Abstract
Amutation in the small GTPase Rab38 gives rise to the mouse coat color phenotype “chocolate” (cht), implicating Rab38 in the regulation of melanogenesis. However, its role remains poorly characterized. We report that cht Rab38G19V is inactive and that the nearly normal pigmentation in cht melanocytes results from functional compensation by the closely related Rab32. In cht cells treated with Rab32-specific small interfering RNA, a dramatic loss of pigmentation is observed. In addition to mature melanosomes, Rab38 and Rab32 localize to perinuclear vesicles carrying tyrosinase and tyrosinase-related protein 1, consistent with a role in the intracellular sorting of these proteins. In Rab38/Rab32-deficient cells, tyrosinase appears to be mistargeted and degraded after exit from the trans-Golgi network (TGN). This suggests that Rab38 and Rab32 regulate a critical step in the trafficking of melanogenic enzymes, in particular, tyrosinase, from the TGN to melanosomes. This work identifies a key role for the Rab38/Rab32 subfamily of Rab proteins in the biogenesis of melanosomes and potentially other lysosome-related organelles.
Introduction
Melanocytes are specialized pigment-producing cells residing in the skin and eyes of mammals. Melanin is synthesized and stored in melanosomes, membrane-bound subcellular organelles. These share some characteristics with lysosomes, but also possess several melanosome-specific components, including matrix proteins and melanin-synthesizing enzymes. Melanosome biogenesis can be divided into four morphologically distinct stages. Stage I premelanosomes are nonpigmented vacuoles derived from endosomes, which then acquire internal striations (stage II). Melanin formation results in pigment being deposited onto these striations, giving rise to stage III, and eventually fully melanized electron-dense stage IV melanosomes. The highly dendritic architecture of melanocytes in the skin allows transfer of mature pigment granules to a large number of keratinocytes, resulting in normal pigmentation (Marks and Seabra, 2001; Raposo and Marks, 2002; Hearing, 2005).
The study of mouse mutants displaying alterations in coat color has identified a large number of gene products that affect pigmentation (Bennett and Lamoreux, 2003). Many of these are involved in melanosome function. They include the enzymes required for melanin synthesis, primarily tyrosinase, as well as tyrosinase-related protein (Tyrp/TRP) 1 and Dct (TRP2; Hearing, 2005), and molecules with structural roles in melanosome formation, such as Pmel17 (Theos et al., 2005b). Others are involved in the regulation of intracellular protein trafficking and organelle biogenesis. Most mouse models for Hermansky-Pudlak syndrome (HPS), a heterogeneous group of disorders associated with albinism in humans, fall into this category. They include subunits of the biogenesis of lysosome-related organelles complexes (BLOCs), of adaptor protein 3 (AP-3), or of the homotypic vacuolar protein sorting complex (Li et al., 2004; Pietro and Dell'Angelica, 2005; Wei, 2006). Others again are required for correct intracellular distribution of melanosomes, for example, Rab27a, melanophilin, and myosinVa (Seabra and Coudrier, 2004).
Analysis of the coat color mutant “chocolate” (cht) identified the small GTPase Rab38 as another gene involved in the regulation of pigmentation (Loftus et al., 2002). The Rab family of proteins consists of >60 members in mammalian cells (Pereira-Leal and Seabra, 2001). Some are ubiquitously expressed, whereas others are highly tissue specific, reflecting more specialized roles. Each Rab protein shows a characteristic subcellular distribution and may thus represent an important determinant of organelle identity (Pfeffer, 2001; Munro, 2002; Seabra and Wasmeier, 2004). Rabs serve as key regulators of vesicular trafficking between subcellular compartments. They have been implicated in the formation of vesicles at the donor membrane and in the tethering, docking, and fusion of vesicles or organelles with their target membranes (Segev, 2001; Zerial and McBride, 2001). They are also involved in a variety of other processes, for example, the regulation of early endosome motility by Rab5 (Nielsen et al., 1999), the capture of melanosomes at the cell periphery by Rab27a (Seabra and Coudrier, 2004), or the remodeling of the cytoskeleton by Rab8 (Peranen et al., 1996). The regulatory capacity of Rab proteins is based on their ability to act as GTP-dependent molecular switches, with activation coupled to reversible association with intracellular membranes. In this way, Rabs can control the recruitment of downstream effectors to specific subcellular compartments in an activation-dependent way (Seabra and Wasmeier, 2004).
Rab38 is predominantly expressed in melanocytes and retinal pigment epithelial cells and is localized to pigmented melanosomes. In the cht mouse, a recessive Gly19 to Val point mutation was identified in Rab38. The resulting coat color phenotype resembles that of the brown mouse, which carries a mutation in Tyrp1, and reduced levels of Tyrp1 were reported in melanosomes of cultured cht melanocytes, suggesting an involvement of Rab38 in Tyrp1 transport (Loftus et al., 2002). The present study characterizes Rab38 in the cht mutant and defines a role for Rab38 in melanosome biogenesis. We demonstrate that Rab38 and the closely related Rab32 are important, functionally redundant regulators of melanosomal protein trafficking and melanocyte pigmentation.
Results
Pigmentation in cht mutant melanocytes
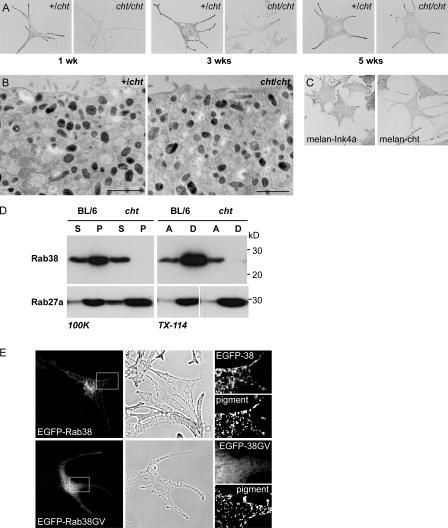
Primary skin melanocytes isolated from homozygous cht/cht mice showed strikingly reduced levels of pigmentation compared with cells from +/cht littermate controls when observed during the initial 2–3 wks in culture. After prolonged culture of 4 or 5 wks, however, cht/cht melanocytes appeared similar to +/cht controls (Fig. 1 A). Ultrastructural analysis of these cells showed no major differences between cht/cht and +/cht in the extent of melanization or melanosome size (Fig. 1 B). Immortal melanocyte cell lines derived from cht/cht mice (melan-cht) are also well pigmented (Fig. 1 C).
Figure 1.
Pigmentation in melanocytes carrying the cht mutation and biochemical characterization of cht mutant Rab38G19V. (A) Brightfield images of control +/cht and homozygous cht/cht primary skin melanocytes at 1–5 wk in culture. (B) Primary melanocytes cultured for 5 wk were processed for conventional EM. Sections show cytoplasmic organelles near the cell periphery. Bar, 500 nm. (C) Brightfield images of immortal melanocyte cell lines derived from BL/6 (melan-Ink4a) or cht (melan-cht) mice carrying an Ink4a deletion. (D) BL/6 or cht melanocyte homogenates were separated into soluble (S) and pelletable (P) fractions by centrifugation at 100,000 g (100K) or into aqueous (A) and detergent (D) fractions by extraction with Triton X-114 (TX-114). The distribution of Rab38, or Rab27a as a control, was analyzed by immunoblotting. (E) BL/6 cells were transiently transfected with EGFP-Rab38 or -Rab38G19V, as indicated. EGFP fluorescence and the corresponding phase-contrast images are shown. (right) Boxed regions at higher magnification, with pigment represented by an inverted phase-contrast image.
Characterization of cht Rab38
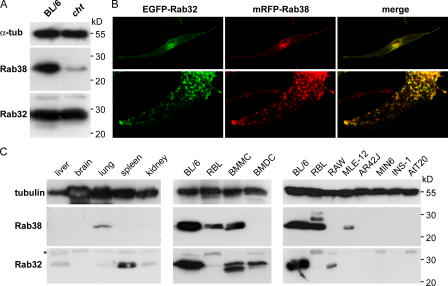
Because the cht phenotype results from a single amino acid change in Rab38, the presence of substantial numbers of mature pigment granules in cht/cht melanocytes could be due to the mutant protein (Rab38G19V) retaining some functional activity. Rabs are peripheral membrane proteins that rely on geranylgeranylation for association with cellular membranes and function (Seabra and Coudrier, 2004). We therefore analyzed the expression and subcellular localization of Rab38 in control BL/6-derived melan-Ink4a melanocytes (BL/6) and in melan-cht melanocytes (cht) by subcellular fractionation (Fig. 1 D). In BL/6 cells, the majority of Rab38 was pelleted by centrifugation at 100,000 g, demonstrating association with cellular membranes, and partitioned into the detergent phase upon extraction with Triton X-114, indicating lipid modification. In cht cells, the total levels of Rab38 were substantially reduced. More important, the protein was exclusively detected in the cytosolic fraction or in the aqueous phase. A control Rab, Rab27a, was pelleted and detergent extracted equally in both cell lines. Recombinant EGFP-tagged Rab38 expressed in BL/6 melanocytes showed a characteristic punctate pattern. It localized primarily to perinuclear membranes but also to the cell periphery, where it was frequently observed overlapping with or in close proximity to pigmented melanosomes (Fig. 1 E; and see Figs. 4, 5, and 6). EGFP-Rab38G19V was diffusely distributed throughout the cytoplasm, with a concentration in the nucleus that is characteristic of soluble EGFP-tagged Rab mutants. These results suggest that cht mutant Rab38 is unstable, lacks posttranslational lipid modification, and does not associate with cellular membranes, rendering it functionally inactive.
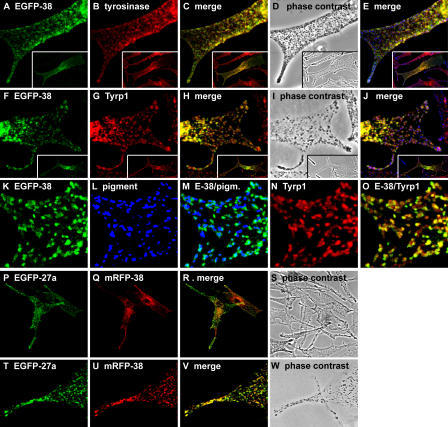
Figure 4.
Rab38 colocalizes with melanosomal proteins in peripheral melanosomes and in perinuclear vesicles. BL/6 melanocytes were transiently transfected with EGFP-Rab38 (EGFP-38; A and F). Cells were fixed, permeabilized, and labeled with antibodies to tyrosinase (B) or Tyrp1 (G), as indicated. (C and H) Merged fluorescent images; (D and I) corresponding phase-contrast images; (E and J) Fluorescent signals and phase contrast (blue) are merged. Panels show regions of cells that include perinuclear and peripheral areas, and insets represent the whole cell. (K–O) Labeled structures at the cell periphery are depicted at higher magnification; EGFP-Rab38 (K), pigment (L), Tyrp1 (N), and merged images (M and O) are shown. (P–W) Melanocytes were cotransfected with EGFP-Rab27a (P and T) and mRFP-Rab38 (Q and U). (R and V) Merged images; (S and W) phase contrast.
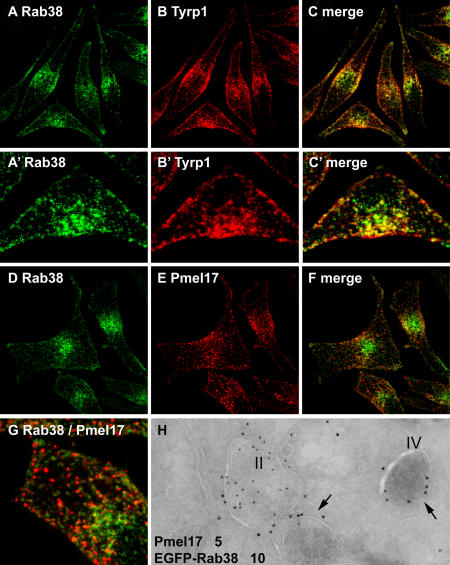
Figure 5.
Rab38-positive structures are distinct from stage II melanosomes. MNT-1 cells were fixed, permeabilized, and double labeled with antibodies to Rab38 (A and D) and either Tyrp1 (B and C) or Pmel17 (HMB45; E–G). (C, F, and G) Merged images; (A′–C′ and G) higher magnifications. (H) MNT-1 cells were transduced with lentivirus to stably express EGFP-Rab38 and were processed for immuno-EM. Ultrathin cryosections were double labeled with antibodies to EGFP (10-nm gold) and Pmel17 (5-nm gold). Stage II and IV melanosomes (arrows) are indicated.
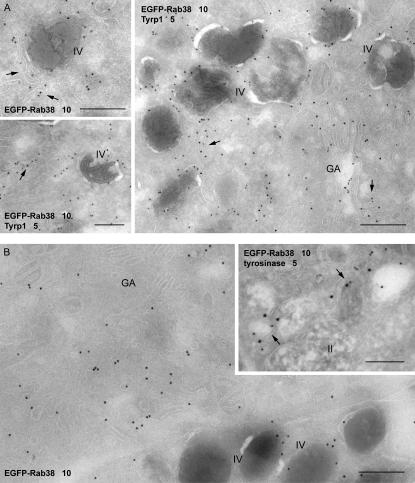
Figure 6.
Distribution of Rab38, Tyrp1, and tyrosinase on small cytoplasmic vesicles and tubules. (A) Ultrathin sections of MNT-1 cells stably expressing EGFP-Rab38 were labeled with antibodies to EGFP or EGFP (10-nm gold) and Tyrp1 (5-nm gold), as indicated. EGFP-Rab38 is present on stage IV melanosomes and on vesicles and tubules (arrows) near melanosomes and the Golgi (GA). (B) BL/6 melanocytes stably expressing EGFP-Rab38 were labeled with antibodies to EGFP or EGFP (10-nm gold) and tyrosinase (5-nm gold), as indicated. Bars, 200 nm.
Rab38 and Rab32 can functionally compensate for each other in pigment biosynthesis
A lower rate of pigment synthesis in cht melanocytes may implicate Rab38 in fine-tuning the kinetics of this pathway. However, it is also possible that Rab38 regulates a more fundamental step in melanosome biogenesis, but that alternative mechanisms can at least partially compensate for the loss of Rab38 function in cht. Rab38 is closely related to Rab32, another member of the Rab family (Jager et al., 2000; Bao et al., 2002; Cohen-Solal et al., 2003). The mouse Rab38 and Rab32 proteins are 67% identical. This is more comparable to the degree of sequence identity between Rab isoforms such as Rab27a and -27b (72%), Rab3a and -3b (78%), or Rab5a and -5b (82%), which are able to functionally compensate for each other, than to that between more distantly related Rabs, which are typically only between 20 and 30% identical. To determine whether there could potentially be redundancy between Rab38 and Rab32, we examined the expression and subcellular localization of Rab32 in melanocytes. Cht cells expressed similar levels of Rab32 to control BL/6 cells (Fig. 2 A). In melanocytes cotransfected with EGFP-tagged Rab38 and mRFP-tagged Rab32, almost complete colocalization was observed, with both Rabs being targeted to the same vesicular structures at the cell periphery, as well as within the cell body (Fig. 2 B).
Figure 2.
Expression pattern and subcellular localization of Rab38 and Rab32. (A) Proteins from BL/6 or cht melanocytes were separated by SDS-PAGE and immunoblotted for Rab38, Rab32, and α-tubulin (α-tub) as loading control. (B) Melanocytes were transiently cotransfected with EGFP-Rab32 and mRFP-Rab38. Green and red fluorescent signals are shown, and colocalization is represented in yellow in the merged images. (bottom) A cell process at higher magnification. (C) Proteins from mouse tissues (50 μg/lane) or from a range of cultured mouse or rat cell lines available in our laboratory (25 μg/lane) were separated by SDS-PAGE and immunoblotted for Rab38, Rab32, and tubulin as loading control. (right and left) α-Tubulin; (middle) γ-tubulin. The asterisk indicates a nonspecific band in the Rab32 panels.
In addition to melanocytes, Rab38 was expressed in bone marrow mast cells and basophil-derived RBL cells and at lower levels in lung and lung alveolar type II–derived MLE-12 cells (Fig. 2 C). It was not detected in liver, brain, spleen, or kidney, or in bone marrow dendritic cells, RAW (macrophage), AR42J (exocrine pancreas), MIN6 and INS-1 (insulinoma), or AtT20 (anterior pituitary) cells. Low levels of Rab32 were observed in liver, lung, and kidney, which may indicate its presence in a subpopulation of cells within these tissues. Rab32 was also seen in spleen, bone marrow mast cells, bone marrow dendritic cells, and macrophage-derived RAW cells. Other cell types tested were negative (Fig. 2 C), suggesting a highly tissue-specific expression pattern for both Rab38 and Rab32.
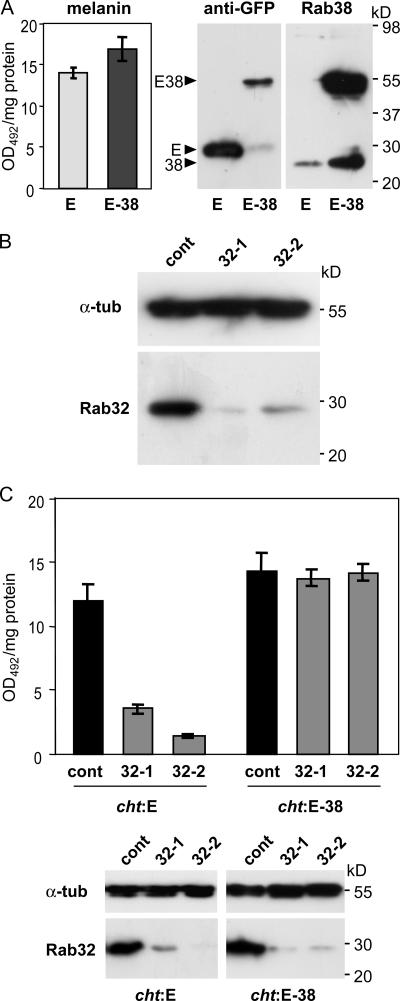
As shown in Fig. 1 C, cht melanocytes possess substantial levels of pigment. Recombinant EGFP-tagged Rab38 was overexpressed in these cells (cht:EGFP-Rab38) using lentiviral vectors. This resulted in a small but reproducible increase in melanin content relative to noninfected (not depicted) or EGFP-expressing cht cells (cht:EGFP; Fig. 3 A), indicating that Rab38 activity is indeed able to stimulate pigment biosynthesis.
Figure 3.
Rab38 and Rab32 are required for melanocyte pigmentation and act in a functionally redundant way. (A) EGFP (E) or EGFP-Rab38 (E-38) were stably overexpressed in cht cells using lentiviral vectors. Cellular proteins were immunoblotted for EGFP or Rab38, as indicated. Melanin content was assayed by measuring optical density at 492 nm (OD492). Assays were performed in triplicate; means and standard deviations are depicted. (B) Cht cells were transfected with either control siRNA oligos or Rab32-specific siRNA oligos (32-1 or 32-2). Cellular proteins were immunoblotted for Rab32 and α-tubulin. (C) Cht cells expressing EGFP (cht:E) or EGFP-Rab38 (cht:E-38) were subjected to two rounds of transfection with control or Rab32-specific siRNA oligos. Melanin content was measured on day 10 after the initial transfection. Assays were performed in triplicate, as above. Cells were also analyzed for expression of Rab32 by immunoblotting (bottom).
We then used Rab32-specific siRNA oligonucleotides to investigate the potential contribution of Rab32 to pigment biogenesis. Transfection with four different siRNAs (including 32–1 and 32–2, and others not depicted) resulted in a substantial reduction in Rab32 protein levels, whereas control oligonucleotides showed no effect (Fig. 3 B). Rab32 siRNA–transfected cht cells, but not cells treated with control siRNA, gradually lost pigmentation. Pigment was measured in cht:EGFP cells 10 d after the first of two rounds of transfection. A 70 or 87% decrease in melanin content, respectively, was observed in cells treated with oligos 32-1 and 32-2 (Fig. 3 C). In contrast, in cht:EGFP-Rab38 cells, where Rab38 function was restored by stably expressing exogenous Rab38, a knock down of Rab32 had no effect on melanin levels (Fig. 3 C). This suggests a role for Rab32 in regulating pigment biogenesis and demonstrates that Rab38 and Rab32 can functionally compensate for each other, whereas the loss of both Rab proteins leads to a dramatic reduction in melanocyte pigmentation.
Subcellular localization of Rab38 and Rab32
Pigment generation requires the formation of an immature melanosome and its subsequent maturation. To analyze further how Rab38 and Rab32 may contribute to this complex process, the subcellular localization of these Rab proteins was examined in more detail. EGFP-tagged Rab38 (and EGFP-Rab32; Fig. 2 B and not depicted) displays a characteristic distribution in melanocytes, where it is present at the cell periphery and on a population of vesicular structures in the perinuclear region (Fig. 4). A similar pattern was observed for endogenous Rab38 (Fig. 5). Labeling of EGFP-Rab38–expressing cells with antibodies to tyrosinase or Tyrp1 showed extensive colocalization with both melanosome markers (Fig. 4). At the cell periphery, EGFP-Rab38 partially colocalized with mature pigmented melanosomes. More frequently, however, Rab38-positive vesicles contained tyrosinase or Tyrp1 but were devoid of pigment, especially in the perinuclear region (Fig. 4, A–O). The distribution of Rab38 overlapped with that of Rab27a, another melanosome marker, but with Rab38 more prominent on perinuclear membranes and Rab27a more concentrated on peripheral, pigmented melanosomes (Fig. 4, P–W).
To determine if nonpigmented Rab38-positive structures could represent newly synthesized, immature melanosomes, we used MNT-1 human melanoma cells, a particularly good model for analyzing early-stage organelles because of their relative abundance (Raposo et al., 2001). Again, Rab38 colocalized extensively with Tyrp1, both in the cell periphery and in the perinuclear region (Fig. 5). MNT-1 cells were also double labeled for Rab38 and the melanosomal matrix protein Pmel17 (Fig. 5). Antibody HMB45 is specific for a maturation-dependent cleavage form of Pmel17 and selectively labels stage II (striated but nonpigmented) melanosomes (Raposo et al., 2001). Little colocalization was observed between Rab38 and Pmel17 by immunofluorescence (IF) microscopy. Immunogold EM on cells stably expressing EGFP-tagged Rab38 did not detect Rab38 on stage II melanosomes either (Fig. 5 H). Instead, in addition to mature pigmented (stage IV) melanosomes, EGFP-Rab38 was present on small cytoplasmic vesicles and tubules, frequently seen near the Golgi apparatus or in close proximity to melanosomes (Fig. 6 A). Many of these vesicular structures also contained Tyrp1. Similarly, EGFP-Rab38 was observed on Tyrp1-positive (not depicted) and on tyrosinase-positive cytoplasmic vesicles in BL/6-derived mouse melanocytes (Fig. 6 B). We detected little colocalization between Rab38 or Rab32 and markers for the Golgi, the TGN, or the early endosome (unpublished data). Collectively, its subcellular localization suggests a possible role for Rab38 in regulating a vesicular transport step involved in the delivery of both Tyrp1 and tyrosinase from the TGN to the maturing melanosome.
Trafficking of tyrosinase and Tyrp1 is disrupted in Rab38/Rab32-deficient cells
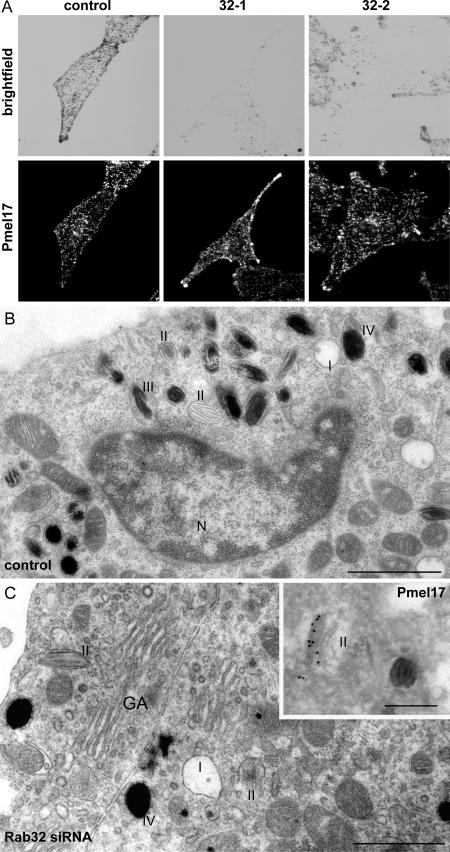
Melanocytes lacking functional Rab38 and Rab32, i.e., cht cells treated with Rab32-specific siRNAs (Rab38/Rab32-deficient cells), are characterized by a drastic reduction in the number of pigmented melanosomes. The abundance and distribution of Pmel17-positive vesicles, however, resembled that in control cells, as assessed by IF microscopy of HMB45-labeled cells (Fig. 7 A). Ultrastructural analysis confirmed that nonpigmented stage II melanosomes were still being formed (Fig. 7, B and C).
Figure 7.
Loss of mature melanosomes but not stage II organelles in Rab38/Rab32-deficient cells. (A) Cht melanocytes subjected to two rounds of transfection with either control or Rab32-specific siRNA oligos were fixed, permeabilized, and labeled for Pmel17 with antibody HMB45. Note the reduction in pigmented structures in the corresponding brightfield images of Rab32 siRNA–treated cells. (B) Cht cells transfected with control siRNA were processed for conventional EM. Melanosomes at different stages of maturation are indicated. (C) Cht cells transfected with Rab32-specific siRNA were analyzed as in B. The inset shows immunogold labeling for Pmel17 on stage II melanosomes in cht cells treated with Rab32-specific siRNA and processed for immuno-EM. N, nucleus; GA, Golgi. Bars: (B and C) 500 nm; (inset) 200 nm.
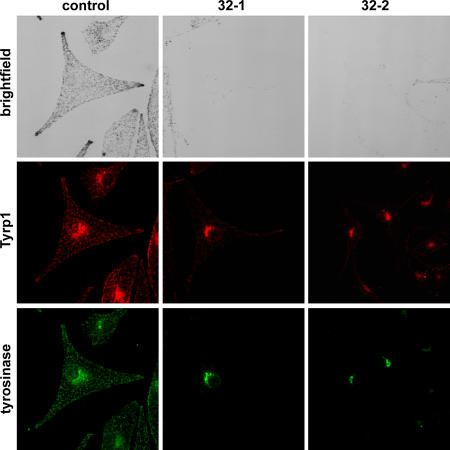
In control cht cells, Tyrp1 and tyrosinase localized to vesicles distributed throughout the cytoplasm and along the cell periphery, with an additional pool in the Golgi region. In contrast, in Rab38/Rab32-deficient cells, Tyrp1 and tyrosinase were almost exclusively restricted to the perinuclear region (Fig. 8). Little Tyrp1, and virtually no tyrosinase, was detected on peripheral structures in these cells. This change in subcellular distribution suggests that the loss of pigmentation induced by Rab32 knockdown is the result of a disruption in the intracellular trafficking of Tyrp1 and, more important, tyrosinase.
Figure 8.
Tyrosinase and Tyrp1 trafficking to the melanosome is blocked in Rab38/Rab32-deficient cells. Cht melanocytes transfected with either control or Rab32-specific siRNA oligos as in Fig. 7 were fixed, permeabilized, and immunolabeled for Tyrp1 and tyrosinase, as indicated. The corresponding brightfield images are shown in the top panels.
The perinuclear pool of tyrosinase in control cht cells as well as tyrosinase in Rab38/Rab32-deficient cells showed a high degree of colocalization with TGN38, a marker for the TGN (Fig. 9). Post-Golgi sorting of tyrosinase is thought to involve trafficking through an endosomal compartment (Theos et al., 2005a). In Rab38/Rab32-deficient cells, some additional tyrosinase-positive structures were frequently observed in close proximity to the TGN38-labeled compartment. However, these did not appear to possess either AP-3 or the transferrin receptor (unpublished data); thus, it is not clear if they could represent such endosomes.
Figure 9.
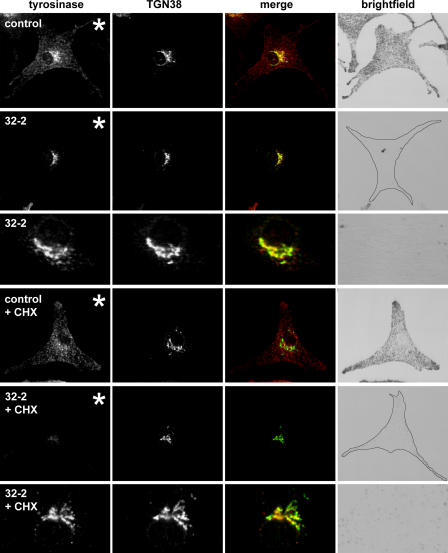
Localization of tyrosinase to the TGN in control and Rab38/Rab32-deficient cells. Control or Rab32 (32-2) siRNA–treated cht cells were either fixed under steady-state conditions (top) or were incubated with cycloheximide for 3 h before fixation (bottom; + CHX). Cells were permeabilized and double labeled for tyrosinase (red) and TGN38 (green), as indicated. Colocalization is shown as yellow in the merged images. The outline of depigmented Rab32 knockdown cells is depicted in the brightfield images. For comparison of tyrosinase levels between panels marked with asterisks, images of tyrosinase labeling were acquired using the same microscope settings; for analysis of colocalization with TGN38 in images showing higher magnifications, signal intensities were adjusted independently.
To distinguish between newly synthesized protein in transit through the Golgi stack and protein retained in this compartment because of a block in transport, protein synthesis was inhibited with cycloheximide for 3 h. In control cells, this resulted in depletion of the perinuclear pool of tyrosinase, whereas protein in peripheral melanosomes was not affected, and no substantial changes in overall levels of tyrosinase were observed. In contrast, the majority of Rab38/Rab32-deficient cells (∼70% of depigmented cells compared with <10% of control cells) showed drastically reduced tyrosinase labeling after incubation with cycloheximide (Fig. 9), indicating degradation of the protein after its exit from the TGN. Any residual tyrosinase seen in these cells remained largely colocalized with TGN38, with no obvious accumulation in a post-TGN compartment. This argues for a critical Rab38/Rab32-dependent step in the trafficking of tyrosinase from the TGN to the melanosome.
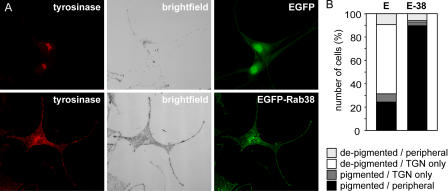
Cht cells were transfected with Rab32 siRNA to induce loss of pigmentation and were subsequently infected with virus introducing either EGFP or EGFP-Rab38. Expression of EGFP alone did not affect levels of pigmentation or the characteristic perinuclear localization of tyrosinase. In contrast, in cells expressing EGFP-Rab38, trafficking of tyrosinase to cytoplasmic and peripheral vesicles was restored, concomitant with the cells recovering normal levels of pigmentation (Fig. 10 A). Approximately 59% of EGFP-expressing cells in this experiment were depigmented and showed restriction of tyrosinase to the TGN region, whereas only 10% of EGFP-Rab38–positive cells had not recovered both pigmentation and a normal subcellular distribution of tyrosinase (Fig. 10 B). This further supports the conclusion that Rab38 can rescue melanosome biogenesis in Rab38/Rab32-deficient cells and that it restores pigment biosynthesis by mediating tyrosinase transport from a perinuclear compartment to the melanosome.
Figure 10.
Expression of exogenous Rab38 rescues tyrosinase trafficking in Rab38/Rab32-deficient cells. (A) Cht melanocytes were subjected to two rounds of transfection with Rab32-specific siRNA oligos. On day 6 after the initial transfection, cells were transduced with lentivirus expressing either EGFP or EGFP-Rab38, as indicated. 4 d later, cells were fixed, permeabilized, and labeled for tyrosinase (red). The corresponding brightfield images and EGFP fluorescence (green) are also shown. (B) EGFP (E) or EGFP-Rab38 (E-38) positive cells were scored as either pigmented or depigmented, and tyrosinase was categorized as either confined to the perinuclear region (TGN only) or present in vesicular structures at the cell periphery (peripheral). Numbers of cells in each category are plotted as a percentage of the total number of cells counted (n = 197 cells for EGFP; n = 126 cells for EGFP-Rab38).
Discussion
We show here for the first time that Rab38 and Rab32 act in a functionally redundant way in regulating skin melanocyte pigmentation. These Rab proteins control an important post-Golgi step in the trafficking of key melanogenic enzymes and are therefore critical for melanosome maturation.
The identification of the cht mutation as Rab38G19V implicated Rab38 in the regulation of pigment biogenesis (Loftus et al., 2002), but the effect of this mutation on Rab38 function remained unclear. Although not an invariant residue within the Rab family, glycine 19 is conserved in >50% of Rabs and is situated within the generally highly conserved GTP binding pocket. The corresponding Rab5 mutant, Rab5A30V, showed increased activity (Li and Liang, 2001). The biochemical properties of Rab38G19V, however, are not compatible with a functionally active Rab, and we therefore regard the cht mouse as equivalent to a Rab38-null mutant.
In comparison with other mouse pigmentation mutants (Li et al., 2004), the cht phenotype is very mild. The similarity in coat color, and reduced Tyrp1 levels in cht melanosomes, led to the suggestion that the cht mouse may be a phenocopy of the brown mouse, with the cht phenotype arising from a defect in Tyrp1 trafficking (Loftus et al., 2002). We now demonstrate that pigmentation in cht melanocytes is dependent on Rab32. The dramatic loss of pigment in the absence of both Rab38 and Rab32 is consistent with a critical role not only in the trafficking of Tyrp1 but also of tyrosinase, the key enzyme in melanin synthesis, which requires the melanosomal environment for catalytic activity (Watabe et al., 2004; Hearing, 2005). On the basis of these observations, we would predict a Rab38/Rab32 double-knockout mouse to show severe hypopigmentation. Compensation by Rab32 may also explain why mutations in Rab38 have not been identified in human patients with oculocutaneous albinism (Suzuki et al., 2003). However, the presence of a detectable coat color phenotype in the cht mouse may indicate subtle functional differences between the two Rab proteins, possibly accounting for the differential expression patterns observed in some specialized cell types.
Functional redundancy between mammalian Rab38 and Rab32 is consistent with the presence of only a single homologue in other species, such as Rab-RP1 in Drosophila melanogaster (Fujikawa et al., 2002) or Glo-1 in Caenorhabditis elegans (Hermann et al., 2005). Rab-RP1 is mutated in lightoid, a D. melanogaster eye color phenotype exhibiting defects in pigment granule synthesis (Ma et al., 2004), and C. elegans Glo-1 mutants lack lysosome-like gut granules (Hermann et al., 2005). These observations suggest an evolutionarily conserved role for the Rab38/Rab32-related subgroup of Rab proteins in the biogenesis of specialized lysosome-related organelles (LROs) such as gut granules in C. elegans, eye pigment granules in D. melanogaster, and mammalian melanosomes.
Deficiencies in the biogenesis of LROs are also characteristic of HPS and the mouse models for HPS, with melanocytes and platelets most severely affected. The corresponding proteins (AP-3, Vps33, and the BLOC subunits), however, are expressed ubiquitously, suggesting more general roles in the biogenesis of lysosomes (Li et al., 2004; Pietro and Dell'Angelica, 2005). In contrast, Rab38 and Rab32 (Jager et al., 2000; Osanai et al., 2001; Cohen-Solal et al., 2003) were restricted to cell types characterized by the presence of LROs, a morphologically and functionally diverse group of organelles that includes melanosomes, platelet-dense granules, mast cell granules, lamellar bodies of lung epithelial cells, lytic granules of cytotoxic T-lymphocytes, and MHC class II compartments of antigen-presenting cells (Marks and Seabra, 2001; Stinchcombe et al., 2004). This further supports a highly specialized role for Rab38 and Rab32 in LRO function.
Recent studies investigating the intracellular trafficking of melanosomal integral membrane proteins like Pmel17, tyrosinase, and Tyrp1 have revealed much about the formation of melanosomes and the complex sorting pathways involved (Raposo and Marks, 2002; Theos et al., 2005a,b; Valencia et al., 2006). Premelanosomes appear to be largely derived from endosomal precursors, which progress to stage II organelles through the Pmel17-dependent formation of lumenal striations. These provide the fibrillar matrix for the subsequent deposition of melanin, initiated by the delivery of melanogenic enzymes, i.e., tyrosinase and tyrosinase-related proteins. The differential enrichment for Pmel17 in early-stage organelles and tyrosinase and Tyrp1 in later-stage pigmented structures provides evidence for the existence of distinct sorting pathways to premelanosomes and to more mature organelles (Raposo et al., 2001). Our results suggest that Rab38 and Rab32 are not required for the formation of early-stage melanosomes. Trafficking of tyrosinase and Tyrp1 to these organelles, on the other hand, is dependent on Rab38 or Rab32.
The subcellular localization of these Rab proteins argues for their recruitment to post-TGN transport vesicles and a role in regulating the subsequent delivery of such vesicles to maturing melanosomes. Given the loss of tyrosinase in the absence of Rab38 and Rab32, this may represent a tissue-specific trafficking route critical for diverting proteins destined for LROs away from the degradative pathway to lysosomes. The TGN localization of tyrosinase in Rab38/Rab32-deficient cells is consistent with the recruitment of these Rabs to carrier vesicles derived directly from the TGN. Melanosomal targeting of tyrosinase is thought to involve transit through an early endosomal compartment (Theos et al., 2005a), which may implicate Rab38/Rab32 in TGN to endosome trafficking. Alternatively, tyrosinase may traverse the endosome normally in the absence of Rab38 and Rab32, but may require their subsequent recruitment to endosome-derived transport vesicles. The latter scenario is supported by the continued presence of Rab38 on or in close proximity to mature melanosomes. A detailed analysis of the different subpopulations making up the endosomal system should prove informative with regard to pinpointing the precise site of action of Rab38 and Rab32.
Morphological studies of melanosomes in a range of HPS mutants documented disruption of organelle maturation at distinct stages in different mutants (Nguyen et al., 2002; Nguyen and Wei, 2004; Wei, 2006). The molecular mechanisms and sites of action of most of the corresponding proteins, in particular the BLOC components, are still unknown. However, aberrant localization of tyrosinase and Tyrp1 is seen in many forms of HPS (Gwynn et al., 2004; Richmond et al., 2005). A more detailed comparison of melanosomal protein trafficking in the different mutants should shed light on the potential interplay between Rab38/Rab32 and other HPS proteins during melanosome biogenesis.
D. melanogaster lightoid (Rab38/Rab32 homologue) and ruby or garnet (AP-3) double mutants show considerably reduced eye pigmentation compared with the corresponding single mutants, indicating that lightoid may function in an AP-3–independent pathway (Ma et al., 2004). The association of Rab38 with Tyrp1 trafficking (Loftus et al., 2002) and the mistargeting of tyrosinase but not Tyrp1 observed in melanocytes lacking AP-3 (Huizing et al., 2001) raised the possibility that tyrosinase may traffic via an AP-3–dependent pathway, whereas Tyrp1 followed a separate Rab38-dependent route. Our results, however, demonstrate a role for Rab38/Rab32 in tyrosinase trafficking as well. The lack of colocalization between Rab38/Rab32 and AP-3 (unpublished data) is consistent with the regulation of distinct steps. The plasticity of the sorting pathways involved, though (Theos et al., 2005a), could certainly result in additive effects upon removal of two components that normally act sequentially within the same pathway. Melanosome targeting of tyrosinase was more severely impaired than that of Tyrp1 upon loss of Rab38/Rab32, possibly indicating that the latter can access alternative pathways more efficiently. For a better understanding of Rab38/Rab32 function and the trafficking pathways regulated by this subfamily of Rab proteins in melanocytes and other cell types, the identification of interacting partners for Rab38 and Rab32 will be of great interest.
Materials and methods
Mice, melanocytes, and cell culture
C57BL/6J-Rab38cht (+/cht) mice were obtained from The Jackson Laboratory and were maintained and propagated under UK project licenses 70/5071 and 70/6210 at the Central Biomedical Services of Imperial College London. Primary mouse melanocytes were derived as described previously (Hume et al., 2002) and were maintained in RPMI-1640 supplemented with 5% FCS, 200 nM phorbol 12-myristate 13-acetate, 200 pM cholera toxin, 100 U/ml penicillin G, and 100 U/ml streptomycin at 37°C with 10% CO2. The immortal melanocyte cell line melan-Ink4a was derived from a/a C57BL/6J mice homozygous for an Ink4a-Arf exon 2 deletion (Sviderskaya et al., 2002). Eight independent melan-cht lines (melan-cht-1–8) were generated by crossing C57BL/6J-Rab38cht/Rab38cht mice with Ink4a-Arf–null mice (to circumvent cell senescence). Melanocyte cultures were prepared from individual F2 neonatal mice homozygous for both mutations (provided by L. Lamoreux, Texas A & M University, College Station, TX) as described previously (Sviderskaya et al., 2002). Cells were maintained in RPMI-1640 supplemented with 10% FCS, 200 nM phorbol 12-myristate 13-acetate, and 200 pM cholera toxin at 37°C with 10% CO2. MNT-1 human melanoma cells were maintained in DME supplemented with 10% AIM-V medium, 20% FCS, nonessential amino acids, and sodium pyruvate.
Tissue and cell preparations and subcellular fractionation
Mouse tissues were collected from C57BL/6J mice perfused with PBS and were homogenized in 3 volumes of 50 mM Hepes, pH 7.2, 10 mM NaCl, 1 mM dithiothreitol, and protease inhibitor cocktail (Roche). Homogenates were centrifuged at 1,000 g for 10 min at 4°C to sediment unbroken cells and nuclei. Cultured cells were pelleted and disrupted by sonication in the aforementioned buffer. Protein concentrations of postnuclear supernatants or whole cell lysates were determined using the Bio-Rad protein assay (Bio-Rad Laboratories). For subcellular fractionation, cells were disrupted as above. Membranes were pelleted by centrifugation at 100,000 g for 1 h at 4°C. Alternatively, Triton X-114 (Calbiochem) was added to 1%, samples were incubated on ice for 10 min, followed by 5 min at 37°C, and centrifuged at 16,000 g for 3 min at room temperature. Aqueous and detergent phases were recovered, and the detergent phase was reextracted once with buffer. All fractions were adjusted to the same volume, and equal volumes were analyzed by SDS-PAGE and immunoblotting.
Antibodies
To generate polyclonal antibodies to Rab38 and Rab32, the C-terminal hypervariable regions of rat Rab38 (residues 162–211) or mouse Rab32 (residues 175–223) were fused to GST and purified on glutathione–Sepharose beads as previously described (Hume et al., 2002). Sera from rabbits immunized with these fusion proteins were preabsorbed against GST and affinity purified using the same antigens immobilized on AminoLink Coupling Gel (Pierce Chemical Co.) as described previously (Seabra et al., 1995). Anti-Rab27a monoclonal antibody 4B12 was described previously (Hume et al., 2001). Rabbit polyclonal anti-tyrosinase PEP7 (IF, 1:150) was a generous gift from V. Hearing (National Institutes of Health, Bethesda, MD). Other antibodies were as follows: rabbit anti-tyrosinase (EM; provided by A. Theos and M. Marks, University of Pennsylvania, Philadelphia, PA; Theos et al., 2005a), mouse anti-Tyrp1 TA99 (IF, 1:200 [ID Labs]; EM [American Type Culture Collection]), mouse anti-Pmel17 HMB45 (IF, 1:50 [DakoCytomation]; EM [Labvision]), sheep anti-TGN38 (1:100; Serotec), rabbit anti-EGFP (EM; Invitrogen), mouse anti-EGFP (immunoblot; 1:1,000; Roche), mouse anti–α tubulin and mouse anti–γ tubulin (1:1,000; Sigma-Aldrich).
Immunoblotting
Samples were fractionated on 13% SDS-PAGE gels and transferred to polyvinylidene difluoride membrane (Millipore). Membranes were blocked in PBS/0.1% Tween-20 (PBS/T) with 4% nonfat dried milk, incubated with primary antibody in PBS/T, washed four times in blocking solution, and incubated with horseradish peroxidase–conjugated secondary antibody (anti–rabbit or anti–mouse; 1:5,000; DakoCytomation) followed by washing in PBS/T. Bound antibody was detected using the ECL Plus Western blotting detection system (GE Healthcare).
Plasmid constructs
pEGFP-Rab27a, -Rab38, and -Rab32 were generated by subcloning cDNA for rat Rab27a, rat Rab38, or human Rab32 into pEGFP (CLONTECH Laboratories, Inc.), using standard cloning techniques. pEGFP-Rab38G19V was generated from pEGFP-Rab38 using the QuikChange site-directed mutagenesis system (Stratagene) to introduce a single nucleotide substitution in codon 19 (GGT to GTT). For mRFP-Rab38, Rab38 was amplified from pEGFP-Rab38 by PCR using Pfu polymerase (Stratagene) and was subcloned into pRFP-C (monomeric red fluorescent protein; a gift from R. Tsien, University of California, San Diego, La Jolla, CA).
Transfection of cells with plasmids and oligonucleotides
For the introduction of plasmid constructs, cells were transfected with Fugene 6 (Roche) according to the manufacturer's recommendations. Per 16-mm well, 0.3 μg DNA and 1 μl transfection reagent were used. Cells were analyzed after 48 h. For siRNA oligonucleotides, mouse melanocytes were transfected with Oligofectamine (Invitrogen). Per 16-mm well, 0.625 μl of reagent was used, and the final concentration of siRNA oligos was 100 nM. For most experiments shown, cells were subjected to two rounds of transfection on days 0 and 5, were passaged as required, and were analyzed on day 10 or 11. siRNAs were purchased from Dharmacon. Oligo 32-1 corresponded to nucleotides 261–280 and oligo 32-2 to nucleotides 602–621 of mouse Rab32. As control, a pool of four nontargeting control siRNAs (Dharmacon) was used.
Lentivirus production and infection of melanocytes
The lentivirus vector pHR′SIN-cPPT-SEW (a gift from A. Thrasher, Institute of Child Health, London, UK) expresses the EGFP gene under the control of the spleen focus forming virus U3 promoter/enhancer and carries a modified Woodchuck posttranscriptional regulatory element to improve virus titre, and the central polypurine tract sequence of HIV-1 to aid nuclear entry (Demaison et al., 2002). EGFP-Rab38 was amplified from pEGFP-Rab38 by PCR using Pfu polymerase and was subcloned into this plasmid using standard techniques. Lentivirus particles were produced and titred as previously described (Waddington et al., 2004). Cells were plated in RPMI-1640 with 5% FCS (5 × 104 cells per well) and were infected with virus at an approximate MOI of 50 for MNT-1 cells and MOI of 100 for mouse melanocytes.
Melanin assay
Cells were disrupted by sonication in 50 mM Tris-HCl, pH 7.4, 2 mM EDTA, 150 mM NaCl, 1 mM dithiothreitol, and protease inhibitors. Pigment was pelleted at 20,000 g for 15 min at 4°C, rinsed once in ethanol/ether (1:1), and dissolved in 2 M NaOH/20% dimethylsulfoxide at 60°C. Melanin content was measured as optical density at 492 nm.
IF microscopy
Cells were grown on glass coverslips (coated with polylysine for MNT-1 cells) and transfected with plasmid constructs where indicated. For inhibition of protein synthesis, cycloheximide was added to the growth medium to 100 μg/ml, and cells were incubated for a further 3 h. Cells on coverslips were rinsed in PBS, fixed with 3% PFA in PBS for 30 min, rinsed again, and permeabilized with 0.05% saponin in PBS. Coverslips were blocked for 30 min, incubated with primary antibodies for 1.5 h, washed four times and incubated with Alexa 488– or Alexa 568–conjugated anti–mouse, anti–rabbit or anti–sheep secondary antibodies (1:200; Invitrogen), and washed again. All incubations and wash steps were in PBS/BSA/0.01% saponin. Coverslips were mounted in Mowiol (Calbiochem) mounting medium and viewed on a microscope (DM-IRBE; Leica) with a PL Fluotar 40× 1.0 oil objective. Images were acquired using a confocal system (TCS NT; Leica) and processed with Photoshop (Adobe).
EM
For conventional EM, cells grown on coverslips were fixed with 2.5% glutaraldehyde in 0.1 M cacodylate buffer for 24 h. After several washes with 0.1 M cacodylate buffer, the cells were postfixed with 2% OsO4, dehydrated in ethanol, and embedded in Epon while on the coverslips. Ultrathin sections were prepared and counterstained with uranyl acetate and lead citrate before observation. For immunogold labeling, cells were fixed with 2% PFA or with a mixture of 2% PFA and 0.2% glutaraldehyde in 0.1 M phosphate buffer, pH 7.4. Cells were processed for ultracryomicrotomy as described previously (Raposo et al., 1997). Ultrathin sections were prepared with an Ultracut FCS ultracryomicrotome (Leica), and single- or double-immunogold labeled with antibodies and protein A coupled to 5 or 10-nm gold, as indicated in the figure legends. Sections were observed and photographed under an electron microscope (Philips CM120; FEI Company). Digital acquisitions were made with a numeric camera (Keen View; Soft Imaging System).
Acknowledgments
We thank other laboratory members for help and support; Molly Strom for cloning the original Rab38 plasmid; Bill Pavan for contributing the cht mouse strain for derivation of melan-cht cells; Lynn Lamoreux for crossing Rab38cht- and Ink4a-Arf–null mice and providing skin for cultures; Vincent Hearing, Alex Theos, and Mickey Marks for antibodies; Adrian Thrasher for lentivirus plasmids; and Mike Themis, Brian Bigger, and Maxine Holder for help with lentivirus production.
This work was supported by grants from the Biotechnology and Biological Sciences Research Council (C19747) and the Wellcome Trust (075498).
Abbreviations used in this paper: AP-3, adaptor protein 3; BLOC, biogenesis of lysosome-related organelles complex; HPS, Hermansky-Pudlak syndrome; IF, immunofluorescence; LRO, lysosome-related organelle; Tyrp1, tyrosinase-related protein 1.
References
- Bao, X., A.E. Faris, E.K. Jang, and R.J. Haslam. 2002. Molecular cloning, bacterial expression and properties of Rab31 and Rab32. Eur. J. Biochem. 269:259–271. [DOI] [PubMed] [Google Scholar]
- Bennett, D.C., and M.L. Lamoreux. 2003. The color loci of mice—a genetic century. Pigment Cell Res. 16:333–344. [DOI] [PubMed] [Google Scholar]
- Cohen-Solal, K.A., R. Sood, Y. Marin, S.M. Crespo-Carbone, D. Sinsimer, J.J. Martino, C. Robbins, I. Makalowska, J. Trent, and S. Chen. 2003. Identification and characterization of mouse Rab32 by mRNA and protein expression analysis. Biochim. Biophys. Acta. 1651:68–75. [DOI] [PubMed] [Google Scholar]
- Demaison, C., K. Parsley, G. Brouns, M. Scherr, K. Battmer, C. Kinnon, M. Grez, and A.J. Thrasher. 2002. High-level transduction and gene expression in hematopoietic repopulating cells using a human immunodeficiency [correction of immunodeficiency] virus type 1-based lentiviral vector containing an internal spleen focus forming virus promoter. Hum. Gene Ther. 13:803–813. [DOI] [PubMed] [Google Scholar]
- Fujikawa, K., A.K. Satoh, S. Kawamura, and K. Ozaki. 2002. Molecular and functional characterization of a unique Rab protein, RABRP1, containing the WDIAGQE sequence in a GTPase motif. Zoolog. Sci. 19:981–993. [DOI] [PubMed] [Google Scholar]
- Gwynn, B., J.A. Martina, J.S. Bonifacino, E.V. Sviderskaya, M.L. Lamoreux, D.C. Bennett, K. Moriyama, M. Huizing, A. Helip-Wooley, W.A. Gahl, et al. 2004. Reduced pigmentation (rp), a mouse model of Hermansky-Pudlak syndrome, encodes a novel component of the BLOC-1 complex. Blood. 104:3181–3189. [DOI] [PubMed] [Google Scholar]
- Hearing, V.J. 2005. Biogenesis of pigment granules: a sensitive way to regulate melanocyte function. J. Dermatol. Sci. 37:3–14. [DOI] [PubMed] [Google Scholar]
- Hermann, G.J., L.K. Schroeder, C.A. Hieb, A.M. Kershner, B.M. Rabbitts, P. Fonarev, B.D. Grant, and J.R. Priess. 2005. Genetic analysis of lysosomal trafficking in Caenorhabditis elegans. Mol. Biol. Cell. 16:3273–3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizing, M., R. Sarangarajan, E. Strovel, Y. Zhao, W.A. Gahl, and R.E. Boissy. 2001. AP-3 mediates tyrosinase but not TRP-1 trafficking in human melanocytes. Mol. Biol. Cell. 12:2075–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume, A.N., L.M. Collinson, A. Rapak, A.Q. Gomes, C.R. Hopkins, and M.C. Seabra. 2001. Rab27a regulates the peripheral distribution of melanosomes in melanocytes. J. Cell Biol. 152:795–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume, A.N., L.M. Collinson, C.R. Hopkins, M. Strom, D.C. Barral, G. Bossi, G.M. Griffiths, and M.C. Seabra. 2002. The leaden gene product is required with Rab27a to recruit myosin Va to melanosomes in melanocytes. Traffic. 3:193–202. [DOI] [PubMed] [Google Scholar]
- Jager, D., E. Stockert, E. Jager, A.O. Gure, M.J. Scanlan, A. Knuth, L.J. Old, and Y.T. Chen. 2000. Serological cloning of a melanocyte rab guanosine 5′-triphosphate-binding protein and a chromosome condensation protein from a melanoma complementary DNA library. Cancer Res. 60:3584–3591. [PubMed] [Google Scholar]
- Li, G., and Z. Liang. 2001. Phosphate-binding loop and Rab GTPase function: mutations at Ser29 and Ala30 of Rab5 lead to loss-of-function as well as gain-of-function phenotype. Biochem. J. 355:681–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, W., M.E. Rusiniak, S. Chintala, R. Gautam, E.K. Novak, and R.T. Swank. 2004. Murine Hermansky–Pudlak syndrome genes: regulators of lysosome-related organelles. Bioessays. 26:616–628. [DOI] [PubMed] [Google Scholar]
- Loftus, S.K., D.M. Larson, L.L. Baxter, A. Antonellis, Y. Chen, X. Wu, Y. Jiang, M. Bittner, J.A. Hammer III, and W.J. Pavan. 2002. Mutation of melanosome protein RAB38 in chocolate mice. Proc. Natl. Acad. Sci. USA. 99:4471–4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, J., H. Plesken, J.E. Treisman, I. Edelman-Novemsky, and M. Ren. 2004. Lightoid and Claret: A rab GTPase and its putative guanine nucleotide exchange factor in biogenesis of Drosophila eye pigment granules. Proc. Natl. Acad. Sci. USA. 101:11652–11657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks, M.S., and M.C. Seabra. 2001. The melanosome: membrane dynamics in black and white. Nat. Rev. Mol. Cell Biol. 2:738–748. [DOI] [PubMed] [Google Scholar]
- Munro, S. 2002. Organelle identity and the targeting of peripheral membrane proteins. Curr. Opin. Cell Biol. 14:506–514. [DOI] [PubMed] [Google Scholar]
- Nguyen, T., and M.L. Wei. 2004. Characterization of melanosomes in murine Hermansky-Pudlak syndrome: mechanisms of hypopigmentation. J. Invest. Dermatol. 122:452–460. [DOI] [PubMed] [Google Scholar]
- Nguyen, T., E.K. Novak, M. Kermani, J. Fluhr, L.L. Peters, R.T. Swank, and M.L. Wei. 2002. Melanosome morphologies in murine models of Hermansky-Pudlak syndrome reflect blocks in organelle development. J. Invest. Dermatol. 119:1156–1164. [DOI] [PubMed] [Google Scholar]
- Nielsen, E., F. Severin, J.M. Backer, A.A. Hyman, and M. Zerial. 1999. Rab5 regulates motility of early endosomes on microtubules. Nat. Cell Biol. 1:376–382. [DOI] [PubMed] [Google Scholar]
- Osanai, K., M. Iguchi, K. Takahashi, Y. Nambu, T. Sakuma, H. Toga, N. Ohya, H. Shimizu, J.H. Fisher, and D.R. Voelker. 2001. Expression and localization of a novel Rab small G protein (Rab38) in the rat lung. Am. J. Pathol. 158:1665–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peranen, J., P. Auvinen, H. Virta, R. Wepf, and K. Simons. 1996. Rab8 promotes polarized membrane transport through reorganization of actin and microtubules in fibroblasts. J. Cell Biol. 135:153–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira-Leal, J.B., and M.C. Seabra. 2001. Evolution of the Rab family of small GTP-binding proteins. J. Mol. Biol. 313:889–901. [DOI] [PubMed] [Google Scholar]
- Pfeffer, S.R. 2001. Rab GTPases: specifying and deciphering organelle identity and function. Trends Cell Biol. 11:487–491. [DOI] [PubMed] [Google Scholar]
- Pietro, S.M.D., and E.C. Dell'Angelica. 2005. The cell biology of Hermansky–Pudlak syndrome: recent advances. Traffic. 6:525–533. [DOI] [PubMed] [Google Scholar]
- Raposo, G., and M.S. Marks. 2002. The dark side of lysosome-related organelles: specialization of the endocytic pathway for melanosome biogenesis. Traffic. 3:237–248. [DOI] [PubMed] [Google Scholar]
- Raposo, G., M.J. Kleijmeer, G. Posthuma, J.W. Slot, and H.J. Geuze. 1997. Immunogold labeling of ultrathin cryosections: application in immunology. In Handbook of Experimental Immunology. Vol. 4. L.A. Herzenberg and C. Blackwell, editors. Blackwell Science, Inc., Cambridge, MA. 1–11.
- Raposo, G., D. Tenza, D.M. Murphy, J.F. Berson, and M.S. Marks. 2001. Distinct protein sorting and localization to premelanosomes, melanosomes, and lysosomes in pigmented melanocytic cells. J. Cell Biol. 152:809–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond, B., M. Huizing, J. Knapp, A. Koshoffer, Y. Zhao, W.A. Gahl, and R.E. Boissy. 2005. Melanocytes derived from patients with Hermansky-Pudlak syndrome types 1, 2, and 3 have distinct defects in cargo trafficking. J. Invest. Dermatol. 124:420–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seabra, M.C., and E. Coudrier. 2004. Rab GTPases and myosin motors in organelle motility. Traffic. 5:393–399. [DOI] [PubMed] [Google Scholar]
- Seabra, M.C., and C. Wasmeier. 2004. Controlling the location and activation of Rab GTPases. Curr. Opin. Cell Biol. 16:451–457. [DOI] [PubMed] [Google Scholar]
- Seabra, M.C., Y.K. Ho, and J.S. Anant. 1995. Deficient geranylgeranylation of Ram/Rab27 in choroideremia. J. Biol. Chem. 270:24420–24427. [DOI] [PubMed] [Google Scholar]
- Segev, N. 2001. Ypt and Rab GTPases: insight into functions through novel interactions. Curr. Opin. Cell Biol. 13:500–511. [DOI] [PubMed] [Google Scholar]
- Stinchcombe, J., G. Bossi, and G.M. Griffiths. 2004. Linking albinism and immunity: the secrets of secretory lysosomes. Science. 305:55–59. [DOI] [PubMed] [Google Scholar]
- Suzuki, T., Y. Miyamura, K. Inagaki, and Y. Tomita. 2003. Characterization of the human RAB38 and RAB7 genes: exclusion of new major pathological loci for Japanese OCA. J. Dermatol. Sci. 32:131–136. [DOI] [PubMed] [Google Scholar]
- Sviderskaya, E.V., S.P. Hill, T.J. Evans-Whipp, L. Chin, S.J. Orlow, D.J. Easty, S.C. Cheong, D. Beach, R.A. DePinho, and D.C. Bennett. 2002. p16(Ink4a) in melanocyte senescence and differentiation. J. Natl. Cancer Inst. 94:446–454. [DOI] [PubMed] [Google Scholar]
- Theos, A.C., D. Tenza, J.A. Martina, I. Hurbain, A.A. Peden, E.V. Sviderskaya, A. Stewart, M.S. Robinson, D.C. Bennett, D.F. Cutler, et al. 2005. a. Functions of adaptor protein (AP)-3 and AP-1 in tyrosinase sorting from endosomes to melanosomes. Mol. Biol. Cell. 16:5356–5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theos, A.C., S.T. Truschel, G. Raposo, and M.S. Marks. 2005. b. The Silver locus product Pmel17/gp100/Silv/ME20: controversial in name and in function. Pigment Cell Res. 18:322–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valencia, J.C., H. Watabe, A. Chi, F. Rouzaud, K.G. Chen, W.D. Vieira, K. Takahashi, Y. Yamaguchi, W. Berens, K. Nagashima, et al. 2006. Sorting of Pmel17 to melanosomes through the plasma membrane by AP1 and AP2: evidence for the polarized nature of melanocytes. J. Cell Sci. 119:1080–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddington, S.N., M.S. Nivsarkar, A.R. Mistry, S.M. Buckley, G. Kemball-Cook, K.L. Mosley, K. Mitrophanous, P. Radcliffe, M.V. Holder, M. Brittan, et al. 2004. Permanent phenotypic correction of hemophilia B in immunocompetent mice by prenatal gene therapy. Blood. 104:2714–2721. [DOI] [PubMed] [Google Scholar]
- Watabe, H., J.C. Valencia, K. Yasumoto, T. Kushimoto, H. Ando, J. Muller, W.D. Vieira, M. Mizoguchi, E. Appella, and V.J. Hearing. 2004. Regulation of tyrosinase processing and trafficking by organellar pH and by proteasome activity. J. Biol. Chem. 279:7971–7981. [DOI] [PubMed] [Google Scholar]
- Wei, M.L. 2006. Hermansky-Pudlak syndrome: a disease of protein trafficking and organelle function. Pigment Cell Res. 19:19–42. [DOI] [PubMed] [Google Scholar]
- Zerial, M., and H. McBride. 2001. Rab proteins as membrane organizers. Nat. Rev. Mol. Cell Biol. 2:107–117. [DOI] [PubMed] [Google Scholar]