakai were used. (D) Double immunoprecipitation of mLgl2 and p32 to analyze complex formation with PKCζ. FLAG-mLgl2-WT and p32-GFP were expressed in HEK293 cells. An initial immunoprecipitation was performed using anti-FLAG antibody, followed by elution of immunoprecipitates with FLAG peptide. The eluates were subjected to a second immunoprecipitation using anti-GFP antibody. The first and second immunoprecipitates were examined by Western blotting using anti-FLAG, anti-GFP, and anti-PKCζ antibodies.
mLgl2 binds to and is phosphorylated by aPKC (PKCλ and PKCζ). p32 has also been shown to interact with and regulate PKCs, including aPKC (Storz et al., 2000; Robles-Flores et al., 2002). We therefore examined whether mLgl2, p32, and PKCζ form a trimeric complex. FLAG-mLgl2-WT and p32-GFP were coexpressed in HEK293 cells, and double immunoprecipitation experiments were performed. We first immunoprecipitated with anti-FLAG antibody, which pulled down both endogenous PKCζ and p32-GFP together with FLAG-mLgl2 (Fig. 1 D). The immunoprecipitate was then eluted from the beads with FLAG peptide, and a second immunoprecipitation was performed using anti-GFP antibody. After the second immunoprecipitation, p32 remained bound to mLgl2 and PKCζ (Fig. 1 D). These results indicate that the three proteins form a trimeric complex. Using several truncation mutants, we showed that amino acids 544–1027 of mLgl2 and the C terminus of p32 are responsible for the interaction (Fig. S1, D–F).
p32 binds to mLgl2 transiently and enhances PKCζ activity
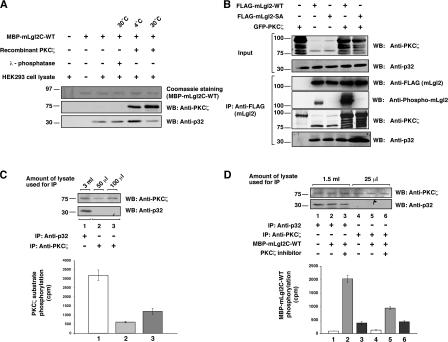
To investigate whether the phosphorylation status of mLgl2 influences its interaction with p32, we performed in vitro binding experiments (Fig. 2 A). Untreated and λ-phosphatase–treated MBP-mLgl2C-WT bound to endogenous p32 from HEK293 cell lysates to a similar extent. Interestingly, MBP-mLgl2C-WT that had been incubated with PKCζ at 4°C pulled down more p32 (Fig. 2 A). However, MBP-mLgl2C-WT incubated with PKCζ at 30°C did not show increased interaction with p32 (Fig. 2 A). These data suggest that PKCζ enhances the interaction between mLgl2 and p32, but once mLgl2 is phosphorylated by PKCζ, p32 is no longer able to efficiently interact with the complex. Comparable results were obtained using a recombinant p32 protein (Fig. S2 A, available at http://www.jcb.org/cgi/content/full/jcb.200612022/DC1).
Figure 2.
p32 interacts with mLgl2 transiently and enhances PKCζ activity. (A and B) The interaction of p32 with mLgl2 was examined in the presence or absence of PKCζ under different mLgl2 phosphorylation conditions. (A) MBP-mLgl2C-WT was incubated with PKCζ or λ-phosphatase at 30 or 4°C, followed by incubation with HEK293 cell lysates and pull-down assay using amylose resin beads. PKCζ and endogenous p32 bound to MBP-mLgl2C-WT were examined by Western blotting using anti-PKCζ and anti-p32 antibodies, respectively. The membrane was stained with Coomassie brilliant blue to visualize MBP-mLgl2C-WT. (B) FLAG-mLgl2-WT or -SA was expressed in HEK293 cells with or without coexpression of GFP-PKCζ. Immunoprecipitation was performed using anti-FLAG antibody, followed by Western blotting with anti-FLAG, anti–phospho-mLgl2, anti-PKCζ, and anti-p32 antibodies. (C) Binding of p32 enhances the activity of PKCζ to phosphorylate a PKCζ peptide substrate. PKCζ was immunoprecipitated from the indicated amounts of HEK293 cell lysate by using either anti-p32 or anti-PKCζ antibody. In vitro kinase assays were performed by incubating immunoprecipitates with PKCζ peptide substrate and γ-[32P]ATP. Peptide substrates were then collected by incubation with streptavidin beads, and the radioactivity of the beads was measured. Without peptide substrate, negligible radioactivity was measured using streptavidin beads alone. (D) Binding of p32 enhances the activity of PKCζ to phosphorylate mLgl2. Immunoprecipitations and in vitro kinase assays were performed as described, except that MBP-mLgl2C-WT was used as PKCζ substrate, and incubations were performed in the presence or absence of a PKCζ inhibitor. (C and D) The radioactive count by control IgG immunoprecipitate was subtracted from that by p32 and PKCζ immunoprecipitates. Phosphorylation reactions were duplicated for each experiment, and the results shown are representative of three independent experiments. Error bars indicate mean ± SD.
To further investigate the interaction between mLgl2, PKCζ, and p32, we performed immunoprecipitation experiments using WT (mLgl2-WT) and nonphosphorylatable (mLgl2-SA) forms of mLgl2, in the presence or absence of overexpressed PKCζ (Fig. 2 B). In the mLgl2-SA mutant, three serines in the conserved aPKC phosphorylation site were changed to alanine. Endogenous p32 from HEK293 cell lysates was coimmunoprecipitated with both mLgl2-WT and mLgl2-SA (Fig. 2 B). Coexpression of GFP-PKCζ enhanced the interaction of p32 with mLgl2-SA, but not with mLgl2-WT (Fig. 2 B). These data further support a key role of PKCζ and the phosphorylation status of mLgl2 in the transient interaction between mLgl2 and p32. Overexpression of mLgl2 did not substantially affect the interaction between p32 and PKCζ, suggesting that the binding of p32 to PKCζ is not affected by the presence of mLgl2 (Fig. S2 B).
We next examined the effect of p32 binding on the activity of PKCζ using in vitro kinase assays. Approximately equal amounts of PKCζ were pulled down by immunoprecipitation with anti-p32 antibody from 3 ml of HEK293 cell lysate and with anti-PKCζ antibody from 100 μl of lysate (Fig. 2 C, top). Immunoprecipitates were incubated with a biotinylated PKCζ-specific peptide substrate and γ-[32P]ATP. PKCζ immunoprecipitated with anti-p32 antibody phosphorylated the substrate ∼2.5 times more efficiently than PKCζ immunoprecipitated with anti-PKCζ antibody (Fig. 2 C), suggesting that the interaction with p32 enhances the catalytic activity of PKCζ. A specific PKC inhibitor, Gö6983, suppressed phosphorylation of the peptide by p32 immunoprecipitates (Fig. S2 C) and other types of PKCs, such as PKCα (classical PKC) and PKCδ (novel PKC), did not efficiently phosphorylate the substrate under the conditions used (Fig. S2 D). These data indicate that the increased phosphorylation of the peptide in the p32 immunoprecipitates is indeed due to enhanced aPKC activity.
As mLgl2 is a crucial target for phosphorylation by PKCζ, we examined whether the interaction with p32 affected the ability of PKCζ to phosphorylate mLgl2. We first immunoprecipitated PKCζ with either anti-p32 or anti-PKCζ antibody as described in the previous paragraph. The immunoprecipitates were then incubated with MBP-mLgl2C-WT and γ-[32P]ATP in the presence or absence of a PKCζ inhibitor. As seen with the peptide substrate, PKCζ bound to p32 was significantly more efficient at phosphorylating MBP-mLgl2C-WT than PKCζ alone (Fig. 2 D). Furthermore, the phosphorylation of MBP-mLgl2C- WT was shown to be mediated by PKCζ, as the addition of the PKCζ inhibitor efficiently blocked the phosphorylation of MBP-mLgl2C-WT (Fig. 2 D). In the absence of immunoprecipitates, MBP-mLgl2C-WT protein was not substantially phosphorylated, indicating that there is no contaminating kinase present with the protein (unpublished data). We also showed that the PKCζ inhibitor did not suppress phosphorylation by other types of PKC, such as PKCα and PKCδ (Fig. S2 E), confirming the specificity of the inhibitor. These data indicate that p32 enhances the activity of PKCζ to phosphorylate mLgl2.
It was recently shown that one mechanism of regulating aPKC activity involves the small GTPase Cdc42; binding of GTP-bound Cdc42 and Par-6 to aPKC enhances the activity of aPKC (Joberty et al., 2000; Lin et al., 2000; Qiu et al., 2000). In Drosophila melanogaster, however, Lgl is still phosphorylated by aPKC, even if the ability of Par-6 to bind to Cdc42 is abolished, indicating that Cdc42 is not required for the activation of aPKC in this system (Hutterer et al., 2004). We propose that p32 binding to mLgl2 and PKCζ is a novel mode of aPKC regulation that enhances its catalytic activity, leading to increased mLgl2 phosphorylation.
p32 partially colocalizes with mLgl2, and overexpression of p32 affects the localization of mLgl2 in MDCK cells
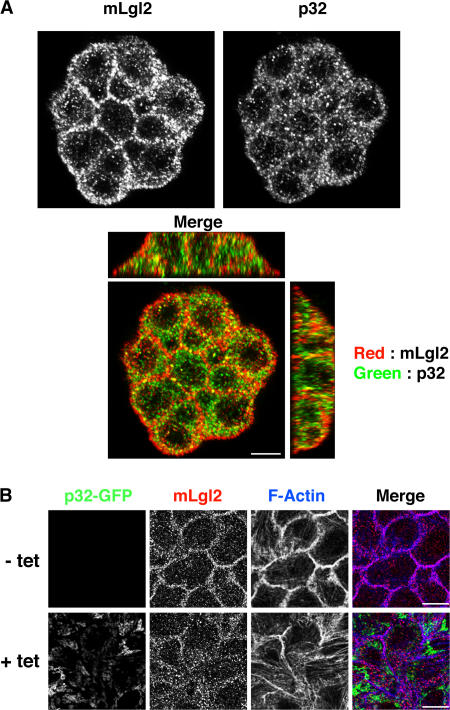
Next, we examined the subcellular localization of endogenous p32 and mLgl2 proteins in MDCK cells. mLgl2 localized at the plasma membrane as well as in the cytosol observed as small puncta, whereas p32 was accumulated along the plasma membrane with some cytosolic localization (Fig. 3 A, top). We found that both proteins partially colocalized at the cortex region of the plasma membrane (Fig. 3 A, bottom), suggesting that the interaction may occur at the cortical region rather than in the cytosol.
Figure 3.
p32 partially colocalizes with mLgl2, and overexpression of p32 affects the localization of mLgl2 in MDCK cells. (A) Immunofluorescence analysis of endogenous mLgl2 and p32 proteins in MDCK cells. (B) Immunofluorescence analysis of p32-GFP, mLgl2, and F-actin in MDCK cells stably expressing p32-GFP in a tetracycline-inducible manner. These results are representative of results from two independent clones of p32-GFP MDCK cells. Bars, 10 μm.
We also examined the effect of overexpression of p32 on the localization of mLgl2. We used MDCK cell lines that stably express p32-GFP in a tetracycline-inducible manner (Fig. S3 A, available at http://www.jcb.org/cgi/content/full/jcb.200612022/DC1). In the absence of tetracycline, expression of p32-GFP was not induced and mLgl2 localized at the plasma membrane (Fig. 3 B, top), as observed in parental nontransfected MDCK cells (not depicted). Upon the addition of tetracycline, the expression of p32-GFP was induced and mLgl2 dissociated from the cell cortex and localized to the cytosol (Fig. 3 B, bottom). These results further support a role for p32 as a regulator of mLgl2.
Overexpression and knockdown of p32 disturb the normal polarization of MDCK cells
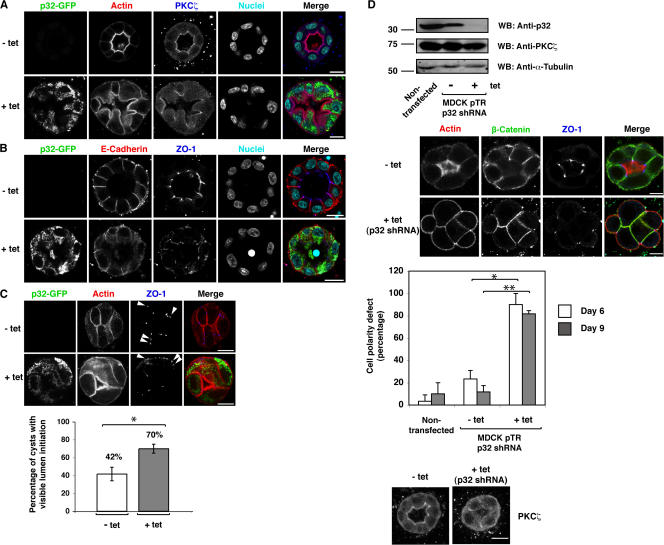
To investigate the functional role of p32 in cell polarity, we used a 3D MDCK cell culture system. First, we used the MDCK cell lines expressing p32-GFP in a tetracycline-inducible manner described in the previous paragraph. Noninduced MDCK cells grown in a collagen gel formed cysts with a regular lumen and well-defined, F-actin–rich apical membrane domains (Fig. 4 A, top). PKCζ also localized to the apical membrane domains (Fig. 4 A, top). However, upon the induction of p32-GFP expression, cysts showed striking morphological changes, with expanded apical membrane domains and irregularly shaped lumens (Fig. 4 A, bottom). PKCζ no longer localized to the apical membrane domains, but instead concentrated at apical sites of cell–cell contacts. Interestingly, an expansion of the apical membrane domains into the basal membrane domains was also observed in mLgl1/2 knockdown MDCK cells (Yamanaka et al., 2006).
Figure 4.
p32 expression level affects cell polarity of MDCK cells in 3D culture. (A and B) Immunofluorescence analysis of cell polarity markers in p32-GFP MDCK cell cysts. MDCK cells stably expressing p32-GFP in a tetracycline-inducible manner were seeded in a collagen gel. Cells were either treated or not treated with tetracycline to induce p32-GFP expression. (A) Immunostaining was performed using anti-GFP and anti-PKCζ antibodies. Actin was visualized with TRITC-labeled phalloidin. Nuclei were stained with Hoechst dye. (B) Immunostaining was performed using anti-GFP, anti-E-cadherin, and anti–ZO-1 antibodies. (C) Cell polarity defects in p32-overexpressing MDCK cells are linked to PKCζ activation. A cell-permeable PKCζ pseudosubstrate inhibitor was added to noninduced or p32-GFP–overexpressing cells. Immunostaining was performed using anti-GFP and –ZO-1 antibodies. Arrowheads indicate ectopic localizations of ZO-1. These results are representative of results from two independent clones of p32-GFP MDCK cells. *, P < 0.05. (D) A knockdown of p32 induces cell polarity defects in p32 shRNA–expressing MDCK cells. (top) Western blotting with anti-p32, anti-PKCζ, or anti–α-tubulin antibody was performed using cell lysates from parental or p32 shRNA cells. (second and bottom panels) Immunofluorescence analysis of cell polarity markers in p32 shRNA MDCK cell cysts. Immunostaining was performed using TRITC-labeled phalloidin and anti–β-catenin, anti–ZO-1, and anti-PKCζ antibodies. These results are representative of results from two independent clones of p32 shRNA MDCK cells. (third panel) For statistical analysis, 20 cysts were examined in each experiment at day 6 or 9 after seeding, and the results represent the means ± SD of three independent experiments. *, P < 0.0015; **, P < 0.0005. Cysts were analyzed by confocal microscopy. Bars, 10 μm.
We next examined the effect of p32 overexpression on the localization of the adherens junction marker E-cadherin and the tight junction component zonula occludens-1 (ZO-1). Under noninduced conditions, E-cadherin localized in a well-defined strip at the basolateral membrane. ZO-1 localized at the tight junction as a distinctive spot in close proximity to the internal lumen (Fig. 4 B, top). In cells induced to overexpress p32-GFP, the linear localization of E-cadherin at the basolateral membrane domain was lost and E-cadherin localized more diffusely (Fig. 4 B, bottom). ZO-1 localization was also disturbed and no longer restricted to the tight junctions (Fig. 4 B, bottom). We quantified the cell polarity defects in p32-overexpressing cells by determining the percentage of cysts with regular apical actin enrichment and normal lumen formation (Fig. S3 B). Although 77% of noninduced cysts had this normal phenotype, only 15% of the induced cysts did, supporting an involvement of p32 in cell polarity.
Because aPKC inhibition using dominant-negative mutants in epithelial cells has been shown to cause a loss of apical membrane domain identity (Chalmers et al., 2005), we examined the effect of a cell-permeable PKCζ inhibitor on cell polarity. In the presence of the PKCζ inhibitor, cysts failed to form normally, as indicated by the lack of apical actin enrichment, mislocalization of ZO-1, and failure to form lumens (Fig. 4 C, top images). To investigate whether the effect of p32 overexpression on cell polarity involves PKCζ activation, we studied whether p32 overexpression could rescue the phenotype induced by the PKCζ inhibitor. As shown in Fig. 4 C (bottom images), overexpression of p32 partially reverted the defects in apical actin enrichment and lumen formation, but not in ZO-1 localization. We conclude that p32 regulates cell polarity at least in part by modulating PKCζ activity.
Finally, we examined whether the knockdown of p32 affects epithelial cell polarity. We established MDCK cell lines stably expressing p32 short hairpin RNA (shRNA) in a tetracycline-inducible manner. In these cells, the level of endogenous p32 protein decreased by >90% in the presence of tetracycline (Fig. 4 D and Fig. S3 C). Even in the absence of tetracycline, a slight decrease in p32 expression was observed compared with nontransfected parental cells, which can sometimes be seen with the Tet-on expression system (Fig. 4 D, top). In the absence of tetracycline, p32 shRNA cells formed cysts where ZO-1 localized at the subapical region (Fig. 4 D, second from top). In contrast, the addition of tetracycline induced cell polarity defects, as indicated by the mislocalization of ZO-1 to the basolateral and basal membrane domains (Fig. 4 D, second from top; more images are shown in Fig. S3 D). A similar mislocalization of ZO-1 to the basolateral membrane domain was also reported in MDCK cells overexpressing mLgl2 (Yamanaka et al., 2003). Statistical analysis showed a significant difference between tet (−) and tet (+) in p32 shRNA cells (Fig. 4 D, third panel). The slight increase of cell polarity defects in tet (−) p32 shRNA cells compared with those in parental cells may be due to decreased p32 expression (Fig. 4 D, third panel). The expression of p32 shRNA also induced mislocalization of PKCζ to the basolateral membrane domain or the cytosol (Fig. 4 D, bottom), further suggesting a role of p32 in regulating aPKC. Collectively, these data indicate a physiological role of p32 in cell polarity.
The deregulation of cell polarity proteins often induces a more invasive phenotype in malignant tumors (Bilder, 2004; Igaki et al., 2006). In this study, we show that overexpression and knockdown of p32 disturb the normal polarization of MDCK cells (Fig. 4). Interestingly, p32 is overexpressed in thyroid, colon, pancreatic, gastric, esophageal, and lung adenocarcinoma, but not in their nonmalignant counterparts (Rubinstein et al., 2004). Furthermore, p32 is differentially expressed during the progression of epidermal carcinoma and accumulates in metastatic islands (Ghosh et al., 2004). Our results suggest that p32 not only serves as a marker of malignant cells but also may function as an oncoprotein. The cell polarity defects observed in p32 knockdown cells further suggest that precisely controlled levels of p32 protein may be essential for normal polarization. In conclusion, we have identified p32 as a novel mLgl2-interacting protein that forms a transient complex with mLgl2 and PKCζ. In this protein complex, p32 regulates cell polarity through its ability to enhance the kinase activity of PKCζ.
Materials and methods
Purification of mLgl2 binding proteins from rat kidney
For each experiment, five adult rats were killed and kidneys were dissected and homogenized in buffer A (5 mM Tris/HCl, pH 7.5, 320 mM sucrose, and 10 μM PMSF). The homogenate was fractionated as previously described (Fujita et al., 1998), and the detergent fraction was applied to 250 μl amylose resin beads (New England Biolabs, Inc.) coupled to 30 μg MBP-mLgl2C-WT or -SD. Beads were washed three times in buffer B (20 mM Hepes/NaOH, pH 7.4, 1 mM DTT, 5 mM MgCl2, 2% NP-40, 150 mM NaCl, and 10 μM PMSF) followed by elution using buffer B with 10 mM maltose. The eluted samples were separated by SDS-PAGE, and proteins were visualized using SYPRO Ruby protein gel stain (Invitrogen). MBP-mLgl2–interacting proteins were excised from the gel, and the amino acid sequences were determined by LC-MS/MS.
LC-MS/MS procedure
mLgl2-interacting protein gel bands were excised and in-gel digested with trypsin. The digest mixtures were separated by nanoscale liquid chromatography (LC Packings) on reverse-phase C18 column (150 × 0.075 mm ID; flow rate 0.15 ml/min). The eluate was introduced directly into a Q-STAR Pulsar-i-hybrid quadruple time of flight mass spectrometer (MDS Sciex). The spectra were searched against a National Center for Biotechnology Information nonredundant database with MASCOT MS/MS Ions search (Matrix Science).
Immunoprecipitation, MBP-mLgl2 pull down, and Western blotting
Immunoprecipitation was performed as described before (Hogan et al., 2004). When indicated, immunoprecipitates were incubated with 400 U λ-phosphatase (New England Biolabs, Inc.) in λ-phosphatase buffer (50 mM Tris/HCl, pH 7.5, 100 mM NaCl, 0.1 mM EGTA, 0.01% Brij 35, and 2 mM MgCl2) or 125 ng recombinant PKCζ (Calbiochem) in phosphorylation buffer (20 mM Tris/HCl, pH 7.5, 5 mM MgCl2, 1 mM EGTA, and 40 μM ATP) for 30 min at the indicated temperature. pFLAG-CMV2-mLgl1 was provided by T. Pawson (Mount Sinai Hospital, Toronto, Canada).
MBP-mLgl2 pull down from HEK293 lysates was performed as described for immunoprecipitation, except that 10 μg MBP-mLgl2C-WT were conjugated to 50 μl amylose resin beads. Western blotting was performed as described previously (Hogan et al., 2004) with the exception that blots in Fig. 1 (A, B, and D), Fig. 2 (A, C, and D), Fig. S1 (A, C, and F), Fig. S2 (A and B), and Fig. S3 (A and C) were visualized using an infrared detection system (Odyssey; Licor Bioscience). In experiments with recombinant proteins, we used 1.6 μg His6-p32 and His6-Hakai and 10 μg MBP-mLgl2C-WT and MBP-Rac-WT proteins.
Cell culture
To obtain MDCK cell lines stably expressing p32-GFP in an inducible manner, we used the tet-on system (Invitrogen). First, MDCK cells were transfected with pcDNA6/TR, followed by selection in a medium containing 5 μg ml−1 blasticidin. Then, pcDNA4/TO/p32-GFP was used for the second transfection, and doubly transfected cells were selected in a tetracycline-free medium containing 5 μg ml−1 blasticidin and 400 μg ml−1 Zeocin. Induction of p32-GFP expression after addition of 2 μg/ml tetracycline was monitored over time and confirmed by Western blotting. Four independent clones were obtained. To obtain MDCK cell lines stably expressing p32 shRNA in a tetracycline-inducible manner, the same procedure was performed, except that pSUPERIOR-p32 shRNA was used for the second transfection and 800 μg ml−1 G418 was added to the medium for selection. Three independent clones were obtained.
Immunofluorescence
Cysts were processed for immunofluorescence staining as described previously (O'Brien et al., 2001). Rabbit anti–mLgl2-S653P-2 antibody was provided by S. Ohno (Yokohama City University, Yokohama, Japan). Cysts were mounted using ProLong Gold Antifade reagent (Invitrogen) and analyzed on a confocal microscope (TCS SP5; Leica) with a 63× NA 1.4 oil-immersion objective at room temperature (Leica). 65 z sections at 1-μm intervals were captured per cyst, and images were acquired using the Leica Application Suite. Images shown are single sections through the center of the cysts. Images were colorized and contrast was enhanced linearly using the Volocity software package (Improvision), and brightness was adjusted using Photoshop CS (Adobe). For the statistical analysis of cell polarity defects, noninduced and p32-GFP–overexpressing MDCK cell cysts were analyzed at room temperature using a microscope (DM IRB; Leica) with a 10× 0.25 air objective (Leica). Images were captured using a camera (Orca; Hamamatsu) and Openlab software (Improvision). Secondary antibodies used were goat anti-mouse Alexa 488 and goat anti-rabbit Alexa 647 (Invitrogen), as well as goat anti-rat Rhodamine Red-X (Jackson ImmunoResearch Laboratories). To visualize actin and nuclei, we used TRITC-labeled phalloidin (Sigma-Aldrich) and Hoechst 33342 (Invitrogen), respectively.
MDCK 3D cell culture and PKCζ inhibition
3D cell culture was performed as described previously (O'Brien et al., 2001) except that chamberslides (BD Biosciences) were used instead of filter inserts. In brief, MDCK cells were seeded into a 2 mg/ml collagen-I gel at a concentration of 2.5 × 105 cells/ml, covered with 500 μl of culture medium and cultured for 6 or 9 d. For induction of p32-GFP expression, the culture medium was supplied with 2 μg/ml tetracycline from day 1, and the medium was replaced daily. For inhibition of PKCζ, a cell-permeable myristoylated PKCζ pseudosubstrate inhibitor (Biosource International) was added to cysts at a concentration of 25 μM from day 1. Before these experiments, we titrated the concentration of the inhibitor (25, 50, and 100 μM) to obtain an optimal condition where cell polarity was disturbed without cytotoxicity. For p32 knockdown, cells were supplied with tetracycline for 3 d before seeding into a collagen gel, and the medium was replaced daily.
In vitro kinase assays
After immunoprecipitation, samples were incubated in 60 μl phosphorylation buffer with 9 μCi γ-[32P]ATP and either 40 μM biotinylated PKCζ peptide substrate or 200 ng MBP-mLgl2C-WT at 30°C for 5 min. 100 nM Gö6983 (Calbiochem) or 0.5 mM PKCζ pseudosubstrate inhibitor (Calbiochem) was added when indicated. The kinetics of mLgl2 phosphorylation by endogenous PKCζ was first determined, and a 5-min incubation time was used for all experiments. After the kinase reaction, 50 μl of the reaction buffer was incubated with either 20 μl streptavidin agarose (Sigma- Aldrich) or 50 μl amylose resin (New England Biolabs, Inc.) at 4°C for 30 min, followed by intensive washing. Radioactivity of the beads was measured using Cerenkov counting in a liquid scintillation analyzer (1900-TR; Packard Instrument Co.). For Fig. S2 E, phosphorylation by PKCα and PKCδ was performed as described previously (Fujita et al., 1996). After phosphorylation, the reaction was stopped by addition of 30 mM NaF and 100 mM EDTA, and the phosphorylated substrate was spotted onto P81 paper (Whatman), followed by intensive washing in 75 mM H3PO4 and Cerenkov counting. The recombinant PKCα and PKCζ were purchased from Calbiochem, and PKCδ was obtained from Sigma-Aldrich.
Statistical analysis
t tests assuming equal or unequal variance were performed for statistical analysis.
Online supplemental material
Fig. S1 shows characterization of the interaction between mLgl2 and p32. Fig. S2 shows that p32 interacts with mLgl2 transiently and enhances PKCζ activity. Fig. S3 shows that p32 expression level affects cell polarity of MDCK cells in 3D culture. The supplemental text provides details about the generation of plasmid constructs and sources of antibodies. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200612022/DC1.
Supplementary Material
Acknowledgments
We thank Martin Raff for critical reading of the manuscript. We also thank Tony Pawson for the pFLAG-CMV2-mLgl1 construct and Shigeo Ohno for anti–mLgl2-S653P-2 antibody.
This work is supported by Medical Research Council funding to the Cell Biology Unit.
Abbreviations used in this paper: aPKC, atypical PKC; HEK, human embryonic kidney; Lgl, lethal giant larvae; MBP, maltose binding protein; mLgl, mammalian Lgl; shRNA, short hairpin RNA; WT, wild type; ZO-1, zonula occludens-1.
References
- Betschinger, J., K. Mechtler, and J.A. Knoblich. 2003. The Par complex directs asymmetric cell division by phosphorylating the cytoskeletal protein Lgl. Nature. 422:326–330. [DOI] [PubMed] [Google Scholar]
- Betschinger, J., F. Eisenhaber, and J.A. Knoblich. 2005. Phosphorylation- induced autoinhibition regulates the cytoskeletal protein Lethal (2) giant larvae. Curr. Biol. 15:276–282. [DOI] [PubMed] [Google Scholar]
- Bilder, D. 2004. Epithelial polarity and proliferation control: links from the Drosophila neoplastic tumor suppressors. Genes Dev. 18:1909–1925. [DOI] [PubMed] [Google Scholar]
- Bilder, D., M. Li, and N. Perrimon. 2000. Cooperative regulation of cell polarity and growth by Drosophila tumor suppressors. Science. 289:113–116. [DOI] [PubMed] [Google Scholar]
- Chalmers, A.D., M. Pambos, J. Mason, S. Lang, C. Wylie, and N. Papalopulu. 2005. aPKC, Crumbs3 and Lgl2 control apicobasal polarity in early vertebrate development. Development. 132:977–986. [DOI] [PubMed] [Google Scholar]
- Dollar, G.L., U. Weber, M. Mlodzik, and S.Y. Sokol. 2005. Regulation of Lethal giant larvae by Dishevelled. Nature. 437:1376–1380. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville, S., and A. Hall. 2003. Cdc42 regulates GSK-3β and adenomatous polyposis coli to control cell polarity. Nature. 421:753–756. [DOI] [PubMed] [Google Scholar]
- Fujita, Y., T. Sasaki, K. Fukui, H. Kotani, T. Kimura, Y. Hata, T.C. Sudhof, R.H. Scheller, and Y. Takai. 1996. Phosphorylation of Munc-18/n-Sec1/rbSec1 by protein kinase C: its implication in regulating the interaction of Munc-18/n-Sec1/rbSec1 with syntaxin. J. Biol. Chem. 271:7265–7268. [DOI] [PubMed] [Google Scholar]
- Fujita, Y., H. Shirataki, T. Sakisaka, T. Asakura, T. Ohya, H. Kotani, S. Yokoyama, H. Nishioka, Y. Matsuura, A. Mizoguchi, et al. 1998. Tomosyn: a syntaxin-1-binding protein that forms a novel complex in the neurotransmitter release process. Neuron. 20:905–915. [DOI] [PubMed] [Google Scholar]
- Ghosh, I., A.R. Chowdhury, M.R. Rajeswari, and K. Datta. 2004. Differential expression of hyaluronic acid binding protein 1 (HABP1)/P32/C1QBP during progression of epidermal carcinoma. Mol. Cell. Biochem. 267:133–139. [DOI] [PubMed] [Google Scholar]
- Hogan, C., N. Serpente, P. Cogram, C.R. Hosking, C.U. Bialucha, S.M. Feller, V.M. Braga, W. Birchmeier, and Y. Fujita. 2004. Rap1 regulates the formation of E-cadherin-based cell-cell contacts. Mol. Cell. Biol. 24:6690–6700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honore, B., P. Madsen, H.H. Rasmussen, J. Vandekerckhove, J.E. Celis, and H. Leffers. 1993. Cloning and expression of a cDNA covering the complete coding region of the P32 subunit of human pre-mRNA splicing factor SF2. Gene. 134:283–287. [DOI] [PubMed] [Google Scholar]
- Hurov, J.B., J.L. Watkins, and H. Piwnica-Worms. 2004. Atypical PKC phosphorylates PAR-1 kinases to regulate localization and activity. Curr. Biol. 14:736–741. [DOI] [PubMed] [Google Scholar]
- Hutterer, A., J. Betschinger, M. Petronczki, and J.A. Knoblich. 2004. Sequential roles of Cdc42, Par-6, aPKC, and Lgl in the establishment of epithelial polarity during Drosophila embryogenesis. Dev. Cell. 6:845–854. [DOI] [PubMed] [Google Scholar]
- Igaki, T., R.A. Pagliarini, and T. Xu. 2006. Loss of cell polarity drives tumor growth and invasion through JNK activation in Drosophila. Curr. Biol. 16:1139–1146. [DOI] [PubMed] [Google Scholar]
- Joberty, G., C. Petersen, L. Gao, and I.G. Macara. 2000. The cell-polarity protein Par6 links Par3 and atypical protein kinase C to Cdc42. Nat. Cell Biol. 2:531–539. [DOI] [PubMed] [Google Scholar]
- Lin, D., A.S. Edwards, J.P. Fawcett, G. Mbamalu, J.D. Scott, and T. Pawson. 2000. A mammalian PAR-3-PAR-6 complex implicated in Cdc42/Rac1 and aPKC signalling and cell polarity. Nat. Cell Biol. 2:540–547. [DOI] [PubMed] [Google Scholar]
- Mechler, B.M., W. McGinnis, and W.J. Gehring. 1985. Molecular cloning of lethal(2)giant larvae, a recessive oncogene of Drosophila melanogaster. EMBO J. 4:1551–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien, L.E., T.S. Jou, A.L. Pollack, Q. Zhang, S.H. Hansen, P. Yurchenco, and K.E. Mostov. 2001. Rac1 orientates epithelial apical polarity through effects on basolateral laminin assembly. Nat. Cell Biol. 3:831–838. [DOI] [PubMed] [Google Scholar]
- Ohshiro, T., T. Yagami, C. Zhang, and F. Matsuzaki. 2000. Role of cortical tumour-suppressor proteins in asymmetric division of Drosophila neuroblast. Nature. 408:593–596. [DOI] [PubMed] [Google Scholar]
- Peng, C.Y., L. Manning, R. Albertson, and C.Q. Doe. 2000. The tumour- suppressor genes lgl and dlg regulate basal protein targeting in Drosophila neuroblasts. Nature. 408:596–600. [DOI] [PubMed] [Google Scholar]
- Plant, P.J., J.P. Fawcett, D.C. Lin, A.D. Holdorf, K. Binns, S. Kulkarni, and T. Pawson. 2003. A polarity complex of mPar-6 and atypical PKC binds, phosphorylates and regulates mammalian Lgl. Nat. Cell Biol. 5:301–308. [DOI] [PubMed] [Google Scholar]
- Qiu, R.G., A. Abo, and G.S. Martin. 2000. A human homolog of the C. elegans polarity determinant Par-6 links Rac and Cdc42 to PKCzeta signaling and cell transformation. Curr. Biol. 10:697–707. [DOI] [PubMed] [Google Scholar]
- Robles-Flores, M., E. Rendon-Huerta, H. Gonzalez-Aguilar, G. Mendoza-Hernandez, S. Islas, V. Mendoza, M.V. Ponce-Castaneda, L. Gonzalez-Mariscal, and F. Lopez-Casillas. 2002. p32 (gC1qBP) is a general protein kinase C (PKC)-binding protein; interaction and cellular localization of P32-PKC complexes in ray hepatocytes. J. Biol. Chem. 277:5247–5255. [DOI] [PubMed] [Google Scholar]
- Rubinstein, D.B., A. Stortchevoi, M. Boosalis, R. Ashfaq, B. Ghebrehiwet, E.I. Peerschke, F. Calvo, and T. Guillaume. 2004. Receptor for the globular heads of C1q (gC1q-R, p33, hyaluronan-binding protein) is preferentially expressed by adenocarcinoma cells. Int. J. Cancer. 110:741–750. [DOI] [PubMed] [Google Scholar]
- Sotillos, S., M.T. Diaz-Meco, E. Caminero, J. Moscat, and S. Campuzano. 2004. DaPKC-dependent phosphorylation of Crumbs is required for epithelial cell polarity in Drosophila. J. Cell Biol. 166:549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz, P., A. Hausser, G. Link, J. Dedio, B. Ghebrehiwet, K. Pfizenmaier, and F.J. Johannes. 2000. Protein kinase C μ is regulated by the multifunctional chaperon protein p32. J. Biol. Chem. 275:24601–24607. [DOI] [PubMed] [Google Scholar]
- Suzuki, A., M. Hirata, K. Kamimura, R. Maniwa, T. Yamanaka, K. Mizuno, M. Kishikawa, H. Hirose, Y. Amano, N. Izumi, et al. 2004. aPKC acts upstream of PAR-1b in both the establishment and maintenance of mammalian epithelial polarity. Curr. Biol. 14:1425–1435. [DOI] [PubMed] [Google Scholar]
- Yamanaka, T., Y. Horikoshi, Y. Sugiyama, C. Ishiyama, A. Suzuki, T. Hirose, A. Iwamatsu, A. Shinohara, and S. Ohno. 2003. Mammalian Lgl forms a protein complex with PAR-6 and aPKC independently of PAR-3 to regulate epithelial cell polarity. Curr. Biol. 13:734–743. [DOI] [PubMed] [Google Scholar]
- Yamanaka, T., Y. Horikoshi, N. Izumi, A. Suzuki, K. Mizuno, and S. Ohno. 2006. Lgl mediates apical domain disassembly by suppressing the PAR-3-aPKC-PAR-6 complex to orient apical membrane polarity. J. Cell Sci. 119:2107–2118. [DOI] [PubMed] [Google Scholar]
- Yasumi, M., T. Sakisaka, T. Hoshino, T. Kimura, Y. Sakamoto, T. Yamanaka, S. Ohno, and Y. Takai. 2005. Direct binding of Lgl2 to LGN during mitosis and its requirement for normal cell division. J. Biol. Chem. 280:6761–6765. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.