Abstract
The respiration-deficient, highly glycolytic metabolic phenotype of cancer cells known as the “Warburg effect” has been appreciated for many years. A new study (see Pelicano et al. on p. 913 of this issue) demonstrates that respiration deficiency caused by mitochondrial mutation or hypoxia may directly promote the enormous survival advantage observed in cancer cells by activation of the phosphatidylinositol 3-kinase–Akt survival pathway. We discuss these and other recent findings that show how metabolic changes associated with cancer can play a significant role in tumor biology.
Otto Warburg first described that cancer cells exhibit altered cellular glucose metabolism that relies primarily on glycolysis rather than mitochondrial respiration in 1930 (Warburg, 1930, 1956). This observation, termed the “Warburg effect,” was largely set aside as modern research techniques evolved, and only in recent years has it seen a renaissance in both the clinic and the laboratory. In the clinic, positron emission tomography imaging using labeled glucose tracers takes advantage of cancer cells' increased glucose uptake to image tumors in human patients. In the laboratory, researchers have been attempting to piece together whether the switch from mitochondrial respiration to glycolysis could fit with the survival advantages observed in cancers. Despite the obvious benefits of anaerobic metabolism in areas of tissue hypoxia, the significantly lower energy yield per glucose molecule would appear to be generally detrimental. Nevertheless, increased glycolysis may have other positive effects that outweigh the negative. In this issue, Pelicano et al. (see p. 913 of this issue) describe one mechanism by which loss of mitochondrial respiration may provide a survival advantage by activating the phosphatidylinositol 3-kinase (PI3K)–Akt survival pathway.
Mitochondrial respiration is often disrupted in cancer because of hypoxia or debilitating mutations in the mitochondrial DNA (mtDNA) that impede electron transport. To investigate the possible role that these respiration deficiencies play in enhancing cell survival, Pelicano et al. disrupted mitochondrial respiration by genetic (mtDNA mutation), pharmacological (chemical inhibition), and microenvironmental (hypoxia) methods in human leukemia and lymphoma cell lines. Although these forms of metabolic disruption did not greatly alter the characteristics of the mitochondria, they did provide a survival advantage for the cells upon treatment with several common anticancer agents. In searching for a possible explanation for this survival phenotype, they observed increased levels of activating Akt phosphorylation in respiration-deficient cells. Enhanced phosphorylation of the Akt substrate glycogen synthase kinase-3 supported the conclusion that decreased mitochondrial respiration led to increased activity of the PI3K–Akt pathway. The authors go on to argue that increased NADH levels, which were caused by defective consumption of NADH in mitochondria, and decreased NADPH levels, which were caused by increased flux of glucose through glycolysis at the expense of the pentose phosphate pathway (PPP), led to oxidation of the tumor suppressor PTEN, a lipid phosphatase that negatively regulates the PI3K pathway. An inhibitor of PI3K signaling, along with PTEN-null cell lines, was then used to support their conclusion that the enhanced survival phenotype of respiration-deficient cells is caused by enhanced PI3K–Akt activation.
The role of the PI3K–Akt pathway in cancer has been intensely investigated and has been shown to play roles in cell survival, growth, cell cycle entry, and cell migration—all of which are key characteristics of cancer cells. Indeed, this pathway has been shown to be hyperactivated in many cancers, often by genetic mutation of PTEN, or by activation of oncogenic signaling proteins such as Ras (for review see Cully et al., 2006). Mutations of PI3K–Akt–regulating genes may not, however, fully explain the observed activation of this pathway. It is tempting to speculate that loss of respiration may be responsible for some PI3K–Akt activation observed in cancer cells, as the large majority of tumors display this metabolic phenotype. Importantly, activation of Akt may also play a key role in up-regulating glucose uptake and glycolysis to support the viability and growth of respiration-deficient cells (Plas et al., 2001; Rathmell et al., 2003). This may represent a feed-forward mechanism of loss of mitochondrial respiration, accumulation of NADH, activation of Akt, and increased glucose uptake to thus complete a switch to glycolysis as the primary cellular ATP source.
It is becoming clear that metabolites can play critical roles in cell signaling pathways (Ladurner, 2006), and the NADH/NADPH ratio may be one new example. In addition to this example of metabolite-based regulation of cell survival, glucose flux through the PPP has also been shown recently to play critical roles in cell survival in other systems. Nutt et al. (2005) have shown that PPP substrates and intermediates can act as a metabolic timer for the initiation of apoptosis in the Xenopus laevis oocyte extract system. Normally, X. laevis egg extracts undergo a spontaneous process resembling apoptosis after several hours. Addition of PPP intermediates or NADPH, however, prevented this apoptosis. Mechanistically, NADPH appeared to promote CaMKII-mediated phosphorylation of caspase-2 to prevent its ability to initiate mitochondrial disruption, release of cytochrome c, and activation of caspase 3. Additionally, Bensaad et al. (2006) have identified a novel antiapoptotic protein, TP53-induced glycolysis and apoptosis regulator (TIGAR), which is induced by p53 under low levels of stress and functions to shunt additional glucose away from glycolysis toward the PPP to promote cell viability. These studies exemplify the importance of the PPP in protecting cells from stress and suggest that the effects of PPP modulation may be context dependent. It will be very interesting to see the results as studies further define mechanisms that regulate glucose flux through the PPP and the role this plays in survival of cancer cells under a variety of physiological and drug-induced stress.
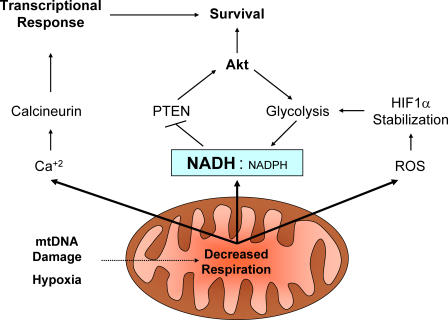
In addition to the regulation of NADH and NADPH, changes in many aspects of cell metabolism can impact mitochondria, and these organelles are becoming increasingly apparent as sensors of metabolic stress that possess the capacity to signal this stress to the cytosol and nucleus (Fig. 1). The Avandhani group has published a series of papers showing that mtDNA mutation can raise intracellular Ca2+ levels and affect a variety of Ca2+-dependent pathways. Mitochondrial stress led to activation of the Ca2+- and calmodulin-dependent phosphatase calcineurin, JNK pathways, inactivation of NF-κB, induction of antiapoptotic Bcl-2 family members Bcl-2 and Bcl-xL, and repression of proapoptotic family members Bid and Bax (Biswas et al., 2005). In addition, mitochondrial generation of reactive oxygen species can play a substantial role in cell physiology, and has recently been shown to be required to allow stabilization of the hypoxia inducible factor-1α transcription factor under hypoxic conditions (Brunelle et al., 2005). These examples of metabolic machinery talking to the rest of the cell through existing signaling pathways display the importance of metabolic processes on an entirely different level than simply serving as a cellular energy source.
Figure 1.
Mitochondrial signaling pathways to regulate cell metabolism and fate. Mitochondria under respiration duress can signal to influence cellular responses via changes in NADH/NADPH ratio, reactive oxygen species (ROS), and intracellular Ca2+.
Although the Warburg effect is well documented and universally accepted, its effect on tumor biology has remained elusive. By artificially reproducing this effect in the absence of other major oncogenic events, we are beginning to appreciate how cancer cells not only survive this metabolic shift, but how it may allow them to thrive in stressful conditions via activation of survival pathways, such as Akt. Understanding the metabolic phenotype of cancer cells and its effects on cell signaling mechanisms not only affords us a better understanding of tumor biology, but may present a plethora of potential targets for advanced cancer therapies.
Abbreviations used in this paper: mtDNA, mitochondrial DNA; PI3K, phosphatidylinositol 3-kinase; PPP, pentose phosphate pathway.
References
- Bensaad, K., A. Tsuruta, M.A. Selak, M.N. Vidal, K. Nakano, R. Bartrons, E. Gottlieb, and K.H. Vousden. 2006. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell. 126:107–120. [DOI] [PubMed] [Google Scholar]
- Biswas, G., M. Guha, and N.G. Avadhani. 2005. Mitochondria-to-nucleus stress signaling in mammalian cells: nature of nuclear gene targets, transcription regulation, and induced resistance to apoptosis. Gene. 354:132–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunelle, J.K., E.L. Bell, N.M. Quesada, K. Vercauteren, V. Tiranti, M. Zeviani, R.C. Scarpulla, and N.S. Chandel. 2005. Oxygen sensing requires mitochondrial ROS but not oxidative phosphorylation. Cell Metab. 1:409–414. [DOI] [PubMed] [Google Scholar]
- Cully, M., H. You, A.J. Levine, and T.W. Mak. 2006. Beyond PTEN mutations: the PI3K pathway as an integrator of multiple inputs during tumorigenesis. Nat. Rev. Cancer. 6:184–192. [DOI] [PubMed] [Google Scholar]
- Ladurner, A.G. 2006. Rheostat control of gene expression by metabolites. Mol. Cell. 24:1–11. [DOI] [PubMed] [Google Scholar]
- Nutt, L.K., S.S. Margolis, M. Jensen, C.E. Herman, W.G. Dunphy, J.C. Rathmell, and S. Kornbluth. 2005. Metabolic regulation of oocyte cell death through the CamKII-mediated phosphorylation of caspase-2. Cell. 123:89–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plas, D.R., S. Talapatra, A.L. Edinger, J.C. Rathmell, and C.B. Thompson. 2001. Akt and Bcl-xL promote growth factor-independent survival through distinct effects on mitochondrial physiology. J. Biol. Chem. 276:12041–12048. [DOI] [PubMed] [Google Scholar]
- Rathmell, J.C., C.J. Fox, D.R. Plas, P. Hammerman, R.M. Cinalli, and C.B. Thompson. 2003. Akt-directed glucose metabolism can prevent Bax conformation change and promote growth factor-independent survival. Mol. Cell. Biol. 23:7315–7328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburg, O. 1930. The Metabolism of Tumors. Constable and Company, Ltd. London. 327 pp.
- Warburg, O. 1956. On the origin of cancer cells. Science. 123:309–314. [DOI] [PubMed] [Google Scholar]