Abstract
Non-homologous DNA end-joining (NHEJ) is a major pathway of double strand break (DSB) repair in human cells. Here we show that vanillin (3-methoxy-4-hydroxybenzaldehyde)—a naturally occurring food component and an acknowledged antimutagen, anticlastogen and anticarcinogen—is an inhibitor of NHEJ. Vanillin blocked DNA end-joining by human cell extracts by directly inhibiting the activity of DNA-PK, a crucial NHEJ component. Inhibition was selective and vanillin had no detectable effect on other steps of the NHEJ process, on an unrelated protein kinase or on DNA mismatch repair by cell extracts. Subtoxic concentrations of vanillin did not affect the ATM/ATR-dependent phosphorylation of Chk2 or the S-phase checkpoint response after ionising radiation. They significantly potentiated the cytotoxicity of cisplatin, but did not affect sensitivity to UVC. A limited screen of structurally related compounds identified two substituted vanillin derivatives that were 100- and 50-fold more potent than vanillin as DNA-PK inhibitors. These compounds also sensitised cells to cisplatin. The inhibition of NHEJ is consistent with the antimutagenic and other biological properties of vanillin, possibly altering the balance between DSB repair by NHEJ and homologous recombination.
INTRODUCTION
DNA double strand breaks (DSBs) are a major threat to cell survival and genomic integrity. They can arise following exposure to exogenous DNA damaging agents as well as through endogenous cellular events such as recombination or replication stalling. Unrepaired DSBs are potentially lethal and their misrepair may lead to chromosome rearrangements, telomere loss and mutation, all of which are common events during the development of cancer (1,2).
Human cells use at least two distinct pathways to rejoin DSBs. Homologous recombination (HR) utilises homologous sequences from an undamaged chromatid to effect repair. HR requires several members of the hRAD51 family of proteins, together with the products of hRAD52, hRAD54, Brca1, Brca2, XRCC2 and XRCC3 genes (3). It seems likely that the complex of RAD50/NBS1/MRE11 proteins is also involved at an early stage of rejoining by HR possibly by tethering the broken DNA ends in close proximity and promoting their resection to provide the substrate for HR (4). Mutational inactivation of the HR pathway has significant effects on the sensitivity of cells to DNA damaging agents as well as the development of cancer (5).
Non-homologous end-joining (NHEJ) is involved in V(D)J recombination to generate antibody diversity (6). It also appears to be the predominant pathway of DSB repair in post-embryonic human cells (7). NHEJ directly ligates severed DNA ends with no apparent requirement for extensive sequence homology. The rejoining process may result in the deletion of short stretches of nucleotides and is therefore potentially mutagenic. NHEJ requires DNA-dependent protein kinase (DNA-PK), which comprises the catalytic subunit DNA-PKcs and the DNA end-binding heterodimer Ku70/Ku80 (8). The Ku complex also binds to inositol hexakisphosphate (IP6) (9) as well as to the DNA ligase IV/XRCC4 heterodimer. It is thought that broken ends are recognised by the Ku heterodimer, which then recruits DNA-PKcs, thereby activating its kinase activity. Several protein components of the NHEJ pathway as well as several non-participants are phosphorylated by this kinase (10). The Ku70/Ku80/DNA-PKcs complex also protects the DNA termini from nuclease attack. After modification to provide suitable 3′OH/5′P termini, end-joining is carried out by XRCC4/DNA ligase IV (2). More recently, the Artemis gene product has also been implicated in NHEJ (11). Since the Artemis protein can cleave DNA hairpins generated by the RAG proteins, its involvement most likely reflects a specific role in V(D)J recombination as well as in 5′–3′ overhang processing in NHEJ. Mutations that inactivate the components of NHEJ confer immune disorders and cellular sensitivity to DSB-inducing agents. They are not generally associated with an increased cancer incidence. It has been suggested that cancer only develops when key signalling proteins, such as ATM (ataxia telagiectasia mutated) and p53, are also mutated (12,13).
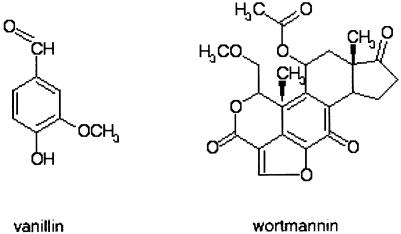
Wortmannin is a sterol fungal metabolite of complex structure (Fig. 1). It strongly inhibits the activity of members of the family of phosphatidylinositol-3 kinase-related kinases (PIKK). This large family includes DNA-PK, the DNA damage signalling proteins ATM, the related ATR and the nutrient-dependent cell growth regulator mTOR (target of rapamycin protein, also known as FRAP, RAFT1 or RAPT) (14–16). Wortmannin inhibits NHEJ catalysed by cell extracts (17) and sensitises cells to agents that produce DSBs (18,19).
Figure 1.
Structures of vanillin and wortmannin.
Several natural plant-derived compounds, including vanillin, cinnamaldehyde, coumarin, umbelliferone, anisaldehyde and tannic acid, have moderate antimutagenic properties. They also sensitise cells to the lethal effects of DNA-damaging agents (20). Vanillin (4-hydroxy-3-methoxybenzaldehyde, Fig. 1) has consistently proven to be the most effective antimutagen amongst the group (21–23). It occurs naturally in the pods of Vanilla planifolia, Vanilla tahitensis and Vanilla pompona and is also synthesised on a large scale in the food industry for use as a flavouring agent. Vanillin itself exhibits little cytotoxicity, mutagenicity or clastogenicity in model systems—including cultured mammalian cells (24,25). It does, however, potentiate the cytotoxicity of some DNA damaging agents including hydrogen peroxide, mitomycin C (MMC), N-methyl-N-nitrosoguanidine and 6-thioguanine (25,26) and suppresses UV- and X-ray-induced chromosome aberrations (27). Vanillin is also an attested anticarcinogen in rats. It decreases the number of small intestinal tumours induced by several carcinogens in a multiple organ bioassay (28) and reduces the number of preneoplastic glutathione S-transferase π isoenzyme-positive foci induced by treatment with 2-amino-3-methylimidazo[4,5]quinoline in a hepatocarcinoma model (29). The mechanisms underlying these various biological effects remain undefined.
Here we report that vanillin inhibits DNA repair by NHEJ and is a selective inhibitor of DNA-PK activity. Unrelated DNA repair reactions and other steps in the NHEJ process were unaffected by vanillin. Sub-toxic concentrations of vanillin produced a dose-dependent sensitisation to cisplatin in a model human tumour cell line. A limited screen of related compounds identified vanillin derivatives that were more potent DNA-PK inhibitors and also sensitised cells to killing by cisplatin. Thus, vanillin is a representative of a family of compounds that might have beneficial antimutagenic effects as well as the ability to potentiate the effectiveness of anticancer drugs.
MATERIALS AND METHODS
Cell culture and toxicity assays
A2780-SC1 human ovarian carcinoma cells were grown in DMEM supplemented with 10% fetal calf serum (FCS) in humidified incubators containing 10% CO2. Vanillin (Sigma) was dissolved in H2O by heating to 70°C for 10 min. For cisplatin survival tests, cells were grown continuously with or without vanillin at 100 or 300 µM. Cells were inoculated onto plates, allowed to adhere in DMEM and treated with cisplatin for 1 h. Cisplatin was removed by replacing with fresh DMEM and cells incubated for 7–10 days. For ionising radiation (IR) survival assays, cells were resuspended in PBS, irradiated and resuspended in fresh DMEM followed by inoculation onto plates. After 7–10 days incubation, surviving colonies were stained with Giemsa and counted.
Human GM00558 lymphoma cells, ATLD-D5037 (MRE11-deficient) and TK6 and WTK1 B-lymphoblastoid cell lines were grown in RPMI supplemented with 10% FCS in 5% CO2. Cell growth assays were performed by treating cells for 1 h with cisplatin or exposing cells to IR in PBSA at 5 × 105 cells/ml. Cells were harvested and resuspended in fresh RPMI and 10% FCS at 5 × 105/ml and inoculated into 24-well plates. Cells were maintained in exponential growth by appropriate dilution and counted daily.
Whole cell extract preparation
Whole cell extracts were made from exponentially growing GM00558 lymphoblasts. Briefly, cells were pelleted and washed by resuspension in 5× pellet volume of hypotonic buffer containing 10 mM Tris–HCl pH 8.0, 1 mM EDTA and 5 mM dithiothreitol (DTT). They were then resuspended in the same buffer (2× pellet volume) and incubated for 20 min on ice followed by dounce homogenisation in the presence of protease inhibitors (2.1 mg/ml aprotinin, 1 mg/ml pepstatin, chymostatin, leupeptin and 0.17 mg/ml AEBSF). The homogenate was then left on ice for 20 min prior to the addition of 1/4 vol of 5× high salt buffer (83.5 mM Tris–HCl pH 7.5, 1.65 M KCl, 3.3 mM EDTA and 1 mM DTT). Homogenate was then separated by centrifugation at 37 000 r.p.m. for 3 h (using SW41 rotor). The resulting supernatant was dialysed overnight against 20 mM Tris–HCl pH 8.0, 0.1 M potassium acetate, 20% glycerol, 0.5 mM EDTA and 1 mM DTT. Extract was finally snap frozen as liquid N2 beads and stored at –80°C.
DNA end-joining assay
End-joining was measured using the plasmid-based in vitro DNA end-joining assay (17). In 10 µl reactions, 10 µg (1–3 µl) of whole cell extract with or without vanillin was mixed with 10 ng of pDEA7Z plasmid linearised with EcoRI restriction enzyme and labelled with [γ-32P]ATP (10 mCi/ml, Amersham). Reactions were incubated for 2 h at 37°C in 50 mM Tris–HCl pH 8.0, 60 mM potassium acetate, 0.5 mM Mg acetate, 1 mM ATP, 1 mM DTT and 0.1 mg/ml BSA. After incubation, 2 µl 5× deproteinisation mix (10 mg/ml proteinase K, 2.5% SDS, 50 mM EDTA and 100 mM Tris–HCl pH 7.5) was added and samples loaded onto a 0.6% agarose gel. Electrophoresis was carried out at 80 V for 2 h and the gel was dried and exposed to Bio-Rad film overnight. Quantitation was carried using a Storm 840 PhosphorImager and IQ software.
Mismatch repair assay
Mismatch repair was assayed in 25 µl reactions containing: 30 mM HEPES–KOH pH 8.0, 7 mM MgCl2, 0.5 mM DTT, 0.1 mM each dNTP (Pharmacia Biotech, Uppsala, Sweden), 4 mM ATP, 40 mM phosphocreatine, 1 mg of creatine phosphokinase (rabbit type I), 40 ng (141 fmol) of substrate and HeLa cell extract. After 20 min incubation at 37°C, the reaction was terminated by the addition of 10 mM EDTA. Following proteinase K digestion and extraction with phenol–chloroform, DNA was digested with 10 U MluI for 1 h. Digested DNA was then separated on a 0.8% agarose gel containing ethidium bromide and the gel was scanned by a CCD camera in the Gel Doc 1000 system (Bio-Rad Laboratories, Hercules, CA).
DNA-dependent protein kinase activity
Protein kinase activation was assayed using the SignaTECT® DNA-Dependent Protein Kinase Assay System (Promega). Following the manufacturer’s protocol, reactions (25 µl) contained purified DNA-PK or whole cell extract, DNA-PK activation buffer, reaction buffer, a DNA-PK biotinylated p53-derived peptide substrate and 0.5 µCi [γ-32P]ATP (10 mCi/ml). Appropriate concentrations of vanillin or its derivatives were included throughout the incubation. Samples were incubated at 30°C for 5 min. Termination buffer was then added and 10 µl of each reaction mixture was spotted onto SAM2® capture membrane. After washing with 2 M NaCl membranes were dried and incorporated 32P-phosphorylated substrate measured by scintillation counting.
Ligase adenylation
Adenylation was determined by the method of Tomkinson et al. (30). [α-32P]ATP incorporation during the adenylation of purified Ligase I was measured by incubating appropriate concentrations of Ligase I and 1 µCi [α-32P]ATP (10 mCi/ml, Amersham) with or without vanillin for 10 min at room temperature in 60 mM Tris pH 8.0, 10 mM MgCl2, 5 mM DTT and 50 µg/ml BSA. Ten micrograms of GM00558 extract was used as a source of other ligases for adenylation. Reactions were stopped by adding SDS and heating to 95°C for 5 min. Products were analysed by 8% SDS–polyacrylamide gel electrophoresis. The gel was fixed in 10% acetic acid, dried and exposed to film.
Ku binding
The assay used to measure the binding of Ku to IP6 was developed by Dr L. Hanakahi (personal communication). Briefly, using Bio-Rad BioSpin 30 columns, Ku bound to 3HTdR-labelled IP6 was eluted and measured by scintillation counting.
Radioresistant DNA synthesis (RDS)
Exponentially growing cells were grown in DMEM supplemented with FCS. Irradiated cells (1 ml) were incubated for 3 h at 37°C in the presence of 1 µCi 3HTdR (1 mCi/ml Amersham). Aliquots were placed onto separate 1.5 × 1.5 cm 3 MM filter papers. 50 µl 10% SDS and 1 mM EDTA was then added to each filter paper. All filters were then washed twice in 10% cold TCA, twice in ethanol and dried. DNA synthesis was measured by incorporation of 3HTdR by scintillation counting.
Phosphorylation of Chk2
Phosphorylation of Chk2 was analysed by western blot using phospho-Chk2 (Thr68) antibody (Cell Signaling NEB, Herts, UK). GM00558 cells treated with and without vanillin were used to make extracts 2 h post-IR exposure. Electrophoresis of protein extracts was then carried out using 10% SDS–PAGE. Protein blots were incubated with a 1000× dilution of primary phospho-Chk2 antibody followed by incubation with a 3000× dilution of secondary antibody (Bio-Rad). Blots were visualised by ECL solution and exposed to film.
Protein kinase C (PKC) activity
PKC was assayed using the SignaTECT® Protein kinase C Assay System (Promega). Reactions (25 µl) contained PKC, PKC activation and co-activation buffers and a biotinylated PKC peptide substrate with or without appropriate concentrations of vanillin or derivatives. The reaction was started by addition of 0.5 µCi [γ-32P]ATP (10 mCi/ml) and incubation was at 30°C for 5 min. After adding termination buffer, 10 µl of each reaction mixture was spotted onto SAM2® capture membrane. Each membrane was washed with 2 M NaCl and dried. Radioactivity was determined by scintillation counting. 50 µM of the known PKC inhibitor, Myristoylated peptide (Myr-RFARKGALRQKNV; Promega), was used as a positive control for inhibition.
Immunofluorescence studies
A2780-SC1 cells were grown on cover slips in DMEM supplemented with 10% fetal bovine serum for 36 h. Vanillin was added at a final concentration of 300 µM. Cisplatin (5 µM) was added for 1 h. When wortmannin was used, it was added for the last 30 min of cisplatin treatment. Cells were then washed with PBS and incubated for a further 24 h in the presence or absence of vanillin. Cells were then washed three times in PBS, treated with 0.5% NaCl pH 7.0, fixed in 4% paraformaldehyde for 30 min and treated with 0.5% Triton X-100 for 10 min. Cover slips were incubated for 2 h at room temperature in primary antibody (rabbit polyclonal α-Rad51-ICRF-FBE2 and mouse monoclonal α-RPA ICRF 70A from R. Wood) followed by 1 h at room temperature with the secondary antibodies (Alexa 488-conjugated goat anti-rabbit and Alexa 546-conjugated goat anti-mouse, Molecular Probes). Nuclei were visualised using To-Pro DNA stain. Images were captured using a Zeiss Laser Scanning Microscope LSM 510 equipped with photomultiplier.
Vanillin-derivative screen
A total of 21 candidate compounds were selected from a cohort of vanillin-like structures identified in the Maybridge Chemicals database. These candidate compounds were selected on the basis of (i) benzaldehyde-like structure and (ii) a similar molecular weight. Compounds (100 µM) were tested for inhibition in the SignaTect DNA-PK assay.
RESULTS
Inhibition of DNA end-joining by vanillin
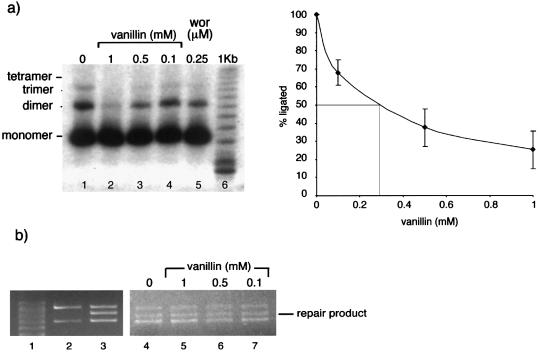
Extracts of GM00558 lymphoma cells were used to assay DNA end-joining of a linearised plasmid. After a 2-h incubation with extract, the predominant product was linear dimer DNA molecules with some trimer and tetramer forms (Fig. 2a). Inclusion of vanillin at a range of concentrations between 100 µM and 1 mM resulted in a progressive decrease in the yield of ligated products. At 100 µM vanillin, no trimers were detectable and a concentration of 1 mM effectively abolished the formation of dimer molecules. The extent of inhibition by 100 µM vanillin was comparable to that produced by 0.25 µM wortmannin. By computing the combined yield of dimers and higher order multimers from six independent assays, the IC50 value for inhibition of rejoining by vanillin was estimated to be 300 µM (right panel).
Figure 2.
DNA end-joining inhibition by vanillin. (a) End-joining activity of 10 µg GM00558 extract. Lane 1, extract alone; lanes 2–4, extract pre-incubated with vanillin for 10 min at 4°C; lane 5, extract pre-incubated with 0.25 µM wortmannin for 10 min at 4°C; lane 6, 1 kb DNA ladder. The right panel shows end-joining activity as measured by intensity of ligated products (measured using Storm 840 IQ software) as a percentage of no vanillin treatment plotted against vanillin concentration. Vanillin has an IC50 of 300 µM. (b) Mismatch repair activity. Activity of HeLa cell extract in repair of an A.C mismatched plasmid substrate. Lane 1, marker; lane 2, no extract; lane 3, extract; lanes 4–7, extract pre-incubated for 5 min at room temperature with a range of concentrations of vanillin shown.
The same concentrations of vanillin did not detectably affect mismatch repair. hMutSα- and hMutLα-dependent correction of a T:C mispair by HeLa cell extracts was unaffected by vanillin concentrations up to 1 mM (Fig. 2b). Similarly, inclusion of wortmannin (0.25 and 2.5 µM) was without detectable effect (data not shown). We conclude that the effect of vanillin on NHEJ by cell extracts does not reflect a general inhibition of in vitro DNA repair.
Inhibition of enzyme reaction by vanillin
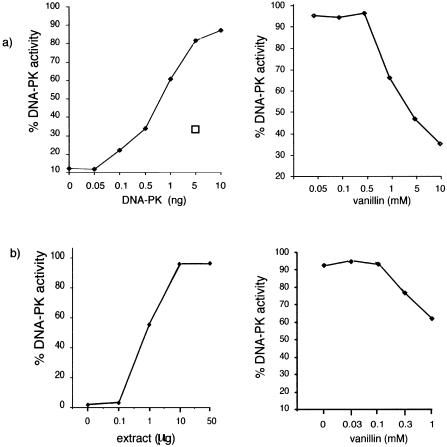
We analysed the effects of vanillin on individual steps in NHEJ. The ability of purified DNA-PK to phosphorylate a target p53 peptide with [γ-32P]ATP was assayed in the presence and absence of vanillin. In the absence of inhibitor, the extent of phosphorylation increased linearly with purified DNA-PK concentration up to 10 ng (Fig. 3a). A value of 1.5 ng was selected for inhibitor studies. Vanillin produced significant kinase inhibition at concentrations of >0.5 mM (right panel). At 10 mM vanillin, inhibition approached 90%. From these data, the IC50 value for vanillin was estimated to be 1.5 mM. As expected, wortmannin (0.25 µM) also significantly reduced DNA-PK activity (Fig. 3a, left panel). Peptide phosphorylation by the extracts of GM00558 cells used for the rejoining assays was also inhibited by vanillin (Fig. 3b) and the extent of inhibition was consistent with an approximate IC50 value of 1.5 mM. These results indicate that the DNA-PK component of NHEJ is a target for inhibition by vanillin.
Figure 3.
DNA-PK activity. (a) Purified DNA-PK activity. Left panel, dose response of increasing concentrations of purified DNA-PK. Open box represents 0.25 µM wortmannin incubated with 5 ng DNA-PK. Right panel, activity of 1.5 ng DNA-PK pre-incubated for 5 min at 4°C with vanillin at varying concentrations. (b) DNA-PK activity using GM00558 extract. Left panel, dose response of increasing concentrations of extract. Right panel, activity of 1 µg extract pre-incubated with vanillin at increasing concentrations.
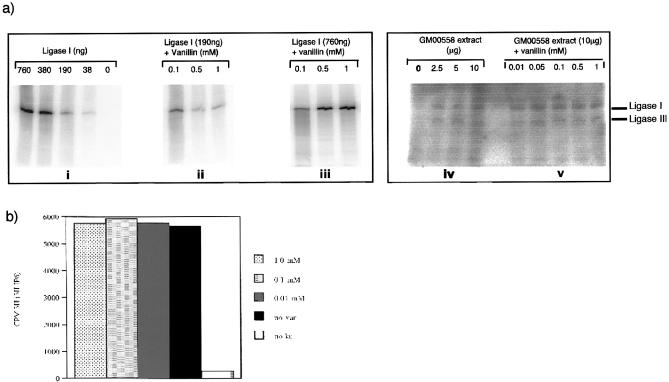
The final rejoining step of NHEJ is catalysed by DNA ligase IV. All ligases require adenylation for activation. Because ligase IV adenylation is particularly difficult to measure in biochemical assays, we analysed the more easily detectable DNA ligase I and III activities. Adenylation of purified DNA ligase I by [α-32P]ATP was unaffected by vanillin concentrations of up to 1 mM—a concentration which produced significant inhibition of DNA-PK (Fig. 4a). Furthermore, the extent of adenylation of DNA ligase I and DNA ligase III by GM00558 cell extracts was similar in the presence and absence of 1 mM vanillin. Vanillin did not detectably affect other components of NHEJ. IP6 binds to Ku70/Ku80 during NHEJ. IP6 binding to purified Ku heterodimer was undiminished by vanillin concentrations up to 1 mM (Fig. 4b).
Figure 4.
Ligase adenylation and Ku-IP6 binding. (a) Ligase adenylation. (i) Incorporation of [α-32P]ATP by a purified Ligase I titration. (ii) Effect of vanillin on Ligase I at 190 ng. A weak signal was generated, therefore (iii) shows effect of vanillin titration on 760 ng Ligase I. (iv) Incorporation of [α-32P]ATP by GM00558 extract. (v) Ten microgram extract pre-incubated (10 min at 4°C) with vanillin. (b) Ku binding to IP6. Effect of vanillin on Ku binding to 3H-labelled IP6. Counts per minute (c.p.m.) of labelled IP6 eluted plotted against vanillin concentration.
We conclude that the ability of vanillin to reduce NHEJ by human cell extracts reflects the ability of this simple benzaldehyde derivative to inhibit DNA-PK and that other reactions in the NHEJ process are largely insensitive. Vanillin shares this property of selective DNA-PK inhibition with the complex fungal derivative wortmannin.
Vanillin and other protein kinases
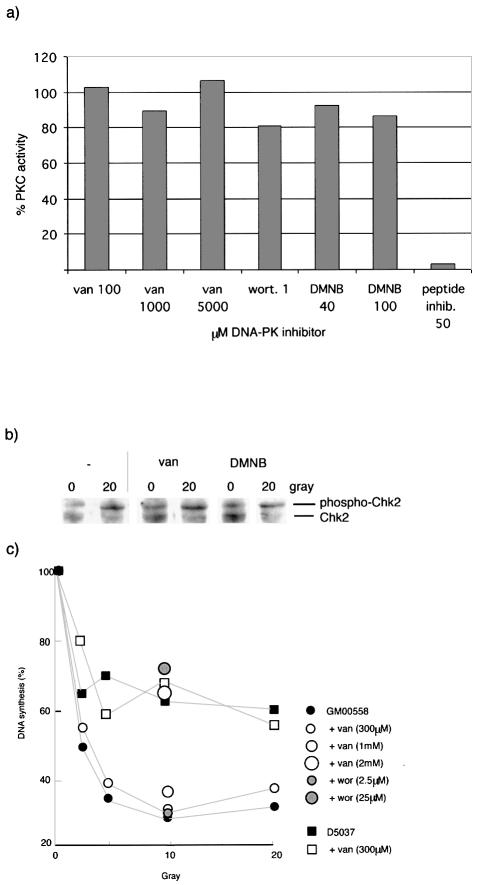
DNA-PK is a PI3-like kinase. We investigated whether vanillin is a general PI3 kinase inhibitor. Checkpoint activation after DNA damage requires PI3-like kinases ATM and ATR. We determined the effect of vanillin on ability of cells to regulate DNA synthesis after exposure to IR (Fig. 5a). DNA synthesis was reduced to 30% in wild type GM00558 cells after 10Gy IR. As expected, the response of MRE11-deficient D5037 cells was reduced consistent with the role of MRE11 in activating the S-phase checkpoint. Both GM00558 and D5037 cells proliferated normally in concentrations of vanillin up to 300 µM. Continuous exposure to sub-toxic concentrations of the inhibitor did not affect the response of either cell line to IR. IR-induced inhibition of DNA synthesis was only affected by toxic concentrations (2 mM) of vanillin. Wortmannin (25 µM) also effectively abolished the IR-induced inhibition of DNA synthesis.
Figure 5.
Kinase activity. (a) Effect of DNA-PK inhibitors on PKC activity. Using 10 ng of PKC vanillin, DMNB, wortmannin (wort) and myristoylated peptide inhibitor was added at increasing concentrations. (b) Phosphoryl ation of Chk2. Extracts made from GM00558 cells treated with 0, 10 and 20 Gy IR and incubated with 300 µM vanillin or 15 µM DMNB were used to measure Phospho-Chk2 by western blot. (c) Radio-resistant DNA synthesis. GM00558 and D5037 (MRE11-deficient) cells incubated with 3H TdR for 3 h. Vanillin was added as a continuous exposure while wortmannin was added during the first hour of incubation. Curves show levels of DNA synthesis with and without 300 µM vanillin. Single points indicate levels of DNA synthesis after exposure to 10 Gy IR at higher concentrations of vanillin and wortmannin.
Chk2 is phosphorylated by the kinases ATM and ATR after genotoxic stress. We measured phospho-Chk2 phosphorylation in extracts from GM00558 cells that had been exposed to IR with and without vanillin treatment. Figure 5c shows that the extent of Chk2 phosphorylation after 10 and 20 Gy IR was not reduced by continuous exposure of cells to vanillin.
To examine whether vanillin is a general inhibitor of protein kinases we assayed its effect on PKC. Phosphorylation of a neurogranin substrate by purified PKC and [γ-32P]ATP was not detectably affected by vanillin or by wortmannin even at very high concentrations (100.5% PKC activity with 5 mM vanillin). As a positive control, myristoylated peptide which is a known PKC inhibitor, reduced phosphorylation by >90% at a concentration of 50 µM (Fig. 5b).
Potentiation of cisplatin induced cell killing by vanillin
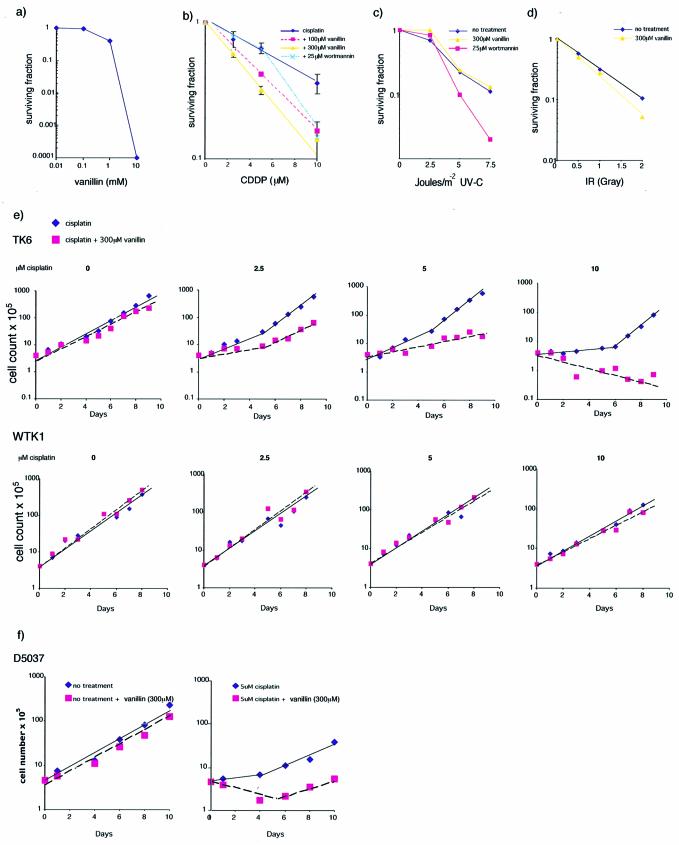
Continuous exposure of A2780 cells to high concentrations (≥1 mM) of vanillin was cytotoxic (Fig. 6a). Two subtoxic concentrations, 100 and 300 µM were used to investigate the effects of the inhibitor on the survival of A2780 cells treated with cisplatin. These non-toxic concentrations of vanillin produced a dose-dependent potentiation of cisplatin toxicity (Fig. 6b). The D37 value for cisplatin in the absence of vanillin was 9.7 µM. This value was reduced to 5.9 and 4.3 µM at 100 and 300 µM vanillin, respectively (P < 0.05 and P < 0.01, Student’s t-test). A concentration of 25 µM wortmannin produced a similar degree of sensitisation to cisplatin although wortmannin alone was slightly toxic (∼70% survival) at this concentration.
Figure 6.
Cytotoxic and growth responses to vanillin. A2780 responses to: (a) vanillin titrations and combinations of vanillin with (b) cisplatin, (c) UV-C and (d) IR exposure. (e) TK6 and WTK1 (p5-defective) growth responses to cisplatin with and without (300 µM) vanillin. (f) Growth responses of D5037 (MRE11-deficient) cells to cisplatin and 15 µM DMNB.
Vanillin at 300 µM had no significant effect on the sensitivity of A2780 cells to UVC doses up to 10 J/m2 (Fig. 6c) or to IR (Fig. 6d). D37 values in the presence and absence of inhibitor were 2.5 and 2.4 J/m2, and 0.7 and 0.8 Gy, respectively.
Vanillin also sensitised TK6 lymphoid cells to cisplatin (Fig. 6e). Treatment with 2.5 or 5 µM cisplatin produced little detectable effect on TK6 cell proliferation. Cell growth was significantly impaired at 10 µM cisplatin although surviving cells resumed growth after ∼5 days. Inclusion of 300 µM vanillin after treatment with 2.5 and 5 µM cisplatin resulted in detectable log-term growth inhibition or death and after treatment with 10 µM cisplatin, no outgrowth of surviving cells was observed in the presence of vanillin. WTK1 cells are related to TK6 and are more resistant to cisplatin. Vanillin did not alter the sensitivity of WTK1 cells using the same range of cisplatin concentrations. The MRE11-deficient cell line (D5037) was sensitive to the cytotoxic effects of a 1-h treatment with 2.5 µM cisplatin (Fig. 6f). The inclusion of a continuous exposure to 300 µM vanillin also sensitised these cells to cisplatin.
Rad51 foci formation
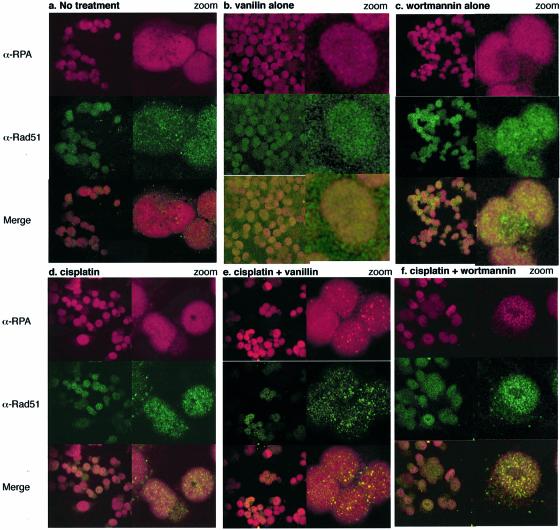
We examined the effects of vanillin on the formation of Rad51-RPA foci in A2780 cells treated with cisplatin (Fig. 7). In untreated cells, <5% (of ∼200 cells) contained Rad51/RPA foci (Fig. 7a). Similar scores were obtained when cells had been exposed continually to 300 µM vanillin (Fig. 7b) or following a 1-h treatment with 25 µM wortmannin (Fig. 7c). Twenty-four hours after a 1-h treatment with 5 µM cisplatin, rad51-RPA foci were present in ∼30% of cells (Fig. 7d). A similar (30–50%) fraction of cells contained Rad51/RPA foci after 5 µM cisplatin and continuous exposure to 300 µM vanillin (Fig. 7e) or 25 µM wortmannin (Fig. 7f). Thus, neither vanillin nor wortmannin reduced the frequency of cisplatin-induced Rad51/RPA focus formation and both inhibitors may have had a marginally stimulatory effect.
Figure 7.
Confocal microscopy. RPA/Rad51 foci formation in the nuclei of A2780 cells: (a) untreated, (b) treated with 300 mM vanillin, (c) exposed for 1 h to 25 µM wortmannin followed by 20 h incubation, (d) exposed to 5 µM cisplatin for 1 h followed by 20 h incubation, (e) exposed to cisplatin while in continuous exposure to vanillin (300 µM) and (f) exposed to cisplatin followed by a 1 h exposure to wortmannin 20 h later. RPA (red), Rad51 (green) and merge (yellow).
Identification of active vanillin derivatives
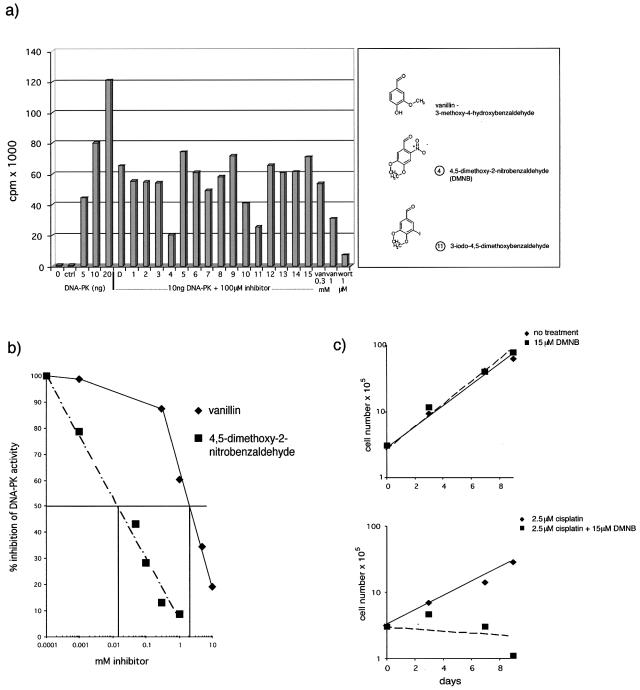
Vanillin is a relatively simple chemical structure. In order to investigate the possible existence of more potent vanillin derivatives a database, from the Maybridge HTS collection (Maybridge Chemicals, Tintagel, UK) consisting of over 53 000 organic synthetic drug-like compounds, was interrogated for the presence of structurally related benzaldehyde derivatives. An initial search identified 15 candidate compounds. Two of which, numbers 4 and 11, were DNA-PK inhibitors when tested at an arbitary concentration of 100 µM (Fig. 8a). Both active compounds were 4,5-dimethoxy derivatives of benzaldehyde. Non-aldehyde analogues were ineffective (data not shown), indicating that the aldehyde group is essential for inhibitory activity. The most active compound was 4,5-dimethoxy-2-nitrobenzaldehyde (DMNB), which had an IC50 of 15 µM for DNA-PK inhibition (Fig. 8b). This is 100 times more potent than vanillin. We incubated HeLa cell extract with DMNB (at 15 and 50 µM) prior to carrying out the mismatch repair assay. DMNB did not detectably affect mismatch repair at any of these concentrations (data not shown). The second active compound, 2-bromo-4,5-dimethoxybenzaldehyde was 50× more potent as a DNA-PK inhibitor (IC50 30 µM, not shown). A second screen using 4,5-dimethoxybenzaldehyde as the search structure identified a further six derivatives. None of these demonstrated DNA-PK inhibitory activity (data not shown).
Figure 8.
Isolation and characterisation of vanillin-derived DNA-PK inhibitors. (a) Left panel, DNA-PK inhibition by vanillin derivatives. Each compound was included in the DNA-PK assay at 100 µM. Structures of active compounds 4 and 11 shown in the right panel. (b) DNA-PK inhibition by vanillin and DMNB. (c) Growth inhibition of D5037 cells exposed for 1 h to 15 µM DMNB 20 h after a 1-h exposure to 2.5 µM cisplatin.
The effect of DMNB on cisplatin-treated D5037 cells was examined. Continuous exposure of DMNB (at its IC50 for DNA-PK of 15 µM) was lethal to the cells (data not shown). Therefore, cells were not treated with DMNB until 20 h after cisplatin treatment (approximately one cell doubling time). DMNB was added for 1 h only. This treatment did not affect cell growth but significantly sensitised the cells to cisplatin. None treated with cisplatin and DMNB was viable (Fig. 8c).
DISCUSSION
The genoprotective properties of vanillin have been acknowledged for a number of years. Its antimutagenic, anticlastogenic and anticancer effects are all well documented in cell culture and animal models. Vanillin also enhances the toxicity of a range of structurally diverse DNA damaging agents in a variety of cell lines. The mechanistic basis for these biological effects has remained undefined. It has been postulated that vanillin exerts its antimutagenic activity in damaged cells by promoting recombination and rejoining of DNA at homologous sites (25). It has also been proposed that vanillin may inhibit a DNA repair process, such that enhancement of lethality decreases mutant yield by killing cells with potentially mutagenic lesions (23). Our demonstration that vanillin selectively blocks DNA repair by the relatively error-prone NHEJ pathway by inhibiting DNA-PK provides a plausible explanation for its antimutagenic and genotoxic enhancing effects. Reducing DSB misrepair by the potentially mutagenic NHEJ pathway and imposing a greater reliance on HR recombination might explain the antimutagenic effect in normal cells. The impairment of NHEJ pathway of DSB repair is consistent with a potentiation of the cytotoxicity of genotoxic agents.
It is presently unclear whether vanillin is a general phosphatidylinositol kinase-related kinase inhibitor like wortmannin. Wortmannin is known to inhibit, albeit less effectively, the PI3KKs ATM and ATR, components inextricably linked to checkpoint activation and DSB repair (31). However, the observation that radiation-sensitivity of XRCC4 defective cells is not further enhanced by wortmannin suggests inhibition of DNA-PK is likely to be its most significant biological property (32). Our observations on radiation-resistant DNA synthesis suggest that the checkpoint protein ATM is not affected by non-toxic concentrations of vanillin and only affected at toxic concentrations of the inhibitor. However, the concentration of wortmannin (25 µM) generally used to sensitise cells to IR did abrogate the S phase checkpoint in our wild-type cells. It restored post-radiation levels of replication comparable to those of MRE11 cells. The extent to which inhibition of the intra-S checkpoint contributes to the sensitising effect of wortmannin is currently unclear. IR-induced phosphorylation of the down-stream effector Chk2, which is dependent on functional ATM/ATR after DNA damage, was not inhibited by vanillin at its IC50 value for DNA-PK inhibition. This provides direct evidence that vanillin does not significantly impair ATM/ATR-dependent kinase activity.
Vanillin, like wortmannin, reacts preferentially with protein lysine residues. Wortmannin binds irreversibly to lysine in the phosphotransferase domain of the phosphatidylinositol kinase-related kinases. (16,33). Lysine 3751 of DNA-PKcs, is likely to be the site of nucleophilic attack by wortmannin (34). Vanillin also binds preferentially to lysine, although in this case via Schiff Base formation (35). It is likely therefore that vanillin can also modify the key active site lysine of DNA-PK. Non-aldehyde analogues did not inhibit DNA-PK, suggesting that the aldehyde group plays a critical role in inhibition. In agreement with this idea, acrolein—a simple α,β unsaturated aliphatic aldehyde—also inhibits DNA-PK albeit with a relatively high IC50 (S.D., unpublished).
Wortmannin is more potent than vanillin as an inhibitor of end-joining and of DNA-PK. The IC50 for vanillin is ∼5000-fold higher. The biological effects of the inhibitors do not reflect this difference, however. The wortmannin concentration typically used to sensitise cells to IR (25 µM) is 100 times higher than its biochemical IC50 (18,36). In contrast, vanillin produced a detectable sensitisation at or considerably below its biochemical IC50 for end-joining and DNA-PK inhibition. It seems likely that these differences reflect the relative sizes of the vanillin and wortmannin molecules. Vanillin is more likely to enter cells freely whereas the more complex wortmannin probably requires active transport. In addition, although we did not find evidence of inhibition of other DNA repair reactions, we cannot exclude the possibility that vanillin may affect cell sensitivity through interference with other components of the cellular responses to DNA damage.
Previous cytotoxicity studies have shown that vanillin sensitised rodent cells to several DNA damaging treatments. We observed that non-toxic concentrations of vanillin produced a significant sensitisation to cisplatin in human A2780 cells. However, we did not observe any significant effects of vanillin on IR or UV sensitivity. We did see evidence of a vanillin-induced increase in sensitisation to IR at higher doses but the reason for a considerably weaker effect compared to that seen for cisplatin is presently unclear. It may reflect the differences between IR and cisplatin in the way they produce DSBs. Cisplatin produces predominantly DNA intrastrand crosslinks. The majority of these (>90%) are between adjacent purines (1,2 adducts) (37) and are relatively refractory to removal by nucleotide excision repair (NER) (38). The remainder are 1,3 crosslinks that are efficiently excised by NER (39). Cisplatin therefore introduces many lesions that are likely to persist until encountered by replication forks in dividing cells. The collapse of aberrant or stalled replication forks is likely to form DSBs (40).
Furthermore, the diversity of DNA damaging agents that vanillin sensitises cells to suggests that vanillin affects the repair of a common intermediate DNA lesion, most likely a DSB. Consistent with this notion, Rad52 (41), Rad51 and BRCA1, all participants in DSB rejoining via the recombinational repair pathway, are implicated in processing cisplatin-induced DNA damage (42,43). Our confocal microscopy data indicate that cisplatin induces Rad51/RPA foci, suggesting that cisplatin-induced lesions, most likely DSBs, are processed by HR. Vanillin did not reduce the frequency of Rad51/RPA foci in agreement with its selective inhibition of NHEJ. Indeed, it induced a reproducible, modest, although not statistically significant increase in Rad51/RPA foci in cisplatin-treated cells. This is consistent with an increased DSB processing by HR being a consequence of inhibition of the alternative NHEJ pathway.
Vanillin sensitised A2780, TK6 and GM00558 cells to cisplatin. In contrast, neither the TK6 derivative, WTK1 nor Raji Burkitt’s lymphoma cells (data not shown) were affected by vanillin. Both WTK1 and Raji are more resistant to cisplatin than GM00558, most likely because of a defective p53. The possibility that the sensitising effects of NHEJ inhibition are confined to cells expressing WT p53 protein would be an interesting area for future investigation.
The simplicity of the vanillin molecule makes it an attractive candidate for modification in search of more active formulations. Our limited analysis of benzaldehyde derivatives identified two additional methoxybenzaldehydes that were significantly better than vanillin as inhibitors of DNA-PK. One was a 2-nitro compound, the other contained a 3-iodo group. In the case of DMNB, the difference in IC50 was 100-fold. It seems likely that the increased activity of DMNB is possibly due, at least in part, to the electron-withdrawing properties of the 2-nitro group that would increase aldehyde reactivity towards protein amino groups. The iodo group does not share this property, however, and it is possible that the hydrophobicity of both these groups is a significant influence—possibly by facilitating the reaction in relatively hydrophobic areas of the protein.
DMNB, like vanillin did not affect PKC activity and did not inhibit Chk2 phosphorylation after IR, indicating that DMNB retains the relative specificity after modification. However, since vanillin and its more active derivatives are simple benzaldehydes it is unlikely that their effects are completely specific for DNA-PK. Indeed, some benzaldehyde derivatives can protect cells from cisplatin-mediated cell inactivation (44). This is apparently due to the ability of benzaldehydes to bind reversibly to outer surface membrane proteins (45), therefore interfering with cellular transport and reducing drug accumulation. However, substituted benzaldehydes with electron-donating groups at the 2-position have the greatest protective effect and no protection was seen using benzaldehydes without these electron-donating groups. Therefore the inhibitory action on DNA-PK specifically by vanillin and the more potent Schiff base-strengthening electrophilic groups on dimethoxybenzaldehydes might explain the cisplatin- sensitising effect of this particular group of compounds.
In conclusion, we have shown that specific members of the vanillin family are simple and relatively specific low molecular weight inhibitors of DNA-PK. This family of novel vanillin-based molecules should be useful tools in assessing the biochemical mechanism of DNA-PK and the relative contribution of NHEJ and other pathways to DSB repair. In view of the already established biological effects of vanillin, the possible long-term beneficial effects of these compounds in preventing genomic instability and cancer might warrant further investigation.
Acknowledgments
ACKNOWLEDGEMENTS
We would like to thank Pauline Branch and Peter Macpherson for assistance with tissue culture and the mismatch repair assay, respectively. We are also grateful to Les Hanakahi for kindly donating purified DNA-PK samples and assistance with the Ku-binding assay, to Peter Robbins and Debbie Barnes for kindly supplying purified Ligase I samples and to Professor A. Taylor at the University of Birmingham for supplying the MRE11-deficient D5037 lymphoblastoid cell line. Our thanks also go to Todd Duncan for assistance on compound structure searches from the Maybridge Chemicals database.
REFERENCES
- 1.Haber J.E. (2000) Partners and pathways repairing a double-strand break. Trends Genet., 16, 259–264. [DOI] [PubMed] [Google Scholar]
- 2.Khanna K.K. and Jackson,S.P. (2001) DNA double-strand breaks: signalling, repair and the cancer connection. Nature Genet., 27, 247–254. [DOI] [PubMed] [Google Scholar]
- 3.Karran P. (2000) DNA double strand break repair in mammalian cells. Curr. Opin. Genet. Dev., 10, 144–150. [DOI] [PubMed] [Google Scholar]
- 4.de Jager M., van Noort,J., van Gent,D.C., Dekker,C., Kanaar,R. and Wyman,C. (2001) Human Rad50/Mre11 is a flexible complex that can tether DNA ends. Mol. Cell, 8, 1129–1135. [DOI] [PubMed] [Google Scholar]
- 5.Godthelp B.C., Wiegant,W.W., van Duijn-Goedhart,A., Scharer,O.D., van Buul,P.P., Kanaar,R. and Zdzienicka,M.Z. (2002) Mammalian Rad51C contributes to DNA cross-link resistance, sister chromatid cohesion and genomic stability. Nucleic Acids Res., 30, 2172–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fugmann S.D., Lee,A.I., Shockett,P.E., Villey,I.J. and Schatz,D.G. (2000) The RAG proteins and V(D)J recombination: complexes, ends and transposition. Annu. Rev. Immunol., 18, 495–527. [DOI] [PubMed] [Google Scholar]
- 7.van Gent D.C., Hoeijmakers,J.H.J. and Kanaar,R. (2001) Chromosomal stability and the DNA double strand break connection. Nature Rev. Genet., 2, 196–206. [DOI] [PubMed] [Google Scholar]
- 8.Jackson S.P. (2002) Sensing and repairing DNA double-strand breaks. Carcinogenesis, 23, 687–696. [DOI] [PubMed] [Google Scholar]
- 9.Hanakahi L.A., Bartlet-Jones,M., Chappell,C., Pappin,D. and West,S. (2000) Binding of inositol phosphate to DNA-PK and stimulation of double-strand break repair. Cell, 102, 721–729. [DOI] [PubMed] [Google Scholar]
- 10.Karmaker P., Piotrowski,J., Brosh,R.M., Sommers,J.A., Lees Millers,S.P., Cheng,W.-H., Snowden,C.M., Ramsden,D.A. and Bohr,V.A. (2002) Werner protein is a target of DNA-dependent protein kinase in vivo and in vitro and its catalytic activities are regulated by phosphorylation. J. Biol. Chem., 277, 18291–18302. [DOI] [PubMed] [Google Scholar]
- 11.Moshous D., Callebaut,I., de Chasseval,R., Corneo,B., Cavazzano-Calvo,M., Le Deist,F., Tezcan,I., Sanal,O., Bertrand,S.Y., Phillipe,N., Fischer,A. and de Villartey,J.P. (2001) Artemis a novel DNA double-strand break repair/V(D)J recombination protein, is mutated in human severe combined immune deficiency. Cell, 105, 177–186. [DOI] [PubMed] [Google Scholar]
- 12.Luo C.-M., Tang,W., Mekeel,K.L., DeFrank,J.S., Anne,R. and Powell,S.N. (1996) High frequency and error-prone DNA recombination in Ataxia Telangiecstasia cell lines. J. Biol. Chem., 271, 4497–4503. [DOI] [PubMed] [Google Scholar]
- 13.O'Driscoll M., Cerosaletti,K.M., Girard,P.M., Dai,Y., Stumm,M., Kysela,B., Hirsch,B., Gennery,A., Palmer,S.E., Seidel,J. et al. (2001) DNA ligase IV mutations identified in patients exhibiting developmental delay and immunodeficiency. Mol. Cell, 8, 1175–1185. [DOI] [PubMed] [Google Scholar]
- 14.Zakian V.A. (1995) ATM-related genes: what do they tell us about about functions of the human gene? Cell, 82, 685–687. [DOI] [PubMed] [Google Scholar]
- 15.Hunter T. (1995) When is a lipid kinase not a lipid kinase? When it is a protein kinase. Cell, 83, 1–4. [DOI] [PubMed] [Google Scholar]
- 16.Sarkaria J.N., Tibbetts,R.S., Busby,E.C., Kennedy,A.P., Hill,D.E. and Abraham,R.T. (1998) Inhibition of phosphoinositide 3-kinase related kinases by the radiosensitizing agent wortmannin. Cancer Res., 58, 4375–4382. [PubMed] [Google Scholar]
- 17.Baumann P. and West,S.C. (1998) DNA end-joining catalyzed by human cell-free extracts. Proc. Natl Acad. Sci. USA, 95, 14066–14070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Gorman D.M., McKenna,S.L., McGahon,A.J., Knox,K.A. and Cotter,T.G. (2000) Sensitisation of HL60 human leukaemia cells to cytotoxic drug-induced apoptosis by inhibition of PI3-kinase survival signals. Leukemia, 4, 602–611. [DOI] [PubMed] [Google Scholar]
- 19.Chernikova S.B., Lindquist,K.L. and Elkind,M.M. (2001) Cell cycle-dependent effects of wortmannin on radiation survival and mutation. Radiat. Res., 155, 826–831. [DOI] [PubMed] [Google Scholar]
- 20.Ohta T. (1993) Modification of genotoxicity by naturally occurring flavorings and their derivatives. Crit. Rev. Toxicol., 23, 127–146. [DOI] [PubMed] [Google Scholar]
- 21.Watanabe K., Ohta,T., Watanabe,M., Kato,T. and Shirasu,Y. (1990) Inhibition of induction of adaptive response by o-vanillin in Escherichia coli B. Mutat. Res., 243, 273–280. [DOI] [PubMed] [Google Scholar]
- 22.Keshava C., Keshava,N., Ong,T. and Nath,J. (1998) Protective effect of vanillin on radiation-induced micronuclei and chromosomal aberrations in V79 cells. Mutat. Res., 397, 149–159. [DOI] [PubMed] [Google Scholar]
- 23.Gustafson D.L., Franz,H.R., Ueno,A.M., Smith,C.R., Doolittle,D.J. and Waldren,C.C. (2000) Vanillin (3-methoxy-4-hydroxybenzaldehyde) inhibits mutation induced by hydrogen peroxide, N-methyl-N-nitrosoguanidine and mitomycin C but not 137Cs γ-radiation at the CD59 locus in human–hamster hybrid AL cells. Mutagenesis, 15, 207–213. [DOI] [PubMed] [Google Scholar]
- 24.Jansson T. and Zech,L. (1987) Effects of vanillin on sister-chromatid exchanges and chromosome aberrations in human lymphocytes. Mutat. Res., 190, 221–224. [DOI] [PubMed] [Google Scholar]
- 25.Tamai K., Tezuka,H. and Kuroda,Y. (1992) Direct modifications by vanillin in cytotoxicity and genetic changes induced by EMS and H2O2 in cultured chinese hamster cells. Mutat. Res., 268, 231–237. [DOI] [PubMed] [Google Scholar]
- 26.Imanishi H., Sasaki,Y.F., Matsumoto,K., Watanabe,M., Ohta, T., Shirasu,Y. and Tutikawa,K. (1990) Suppression of 6-TG-resistant mutations in V79 cells and recessive spot formations in mice by vanillin. Mutat. Res. 243, 151–158. [DOI] [PubMed] [Google Scholar]
- 27.Sasaki Y.F., Imanishi,H., Watanabe,M., Ohta,T. and Shirasu,Y. (1990) Suppressing effects of antimutagenic flavorings on chromosome aberrations induced by UV-light or X-rays in cultured Chinese hamster cells. Mutat. Res., 229, 1–10. [DOI] [PubMed] [Google Scholar]
- 28.Akagi K., Hirose,M., Hoshiya,T., Mizoguchi,Y., Ito,N. and Shrai,T. (1995) Modulating effects of elagic acid, vanillin and quercetin in a rat medium term multi-organ carcinogenesis model, Cancer Lett., 94, 113–121. [DOI] [PubMed] [Google Scholar]
- 29.Tsuda H., Uehara,N., Iwahori,Y., Asamoto,M., Iigo,M., Nagao,M., Matsumoto,K., Ito,M. and Hirono,I. (1994) Chemopreventive effects of beta-carotene, alpha-tocopherol and five naturally occurring antioxidants on initiation of hepatocarcinogenesis by 2-amino-3-methylimidazo[4,5-f]quinoline in the rat. Jpn J. Cancer Res., 85, 1214–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tomkinson A.E., Lasko,D.D., Daly,G. and Lindahl,T. (1990) Mammalian DNA ligases. Catalytic domain and size of DNA ligase I. J. Biol. Chem., 265, 12611–12617. [PubMed] [Google Scholar]
- 31.Boulton S., Kyle,S., Yalcintepe,L. and Durkacz,B.W. (1996) Wortmannin is a potent inhibitor of DNA double strand break but not single strand break repair in Chinese hamster ovary cells. Carcinogenesis, 17, 2285–2290. [DOI] [PubMed] [Google Scholar]
- 32.Delacôte F., Han,M., Stamato,T.D., Jasin,M. and Lopez,B.S. (2002) An xrcc4 defect or Wortmannin stimulates homologous recombination specifically induced by double-strand breaks in mammalian cells. Nucleic Acids Res., 30, 3454–3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wymann M.P., Bulgarelli-Leva,G., Zvelebil,M.J., Pirola,L., Vanhaesebroeck,B., Waterfield,M.D. and Panayotou,G. (1996) Wortmannin inactivates phosphoinositide 3-kinase by covalent modification of Lys-802, a residue involved in the phosphate transfer reaction. Mol. Cell. Biol., 16, 1722–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Izzard R.A., Jackson,S.P. and Smith,G.C.M. (1999) Competitive and noncompetitive inhibition of the DNA-dependent protein kinase. Cancer Res., 59, 2581–2586. [PubMed] [Google Scholar]
- 35.Chobpattana W., Jeon,I.J. and Smith,J.S. (2000) Kinetics of interaction of vanillin with amino acids and peptides in model systems. J. Agric. Food Chem., 48, 3885–3889. [DOI] [PubMed] [Google Scholar]
- 36.Price B.D. and Youmell,M.B. (1996) The phosphatidylinositol 3-kinase inhibitor wortmannin sensitizes murine fibroblasts and human tumor cells to radiation and blocks induction of p53 following DNA damage. Cancer Res., 56, 246–250. [PubMed] [Google Scholar]
- 37.Fichtinger-Schepman A.M., van der veer,J.L., den Hartog,J.H., Lohman,P.H. and Reedijk,J. (1985) Adducts of the anticancer drug cis-diamminedichloroplatinum(II) with DNA: formation, identification and quantification. Biochemistry, 24, 707–713. [DOI] [PubMed] [Google Scholar]
- 38.Szymkowski D.E., Yarema,K., Essigman,J.M., Lippard,S.J. and Wood,R.D. (1992) An intrastrand d(GpG) platinum cross-link in duplex M13 DNA is refractory to repair by human cell extracts. Proc. Natl Acad. Sci. USA, 89, 10772–10776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moggs J.G., Yarema,K.J., Essigmann,J.M. and Wood,R.D. (1996) Analysis of incision sites produced by human cell extracts and purified proteins during nucleotide excision repair of a 1,3-intrastrand d(GpTpG)-cisplatin adduct. J. Biol. Chem., 271, 7177–7186. [DOI] [PubMed] [Google Scholar]
- 40.Morishita T., Tsutsui,Y., Iwasaki,H. and Shinagawa,H. (2002) The Schizosaccharomyces pombe rad60 gene is essential for repairing double-strand DNA breaks spontaneously occurring during replication and induced by DNA-damaging agents. Mol. Cell. Biol., 22, 3537–3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Durant S.T., Morris,M.M., Illand,M., McKay,H.J., McCormick,C., Hirst,G.L., Borts,R.H. and Brown,R. (1999). Dependence on RAD52 and RAD1 for anticancer drug resistance mediated by inactivation of mismatch repair genes. Curr. Biol., 9, 51–54. [DOI] [PubMed] [Google Scholar]
- 42.Britten R.A., Kuny,S. and Perdue,S. (1999) Modification of non-conservative double-strand break (DSB) rejoining activity after the induction of cisplatin resistance in human tumour cells. Br. J. Cancer, 79, 843–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Venkitaraman A.R. (2001) Functions of BRCA1 and BRCA2 in the biological response to DNA damage. J. Cell Sci., 114, 3591–3598. [DOI] [PubMed] [Google Scholar]
- 44.Dornish J.M. and Pettersen,E.O. (1989) Modulation of cis-diammineplatinum(II)-induced cytotoxicity by benzaldehyde derivatives. Cancer Lett., 46, 63–68. [DOI] [PubMed] [Google Scholar]
- 45.Miyakawa T., Zundel,J.-L. and Sakaguchi,K. (1979) Selective inhibitory effect of benzaldehyde on the growth of simian virus 40-transformed cells. Biochem. Biophys. Res. Commun., 87, 1024–1030. [DOI] [PubMed] [Google Scholar]