Abstract
MyoD mRNA is expressed in a subpopulation of cells within the embryonic epiblast. Most of these cells are incorporated into somites and synthesize Noggin. Ablation of MyoD-positive cells in the epiblast subsequently results in the herniation of organs through the ventral body wall, a decrease in the expression of Noggin, MyoD, Myf5, and myosin in the somites and limbs, and an increase in Pax-3–positive myogenic precursors. The addition of Noggin lateral to the somites compensates for the loss of MyoD-positive epiblast cells. Skeletal muscle stem cells that arise in the epiblast are utilized in the somites to promote muscle differentiation by serving as a source of Noggin.
Introduction
Skeletal muscle differentiation begins in the embryonic somites. Soon after their separation from the presomitic mesoderm, somites become partitioned into the dermomyotome and sclerotome (Christ and Ordahl, 1995; Stockdale et al., 2000; Pownall et al., 2002). Sclerotome cells form the cartilages of the vertebral bodies and ribs. The dermomyotome gives rise to the differentiated skeletal muscle of the myotome and the connective tissues of the dermatome. The dorsomedial region of the dermomyotome is the site of early expression of the skeletal muscle–specific transcription factors MyoD and Myf5 (Sassoon et al. 1989; Ott et al., 1991; Pownall and Emerson 1992) and is a source of cells for the myotome (Christ et al., 1978; Ordahl et al., 2000; Kalcheim and Ben-Yair, 2005).
Skeletal muscle differentiation in the somites is promoted by members of the Wnt family released from the neural tube and overlying ectoderm and by Sonic Hedgehog produced in the notochord (Stern et al., 1995; Munsterberg et al., 1995; Fan et al., 1997; Borycki et al., 1998, 1999; Tajbakhsh et al., 1998; Wagner et al., 2000). Myogenesis is also regulated by Noggin and Wnt5b that are synthesized within the segmental plate and somites (Pourquie et al., 1996; Hirsinger et al., 1997; Marcelle et al., 1997; Reshef et al., 1998; Tonegawa and Takahashi, 1998; Amthor et al., 1999; Sela-Donenfield and Kalcheim, 2002; Linker et al., 2003). Noggin promotes myogenesis by inhibiting bone morphogenetic proteins (BMPs) diffusing from the lateral plate mesoderm (Zimmerman et al., 1996; Pourquie et al., 1996; Hirsinger et al., 1997; Marcelle et al., 1997; Dietrich et al., 1998; Reshef et al., 1998; Tonegawa and Takahashi, 1998; Amthor et al., 1999).
Although inductive molecules are required for the up-regulation of MyoD and Myf5 in the somite and the onset of skeletal muscle differentiation (Pownall et al., 2002), both transcription factors are weakly expressed in the presomitic mesoderm (George-Weinstein et al. 1996; Gerhart et al., 2000; Hirsinger et al., 2001; Kiefer and Hauschka, 2001). Cells expressing MyoD mRNA are also present in the epiblast of the chick embryo (George-Weinstein et al., 1996; Gerhart et al., 2000; Strony et al., 2005). The epiblast gives rise to all tissues of the embryo (Fontaine and Le Douarin, 1977; Bellairs, 1986; Stern and Canning, 1990) and is a source for embryonic stem cell lines (Smith, 2001). When MyoD-positive (MyoDpos) cells are isolated from the epiblast and placed in culture, nearly all differentiate into skeletal muscle (Gerhart et al., 2004a). This population recruits pluripotent epiblast cells to the skeletal muscle lineage in vitro by releasing an inhibitor of the BMP signaling pathway (Gerhart et al., 2004a). In this study, we examined the role that MyoD-expressing epiblast cells play in regulating myogenesis in vivo.
Results
Expression of Noggin by MyoDpos epiblast cells
Given that MyoD-expressing epiblast cells produce an inhibitor of the BMP signaling pathway in vitro, and that Noggin is important for muscle differentiation in vivo, we hypothesized that MyoDpos cells would be incorporated into the somites and produce Noggin. To test this hypothesis, we examined the sites of incorporation of MyoDpos epiblast cells in the developing chick embryo and determined whether they expressed Noggin. MyoDpos cells were tracked in the embryo by tagging them with the G8 mAb. G8 recognizes a surface antigen specifically expressed in cells that express MyoD mRNA in the epiblast and fetal organs (Gerhart et al., 2001, 2004a; Strony et al., 2005).
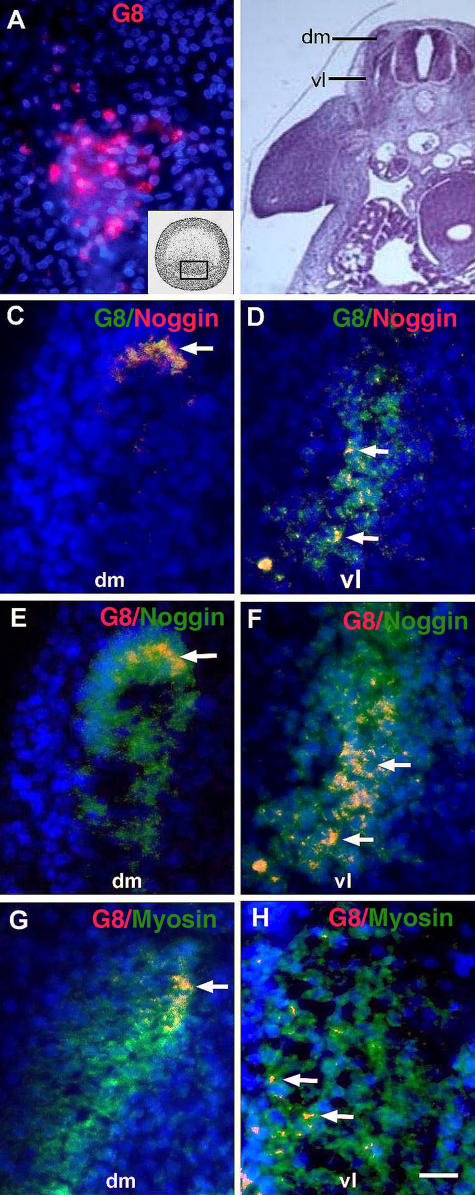
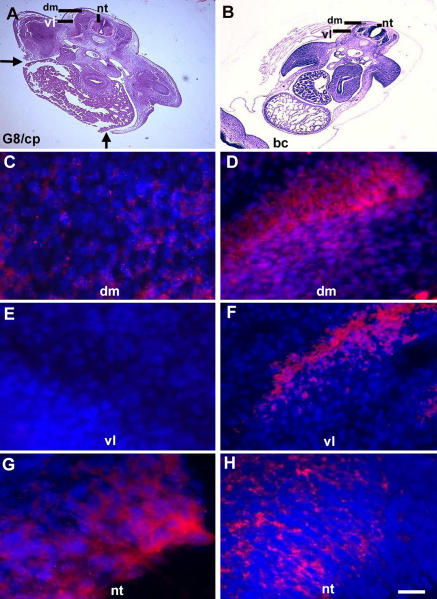
Most cells labeled with the G8 mAb in the epiblast (Fig. 1 A) were later found in the somites (Fig. 1, C–H). G8-positive (G8pos) cells were concentrated in the dorsomedial and ventrolateral regions of the dermomyotome and myotome (Fig. 1, C–F), and some expressed sarcomeric myosin, which is a marker for differentiation (Fig. 1, G and H).
Figure 1.
Expression of Noggin and myosin in MyoDpos cells originating in the epiblast. MyoDpos cells labeled with the G8 mAb were present in the posterior region of the stage 2 epiblast (red cells in A). 4–5 d after labeling with G8, stage 25 embryos were examined for expression of Noggin and sarcomeric myosin. Regions indicated on the right side of the embryo in the hematoxylin and eosin–stained section are shown at higher magnification in fluorescence photomicrographs of merged images of G8 mAb (labeled with Alexa Fluor 488 [green in C and D] and rhodamine [red in E–H]) and either Cy3/red-labeled dendrimers to Noggin mRNA (C and D) or Alexa Fluor 488/green–labeled antibodies to Noggin (E and F) or myosin (G and H). Nuclei were stained with Hoechst dye (blue). Double-labeled cells (overlay of red and green) appear yellow (arrows). (C–F) G8pos/ Nogginpos cells were observed in the dorsomedial (dm) and ventrolateral (vl) dermomyotome and myotome. (G and H) G8pos/myosinpos cells were present in the myotome. Bar: (A and C–H) 9 μm; (B) 135 μm.
The majority of cells that had been prelabled with G8 in the stage 2 embryo expressed Noggin mRNA and protein in the somites, and most cells expressing Noggin mRNA were labeled with G8 (Fig. 1, C–F). Labeling for Noggin protein was more extensive than the distribution of G8pos cells (Fig. 1, E and F), most likely a reflection of diffusion. A few G8pos/Noggin-positive (Nogginpos) cells were also found in the mesenchyme of the head, neural tube, and eyes (Fig. 2, B, D, and E). The neural tube contained cells that expressed Noggin, but not G8 (Fig. 2 B). This pattern of Noggin expression is similar to that reported by Reshef et al. (1998). Our double-labeling experiments suggest that cells that express MyoD mRNA in the epiblast become a primary source of Noggin in the somite.
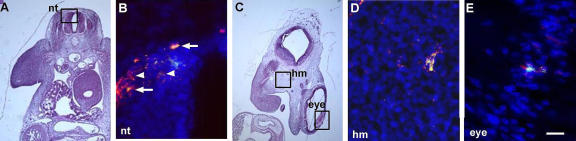
Figure 2.
Expression of Noggin in G8pos epiblast cells incorporated into the head and neural tube. (A and C) Areas outlined in hematoxylin and eosin–stained sections are shown at higher magnification in fluorescence photomicrographs that are the merged images of Hoechst-stained nuclei, Alexa Fluor 488–labeled G8 mAb, and Cy3-labeled dendrimers to Noggin mRNA. Double-labeled cells (overlay of red and green) appear yellow. G8pos cells expressed Noggin mRNA in the neural tube (nt; B, arrows), head mesenchyme (hm; D), and eye (E) of the stage 25 embryo. G8neg/Nogginpos cells were present in the neural tube (arrowheads). Bar: (A and C) 135 μm; (B, D, and E) 9 μm.
Effects of ablating MyoDpos cells in the epiblast on morphogenesis
To determine if MyoDpos epiblast cells are critical for skeletal myogenesis, they were ablated in the embryo by labeling them with the G8 mAb, followed by lysis with complement. Approximately 70 cells (∼0.4% of the total epiblast cells) were ablated in the posterior epiblast of the stage 2 embryo (Fig. 3 A), which is where MyoD/G8-expressing cells are located (Fig. 1 A). Embryos treated with complement (Fig. 3 B) or G8 alone (not depicted) had only a few dead cells throughout the entire epiblast. The specificity of G8/complement treatment was demonstrated by incubating embryos with the E12 mAb and complement. E12 labels a subpopulation of epiblast cells that expresses the neurogenic transcription factor NeuroM, but not MyoD (Strony et al., 2005). Cells lysed with E12 and complement were located in the central/anterior and posterior regions of the epiblast (Fig. 3, C and D).
Figure 3.
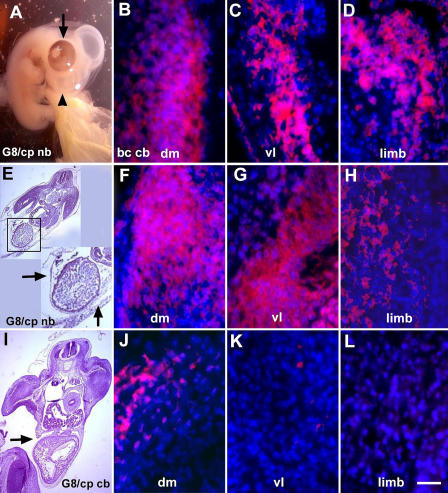
Morphological effects of ablating MyoDpos/G8pos cells in the epiblast. Stage 2 embryos were treated with the G8 or E12 mAb and complement (G8/cp and E12/cp), or complement (cp) or Hanks' buffer (bc) only. Embryos were incubated in trypan blue to reveal dead cells (blue cells and arrows in A–D). Other embryos were grown for 5 (E–G) or 7 d (H; stages 26 and 30, respectively). Sections were stained with hematoxylin and eosin (I–L). The ventral body wall was discontinuous (E, H, and I, arrows) and the dermatome (J–L, arrows) and dermomyotome/myotome (dm; J, double-headed arrow; K and L, diamond) were thickened in G8/complement-treated embryos (J) compared with embryos treated with buffer alone (K) or E12 and complement (L). G8/complement-treated embryos also exhibited malformations of the eye (F and H, black arrowheads) and facial prominences (E, white arrowhead). The notochord (nc) and neural tube (nt) appeared normal in G8/complement-treated embryos (J). The neural tube was kinked in E12/complement-treated embryos (L). Bar: (A–D and J–L) 56 μm; (I) 135 μm.
Morphogenesis appeared to progress normally for the first 2 d after ablation of MyoDpos epiblast cells with G8 and complement (unpublished data). Similar numbers of somites formed in treated and control embryos. However, differences were observed as development progressed. Whereas the ventral body wall was closed in 5-d control embryos, it remained open in G8/complement-treated embryos (Fig. 3, E, H, and I). Embryos died between the fifth and seventh day after elimination of MyoDpos cells in the epiblast, and all had herniations of the heart and abdominal organs through the ventral body wall. Treated embryos also exhibited malformations of both eyes, or more commonly, the right eye only (Fig. 3, E and H), and twisting of the neural tube at the cervical or sacral level (not depicted). In some embryos, abnormalities were observed in the facial prominences (Fig. 3 E). None of the control embryos had ventral body wall, eye, or facial defects (Fig. 3, F and G). The malformations resulting from ablation of MyoDpos cells in the epiblast are consistent with their locations in older embryos, as determined by cell-tracking experiments (Fig. 1).
Histological analyses of embryos treated with G8 and complement revealed that the somites partitioned into the dermomyotome, myotome, and sclerotome. However, abnormalities were observed in the morphology of the dermomyotome and myotome, ranging from an enlargement of the dorsomedial and ventrolateral regions (not depicted) to a thickening along the entire length (Fig. 3 J) compared with controls (Fig. 3, K and L). The boundary between the dermomyotome and myotome in treated embryos was less discrete than that of control embryos. These morphological differences were paralleled by an increase in cell number in the dermomyotome and myotome-like structure of G8/complement-treated embryos (Table I). The dermatomes of treated embryos were also expanded, whereas the sclerotomes of control embryos contained more cells than those of treated embryos (Fig. 3 J; Table I). The notochord (Fig. 3 J) and cartilage rudiments of the limbs (not depicted) appeared similar in treated and control embryos. The neural tube was properly positioned in ablated embryos (Fig. 3, I and K), except for twisting at cranial or sacral levels (not depicted). In contrast, the somites of embryos treated with the E12 mAb and complement were similar to other control embryos, except that the neural tube was kinked along its length (Fig. 3 L). These experiments demonstrate that ablating separate subpopulations of cells in the epiblast results in distinctly different malformations.
Table I.
Number of cells in somite compartments of G8/complement-treated and control embryos
| G8/complement | Dermatome | Dermomyotome | Myotome | Sclerotome |
|---|---|---|---|---|
| right | 309 ± 115 | 192 ± 46 | 828 ± 370 | 228 ± 67 |
| left | 289 ± 110 | 178 ± 42 | 704 ± 221 | 271 ± 55 |
| Buffer | ||||
| right | 169 ± 34 | 144 ± 22 | 246 ± 47 | 503 ± 61 |
| left | 144 ± 26 | 138 ± 9 | 246 ± 60 | 516 ± 107 |
Embryos were incubated with the G8 mAb and complement or Hanks' buffer at stage 2 and fixed 4.5 d after treatment. The number of nuclei in each compartment of the somite was determined by microscopy using the Image-Pro Plus image analysis software. Values are the mean ± the SD of 20 and 24 transverse sections through the wing levels of two treated and two control embryos, respectively. More cells were present in the dermatome, dermomyotome, and myotome-like regions of embryos treated with G8 and complement than embryos incubated with buffer alone (P ≤ 0.0005). The reverse was found for the sclerotome region of the somite (P ≤ 0.0005).
Effects of ablating MyoDpos cells in the epiblast on myogenesis
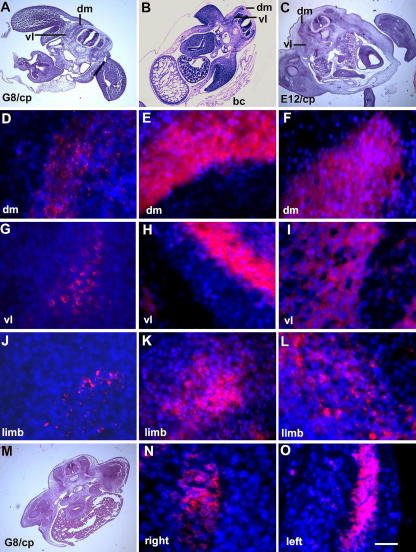
The effect of ablating G8pos/MyoDpos cells in the epiblast on skeletal muscle differentiation was examined by staining for sarcomeric myosin. Whereas control embryos contained an abundance of muscle in the dorsomedial and ventrolateral myotomes and limbs, G8/complement-treated embryos had reduced myosin staining in the dorsomedial myotome, and severely diminished or no detectable myosin in the ventrolateral myotome (Fig. 4, D–I; Table II). Differentiated skeletal muscle was also decreased in the limbs (Fig. 4, J–L). In all G8/complement-treated embryos, differentiation was affected more severely on the right side of the embryo than the left (Fig. 4, N and O; Table II).
Figure 4.
Effect of ablating MyoDpos/G8pos epiblast cells on skeletal muscle differentiation in the somites and limbs. Stage 2 G8pos epiblast cells were lysed with complement (G8/cp). Controls included embryos treated with E12 and complement (E12/cp) or Hanks' buffer only (bc). Embryos were incubated for 4.5–5 d (stages 25–26). Regions on the right side of the embryo in hematoxylin and eosin–stained sections (A–C) are shown at higher magnification in fluorescence photomicrographs of merged images of Hoechst-stained nuclei and rhodamine-labeled antibodies to sarcomeric myosin. Less myosin was detected in the dorsomedial (dm) and ventrolateral (vl) portions of the myotome and limb of G8/complement-treated embryos (D, G, and J) than in those treated with buffer (E, H, and K) or E12 mAb/complement (F, I, and L). (M) A hematoxylin and eosin–stained section shown at higher magnification in N and O. Myosin expression was affected more severely on the right side of the embryo than on the left. Bar: (A–C and M) 135 μm; (N and O) 9 μm in D–L.
Table II.
Number of differentiated skeletal muscle cells in the myotomes of G8/complement-treated and control embryos
| G8/complement | Number of myosin-positive cells |
|---|---|
| right (n = 12) | 18 ± 13 |
| left (n = 11) | 32 ± 5 |
| Buffer | |
| right (n = 16) | 95 ± 9 |
| left (n = 17) | 94 ± 11 |
Embryos were incubated with the G8 mAb and complement or Hanks' buffer at stage 2 and fixed 4.5 d after treatment. Transverse sections were stained with the MF20 mAb to sarcomeric myosin. The number of nuclei in the myotome-like structure of two G8/complement-treated embryos and the myotome of two buffer-treated embryos in sections through the wing level was determined by microscopy using the Image-Pro Plus image analysis software. Values are the mean ± the SD of the number of sections (n) indicated in parentheses. Fewer myosin-positive cells were present in G8/complement-treated than control embryos (P ≤ 0.0005). More myosin-positive cells were present in the myotome-like structure on the left side of G8/complement treated embryos than the right (P ≤ 0.005).
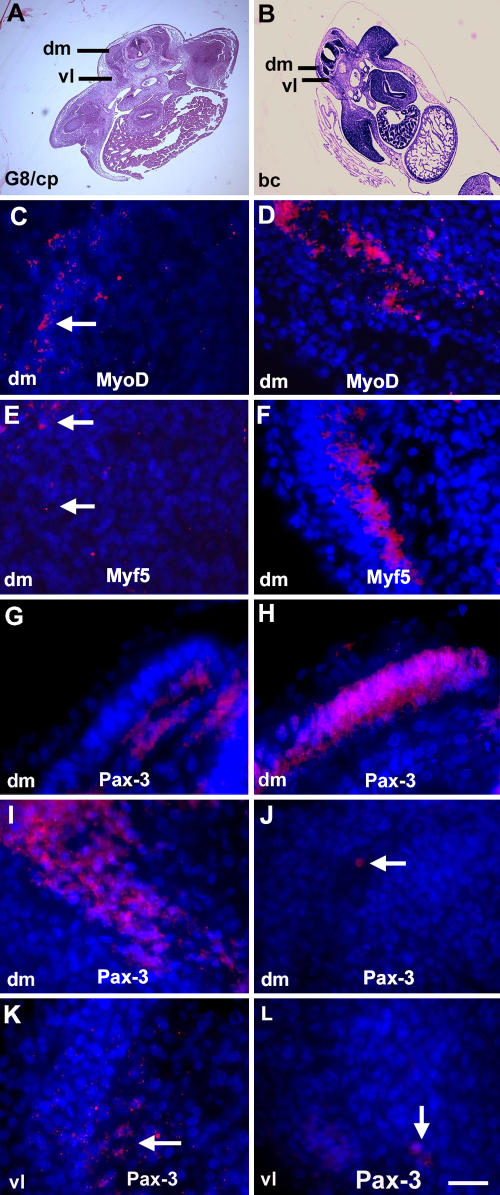
The effect of ablating G8pos/MyoDpos epiblast cells on muscle differentiation was accompanied by a decrease in MyoD and Myf5 mRNAs in the 5-d embryo (Fig. 5, C–F). At this time, few cells in control embryos expressed the marker for myogenic precursor cells Pax-3 (Stockdale et al., 2000) in the dermomyotome; however, there was an abundance of Pax3-positive cells in the dermomyotome of treated embryos (Fig. 5, I–L). In contrast, in the 2.5-d embryo, more Pax-3–positive cells were present in the somites of control embryos than those treated with G8 and complement (G and H).
Figure 5.
Effect of ablating MyoDpos/G8pos epiblast cells on MyoD, Myf5, and Pax-3 expression. Stage 2 embryos were treated with the G8 mAb and complement (G8/cp) or Hanks' buffer (bc) and grown for 2 d (stage 14; G and H) or 4.5 d (stage 25; C–F and I–L). Areas outlined in hematoxylin and eosin–stained sections (A and B) are shown at higher magnification in fluorescence photomicrographs that are the merged images of Hoechst-stained nuclei and Cy3-labeled dendrimers to MyoD or Myf5 mRNA, or rhodamine-labeled antibody to Pax-3. Less MyoD and Myf5 mRNAs were detected in the somites of G8/complement-treated embryos (C and E) than in embryos treated with buffer only (D and F). More Pax-3–positive cells were present in stage 14 control embryos than treated embryos (G and H), whereas the reverse was found in dorsomedial (dm) and ventrolateral (vl) dermomyotomes/myotomes of stage 25 embryos (I–L). Bar: (A and B) 135 μm; (C–L) 9 μm.
Ablation of MyoDpos epiblast cells also resulted in a decreased expression of Noggin in the somites compared with control embryos (Fig. 6, C–F). The reduction in Noggin was more pronounced on the right side of the embryo than on the left (not depicted). In contrast, Noggin expression in the neural tube appeared increased in treated embryos compared with controls (Fig. 6, G and H).
Figure 6.
Effect of ablating G8pos/MyoDpos epiblast cells on Noggin expression. Noggin expression was analyzed 4.5 d after ablating stage 2 MyoDpos epiblast cells. Regions indicated in hematoxylin and eosin–stained sections (A and B) are shown in fluorescence photomicrographs of merged images of Hoechst-stained nuclei and Cy3-labeled dendrimers to Noggin mRNA (C–H). Less Noggin mRNA was detected in the dorsomedial (dm) and ventrolateral (vl) dermomyotome and myotome in G8/complement-treated embryos (G8/cp) (C and E) than in buffer-treated embryos (bc; D and F). (G and H) The reverse was found for Noggin expression in the neural tube (nt). Bar: (A and B) 135 μm; (C–H) 9 μm.
Overall, these results complement those of published studies. First, an inverse relationship was found between expression of MyoD/Myf5 and Pax-3 in the somite (Goulding et al., 1994; Williams and Ordahl, 1994; Marcelle et al., 1995). Second, inhibition of MyoD and Myf5 expression in Pax-3-positive cells occurs in response to BMP signaling (Reshef et al., 1998). Third, Pax-3–positive cells are present in Noggin-null mice (McMahon et al., 1998).
Addition of exogenous Noggin to ablated embryos
To test whether exogenous Noggin could compensate for the loss of MyoDpos epiblast cells, Noggin-soaked beads were implanted into embryos 2 d after ablating G8pos/MyoDpos cells in the epiblast. 4 d later, the aforementioned gross malformations of the ventral body wall, neural tube, and facial prominences were not observed (Fig. 7, A and E). The eyes had normal pigmentation after Noggin supplementation, although in some embryos the right eye remained smaller than the left (Fig. 7 A).
Figure 7.
Effect of exogenous Noggin on muscle differentiation in G8/complement-treated embryos. Noggin-soaked beads were implanted lateral to the somites 2 d after ablating MyoDpos/G8pos cells in the stage 2 epiblast. Exogenous Noggin prevented body wall herniations (arrows in inset of E) and facial defects (arrowhead in A), and promoted normal eye pigmentation (arrow in A) and myosin synthesis in the myotome (dm/vl) and limb (F–H). The body wall remained incomplete (arrow in I) and myosin staining was reduced (J–L) in G8/complement-treated embryos implanted with PBS-soaked beads. Staining for myosin in buffer-treated embryos implanted with PBS-soaked beads is shown in B–D. Bar: (A, E, and I) 135 μm; (B–D, F–H, and J–L) 9 μm.
The amount of myosin staining in the ventrolateral and dorsolateral myotomes of G8/complement-treated embryos supplemented with Noggin appeared similar to, or increased, compared with that of buffer-treated embryos implanted with control beads (Fig. 7, B, C, F, and G). Enhanced myogenesis in the myotomes may reflect the accumulation of Pax-3– positive cells before the addition of Noggin. Exogenous Noggin also promoted muscle differentiation in the limbs of ablated embryos, but not to the level of that observed in control embryos (Fig. 7, D and H). These results demonstrate that Noggin can, to a significant extent, replace the ablated MyoDpos epiblast cells to promote both skeletal myogenesis and normal morphogenesis.
Discussion
The purpose of this study was to determine whether cells that express MyoD mRNA in the epiblast play a role in regulating skeletal myogenesis in the somites. Initially, these cells were found in the posterior epiblast of the two-layered embryo (Gerhart et al., 2000; Strony et al., 2005). A few hours later, MyoDpos cells were present within and lateral to the primitive streak (Gerhart et al., 2000; Strony et al., 2005), in a pattern similar to that revealed by fate map analyses of prospective paraxial mesoderm cells (Rosenquist, 1966; Bortier and Vakaet, 1992; Hatada and Stern, 1994; Nicolas et al., 1996; Jouve et al., 2002). As expected, most MyoDpos epiblast cells were incorporated into the somites.
A few MyoDpos cells were observed in areas of the epiblast fated for nonsomitic tissues, and later, in the sclerotome, neural tube, and fetal organs lacking skeletal muscle (Gerhart et al., 2000, 2001; Strony et al., 2005). Cells expressing MyoD and Myf5 outside of skeletal muscle remain undifferentiated (Gerhart et al., 2000, 2001; Tajbakhsh and Buckingham, 1995). This raises the possibility that ectopically placed myogenic precursors, possibly originating in the epiblast, could be a potential source of rhabdomyosarcomas. These malignancies are characterized by the expression of myogenic genes and often arise in structures lacking skeletal muscle (Dagher and Helman, 1999).
MyoD-expressing cells of the epiblast become a major source of Noggin within the somites. Elimination of this population in the epiblast resulted in a reduction of Noggin and skeletal muscle in the myotomes and limbs in older embryos. These results are consistent with previous studies demonstrating that Noggin regulates myogenesis in the somites by inhibiting BMPs (Pourquie et al., 1996; Zimmerman et al., 1996; Hirsinger et al., 1997; Marcelle et al., 1997; Dietrich et al., 1998; Reshef et al., 1998; Tonegawa and Takahashi, 1998; Amthor et al., 1999; Linker et al., 2003). Although some epiblast cells initiated the expression of MyoD and the G8 antigen after stage 2 (see Materials and methods), they were unable to completely compensate for those that were ablated earlier in development.
Ventrolateral hypaxial muscles were more severely affected by ablating MyoDpos epiblast cells than the dorsomedial epaxial muscles. This is opposite to what was observed in Noggin-deficient mice (McMahon et al., 1998); however, in G8/complement-treated chick embryos, there was an alternative source of Noggin in the neural tube in close proximity to the epaxial myotome. The paucity of muscle in the ventral myotome of G8/complement-treated embryos was the most likely cause of herniation of organs through the body wall. Malformations of the eye and facial prominences arising from ablation of MyoDpos epiblast cells may be secondary to a disturbance in the differentiation of facial and extraocular muscles, although it is possible that Noggin produced by MyoDpos epiblast cells affects nonmyogenic cells.
The malformations that arise as a result of ablation of MyoDpos cells in the epiblast resemble those that are present in humans with Axenfeld-Rieger syndrome, an autosomal dominant haploinsufficiency of the Pitx2 gene (Jorgenson et al., 1978; Semina et al., 1996; Lines et al., 2002). Mutations in Pitx2 affect the development of left-sided structures (Logan et al., 1998; Piedra et al., 1998; Ryan et al., 1998; St. Amand et al., 1998; Yoshioka et al., 1998), whereas elimination of MyoDpos cells in the chick epiblast affected myogenesis, eye development, and Noggin expression more severely on the right side of the embryo. Although Pitx2 is involved in left–right asymmetry, it is expressed symmetrically in myogenic cells of the eye, limb, and myotome of the mouse (Campione et al., 1999; Yoshioka et al., 1998, Kitamura et al., 1999). Pitx2-null mice exhibit dysgenesis of the extraocular muscles and thinning of the abdominal wall (Kitamura et al., 1999). Given the similarities between the malformations in the chick embryo arising from ablation of MyoDpos epiblast cells and those that result from perturbation of Pitx2, it is possible that inhibition of BMP signaling by Noggin is involved in Pitx2 expression in the somite during myogenesis. This notion is supported by the fact that BMP2 induces, and Noggin inhibits, the expression of Snail/snr which represses Pitx2 expression (Isaac et al., 1997; Patel et al., 1999; Piedra and Ros, 2002; Schlange et al., 2002).
One possible explanation for the asymmetric effects of ablating G8/MyoDpos cells in the stage 2 embryo is that cells initiating expression of MyoD in the epiblast after treatment are preferentially incorporated into the left side of the embryo. It also is possible that MyoDpos epiblast cells are involved in signaling before their incorporation into the somites. This notion is consistent with our finding more MyoDpos cells on the right side of Hensen's node (Gerhart et al., 2000), which is a structure located at the rostral end of the primitive streak that is a rich source of signaling molecules regulating laterality in the embryo (Chapman et al., 2002; Raya and Izpisua Belmonte, 2004). The importance of the node in myogenesis was illustrated in the mouse inverted viscerus mutant embryo, in which defects in the flow of molecules across the node reversed the asymmetric expression of α-skeletal actin and myosin light chain 3F in the myotome (Golding et al., 2004). Cell-tracking experiments that follow the pathways of migration of stages 1–3 MyoDpos epiblast cells may shed light on the mechanism whereby muscle differentiation is asymmetrically perturbed after their ablation.
We have identified skeletal muscle stem cells in the epiblast based on their expression of MyoD and demonstrated a critical role for these cells in regulating myogenesis in vitro and in vivo (George-Weinstein et al., 1996; Gerhart et al., 2004a; Strony et al., 2005; this study). Choi et al. (1989) postulated that the early chick embryo contains founder cells for the myogenic lineage based on their observation that skeletal muscle emerges in chick blastoderm cultures. In this context, founder cells were defined as those that give rise to all cells of a given lineage. In contrast, MyoDpos epiblast cells of the stage 2 embryo do not appear to be the sole source of myogenic precursors in the somite. In our cell-tracking experiments, the G8 mAb that had been applied in the stage 2 embryo was not detected in most cells of the myotome. It is possible that asymmetric divisions occurring in the dermomyotome or degradation of the antibody may have reduced the signal in myotome cells. However, cells expressing Pax-3 did emerge in the dermomyotome after ablating MyoDpos cells in the epiblast, and their ability to differentiate was revealed when ablated embryos were supplemented with exogenous Noggin. Therefore, under these experimental conditions, most Pax-3 precursors do not appear to be the direct descendants of MyoDpos epiblast cells.
Muscle founder/pioneer cells also have been defined in the avian embryo as those that are the first to differentiate and enter the myotome (Kahane et al., 1998). In invertebrate embryos, founder/pioneer cells enter the myotome and serve as a scaffold for myoblast fusion (Bate, 1990; Baylies and Michelson, 2001; Dworak and Sink, 2002). Although G8pos/MyoDpos epiblast cells may be involved in both of these processes, their influence clearly extends beyond that of playing a structural role in the myotome. Collectively, the data indicate that the primary function of MyoDpos epiblast cells within the somites is to promote the differentiation of myogenic precursors by releasing Noggin.
Materials and methods
Tracking MyoDpos cells from the epiblast into the somites
White Leghorn chick embryos were obtained from BE Eggs and staged according to the method of Hamburger and Hamilton (1951). Stage 2 embryos were removed from the shell on the yolk and placed in a tissue culture dish. 100 μl of G8 mAb diluted 1:40 in PBS was applied to the embryo for 45 min. After rinsing in PBS, embryos were incubated for 30 min in 100 μl rhodamine-conjugated goat anti–mouse FAb′2 fragments (Jackson ImmunoResearch Laboratories) diluted 1:400 and rinsed. Embryos on the yolk were poured into an empty host shell, covered, and incubated at 37°C for 2–4 d. Embryos were fixed in 4% formaldehyde overnight, embedded in paraffin, sectioned transversely at 10 μm, and applied to gelatin-coated slides. Sections were labeled with an antibody to Noggin (R&D Systems) or processed for expression of Noggin mRNA as described in the following paragraphs. Sections were mounted in Gelmount (Biomeda) and observed with an epifluorescence microscope (Eclipse E800; Nikon) using 4×/0.2 NA and 60×/1.4 NA oil objectives. Photomicrographs were produced with the video camera (Evolution QE; Media Cybernetics) and Image-Pro Plus image analysis software (Phase 3 Imaging Systems). Tracking of G8pos epiblast cells was conducted in three embryos.
To test for the presence of residual unbound G8 mAb after the initial labeling period, we first determined the number of G8pos cells directly after labeling stage 2 embryos. An average of 76 cells in three embryos was labeled with G8. When embryos were labeled with G8 and an Alexa Fluor 488–conjugated secondary antibody, incubated for 3 h at 37°C, and labeled with a rhodamine-conjugated secondary antibody, there was an average of 77 cells labeled with both Alexa Fluor 488 and rhodamine, and no cells were labeled with either fluorochrome alone. A third group of embryos was labeled with G8 and Alexa Fluor 488 secondary antibody, incubated for 3 h, and then exposed to more G8 mAb, followed by the rhodamine secondary antibody. In this case, there was an average of 80 cells with both Alexa Fluor 488 and rhodamine, and 17 cells were labeled with rhodamine alone. These results demonstrate that additional cells are expressing the G8 antigen after the initial labeling period; however, there is insufficient residual G8 mAb present to label those cells. Therefore, unbound G8 mAb does appear to be washed out of the embryo during our labeling procedure, thereby demonstrating the feasibility of tracking MyoDpos cells from the stage 2 epiblast into the mesoderm.
Eliminating MyoDpos cells in the epiblast
Stage 2 embryos were removed from the shell on the yolk, labeled with the G8 mAb, and rinsed as described in the previous section. 100 μl of baby rabbit complement (Cedar Lane, Inc.) diluted 1:40 in Hanks' buffered saline containing 0.1% BSA was applied to the embryo for 30 min at room temperature. Control embryos received Hanks' buffer with BSA, G8 mAb, or complement alone. An additional control involved incubating embryos in the E12 mAb that labels a subpopulation of cells expressing NeuroM mRNA in the epiblast (Strony et al., 2005), followed by the addition of complement. The presence of lysed cells was determined directly after treatment by incubating embryos in 0.2% trypan blue in PBS for 15 min at 37°C and counting the number of blue cells. After treatment, embryos not exposed to trypan blue were poured into an empty shell and incubated for 2–7 d. Embryos were analyzed at the gross level and in 10-μm serial sections after embedding in paraffin. The number of embryos in each treatment group is listed in Table III. Some sections were stained with hematoxylin 2 and eosin-Y (Richard Allan Scientific). Other sections were stained with antibodies or analyzed for mRNA expression by in situ hybridization, as described in In situ hybridization.
Table III.
Numbers of embryos analyzed by gross inspection and tissue sectioning
| Number of Embryos
|
||||||
|---|---|---|---|---|---|---|
| Gross morphology
|
Histology
|
|||||
| 2–3 d | 4–5 d | 7 d | 2–2.5 d | 4–5 d | 7 d | |
| Hanks' buffer | 7 | 7 | 2 | 2 | 3 | 1 |
| G8 mAb | 1 | 6 | 1 | 1 | 1 | |
| E12 mAb | 2 | |||||
| Complement | 3 | 4 | 1 | 1 | ||
| E12 mAb + complement | 5 | 1 | 2 | 1 | ||
| G8 mAb + complement | 9 | 9 | 5 | 2 | 3 | 1 |
| Hanks' Buffer Tx + PBS beads | 3 | 2 | 1 | 2 | ||
| G8/complement Tx + PBS beads | 5 | 2 | 1 | 2 | ||
| G8/complement Tx + Noggin beads | 4 | 6 | 2 | 3 | ||
Embryos were incubated with Hanks' buffer, antibodies or complement alone, or antibodies and complement at stage 2 and fixed for 2–2.5 (stages 14–17), 4–5 (stages 25–26), or 7 d (stage 30) after treatment. Other embryos were treated at stage 2, grown to stages 11–13, and implanted with beads soaked in PBS or Noggin. All embryos were examined morphologically and some embryos were embedded and sectioned (histology). 90% of embryos treated with the G8 mAb and complement survived for 5 d.
Treating embryos with Noggin
MyoDpos cells of the stage 2 epiblast were lysed with G8 and complement and placed in a shell as described in the previous section. Control embryos were incubated in Hanks' buffer only. Embryos were incubated at 37°C to reach stages 11–14. Embryos on the yolk were removed from the shell and placed in a 100-mm tissue culture dish in preparation for addition of Noggin-soaked beads.
1 μl Affigel blue agarose beads (BioRad) was soaked in either 10 μl of a 100-ng human recombinant Noggin (PeproTech) per milliliter of PBS solution or PBS alone (Slavkin et al., 2000). A PBS- or Noggin-soaked 70-μm bead was inserted on the right side of the embryo, lateral to the fifth and sixth rostral somites, 11th and 12th somites, and between the most caudal somite and the rostral end of the presomitic mesoderm. Embryos were placed in 60-ml capacity glass bowls, covered, and cultured at 37°C for 2–4 d. Embryos were analyzed at the gross level and in transverse, 10-μm serial sections. The number of embryos in each treatment group is listed in Table III.
In situ hybridization
Paraffin sections were applied to Teflon-printed, three-well glass slides (Electron Microscopy Sciences) coated with 0.2% gelatin. The in situ hybridization procedure is described in detail in Gerhart et al. (2004b). Messenger RNAs for MyoD, Myf5, and Noggin were detected with DNA dendrimers conjugated with Cy3 and the following antisense oligonucleotide sequences: chicken MyoD, 5′-TTCTCAAGAGCAAATACTCACCATTTGGTGA TTCCGTGTAGTA-3′ (L34006; Dechesne et al., 1994); chicken Noggin, 5′-TCTCGTTAAGATCCTTCTCCTTGGGGTCAAA-3′ (NM_204123; Tonegawa and Takahashi, 1998); and chicken Myf5, 5′-ATATAGTGGATGGCAGAGCTGAGG ATTTCG-3′ (S53719; Neville et al., 1992). Fluorescent dendrimers were obtained from Genisphere, Inc. Nuclei were stained with Hoechst dye. Double labeling with dendrimers and antibodies was performed as previously described (Gerhart et al., 2001, 2004a,b; Strony et al., 2005).
Immunofluorescence localization
Paraffin sections were labeled with the MF20 mAb to sarcomeric myosin heavy chain (Bader et al., 1982) diluted 1:60, a goat anti–mouse polyclonal antiserum to Noggin (R & D Systems) diluted 1:200, or a mAb to Pax-3 (Baker et al., 1999) diluted 1:150. Primary antibodies were labeled with rhodamine-conjugated goat anti–mouse FAb′2 fragments (Jackson ImmunoResearch Laboratories) or fluorescein-conjugated donkey anti–goat IgG (CHEMICON International, Inc.), as previously described (George-Weinstein et al., 1994). The MF20 and Pax-3 mAbs were obtained from the Developmental Studies Hybridoma Bank.
Cell counting
The number of nuclei present in the dermatome, dermomyotome, myotome, and sclerotome of 20–24 sections through the wing level of two embryos treated with the G8 mAb and complement and two embryos incubated with Hanks' buffer was determined in sections stained with Hoechst dye or hematoxylin and eosin using the Image-Pro Plus image analysis software. The accuracy of cell counting via software analysis was validated by comparing cell numbers to those obtained by manually counting cells. The number of myosin-positive cells was determined in 11–17 sections from the wing level of two treated and two control embryos by manually counting MF20-labeled cells via microscopy. Statistically significant differences in populations were determined using the t test.
Acknowledgments
The authors thank Dr. Charles Ordahl and Ms. Christa Smolenski for critically reading the manuscript, and Genisphere, Inc. for generously supplying the dendrimers.
This study was funded by the National Institutes of Health (HD43157 and AR052326 to M. George-Weinstein, and GM61702 and AR052326 to K. Knudsen).
Abbreviation used in this paper: BMP, bone morphogenetic protein.
References
- Amthor, H., B. Christ, and K. Patel. 1999. A molecular mechanism enabling continuous embryonic muscle growth-a balance between proliferation and differentiation. Development. 126:1041–1053. [DOI] [PubMed] [Google Scholar]
- Bader, D., T. Masaki, and D.A. Fischman. 1982. Immunochemical analysis of myosin heavy chain during avian myogenesis in vivo and in vitro. J. Cell Biol. 95:763–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker, C.V.H., M.R. Stark, C. Marcelle, and M. Bronner-Fraser. 1999. Competence, specification and induction of Pax-3 in the trigeminal placode. Development. 126:147–156. [DOI] [PubMed] [Google Scholar]
- Bate, M. 1990. The embryonic development of larval muscles in Drosophila. Development. 110:791–804. [DOI] [PubMed] [Google Scholar]
- Baylies, M.K., and A.M. Michelson. 2001. Invertebrate myogenesis: looking back to the future of muscle development. Curr. Opin. Genet. Dev. 11:431–439. [DOI] [PubMed] [Google Scholar]
- Bellairs, R. 1986. The primitive streak. Anat. Embryol. (Berl.). 174:1–14. [DOI] [PubMed] [Google Scholar]
- Bortier, H., and L.C. Vakaet. 1992. Fate mapping the neural plate and the intraembryonic mesoblast in the upper layer of the chicken blastoderm with xenografting and time-lapse videography. Dev. Suppl. 93–97. [PubMed]
- Borycki, A.G., L. Mendham, and C.P. Emerson. 1998. Control of somite patterning by Sonic hedgehog and its downstream signal response genes. Development. 125:777–790. [DOI] [PubMed] [Google Scholar]
- Borycki, A.G., B. Brunk, S. Tajbakhsh, M. Buckingham, C. Chiang, and C.P. Emerson. 1999. Sonic hedgehog controls epaxial muscle determination through Myf5 activation. Development. 126:4053–4063. [DOI] [PubMed] [Google Scholar]
- Campione, M., H. Steinbeisser, A. Scheickert, K. Deissler, F. van Bebber, L.A. Lowe, S. Nowotschin, C. Viebahn, P. Haffter, M.R. Kuehn, and M. Blum. 1999. The homeobox gene Pitx2: mediator of asymmetric left-right signaling in vertebrate heart and gut looping. Development. 126:1225–1234. [DOI] [PubMed] [Google Scholar]
- Chapman, S.C., F.R. Schubert, G.C. Schoenwolf, and A. Lumsden. 2002. Analysis of spatial and temporal gene expression patters in blastula and gastrula stage chick embryos. Dev. Biol. 245:187–199. [DOI] [PubMed] [Google Scholar]
- Choi, J., T. Schultheiss, M. Lu, F. Wachtler, N. Kuruc, W.W. Franke, D. Bader, D.A. Fischman, and H. Holtzer. 1989. Founder cells for the cardiac and skeletal myogenic lineages. In Cellular and Molecular Biology of Muscle Development. L. Kedes, and F.E. Stockdale, editors. A.R. Liss, New York. 27–36.
- Christ, B., and C.P. Ordahl. 1995. Early stages of chick somite development. Anat. Embryol. (Berl.). 191:381–396. [DOI] [PubMed] [Google Scholar]
- Christ, B., H.J. Jacob, and M. Jacob. 1978. On the formation of myotomes in avian embryos. An experimental and scanning electron microscope study. Experientia. 34:514–516. [Google Scholar]
- Dagher, R., and L. Helman. 1999. Rhabdomyosarcoma: an overview. Oncologist. 4:34–44. [PubMed] [Google Scholar]
- Dechesne, C.A., Q. Wei, J. Eldridge, L. Gannoun-Zaki, P. Millasseau, L. Bougueleret, D. Caterina, and B.M. Paterson. 1994. E-box- and MEF-2- independent muscle-specific expression, positive autoregulation, and cross-activation of the chicken MyoD (CMD1) promoter reveal an indirect regulatory pathway. Mol. Cell. Biol. 14:5474–5486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich, S., F.R. Schubert, C. Healy, P.T. Sharpe, and A. Lumsden. 1998. Specification of the hypaxial musculature. Development. 125:2235–2249. [DOI] [PubMed] [Google Scholar]
- Dworak, H.A., and H. Sink. 2002. Myoblast fusion in Drosophila. Bioessays. 24:591–601. [DOI] [PubMed] [Google Scholar]
- Fan, C.-M., C.S. Lee, and M. Tesslier-Lavigne. 1997. A role for Wnt proteins in induction of dermomyotome. Dev. Biol. 191:160–165. [DOI] [PubMed] [Google Scholar]
- Fontaine, J., and N. Le Douarin. 1977. Analyses of endoderm formation into avian blastoderm by the use of quail-chick chimeras. The problem of the neural ectodermal origin of the cells of the APUD series. J. Embryol. Exp. Morphol. 41:209–222. [PubMed] [Google Scholar]
- George-Weinstein, M., J. Gerhart, F. Foti, and J.W. Lash. 1994. Maturation of myogenic and chondrogenic cells in the presomitic mesoderm of the chick embryo. Exp. Cell Res. 211:263–274. [DOI] [PubMed] [Google Scholar]
- George-Weinstein, M., J. Gerhart, R. Reed, J. Flynn, B. Callihan, M. Mattiacci, C. Miehle, G. Foti, J.W. Lash, and H. Weintraub. 1996. Skeletal myogenesis: The preferred pathway of chick embryo epiblast cells in vitro. Dev. Biol. 173:279–291. [DOI] [PubMed] [Google Scholar]
- Gerhart, J., M. Baytion, S. DeLuca, R. Getts, C. Lopez, R. Niewenhuis, T. Nilsen, S. Olex, H. Weintraub, and M. George-Weinstein. 2000. DNA dendrimers localize MyoD mRNA in presomitic tissues of the chick embryo. J. Cell Biol. 149:825–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhart, J., B. Bast, C. Neely, S. Iem, P. Amegbe, R. Niewenhuis, S. Miklasz, P.F. Cheng, and M. George-Weinstein. 2001. MyoD-positive myoblasts are present in mature fetal organs lacking skeletal muscle. J. Cell Biol. 155:381–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhart, J., C. Neely, B. Stewart, J. Perlman, D. Beckmann, M. Wallon, K. Knudsen, and M. George-Weinstein. 2004. a. Epiblast cells that express MyoD recruit pluripotent cells to the skeletal muscle lineage. J. Cell Biol. 164:739–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhart, J., M. Baytion, J. Perlman, C. Neely, B. Hearon, R. Getts, J. Kadushin, T. Nilsen, and M. George-Weinstein. 2004. b. Visualizing the needle in the haystack: in situ hybridization with fluorescent DNA dendrimers. Biol. Proced. Online. 6:149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding, J., T.A. Partridge, J.R. Beauchamp, T. King, N.A. Brown, M. Gassmann, and P.S. Zammit. 2004. Mouse myotomes pairs exhibit left-right asymmetric expression of MLC3F and α-skeletal actin. Dev. Dyn. 231:795–800. [DOI] [PubMed] [Google Scholar]
- Goulding, M., A. Lumsden, and A.J. Paquette. 1994. Regulation of Pax-3 expression in the dermomyotome and its role in muscle development. Development. 120:957–971. [DOI] [PubMed] [Google Scholar]
- Hamburger, V., and H.L. Hamilton. 1951. A series of normal stages in the development of the chick embryo. J. Morphol. 88:49–92. [PubMed] [Google Scholar]
- Hatada, Y., and C. Stern. 1994. A fate map of the epiblast of the early chick embryo. Development. 120:2879–2889. [DOI] [PubMed] [Google Scholar]
- Hirsinger, E., D. Duprez, C. Jouve, P. Malapert, J. Cooke, and O. Pourquie. 1997. Noggin acts downstream of Wnt and Sonic Hedgehog to antagonize BMP4 in avian somite patterning. Development. 124:4606–4614. [DOI] [PubMed] [Google Scholar]
- Hirsinger, E., P. Malapert, J. Dubrulle, M.C. Delfini, D. Duprez, Henrique, D. Ish-Horowicz, and O. Pourquie. 2001. Notch signaling acts in postmitotic avian myogenic cells to control MyoD activation. Development. 128:107–116. [DOI] [PubMed] [Google Scholar]
- Isaac, A., M.G. Sargent, and J. Cooke. 1997. Control of vertebrate left-right asymmetry by a snail-related zinc finger gene. Science. 275:1301–1304. [DOI] [PubMed] [Google Scholar]
- Jorgenson, R.J., L.S. Levin, H.E. Cross, F. Yoder, and T.E. Kelly. 1978. The Rieger syndrome. Am. J. Med. Genet. 2:307–318. [DOI] [PubMed] [Google Scholar]
- Jouve, C., T. Iimura, and O. Pourquie. 2002. Onset of the segmentation clock in the chick embryo: evidence for oscillations in the somite precursors in the primitive streak. Development. 129:1107–1117. [DOI] [PubMed] [Google Scholar]
- Kahane, N., Y. Cinnamon, and C. Kalcheim. 1998. The origin and fate of pioneer myotomal cells in the avian embryo. Mech. Dev. 74:59–73. [DOI] [PubMed] [Google Scholar]
- Kalcheim, C., and R. Ben-Yair. 2005. Cell rearrangements during development of the somite and its derivatives. Curr. Opin. Genet. Dev. 15:371–380. [DOI] [PubMed] [Google Scholar]
- Kiefer, J.C., and S.D. Hauschka. 2001. Myf-5 is transiently expressed in non-muscle mesoderm and exhibits dynamic regional changes within the presegmented mesoderm and somites I-IV. Dev. Biol. 232:77–90. [DOI] [PubMed] [Google Scholar]
- Kitamura, K., H. Miura, S. Miyagawa-Tomita, M. Yanazawa, Y. Katoh-Fukui, R. Suzuki, H. Ohuchi, A. Suhiro, Y. Moteg, Y. Nakahara, et al. 1999. Mouse Pitx2 deficiency leads to anomalies of the ventral body wall, heart, extra- and periocular mesoderm and right pulmonary isomerism. Development. 126:5749–5758. [DOI] [PubMed] [Google Scholar]
- Lines, M.A., K. Kozlowski, and M.A. Walter. 2002. Molecular genetics of Axenfield-Rieger malformations. Hum. Mol. Genet. 11:1177–1184. [DOI] [PubMed] [Google Scholar]
- Linker, C., C. Lesbros, M. Stark, and C. Marcelle. 2003. Intrinsic signals regulate the initial steps of myogenesis in vertebrates. Development. 130:4797–4807. [DOI] [PubMed] [Google Scholar]
- Logan, M., S.M. Pagan-Westphal, D.M. Smith, L. Paganessi, and C.J. Tabin. 1998. The transcription factor Pitx2 mediates situs-specific morphogenesis in response to left-right asymmetric signals. Cell. 94:307–317. [DOI] [PubMed] [Google Scholar]
- Marcelle, C., J. Wolf, and M. Bronner-Fraser. 1995. The in vivo expression of the FGF receptor FREK mRNA in avian myoblasts suggests a role in muscle growth and differentiation. Dev. Biol. 172:100–114. [DOI] [PubMed] [Google Scholar]
- Marcelle, C., M.R. Stark, and M. Bronner-Fraser. 1997. Coordinate actions of BMPs, Shh and Noggin mediate patterning of the dorsal somite. Development. 124:3955–3963. [DOI] [PubMed] [Google Scholar]
- McMahon, J.A., S. Takada, L.B. Zimmerman, C.-M. Fan, R.M. Harland, and A.P. McMahon. 1998. Noggin-mediated antagonism of BMP signaling is required for growth and patterning of the neural tube and somite. Genes Dev. 12:1438–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munsterberg, A.E., J. Kitajewski, D.A. Bumcrot, A.P. McMahon, and A.B. Lassar. 1995. Combinatorial signaling by Sonic hedgehog and Wnt family members induces myogenic bHLH gene expression in the somite. Genes Dev. 9:2911–2922. [DOI] [PubMed] [Google Scholar]
- Neville, C.M., M. Schmidt, and J. Schmidt. 1992. Response of myogenic determination factors to cessation and resumption of electrical activity in skeletal muscle: a possible role for myogenin in denervation supersensitivity. Cell. Mol. Neurobiol. 12:511–527. [DOI] [PubMed] [Google Scholar]
- Nicolas, J.F., L. Mathis, C. Bonnerot, and W. Saurin. 1996. Evidence in the mouse for self-renewing stem cells in the formation of a segmented longitudinal structure, the myotome. Development. 122:2933–2946. [DOI] [PubMed] [Google Scholar]
- Ordahl, C.P., B.A. Williams, and W. Denteclaw. 2000. Determination and morphogenesis in myogenic progenitor cells: an experimental embryological approach. Curr. Top. Dev. Biol. 48:319–367. [DOI] [PubMed] [Google Scholar]
- Ott, M.O., E. Bober, G. Lyons, H. Arnold, and M. Buckingham. 1991. Early expression of the myogenic regulatory gene, myf-5, in precursor cells of skeletal muscle in the mouse embryo. Development. 111:1097–1107. [DOI] [PubMed] [Google Scholar]
- Patel, K., A. Isaac, and J. Cooke. 1999. Nodal signaling and the roles of the transcription factors SnrR and Pitx2 in vertebrate left-right asymmetry. Curr. Biol. 9:609–612. [DOI] [PubMed] [Google Scholar]
- Piedra, M.E., and M.A. Ros. 2002. BMP signaling positively regulates Nodal expression during left right specification in the chick embryo. Development. 129:3431–3440. [DOI] [PubMed] [Google Scholar]
- Piedra, M.E., J.M. Icardo, M. Albajar, J.C. Rodriguez-Rey, and M.A. Ros. 1998. Pitx2 participates in the late phase of the pathway controlling left-right asymmetry. Cell. 94:319–324. [DOI] [PubMed] [Google Scholar]
- Pourquie, O., C.M. Fan, M. Coltey, E. Hirsinger, E. Watanabe, C. Breant, P. Francis-West, P. Brickell, M. Tessier-Lavigne, and N.M. Le Douarin. 1996. Lateral and axial signals involved in avian somite patterning: a role for BMP4. Cell. 84:461–471. [DOI] [PubMed] [Google Scholar]
- Pownall, M.E., and C.P. Emerson. 1992. Sequential activation of three myogenic regulatory genes during somite morphogenesis in quail embryos. Dev. Biol. 151:67–79. [DOI] [PubMed] [Google Scholar]
- Pownall, M.E., M.K. Gustafsson, and C.P. Emerson. 2002. Myogenic regulatory factors and the specification of muscle progenitors in vertebrate embryos. Annu. Rev. Cell Dev. Biol. 18:747–783. [DOI] [PubMed] [Google Scholar]
- Raya, A., and J.C. Izpisua Belmonte. 2004. Unveiling the establishment of left- right asymmetry in the chick embryo. Mech. Dev. 121:1043–1054. [DOI] [PubMed] [Google Scholar]
- Reshef, R., M. Maroto, and A.B. Lassar. 1998. Regulation of dorsal somatic cell fates: BMPs and Noggin control the timing and pattern of myogenic regulator expression. Genes Dev. 12:290–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenquist, G.C. 1966. A radioautographic study of labeled grafts in the chick blastoderm. Development from primitive streak stages to stage 12. Contrib. Embryol. Carnegie Inst. 38:71–110. [Google Scholar]
- Ryan, A.K., B. Blumber, C. Rodriguez-Esteban, S. Yonei-Tamura, K. Tamura, T. Tsukui, J. de la Pena, W. Sabbagh, J. Greenwald, S. Choe, et al. 1998. Pitx2 determines left-right asymmetry of internal organs in vertebrates. Nature. 394:545–551. [DOI] [PubMed] [Google Scholar]
- Sassoon, D., G. Lyons, W.E. Wright, V. Lin, A. Lassar, H. Weintraub, and M. Buckingham. 1989. Expression of two myogenic regulatory factors myogenin and MyoD1 during mouse embryogenesis. Nature. 341:303–307. [DOI] [PubMed] [Google Scholar]
- Schlange, T., H.H. Arnold, and T. Brand. 2002. Chick CFC controls Left1 expression in the embryonic midline and nodal expression in the lateral plate. Development. 234:376–389. [DOI] [PubMed] [Google Scholar]
- Sela-Donenfeld, D., and C. Kalcheim. 2002. Localized BMP4-noggin interactions generate the dynamic patterning of noggin expression in the somite. Dev. Biol. 246:311–328. [DOI] [PubMed] [Google Scholar]
- Semina, E.V., R. Reiter, N.J. Leysens, W.L.M. Alward, K.W. Small, N.A. Datson, J. Siegel-Batelt, D. Bierke-Nelson, P. Bitoun, B.U. Zabel, et al. 1996. Cloning and characterization of a novel bicoid-related homeobox transcription factor gene, RIEG, involved in Rieger syndrome. Nat. Genet. 14:392–399. [DOI] [PubMed] [Google Scholar]
- Slavkin, H., G. Nuckolls, and L. Shum. 2000. Craniofacial development and patterning. In Developmental Biology Protocols. Methods in Molecular Biology, 136. R.S. Tuan and C.W. Lo, editors. Humana Press, Totowa, NJ. 45–54. [DOI] [PubMed]
- Smith, A.G. 2001. Embryo-derived stem cells: Of mice and men. Annu. Rev. Cell Dev. Biol. 17:435–462. [DOI] [PubMed] [Google Scholar]
- St Amand, T.R., J. Ra, Y. Zhang, Y. Hu, S.I. Baber, M. Qui, and Y. Chen. 1998. Cloning and expression pattern of chicken Pitx2: a new component in the SHH signaling pathway controlling embryonic heart looping. Biochem. Biophys. Res. Commun. 247:100–105. [DOI] [PubMed] [Google Scholar]
- Stern, C., and D.R. Canning. 1990. Origin of cells giving rise to mesoderm and endoderm in chick embryo. Nature. 343:273–275. [DOI] [PubMed] [Google Scholar]
- Stern, H.M., A.M.C. Brown, and S.D. Hauschka. 1995. Myogenesis in paraxial mesoderm: Preferential induction by dorsal neural tube and by cells expression Wnt-1. Development. 121:3675–3686. [DOI] [PubMed] [Google Scholar]
- Stockdale, F.E., W. Nikovits, and B. Christ. 2000. Molecular and cellular biology of avian somite development. Dev. Dyn. 219:304–321. [DOI] [PubMed] [Google Scholar]
- Strony, R., J. Gerhart, D. Tornambe, J. Perlman, C. Neely, J. Dare, B. Stewart, and M. George-Weinstein. 2005. NeuroM and MyoD are expressed in separate subpopulations of cells in the pregastrulating epiblast. Gene Expr. Patterns. 5:387–395. [DOI] [PubMed] [Google Scholar]
- Tajbakhsh, S., and M.E. Buckingham. 1995. Lineage restriction of the my9ogenic convesion factor myf-5 in the brain. Development. 121:4077–4083. [DOI] [PubMed] [Google Scholar]
- Tajbakhsh, S., U. Borello, E. Vivarelli, R. Kelly, J. Papkoff, D. Duprez, M. Buckingham, and G. Cossu. 1998. Differential activation of Myf5 and MyoD by different Wnts in explants of mouse paraxial mesoderm and the later activation of myogenesis in the absence of Myf5. Development. 125:4155–4162. [DOI] [PubMed] [Google Scholar]
- Tonegawa, A., and Y. Takahashi. 1998. Somitogenesis controlled by Noggin. Dev. Biol. 202:172–182. [DOI] [PubMed] [Google Scholar]
- Wagner, J., C. Schmidt, W. Nikowits, Jr., and B. Christ. 2000. Compartmentalization of the somite and myogenesis in chick embryos are influenced by wnt expression. Dev. Biol. 228:86–94. [DOI] [PubMed] [Google Scholar]
- Williams, B.A., and C.P. Ordahl. 1994. Pax-3 expression in segmental mesoderm marks early stages in myogenic cell specification. Development. 120:785–796. [DOI] [PubMed] [Google Scholar]
- Yoshioka, H., C. Meno, K. Koshiba, M. Sughihara, H. Itoh, Y. Ishimaru, T. Inoue, H. Ohuchi, E.V. Semina, J.C. Murray, et al. 1998. Pitx2, a bicoid-type homeobox gene, is involved in a left-signaling pathway in determination of left-right asymmetry. Cell. 94:299–305. [DOI] [PubMed] [Google Scholar]
- Zimmerman, L.B., J.D. Jesus-Escobar, and R.M. Harland. 1996. The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein-4. Cell. 86:599–606. [DOI] [PubMed] [Google Scholar]