Abstract
Over 60 distinct Rab GTPases regulate specific vesicular transport steps in the mammalian central vacuolar system. Wasmeier et al. (this issue, p. 271) reveal a redundant role for two tissue-specific Rab proteins in regulating transport to a tissue-specific lysosome-related organelle, the melanosome.
Rab proteins are small GTPases of the ras superfamily that confer timing and target specificity to vesicle budding, tethering, docking, fusion, and motility within the eukaryotic secretory and endosomal pathways (Zerial and McBride, 2001). They thus ensure accurate delivery of cargo macromolecules to the right target organelle. Most mammalian rabs are broadly expressed and regulate membrane transport between ubiquitous compartments, such as endoplasmic reticulum and Golgi. However, certain cell types in higher eukaryotes harbor additional unique organelles that carry out tissue-specific functions. The best characterized are so-called lysosome-related organelles, which include melanosomes, the pigment organelle of melanocytes and of pigment epithelia in the eye. How are vesicular carriers that ferry cargo destined for melanosomes diverted from ubiquitous organelles that coexist in the same cell? Wasmeier et al. (this issue, p. 271) provide an important clue by showing that tissue-specific rab proteins are required to deliver cargo to mature melanosomes. Surprisingly, not just one but two such rabs play a redundant role in this process.
The authors begin by analyzing pigmentation in melanocytes from chocolate mice. Chocolate is one of many known mouse strains with coat color dilution because of naturally occurring genetic mutations that affect melanocyte biology (Bennett and Lamoreux, 2003). Chocolate mice have a coding mutation within the gene for Rab38 (Loftus et al., 2002), a rab protein limited in expression to melanocytes and a few other cell types (Jager et al., 2000). Wasmeier et al. (2006) found that the mutant Rab38 in chocolate mice is nonfunctional. Nevertheless, melanocytes from chocolate mice are only mildly hypopigmented and harbor melanosomes with normal morphology. They inferred that Rab38 function might be compensated by another rab protein.
Enter Rab32. Rab32 and Rab38 share 67% amino acid identity and are thus as similar to each other as isoforms of other rab proteins. Moreover, although limited in tissue distribution, Rab32 is expressed in melanocytes and platelets (Cohen-Solal et al., 2003). Wasmeier et al. (2006) confirmed that melanocytes express both Rab32 and Rab38. Importantly, siRNA-mediated depletion of Rab32 from chocolate melanocytes results in a dramatic loss of pigment and of ultrastructurally intact melanosomes. Pigmentation and normal morphology are restored by expressing epitope-tagged forms of either Rab32 or Rab38. Thus, the two rab proteins perform a redundant but essential function in melanosome maturation, supporting work in Drosophila melanogaster and Caenorhabditis elegans in which mutations in a single tissue- specific Rab32/38 orthologue block formation of lysosome-related pigment granules and gut granules, respectively (Ma et al., 2004; Hermann et al., 2005).
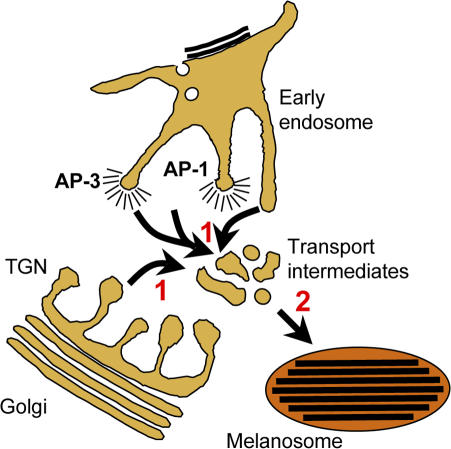
How and where do these Rab proteins function? Wasmeier et al. (2006) show that both Rab32 and Rab38 localize to some extent to melanosomes and primarily to tubulovesicular structures in the vicinity of the TGN and perinuclear endosomes; these latter structures lack labeling for markers of the Golgi, TGN, or sorting endosomes, and thus likely represent an endosomal subcompartment. Moreover, two critical cargo proteins—tyrosinase and the tyrosinase-related protein Tyrp1—are depleted from melanosomes in Rab32/Rab38-deficient melanocytes. Together, the data suggest that Rab32 and Rab38 regulate targeting of biosynthetic cargo to mature melanosomes through transport intermediates (Fig. 1).
Figure 1.
Model for Rab38 function in melanosome protein transport. Cargo destined for mature melanosomes are targeted first (1) to a Rab38-coated tubulovesicular transport intermediate from early endosomes via AP-1– or AP-3–associated clathrin-coated buds or from either early endosomes or the TGN by non–clathrin-coated vesicles. From these intermediates, cargo is delivered to melanosomes (2), which also associate with Rab38. Rab38 may facilitate either or both steps.
These findings have important implications for understanding how cell type–specific transport processes control the formation of tissue-specific organelles. Specific lysosome-related organelles are also affected by a human disease, Hermansky-Pudlak syndrome, and related animal models in which melanosomes, platelet-dense granules, and type II pneumocyte lamellar bodies are malformed, causing albinism, excessive bleeding, and lung pathology (Di Pietro and Dell'Angelica, 2005). The disease is due to mutations in genes encoding subunits of one of four protein complexes, AP-3, BLOC-1, BLOC-2, and BLOC-3, but each complex is ubiquitously expressed. Why their depletion causes tissue-specific pathology is thus unclear. Wasmeier et al. (2006) provide a potential explanation: perhaps the ubiquitous complexes function with tissue-specific rab proteins. Rab38 and Rab32 are each expressed by a distinct, restricted set of cell types, and only rare cell types express both. They thus likely function independently and nonredundantly in nonmelanocytes. Interestingly, although Rab38-deficient rats have platelet and lung dysfunction as well as hypopigmentation (Oiso et al., 2004), platelets are normal in chocolate mice, perhaps reflecting expression of Rab32 but not Rab38 in these cells. Rab38 but not Rab32 is detected in type II pneumocytes (Osanai et al., 2001); thus, chocolate mice are predicted to develop lung disease.
The next challenge is to define the mechanism by which Rab32 and Rab38 function and to distinguish whether either plays a nonredundant role in melanocytes. Despite the functional overlap, chocolate mice are hypopigmented, and Wasmeier et al. (2006) show that development of pigmentation in chocolate melanocytes is delayed. Does this reflect a reduced combined steady-state level of two completely redundant rab proteins, or does Rab38 bind a cohort of effectors that Rab32 does not? Both rab proteins are enriched on transport intermediates but also present on mature melanosomes (Fig. 1). Thus, Rab32 and Rab38 might function at sequential steps, such as tethering endosome-derived vesicles to melanosomal membranes and then fusion, much like Ypt7 or Rab5 in homotypic fusion of vacuoles and endosomes, respectively (Zerial and McBride, 2001). Candidate tethers are the BLOCs and the HOPS (homotypic fusion and vacuole protein sorting) complex, for which various subunits are mutated in Hermansky-Pudlak syndrome–like strains in mice and D. melanogaster (Suzuki et al., 2003).
Another challenge is to define the pathways in which Rab32 and Rab38 function. Wasmeier et al. (2006) show that two melanosome cargoes, tyrosinase and Tyrp1, are mislocalized in Rab32/38-deficient cells and provide evidence that tyrosinase is degraded, most likely by mistargeting to lysosomes. Tyrosinase follows two pathways from early endosomes to the melanosome, one via AP-3 and the other via the related clathrin adaptor, AP-1 (Theos et al., 2005; Fig. 1). Tyrp1 likely follows the AP-1 route primarily, as it colocalizes extensively with AP-1–coated membranes (Raposo et al., 2001) and is not grossly missorted in AP-3–deficient cells (Huizing et al., 2001). Does Rab32/38 participate in both endosome-dependent pathways or, perhaps, in a separate pathway from the TGN (Fig. 1)? Alternatively, might Rab32/38 function in directing cargo from the TGN to endosomal domains from which cargo can be sorted to melanosomes? An intriguing possibility is that Rab32/38 functions to divert AP-1–dependent cargo toward a lysosome-related organelle and away from a default pathway to lysosomes.
Wasmeier et al. (2006) have provided us with a key step that opens the door to many new unanswered questions regarding organelle biogenesis in the endosomal system. We anxiously await the next installment of the story.
Acknowledgments
I thank Juan S. Bonifacino for critical comments on the manuscript.
This paper was supported by National Institutes of Health grants R01 EY015625 and R01 AR048155.
References
- Bennett, D.C., and M.L. Lamoreux. 2003. The color loci of mice—a genetic century. Pigment Cell Res. 16:333–344. [DOI] [PubMed] [Google Scholar]
- Cohen-Solal, K.A., R. Sood, Y. Marin, S.M. Crespo-Carbone, D. Sinsimer, J.J. Martino, C. Robbins, I. Makalowska, J. Trent, and S. Chen. 2003. Identification and ch aracterization of mouse Rab32 by mRNA and protein expression analysis. Biochim. Biophys. Acta. 1651:68–75. [DOI] [PubMed] [Google Scholar]
- Di Pietro, S.M., and E.C. Dell'Angelica. 2005. The cell biology of Hermansky-Pudlak syndrome: recent advances. Traffic. 6:525–533. [DOI] [PubMed] [Google Scholar]
- Hermann, G.J., L.K. Schroeder, C.A. Hieb, A.M. Kershner, B.M. Rabbitts, P. Fonarev, B.D. Grant, and J.R. Priess. 2005. Genetic analysis of lysosomal trafficking in Caenorhabditis elegans. Mol. Biol. Cell. 16:3273–3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizing, M., R. Sarangarajan, E. Strovel, Y. Zho, W.A. Gahl, and R.E. Boissy. 2001. AP-3 mediates tyrosinase but not TRP-1 trafficking in human melanocytes. Mol. Biol. Cell. 12:2075–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager, D., E. Stockert, E. Jager, A.O. Gure, M.J. Scanlan, A. Knuth, L.J. Old, and Y.T. Chen. 2000. Serological cloning of a melanocyte rab guanosine 5′- triphosphate-binding protein and a chromosome condensation protein from a melanoma complementary DNA library. Cancer Res. 60:3584–3591. [PubMed] [Google Scholar]
- Loftus, S.K., D.M. Larson, L.L. Baxter, A. Antonellis, Y.A. Chen, X.S. Wu, Y. Jiang, M. Bittner, J.A. Hammer III, and W.J. Pavan. 2002. Mutation of melanosome protein RAB38 in chocolate mice. Proc. Natl. Acad. Sci. USA. 99:4471–4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, J., H. Plesken, J.E. Treisman, I. Edelman-Novemsky, and M. Ren. 2004. Lightoid and Claret: a rab GTPase and its putative guanine nucleotide exchange factor in biogenesis of Drosophila eye pigment granules. Proc. Natl. Acad. Sci. USA. 101:11652–11657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oiso, N., S.R. Riddle, T. Serikawa, T. Kuramoto, and R.A. Spritz. 2004. The rat Ruby (R) locus is Rab38: identical mutations in Fawn-hooded and Tester-Moriyama rats derived from an ancestral Long Evans rat sub-strain. Mamm. Genome. 15:307–314. [DOI] [PubMed] [Google Scholar]
- Osanai, K., M. Iguchi, K. Takahashi, Y. Nambu, T. Sakuma, H. Toga, N. Ohya, H. Shimizu, J.H. Fisher, and D.R. Voelker. 2001. Expression and localization of a novel Rab small G protein (Rab38) in the rat lung. Am. J. Pathol. 158:1665–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo, G., D. Tenza, D.M. Murphy, J.F. Berson, and M.S. Marks. 2001. Distinct protein sorting and localization to premelanosomes, melanosomes, and lysosomes in pigmented melanocytic cells. J. Cell Biol. 152:809–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki, T., N. Oiso, R. Gautam, E.K. Novak, J.J. Panthier, P.G. Suprabha, T. Vida, R.T. Swank, and R.A. Spritz. 2003. The mouse organellar biogenesis mutant buff results from a mutation in Vps33a, a homologue of yeast vps33 and Drosophila carnation. Proc. Natl. Acad. Sci. USA. 100:1146–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theos, A.C., D. Tenza, J.A. Martina, I. Hurbain, A.A. Peden, E.V. Sviderskaya, A. Stewart, M.S. Robinson, D.C. Bennett, D.F. Cutler, et al. 2005. Functions of AP-3 and AP-1 in tyrosinase sorting from endosomes to melanosomes. Mol. Biol. Cell. 16:5356–5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasmeier, C., M. Romao, L. Plowright, D.C. Bennett, G. Raposo, and M.C. Seabra. 2006. Rab38 and Rab32 control post-Golgi trafficking of melanogenic enzymes. J. Cell Biol. 175:271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerial, M., and H. McBride. 2001. Rab proteins as membrane organizers. Nat. Rev. Mol. Cell Biol. 2:107–119. [DOI] [PubMed] [Google Scholar]