Abstract
Rapamycin (rapa)-induced heterodimerization of the FRB domain of the mammalian target of rapa and FKBP12 was used to translocate a phosphoinositide 5-phosphatase (5-ptase) enzyme to the plasma membrane (PM) to evoke rapid changes in phosphatidylinositol 4,5-bisphosphate (PtdIns(4,5)P 2) levels. Rapa-induced PM recruitment of a truncated type IV 5-ptase containing only the 5-ptase domain fused to FKBP12 rapidly decreased PM PtdIns(4,5)P 2 as monitored by the PLCδ1PH-GFP fusion construct. This decrease was paralleled by rapid termination of the ATP-induced Ca2+ signal and the prompt inactivation of menthol-activated transient receptor potential melastatin 8 (TRPM8) channels. Depletion of PM PtdIns(4,5)P 2 was associated with a complete blockade of transferrin uptake and inhibition of epidermal growth factor internalization. None of these changes were observed upon rapa-induced translocation of an mRFP-FKBP12 fusion protein that was used as a control. These data demonstrate that rapid inducible depletion of PM PtdIns(4,5)P 2 is a powerful tool to study the multiple regulatory roles of this phospholipid and to study differential sensitivities of various processes to PtdIns(4,5)P 2 depletion.
Introduction
Phosphatidylinositol 4,5-bisphosphate (PtdIns(4,5)P 2) is the major phosphoinositide species in mammalian cells and has been associated with numerous molecular events critical for cellular signaling. PtdIns(4,5)P 2 is hydrolyzed by PLC enzymes to generate diacylglycerol and inositol 1,4,5-triphosphate, two pivotal second messengers (Berridge, 1993), and it is also converted by class I phosphoinositol 3-kinases to PtdIns(3,4,5)P 3 (Toker and Cantley, 1997). PtdIns(4,5)P 2 directly interacts with several ion channels, transporters (Fuster et al., 2004; Suh and Hille, 2005), and actin binding proteins (Hilpela et al., 2004) and regulates enzymes such as PLC and PLD (Liscovitch et al., 1994; Lomasney et al., 1996). Several molecules within the receptor internalization machinery also contain inositide binding domains, but the exact lipid species that regulates them in the cell has not been firmly established (Itoh et al., 2001). It is a major challenge to understand how a single type of molecule is able to regulate so many processes simultaneously and perhaps independently within the plasma membrane (PM).
Part of the problem in studying the multiple functions of PtdIns(4,5)P 2 is that it is difficult to manipulate phosphoinositide levels within the cells. For example, most data on channel regulation rely upon the addition of phospholipids to excised patches and the use of inhibitors such as high concentrations of wortmannin to inhibit PtdIns(4,5)P 2 formation (Suh and Hille, 2002; Rohacs et al., 2005). Several attempts have been made to alter the level of PtdIns(4,5)P 2 in intact cells by expressing either phosphatidylinositol 4-phosphate 5-kinase or 5-phosphatase (5-ptase) enzymes (Ono et al., 2004; Chen et al., 2006). However, prolonged changes in PtdIns(4,5)P 2 levels initiate several trafficking and signaling events that will alter the disposition of the cells by the time the effects are analyzed (Brown et al., 2001). This makes it difficult to draw firm conclusions regarding direct effect of the lipid on any single process.
To overcome this problem, we developed a strategy to promptly regulate membrane PtdIns(4,5)P 2 levels by a drug-inducible membrane targeting of a type IV 5-ptase enzyme (Kisseleva et al., 2000; Kong et al., 2000) based on the heterodimerization of the FRB (fragment of mammalian target of rapamycin [mTOR] that binds FKBP12) and FKBP12 (FK506 binding protein 12; Muthuswamy et al., 1999). In this approach, the phosphatase is fused to the FKBP12 protein, and upon addition of rapamycin (rapa; or an analogue that does not interact with endogenous mTOR protein) the enzyme rapidly translocates to the membrane where its binding partner, the FRB domain, is targeted. This method has been successfully used to manipulate small GTP binding proteins at the PM (Inoue et al., 2005) and to study the effects of β-arrestin membrane recruitment (Terrillon and Bouvier, 2004). In the present study we show the use of this approach to manipulate PM PtdIns(4,5)P 2 levels and demonstrate how these manipulations affect selected processes that are regulated by this phosphoinositide species.
Results and discussion
Targeting of type IV 5-ptase alters PM PtdIns(4,5)P 2 levels
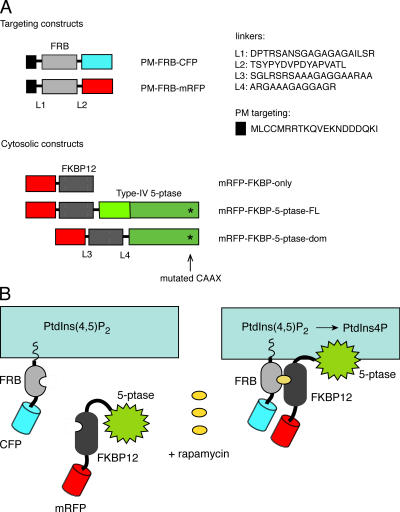
Fig. 1 shows the concept and the constructs used for rapa-induced targeting of the type IV 5-ptase to the PM. For membrane targeting of the FRB domain of mTOR, the palmitoylation sequence of the human GAP43 protein was used (Tanimura et al., 2004). To follow their localization, the FRB protein was also tagged with either CFP or monomeric red fluorescent protein (mRFP). The 5-ptase (either full-length or only the 5-ptase domain) was mutated (C641A) to eliminate its C-terminal lipid modification and membrane targeting and was fused to FKBP12 and also tagged with mRFP (Fig. 1). A mutant form of FRB (T2098L of mTOR) that can be heterodimerized with FKBP with a rapa analogue (AP21967; rapalogue) that does not bind to endogenous mTOR has been recommended. However, because of its easier availability and faster action, we mostly used rapa and the wild-type FRB protein in the present studies. Nevertheless, the mutant FRB and the rapalogue have also been tested and their use is recommended for applications where rapa itself could affect the process being investigated.
Figure 1.
Rapa-induced translocation of the type IV 5-ptase domain to the PM. (A) Outline of the constructs used in the present study. The asterisk shows the C641A mutation that eliminates membrane localization of the 5-ptase. (B) Heterodimerization of the PM-targeted FRB fragment of mTOR with the cytosolic 5-ptase fused to FKBP12 upon rapa addition causes PM recruitment of the enzyme and rapid dephosphorylation of PtdIns(4,5)P 2.
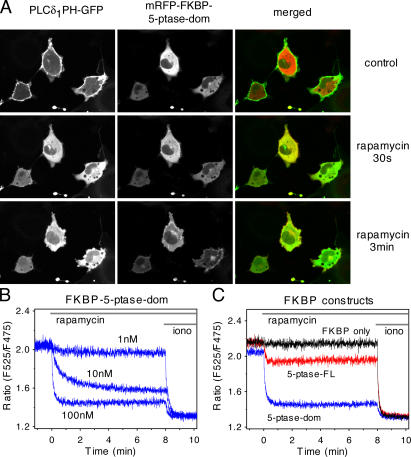
To monitor the effects of the 5-ptase on PtdIns(4,5)P 2 levels in the PM, these constructs were transfected together with the PLCδ1PH-GFP construct in COS-7 cells. Expression of either 5-ptase constructs in the cytosol at a broad range of expression levels caused no apparent change in the localization of the PLCδ1PH-GFP, probably because the cytosolic phosphatase is inefficient to hydrolyze the lipid in the membrane and because cells can make adjustments to maintain their PtdIns(4,5)P 2 levels. However, a small number of cells expressing high levels of the truncated 5-ptase domain showed no PLCδ1PH-GFP localization, indicating that high concentrations of the truncated enzyme could decrease lipid levels even without membrane targeting. 100 nM rapa caused rapid translocation of the fusion protein containing the 5-ptase domain to the PM causing a prompt and complete loss of PLCδ1PH-GFP localization in most cells (Fig. 2 A). Using the full-length phosphatase, however, caused only incomplete translocation of the PLCδ1PH-GFP reporter to the cytosol in some of the cells, and many cells showed no detectable change in PLCδ1PH-GFP localization in spite of efficient enzyme recruitment to the membrane (unpublished data). This important finding is consistent with the notion that a full-length enzyme contains regulatory regions that keep the enzyme activity under control. No changes were observed in PLCδ1PH-GFP distribution upon rapa-induced translocation of the mRFP-FKBP fusion protein that did not contain the 5-ptase. FRET measurements between the CFP- and YFP-tagged PLCδ1PH domain (van Der Wal et al., 2001) used either in single cells (not shown) or in a population of COS-7 cells (Fig. 2 B) have clearly demonstrated the lipid changes evoked by this approach.
Figure 2.
Changes in PtdIns(4,5)P2 levels after rapa-induced membrane targeting of the 5-ptase. (A) COS-7 cells were transfected with the PLCδ1PH-GFP plasmid to monitor PtdIns(4,5)P 2 in the PM along with the membrane-targeted FRB-CFP (CFP channel is not shown) and the mRFP−FKBP−5-ptase domain constructs. Addition of 100 nM rapa induces translocation of the 5-ptase to the membrane, causing a complete loss of PLCδ1PH-GFP localization. Even partial localization of the enzyme (at 30 s) is sufficient to eliminate PtdIns(4,5)P 2. (B) FRET analysis of the PLCδ1PH domain translocation in cell suspensions. COS-7 cells were transfected with the CFP- and YFP-tagged forms of the PLCδ1PH domains together with the membrane-targeted FRB and the FKBP−5-ptase both tagged with mRFP. Cells were trypsinized and analyzed in suspension in a spectrofluorometer as described in Materials and methods. Addition of rapa at the indicated concentrations induces PtdIns(4,5)P 2 depletion resulting in a decreased membrane localization of the PH domain reflected in the decreased FRET signal. 10 μM ionomycin (iono) was used to completely eliminate PtdIns(4,5)P 2 (Várnai and Balla, 1998). (C) Similar experiment as in B except that the FKBP construct did not contain the phosphatase (FKBP only; black trace) or contained the full-length 5-ptase (5-ptase−FL; red trace). Representative data are shown from two identical observations.
Decreasing PM PtdIns(4,5)P 2 levels rapidly terminates Ca2+ influx during ATP stimulation
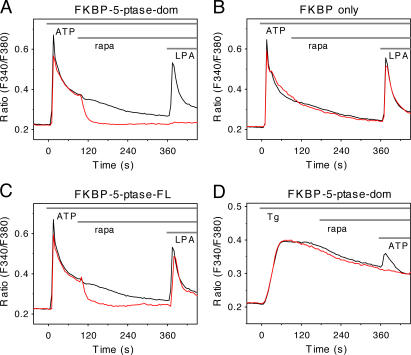
Next, we examined the effects of PtdIns(4,5)P 2 depletion on Ca2+ signaling evoked via the endogenous P2Y receptors in COS-7 cells. Cells were transfected with the PM-targeted FRB-CFP (or -mRFP) together with either the full-length or truncated 5-ptase mRFP-FKBP fusion construct for 1 d. The expression as well as the movements of the 5-ptase were monitored in the red channel simultaneously with single-cell cytoplasmic Ca2+ ([Ca2+]i) measurements with fura-2. Addition of 50 μM ATP evoked a Ca2+ signal in many cells expressing the 5-ptase in the cytosol, but several cells expressing a high level of the phosphatase showed impaired response to ATP. Fig. 3 shows averaged Ca2+ recordings from single cells where the truncated 5-ptase domain was expressed and the cells showed a response to ATP. Here, administration of rapa promptly terminated the plateau phase of [Ca2+]i increase with kinetics similar to those of the PtdIns(4,5)P 2 decrease. Notably, these cells failed to respond to a subsequent stimulation with another Ca2+-mobilizing agonist, lysophosphatidic acid.
Figure 3.
[Ca2+]i changes in single COS-7 cells after stimulation of the endogenous P2Y receptors with ATP or inhibition of the sarcoplasmic and ER Ca2+ ATPase with Tg. Cells were transfected with the membrane-targeted FRB-CFP plasmid together with the mRFP-FKBP fused to the isolated 5-ptase domain (A and D) or the full-length 5-ptase (C) or not containing the 5-ptase (B). Cells were loaded with fura-2/AM and examined at room temperature. Only cells that showed a response to ATP (50 μM) were included in the analysis in A−C. The effects of the constructs on the Ca2+ responses were calculated from cells that fell in the same range of FKBP construct expression (red traces) with a mean fluorescence of (in arbitrary units ± SEM) 1705 ± 150 (n = 44), 1827 ± 295 (n = 18), 1827 ± 217 (n = 22), and 2117 ± 276 (n = 44) for A, B, C, and D, respectively. The black traces are from untransfected cells in the same field (n = 120, 68, 120, and 38 for A, B, C, and D, respectively). Note the lack of effect of rapa on the 200 nM Tg-induced Ca2+ elevation (D) in contrast to the strong effect on the ATP-induced Ca2+ plateau (A). Error bars (<5%) have been omitted for better clarity. These data were reproduced at least in three different cell preparations.
Translocation of the full-length 5-ptase with 100 nM rapa also caused a rapid inhibition of the ATP-induced Ca2+ signal. However, these cells still showed a transient [Ca2+]i response to lysophosphatidic acid, indicating that maintenance of the Ca2+ signal is more sensitive to small depletion of the PtdIns(4,5)P 2 levels than the initial response of Ca2+ mobilization (Fig. 3 C). Because activation of P2Y receptors leads to InsP3 production and Ca2+ release form ER stores, hence activating capacitative Ca2+ influx, we wanted to determine whether capacitative Ca2+ influx itself requires PtdIns(4,5)P 2 in the membrane (Broad et al., 2001). To do this, the effect of lipid depletion on thapsigargin (Tg)-induced Ca2+ response was examined. Tg depletes Ca2+ stores by inhibition of the sarcoplasmic and ER Ca2+ ATPase that keeps Ca2+ stores filled and therefore activates Ca2+ influx without the need for InsP3. Fig. 3 D shows that the sustained [Ca2+]i increase after Tg treatment was not affected by the same manipulations of PtdIns(4,5)P 2 levels that eliminated the ATP-induced sustained Ca2+ elevations. This finding suggests that PtdIns(4,5)P 2 depletion interferes with the sustained generation of InsP3 rather than with the capacitative Ca2+ influx mechanism itself. A more detailed analysis of the relationship between these mechanisms is currently under way.
Several controls were used to rule out that the observed effects are caused by rapa itself or by the transfected constructs and/or their translocation to the membrane. First, the response of cells in the same field of view not expressing the phosphatase were monitored and found to show no change in response to rapa. Second, the Ca2+ response of cells expressing both the targeting construct and mRFP-FKBP12 without the 5-ptase also showed no change in response to rapa addition (Fig. 3 B).
Decreasing PM PtdIns(4,5)P 2 levels affects the activity of transient receptor potential melastatin 8 (TRPM8) channels
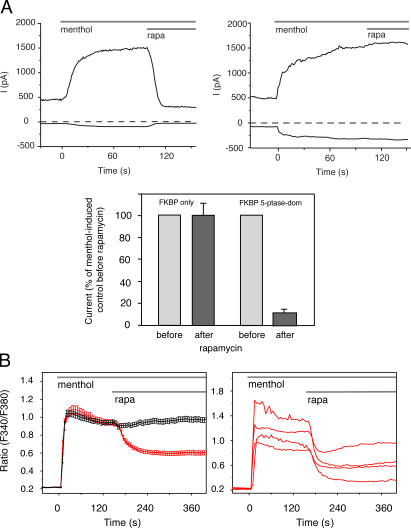
TRPM8 is one of the Ca2+ conductive channels that has been shown to require PtdIns(4,5)P 2 for its activity (Liu and Qin, 2005; Rohacs et al., 2005). Therefore, we chose these channels to study their PtdIns(4,5)P 2 dependence by monitoring either their activity in patch-clamp recordings using the whole-cell configuration or by following the [Ca2+]i signal evoked via their activation by menthol. Fig. 4 A shows that human embryonic kidney 293 (HEK293) cells expressing TRPM8 channels respond to menthol stimulation with a large increase in an outwardly rectifying current. TRPM8 channel activation by menthol is also reflected in the rapid and sustained [Ca2+]i elevation observed in transfected COS-7 (Fig. 4 B) or HEK293 cells (not depicted). In cells also expressing the truncated FKBP12−5-ptase construct together with the PM-targeted FRB domain, addition of 100 nM rapa caused a prompt decrease in the menthol-induced current (Fig. 4 A). A rapid drop in [Ca2+]i was also observed in COS-7 cells (Fig. 4 B) and HEK293 cells (not depicted). None of these changes were observed when rapa was added to cells expressing the PM-targeted FRB and the FKBP12 construct without the phosphatase (Fig. 4, A and B). These results clearly showed that TRPM8 channels require PtdIns(4,5)P 2 for their activity and can report on rapid changes in the level of this lipid in intact cells. Interestingly, in spite of the apparently complete inhibition of the menthol-induced membrane conductance, [Ca2+]i did not return to baseline after rapa addition. This remaining [Ca2+]i elevation observed in both cell types could be explained by a stimulated store-operated Ca2+ entry pathway if functional TRPM8 channels in the ER release ER Ca2+ in response to menthol, as suggested by a recent study (Thebault et al., 2005). This and other possibilities will require further analysis, as do questions on the role of the lipid in determining the menthol sensitivity and gating behavior of these channels.
Figure 4.
Electrophysiological recordings in HEK293 cells and [Ca2+]i changes in single COS-7 cells expressing TRPM8 channels. Cells were also transfected with the membrane-targeted FRB-CFP plasmid and either the mRFP-FKBP−fused isolated 5-ptase domain or mRFP-FKBP only. (A) Current recording measured in the whole-cell configuration, using the ramp protocol described in Materials and methods. The currents at +100 (top curves) and −100 mV (bottom curves) are shown. The averaged responses to rapa from 14 and 4 recordings for 5-ptase and FKBP-only expressing cells, respectively, measured at +100 mV are shown in the bottom histogram. (B) Averaged [Ca2+]i responses from cells expressing the mRFP−FKBP− 5-ptase domain (left, red) or mRFP-FKBP only (left, black) domain (means ± SEM from 79 and 76 cells, respectively). Representative single-cell [Ca2+]i responses from cells expressing the mRFP−FKBP−5-ptase domain are shown at the right (means ± SEM from 79 and 76 cells, respectively). Elimination of PtdIns(4,5)P 2 rapidly inhibits the whole cell current and reduces the Ca2+ signal stimulated by 500 μM menthol.
PM PtdIns(4,5)P 2 is needed for receptor internalization
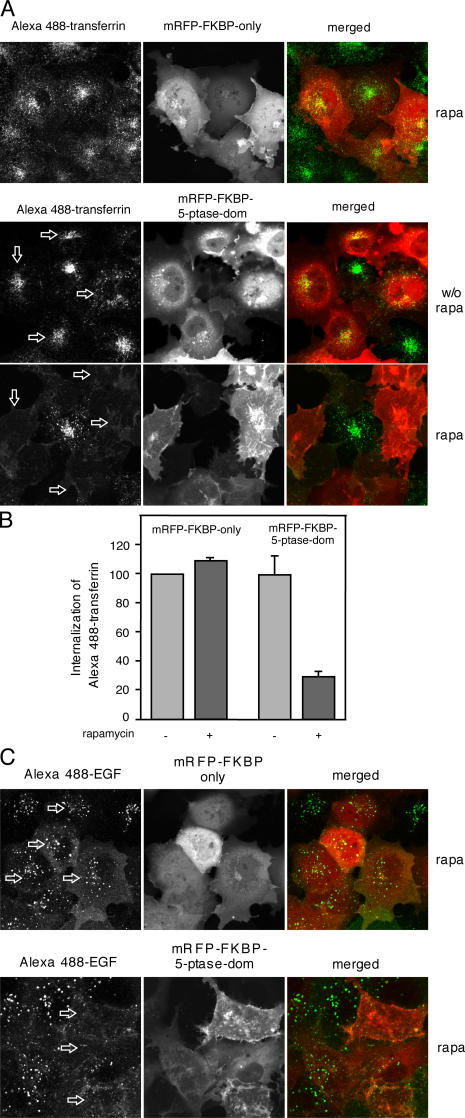
We next examined how PtdIns(4,5)P 2 depletion affects internalization of the transferrin (Tf) and EGF receptors. It is generally believed that PtdIns(4,5)P 2 regulates the recruitment of numerous proteins to the PM that are required for receptor endocytosis (Wenk and De Camilli, 2004). To follow endocytosis, we used fluorescent analogues of Tf and EGF and incubated the cells with or without PtdIns(4,5)P 2 depletion. We also determined the uptake of the fluorescent analogues in cells in which rapa induced the translocation of an mRFP-FKBP12 construct without the phosphatase. As shown in Fig. 5, both Tf and EGF appeared in intracellular vesicular compartments in all cells regardless of their transfection with the constructs. This compartment corresponds to early and recycling endosomes in the case of Tf and to the multivesicular body/late endosomal compartment in the case of EGF (van Dam et al., 2002; Minogue et al., 2006). Elimination of PtdIns(4,5)P 2 with rapa addition completely prevented the uptake of either fluorescent cargo into the cells. These effects were not observed in rapa-treated cells that expressed the same constructs without the 5-ptase domain. A more quantitative assessment of this process was obtained by FACS analysis in the case of Tf. Here, the mean green fluorescent intensity of the cells (a measure of internalized Tf) in the population of cells expressing the red (5-ptase) construct showed the changes observed in the confocal pictures (Fig. 5 B). These data suggested that Tf receptors will not internalize when PtdIns(4,5)P 2 is not available in the PM.
Figure 5.
Internalization of Alexa 488−Tf or Alexa 488−EGF in COS-7 cells transfected with the membrane-targeted FRB and the indicated FKBP constructs. Where indicated, fluorescent ligand was added after 3-min pretreatment with 100 nM rapa. Confocal pictures were taken at 10 min after the addition of the ligand (Alexa 488−Tf in A and B and Alexa 488−EGF in C) and after removing and washing of the unbound protein. Note that the lack of internalization of Tf in cells expressing the 5-ptase domain was evident only after rapa addition (arrows). (B) FACS analysis of COS-7 cells expressing the membrane-targeted FRB-CFP plasmid and the indicated FKBP fusion protein after 5- or 10-min uptake of Alexa 488−Tf after treatment with solvent or rapa. Cells showing mRFP expression in the red channel were selected and their mean green signal intensities were calculated. Means ± SEM of three experiments.
Collectively, these data clearly demonstrate that changes in membrane PtdIns(4,5)P 2 levels by themselves without the generation of second messengers can have multiple consequences on a wide range of cellular processes. In a similar manner, modulation of phosphoinositides in defined membrane compartments can be achieved by recruiting other enzymes (phosphatases and kinases) to the PM or to other cellular membrane compartments to analyze the role of PtdIns(4,5)P 2 or other inositol lipids in specific cellular processes. Although this approach has considerable potential, caution and the use of appropriate controls are essential to avoid possible artifacts. Numerous cellular processes are based on FKBP12 interactions, and overexpression of an FKBP12 construct could alter their properties. Similarly, rapa is an inhibitor of mTOR that can exert several effects on its own. This problem is alleviated with the use of the rapalogue that does not bind to the endogenous protein. Lastly, the targeting of the FRB by itself can have its own effects on selected cellular functions. However, if these possibilities are kept in mind this technique can permit further exploration of the complex regulatory features of phosphoinositides.
Materials and methods
Materials
AP21967 was obtained from Ariad Pharmaceuticals. Rapa and Tg were purchased from Calbiochem. Alexa 488−Tf and Alexa 488−EGF were obtained from Invitrogen. All other chemicals were purchased from Sigma-Aldrich and were of highest analytical grade.
DNA constructs
The PLCδ1PH-GFP construct and its color variants have been previously described (Várnai and Balla, 1998; van Der Wal et al., 2001). For PM tethering, the N-terminal localization sequence (MLCCMRRTKQVEKNDDDQKI) of the human GAP43 (residues 1–20) was fused to the N terminus of the FRB domain of human mTOR1 (residues 2019–2114 amplified from a human EST available from GenBank/EMBL/DDBJ under accession no. 5495577) through a short linker. To visualize the fusion protein, the construct was tagged with CFP or mRFP (mRFP provided by R.Y. Tsien, University of California, San Diego, San Diego, CA). The T2098L mutant version of FRB was generated by exchanging the FRB portion from the plasmid obtained from the Argent heterodimerization kit (Ariad Pharmaceuticals). Three constructs were designed that contained FKBP12 (amplified from a human EST available from GenBank/EMBL/DDBJ under accession no. 3504715). All of them contained mRFP fused to the N terminus of FKBP12. The human type IV 5-ptase enzyme (available from GenBank/EMBL/DDBJ under accession no. NM_019892; provided by P.W. Majerus, Washington University, St. Louis, MO) was then fused to the C terminus of the FKBP12 either as the full-length protein or only its 5-ptase domain (residues 214–644). In both cases, the C641A mutation was introduced to destroy the C-terminal CAAX domain. A construct containing a stop codon at the end of the FKBP12 was also created (FKBP only).
Confocal analysis of single cells and [Ca2+]i measurements
COS-7 cells were cultured on glass coverslips (3 × 105 cells/35-mm dish) and transfected with the various constructs (2 μg of total DNA/dish) using Lipofectamine 2000 for 24 h as described elsewhere (Varnai et al., 2005). For Ca2+ measurements, cells were loaded with 3 μM fura-2/AM (45 min, room temperature). Ca2+ measurements were performed at room temperature in a modified Krebs-Ringer buffer containing 120 mM NaCl, 4.7 mM KCl, 1.2 mM CaCl2, 0.7 mM MgSO4, 10 mM glucose, and 10 mM Na-Hepes, pH 7.4. An inverted microscope (IX70; Olympus) equipped with an illuminator (Lambda-DG4; Sutter Instrument Co.) and a digital camera (MicroMAX-1024BFT; Roper Scientific) and the appropriate filter sets were used for Ca2+ analysis. Data acquisition and processing was performed by the MetaFluor software (Molecular Devices). Confocal analysis was performed in the same solution at 35°C using a confocal microscope (LSM 510-META; Carl Zeiss MicroImaging, Inc.).
Patch-clamp recordings
Patch-clamp experiments were performed on HEK293 cells after 2 d of transfection with the respective DNA constructs. Recordings were made using an amplifier (Axopatch 200B; Axon Instruments, Inc.) in an extracellular solution containing 137 mM NaCl, 5 mM KCl, 1 mM MgCl2, 10 mM Hepes, and 10 mM glucose, pH 7.4. The pipette solution contained 135 mM K gluconate, 5 mM KCl, 1 mM MgCl2, 5 mM EGTA, 10 mM Hepes, and 2 mM ATP (Na)2, pH 7.2. To assess TRPM8 channel activity, voltage ramps were applied from −100 to +100 mV every second. The current values measured at the +100 and −100 mV potential are shown in the recordings. Menthol was used at a concentration of 500 μM and rapa at 100 nM.
Cell suspension FRET measurements
COS-7 cells were cultured in 10-cm dishes (3 × 106 cells) and transfected with equal amounts of PLCδ1PH-CFP and -YFP, as well as the mRFP version of the appropriate FRB and FKBP constructs (10 μg of total DNA/dish) using Lipofectamine 2000 for 24 h. Cells were then trypsinized, centrifuged, and resuspended in the same modified Krebs-Ringer solution used in the Ca2+ experiments. Measurements were performed at 35°C using a Deltascan fluorometer (PTI Technologies, Inc.) with excitation of 425 nm. To monitor the FRET signal, the ratio of the 525- and 475-nm emission was calculated.
FACS measurements
COS-7 cells were cultured in 10-cm dishes (3 × 106 cells) and transfected with equal amounts of the appropriate FRB and FKBP constructs (10 μg of total DNA/dish) using Lipofectamine 2000 for 24 h. Cells were then trypsinized, centrifuged, and resuspended in the same solution that was used in the Ca2+ experiments (106 cells/ml). After treating the cells with rapa (3 min) and then with fluorescent transferrin (5 min) they were fixed with 2% PFA. FACS measurements were performed using a FACScan instrument (Becton Dickinson). To monitor the internalization in the transfected cell populations, the red channel was set to analyze the transfected cells and the mean green fluorescence of these cells was calculated.
Acknowledgments
We are grateful to Dr. Roger Y. Tsien for the mRFP and to Dr. Philip W. Majerus for the human type IV 5-ptase clone. The confocal imaging was performed at the Microscopy & Imaging Core of the National Institute of Child Health and Human Development (NICHD), National Institutes of Health (NIH), with the kind assistance of Drs. Vincent Schram and James T. Russell. The invaluable help of Dr. Jon Marsh with the FACS analysis is greatly appreciated.
The research of T. Balla and P. Varnai was supported by the Intramural Research Program of the NICHD of the NIH and by an appointment of P. Varnai to the Senior Fellowship Program at the NIH. This latter program is administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and the NIH. T. Rohacs was supported by grants from the American Heart Association, the Sinsheimer Foundation, and the University of Medicine and Dentistry of New Jersey Foundation.
Note added in proof. While this paper was under review, Suh et al. (Suh, B.C., T. Inoue, T. Meyer, and B. Hille. 2006. Science. 10.1126/ science.1131163) described a similar approach to chemically manipulate PtdIns(4,5)P 2 levels and KCNQ potassium channels.
Abbreviations used in this paper: [Ca2+]i; cytoplasmic Ca2+; HEK, human embryonic kidney; mRFP, monomeric red fluorescent protein; mTOR, mammalian target of rapamycin; PM, plasma membrane; PtdIns(4,5)P 2, phosphatidylinositol 4,5-bisphosphate; 5-ptase, phosphoinositide 5-phosphatase; rapa, rapamycin; Tf, transferrin; Tg, thapsigargin; TRPM8, transient receptor potential melastatin 8.
References
- Berridge, M.J. 1993. Cell signalling: a tale of two messengers. Nature. 365:388–389. [DOI] [PubMed] [Google Scholar]
- Broad, L.M., F.J. Braun, J.P. Lievremont, G.S. Bird, T. Kurosaki, and J.W. Putney Jr. 2001. Role of the phospholipase C-inositol 1,4,5-trisphosphate pathway in calcium release-activated calcium current and capacitative calcium entry. J. Biol. Chem. 276:15945–15952. [DOI] [PubMed] [Google Scholar]
- Brown, F.D., A.L. Rozelle, H.L. Yin, T. Balla, and J.G. Donaldson. 2001. Phosphatidylinositol 4,5-bisphosphate and Arf6-regulated membrane traffic. J. Cell Biol. 154:1007–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, X., E.M. Talley, N. Patel, A. Gomis, W.E. McIntire, B. Dong, F. Viana, J.C. Garrison, and D.A. Bayliss. 2006. Inhibition of a background potassium channel by Gq protein alpha-subunits. Proc. Natl. Acad. Sci. USA. 103:3422–3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster, D., O.W. Moe, and D.W. Hilgemann. 2004. Lipid- and mechanosensitivities of sodium/hydrogen exchangers analyzed by electrical methods. Proc. Natl. Acad. Sci. USA. 101:10482–10487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilpela, P., M.K. Vartiainen, and P. Lappalainen. 2004. Regulation of the actin cytoskeleton by PI(4,5)P2 and PI(3,4,5)P3. Curr. Top. Microbiol. Immunol. 282:117–163. [DOI] [PubMed] [Google Scholar]
- Inoue, T., W.D. Heo, J.S. Grimley, T.J. Wandless, and T. Meyer. 2005. An inducible translocation strategy to rapidly activate and inhibit small GTPase signaling pathways. Nat. Methods. 2:415–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh, T., S. Koshiba, T. Kigawa, A. Kikuchi, S. Yokoyama, and T. Takenawa. 2001. Role of the ENTH domain in phosphatidylinositol-4,5-bisphosphate binding and endocytosis. Science. 291:1047–1051. [DOI] [PubMed] [Google Scholar]
- Kisseleva, M.V., M.P. Wilson, and P.W. Majerus. 2000. The isolation and characterization of a cDNA encoding phospholipid-specific inositol polyphosphate 5-phosphatase. J. Biol. Chem. 275:20110–20116. [DOI] [PubMed] [Google Scholar]
- Kong, A.M., C.J. Speed, C.J. O'Malley, M.J. Layton, T. Meehan, K.L. Loveland, S. Cheema, L.M. Ooms, and C.A. Mitchell. 2000. Cloning and characterization of a 72-kDa inositol-polyphosphate 5-phosphatase localized to the Golgi network. J. Biol. Chem. 275:24052–24064. [DOI] [PubMed] [Google Scholar]
- Liscovitch, M., V. Chalifa, P. Pertile, C.S. Chen, and L.C. Cantley. 1994. Novel function of phosphatidylinositol 4,5-biphosphate as a cofactor for brain membrane phospholipase D. J. Biol. Chem. 269:21403–21406. [PubMed] [Google Scholar]
- Liu, B., and F. Qin. 2005. Functional control of cold- and menthol-sensitive TRPM8 ion channels by phosphatidylinositol 4,5-bisphosphate. J. Neurosci. 25:1674–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomasney, J.W., H.F. Cheng, L.P. Wang, Y. Kuan, S. Liu, S.W. Fesik, and K. King. 1996. Phosphatidylinositol 4,5-bisphosphate binding to the pleckstrin homology domain of phospholipase C-delta1 enhances enzyme activity. J. Biol. Chem. 271:25316–25326. [DOI] [PubMed] [Google Scholar]
- Minogue, S., M.G. Waugh, M.A. De Matteis, D.J. Stephens, F. Berditchevski, and J.J. Hsuan. 2006. Phosphatidylinositol 4-kinase is required for endosomal trafficking and degradation of the EGF receptor. J. Cell Sci. 119:571–581. [DOI] [PubMed] [Google Scholar]
- Muthuswamy, S.K., M. Gilman, and J.S. Brugge. 1999. Controlled dimerization of ErbB receptors provides evidence for differential signaling by homo- and heterodimers. Mol. Cell. Biol. 19:6845–6857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono, A., S.D. Ablan, S.J. Lockett, K. Nagashima, and E.O. Freed. 2004. Phosphatidylinositol (4,5) bisphosphate regulates HIV-1 Gag targeting to the plasma membrane. Proc. Natl. Acad. Sci. USA. 101:14889–14894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohacs, T., C.M. Lopes, I. Michailidis, and D.E. Logothetis. 2005. PI(4,5)P2 regulates the activation and desensitization of TRPM8 channels through the TRP domain. Nat. Neurosci. 8:626–634. [DOI] [PubMed] [Google Scholar]
- Suh, B.C., and B. Hille. 2002. Recovery from muscarinic modulation of M current channels requires phosphatidylinositol 4,5-bisphosphate synthesis. Neuron. 35:507–520. [DOI] [PubMed] [Google Scholar]
- Suh, B.C., and B. Hille. 2005. Regulation of ion channels by phosphatidylinositol 4,5-bisphosphate. Curr. Opin. Neurobiol. 15:370–378. [DOI] [PubMed] [Google Scholar]
- Tanimura, A., A. Nezu, T. Morita, R.J. Turner, and Y. Tojyo. 2004. Fluorescent biosensor for quantitative real-time measurements of inositol 1,4,5-trisphosphate in single living cells. J. Biol. Chem. 279:38095–38098. [DOI] [PubMed] [Google Scholar]
- Terrillon, S., and M. Bouvier. 2004. Receptor activity-independent recruitment of betaarrestin2 reveals specific signalling modes. EMBO J. 23:3950–3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thebault, S., L. Lemonnier, G. Bidaux, M. Flourakis, A. Bavencoffe, D. Gordienko, M. Roudbaraki, P. Delcourt, Y. Panchin, Y. Shuba, et al. 2005. Novel role of cold/menthol-sensitive transient receptor potential melastatine family member 8 (TRPM8) in the activation of store-operated channels in LNCaP human prostate cancer epithelial cells. J. Biol. Chem. 280:39423–39435. [DOI] [PubMed] [Google Scholar]
- Toker, A., and L.C. Cantley. 1997. Signalling through the lipid products of phosphoinositide-3-OH kinase. Nature. 387:673–676. [DOI] [PubMed] [Google Scholar]
- van Dam, E.M., T. Ten Broeke, K. Jansen, P. Spijkers, and W. Stoorvogel. 2002. Endocytosed transferrin receptors recycle via distinct dynamin and phosphatidylinositol 3-kinase-dependent pathways. J. Biol. Chem. 277:48876–48883. [DOI] [PubMed] [Google Scholar]
- van Der Wal, J., R. Habets, P. Varnai, T. Balla, and K. Jalink. 2001. Monitoring phospholipase C activation kinetics in live cells by FRET. J. Biol. Chem. 276:15337–15344. [DOI] [PubMed] [Google Scholar]
- Várnai, P., and T. Balla. 1998. Visualization of phosphoinositides that bind pleckstrin homology domains: calcium- and agonist-induced dynamic changes and relationship to myo-[3H]inositol-labeled phosphoinositide pools. J. Cell Biol. 143:501–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnai, P., A. Balla, L. Hunyady, and T. Balla. 2005. Targeted expression of the inositol 1,4,5-triphosphate receptor (IP3R) ligand-binding domain releases Ca2+ via endogenous IP3R channels. Proc. Natl. Acad. Sci. USA. 102:7859–7864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenk, M.R., and P. De Camilli. 2004. Protein-lipid interactions and phosphoinositide metabolism in membrane traffic: insights from vesicle recycling in nerve terminals. Proc. Natl. Acad. Sci. USA. 101:8262–8269. [DOI] [PMC free article] [PubMed] [Google Scholar]