Abstract
Focal adhesions are multiprotein assemblages that link cells to the extracellular matrix. The transmembrane protein, integrin, is a key component of these structures. In vertebrate muscle, focal adhesion–like structures called costameres attach myofibrils at the periphery of muscle cells to the cell membrane. In Caenorhabditis elegans muscle, all the myofibrils are attached to the cell membrane at both dense bodies (Z-disks) and M-lines. Clustered at the base of dense bodies and M-lines, and associated with the cytoplasmic tail of β-integrin, is a complex of many proteins, including UNC-97 (vertebrate PINCH). Previously, we showed that UNC-97 interacts with UNC-98, a 37-kD protein, containing four C2H2 Zn fingers, that localizes to M-lines. We report that UNC-98 also interacts with the C-terminal portion of a myosin heavy chain. Multiple lines of evidence support a model in which UNC-98 links integrin-associated proteins to myosin in thick filaments at M-lines.
Introduction
In vertebrate striated muscle cells, the most peripherally located myofibrils are attached to the sarcolemma through costameres, structures compositionally and functionally similar to focal adhesions (Ervasti, 2003; Samarel, 2005). Costameres are thought to laterally transmit the force of muscle contraction across the cell membrane to the ECM and serve to keep sarcomeres in register. The protein assemblies that compose the costameres are located beneath the Z-disks of peripheral myofibrils. Some components of focal adhesions (Porter et al., 1992), including αv integrin (McDonald et al., 1995), have also been found located at peripheral M-lines. For both focal adhesions and Z-disk costameres, integrins are coupled to cytoskeletal actin filaments and myofibrillar thin filaments, respectively. However, the means of attaching myosin thick filaments to the muscle cell membrane is unknown.
In Caenorhabditis elegans muscle, the actin thin filaments are attached to dense bodies (Z-disk analogues) and the myosin thick filaments are organized around M-lines (for review see Moerman and Williams, 2006). All the dense bodies and M-lines appear to be anchored to the cell membrane and, thus, also serve the same function as vertebrate costameres. In C. elegans, clustered on the cytoplasmic side of the sarcolemma at the base of dense bodies and M-lines, is a complex of proteins associated with the cytoplasmic tail of PAT-3 (β-integrin). These proteins include UNC-112 (Mig-2), PAT-4 (integrin-linked kinase), PAT-6 (actopaxin), and UNC-97 (PINCH; Moerman and Williams, 2006). At the dense bodies, vinculin, α-actinin, and talin likely link integrins to actin thin filaments. However, at the M-lines, the identity of the molecule or molecules that directly link the membrane-proximal integrin complex to the myosin thick filaments is unknown. Among UNC-97–interacting molecules is UNC-98, a 310-residue protein containing four C2H2 Zn fingers that localizes by antibodies to the M-lines (Zengel and Epstein, 1980; Hobert et al., 1999; Mercer et al., 2003). The interaction between UNC-97 and UNC-98 requires all four Zn fingers of UNC-98 (Mercer et al., 2003).
Results and discussion
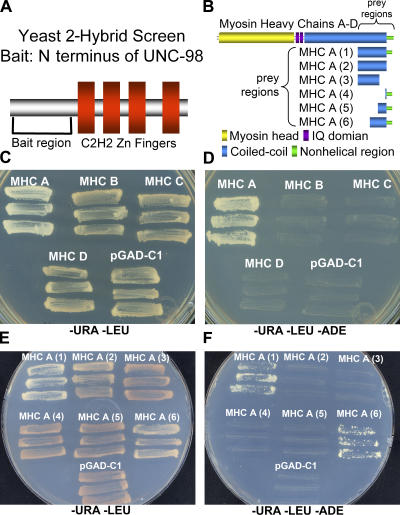
To identify additional functional partners of UNC-98 at the M-line, we screened a yeast two-hybrid library, using as bait the N-terminal, non-Zn finger–containing 112 residues of UNC-98 (Fig. 1 A). 33 positive clones were identified encoding 18 unique proteins that interact with the N terminus of UNC-98 (Table S1, available at http://www.jcb.org/cgi/content/full/jcb.200608043/DC1). Three of the confirmed clones encoded myosin heavy chain (MHC) A, a body wall muscle–specific myosin.
Figure 1.
Yeast two-hybrid analysis shows that aa 1–112 of UNC-98 interact specifically with aa 1771–1969 of MHC A, and not B, C, or D. (A) A yeast two-hybrid bait expressing the first 112 amino acids of UNC-98 excluding the four C2H2 Zn finger domains (pGDBU98-4c) was used to screen approximately four million yeast colonies. 33 confirmed interacting clones comprised 18 unique genes, including the C terminus of MHC A (isolated three times). (B) MHC A, B, C, and D have similar structures, including a myosin head domain (yellow), IQ domains (purple), and a coiled-coil domain (blue). In addition, MHC A and B have a nonhelical region (green). The prey proteins for the first experiment include ∼300 residues of the C termini of each MHC (A, B, C, and D). The prey proteins for the second experiment include the following portions of MHC A: aa 1636–1937, lacking the entire nonhelical region (MHC A (2)); aa 1636–1870, lacking a portion of the coiled-coil region and the nonhelical region (MHC A (3)); aa 1938–1969, including just the nonhelical region (MHC A (4)); aa 1871–1969, including a portion of the coiled-coil region and the nonhelical region (MHC A (5)); and aa 1771–1969, including a larger portion of the coiled-coil region and the nonhelical region (MHC A (6)). (C) Growth on media selecting for the maintenance of the bait (−URA) and the prey (−LEU) plasmids confirms that the yeast harbors both the N-terminal UNC-98 bait and the C terminus of each of the MHCs. (D) When the yeast shown in C were tested for their ability to grow on media excluding adenine (−ADE), growth only occurred when the N terminus of UNC-98 and the C terminus of MHC A, but not MHC B, C, or D were present. (E) Growth on media selecting for the maintenance of the bait and the prey plasmids confirms that the yeast harbors both the N-terminal UNC-98 bait and the preys containing deletion derivatives of the C terminus of MHC A. (F) When the yeast shown in E were tested for their ability to grow on media excluding adenine, growth only occurred when the N terminus of UNC-98 and either the C-terminal 330 (MHC A (1)) or 200 residues (MHC A (6)) of MHC A were present.
C. elegans contains four different muscle MHC genes, each encoding a different myosin isoform, A–D (Schachat et al., 1977; Waterston et al., 1982; Dibb et al., 1989). All four heavy chains have a similar structure, including a myosin head domain, IQ domains, and a coiled-coil domain (Dibb et al., 1989). In addition, the body wall muscle–specific isoforms, MHC A and B, have an ∼30-residue-long C-terminal nonhelical region. The positive clones identified in the screen encoded this nonhelical tail piece and a portion of the coiled-coil domain. To determine whether the N terminus of UNC-98 interacts specifically with MHC A, prey plasmids were generated encoding the analogous region of MHC B, C, and D (Fig. 1 B). The N terminus of UNC-98 interacts with the C terminus of MHC A but not with the equivalent regions of MHC B, C, and D in the yeast two-hybrid system (Fig. 1, C and D). This result is consistent with the lack of expression of UNC-98 in the pharynx (Mercer et al., 2003), where MHC C and D are specifically expressed. Moreover, the interaction of UNC-98 with MHC A and not MHC B is consistent with the different localizations of the two myosins in thick filaments of body wall muscle: MHC B to the polar regions and MHC A to the central region (Miller et al., 1983), near the M-line localization of UNC-98.
To narrow the critical region of MHC A required for interaction with UNC-98, additional prey plasmids encoding a series of deletion derivatives of the C terminus of MHC A were tested (Fig. 1 B). As shown in Fig. 1 (E and F), the N terminus of UNC-98 interacts with the C-terminal 200 residues of MHC A, including the nonhelical region and a portion of the coiled-coil rod (MHC A (6)). Although the 32-residue nonhelical tail contributes to this binding (absence of binding when this region is removed; see MHC A (2)), it is not sufficient for this binding (absence of binding when just this region is tested; see MHC A (4)). The nonhelical region of MHC A, which may be phosphorylated (Schriefer and Waterston, 1989), is not required for MHC A to initiate thick filament assembly (Hoppe and Waterston, 1996). It is possible that the nonhelical region protrudes from the surface of the thick filament shaft and interacts with other proteins, such as UNC-98.
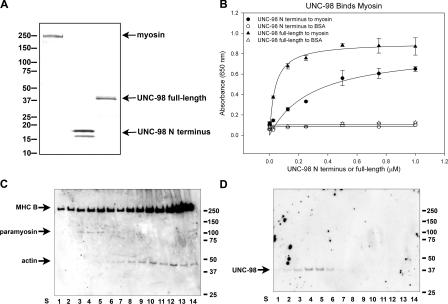
To provide additional evidence that UNC-98 interacts with MHC A, in vitro protein interaction was shown using an ELISA assay. Wild-type myosin II (including MHC A) showed saturable binding to both full-length and the N-terminal portion of UNC-98 expressed in Escherichia coli (Fig. 2, A and B). To obtain evidence that UNC-98 is associated with MHC A in vivo, we sought to determine whether UNC-98 copurifies with native thick filaments using established procedures (Epstein et al., 1988; Deitiker and Epstein, 1993; Fig. S1, B and C, available at http://www.jcb.org/cgi/content/full/jcb.200608043/DC1). Fractions were taken at each step of the preparation and analyzed by Western blot using antibodies specific to the N terminus of UNC-98 (Fig. S1, A and C). UNC-98 is prominent in the fraction in which thick filaments pellet, indicating that UNC-98 copurifies with thick filaments. In contrast to UNC-98, UNC-97 does not copurify with thick filaments (Fig. S1 C). To further isolate intact thick filaments, a fraction containing thick filaments, thin filaments, and ribosomes was fractionated on a sucrose density gradient. Fractions were collected from the bottom of the gradient (starting with S1) and were immunoblotted. Sucrose gradient fractions S3–S5 that contain both myosin and paramyosin (Fig. 2 C) also contain UNC-98 (Fig. 2 D), indicating that UNC-98 copurifies with thick filaments.
Figure 2.
UNC-98 shows saturable binding to nematode myosin in vitro and copurifies with nematode thick filaments. (A) The proteins used in the ELISA assay include total myosin II purified from C. elegans (MHC A, B, C, and D), bacterially expressed N terminus of UNC-98 (112 amino acids), and bacterially expressed UNC-98 (310 amino acids). These purified proteins (1 μg each) were visualized on an SDS-PAGE gel by Coomassie staining. (B) When increasing amounts (at concentrations from 0 to 1.0 μM) of the N-terminal portion of UNC-98 (100 μl) were exposed to a fixed amount of myosin (100 μl at 0.5 μM) in vitro, it bound increasingly until it reached a saturation level. The best-fit ligand binding curves were determined by plotting means and standard errors of three absorbance values (SigmaPlot 9.0). The dissociation constants were calculated from this data to be 0.037 μM for full-length UNC-98 and 0.295 μM for the N-terminal portion of UNC-98. (C and D) Native thick filaments were purified using established procedures from wild-type nematodes (Epstein et al., 1974; MacLeod et al., 1977). A fraction enriched in thick filaments (Fig. S1, B and C, available at http://www.jcb.org/cgi/content/full/jcb.200608043/DC1) was further fractionated on a sucrose density gradient. Gradient fractions S1–S14 were run on duplicate gels and immunoblotted. One blot was exposed to a combination of anti–MHC B, anti-paramyosin, and anti-actin antibodies (C). The other blot was exposed to anti–UNC-98 antibodies (D). UNC-98 is present in sucrose gradient fractions S3–S5 that contain myosin and paramyosin, markers for thick filaments.
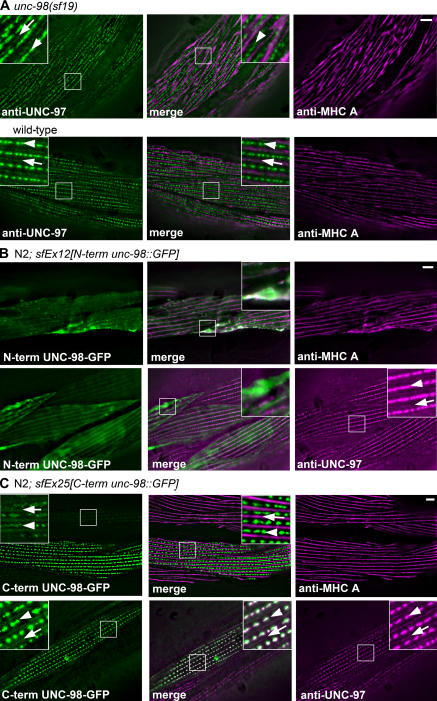
Because UNC-98 interacts with both UNC-97 and MHC A, we asked what effect loss of function of unc-98, myo-3 (encodes MHC A), or unc-97 would have on the in vivo localization of these proteins. To facilitate these studies, antibodies to UNC-97 were generated (Fig. S2, available at http://www.jcb.org/cgi/content/full/jcb.200608043/DC1). unc-98(sf19) is a strong loss-of-function splice site mutation (Mercer et al., 2003) that results in a greatly reduced level of a truncated UNC-98 mutant protein (Fig. S1 A). As shown in Fig. 3 A, compared with wild type, in unc-98(sf19), neither the membrane-associated attachment complexes (visualized by anti–UNC-97) nor the A-bands (visualized with anti–MHC A) are organized in their normal sharply defined patterns. In fact, MHC A seems to be no longer restricted to straight A-bands and sometimes appears to cross over rows of dense bodies (Fig. 3 A, enlarged view in merged panel of unc-98(sf19)).
Figure 3.
Loss of function of unc-98 affects the localization of MHC A but not UNC-97; the myosin binding N-terminal portion of UNC-98 but not the C-terminal portion of UNC-98 affects the localization of MHC A. (A) Immunofluorescence microscopy of unc-98(sf19) shows that the banding pattern of MHC A is not as sharply defined as in wild type, and the localization of UNC-97 is no longer in a sharply defined pattern. In fact, MHC A is no longer restricted to A-bands and can sometimes be seen to cross over rows of dense bodies. (B) When the N terminus of UNC-98 (lacking the Zn fingers) is overexpressed as a GFP fusion in a wild-type background, its localization is dispersed within the myofibril and results in aggregates of MHC A, whereas UNC-97 is normally localized to focal adhesions. (C) When the C terminus of UNC-98 (containing the Zn fingers) is overexpressed as a GFP fusion in a wild-type background, it localizes normally to M-lines and dense bodies, and both MHC A and UNC-97 are normally localized. M-lines are marked with arrows, and dense bodies are marked with arrowheads. Insets show threefold magnifications. Bars, 5 μm.
As noted above, the N-terminal 112 residues of UNC-98 interact with MHC A, a region of UNC-98 that is not required for interaction with UNC-97 (Mercer et al., 2003). Transgenic overexpression of the N terminus of UNC-98 as a GFP fusion protein in a wild-type background results in abnormal aggregates that contain MHC A and the N terminus of UNC-98 (Fig. 3 B). In contrast, UNC-97 is properly localized (Fig. 3 B). This suggests that an excess of the N terminus of UNC-98 competes with endogenous UNC-98 for binding with MHC A, interfering to some degree with the interaction of intact UNC-98 and MHC A. In contrast, when the C-terminal portion of UNC-98 containing all four Zn fingers, which is not necessary for binding with MHC A, is overexpressed in a wild-type background, MHC A and UNC-97 are properly localized (Fig. 3 C). Because overexpression of the myosin binding portion of UNC-98 can disrupt the normal localization of MHC A, this suggests that MHC A localization depends on UNC-98.
We wished to determine the effect of loss-of-function mutations in myo-3, the gene encoding MHC A, on myofibril organization in adults; however, available loss-of-function myo-3 mutations are embryonic lethal (Waterston, 1989). Therefore, a strain was used in which a myo-3 mutant was rescued by a transgenic array containing copies of the wild-type myo-3 gene translationally fused to GFP (Campagnola et al., 2002). Extrachromasomal arrays are occasionally lost upon cell division during development in C. elegans. This resulting “mosaic expression” allowed visualization of body wall muscle cells lacking myo-3 expression in a viable adult animal.
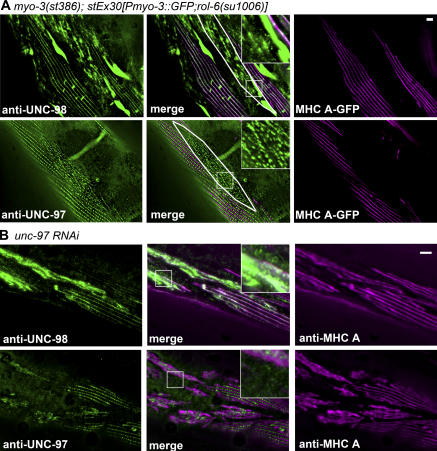
As shown in Fig. 4 A, in adult body wall muscle cells that lack MHC A, UNC-98 aggregates especially at the ends of the spindle-shaped cells and is not associated with focal adhesions. In contrast, in these cells, UNC-97 is not found in aggregates and is still localized to membrane-proximal regions, but in an abnormal pattern. Given that UNC-98 aggregates in cells lacking MHC A, interaction between UNC-98 and MHC A must be critical for anchorage of UNC-98 to thick filaments. The different degree of disruption of UNC-97 and UNC-98 in cells lacking MHC A is consistent with the idea that UNC-97 and UNC-98 can primarily exist in different protein complexes. UNC-97 is part of a four-protein complex associated with the cytoplasmic tail of β-integrin (Moerman and Williams, 2006), whereas thick filaments contain UNC-98 (Fig. 2, C and D) but not UNC-97 (Fig. S1 C). The somewhat disrupted organization of UNC-97 in cells lacking MHC A can be explained by considering that the organization of integrins (and integrin-associated proteins) is directed by transmembrane signals arising from both inside and outside the cell. When MHC A (and thick filaments) are lost, at least some signals originating from the inside of the cell are lost, and thus the organization of UNC-97 is affected.
Figure 4.
The absence of MHC A or the knock down of UNC-97 affects the localization of UNC-98. (A) Within cells lacking MHC A (in a mosaic line expressing myo-3∷gfp to rescue the myo-3(st386) null mutation), UNC-98 localization is severely disrupted and found in aggregates at the ends of muscle cells. In comparison, UNC-97 appears to localize to focal adhesion structures, although in an irregular pattern, and is not present in aggregates. (Representative cells lacking MHC A expression are outlined in white.) (B) In adult body wall muscle cells in which unc-97 has been knocked down by RNAi, both UNC-98 and MHC A are mislocalized. Insets show threefold magnifications. Bars, 5 μm.
What is the effect of loss of function of unc-97 on UNC-98 and MHC A? The previously reported unc-97 loss-of-function mutation su110 produces a slightly truncated protein of approximately normal abundance that retains the UNC-98 binding region, and thus it was not suitable for our studies (Fig. S2 A). Therefore, RNAi was used to examine the loss of function of unc-97. Bacteria expressing double-stranded RNA for unc-97 were fed to worms beginning at the L1 larval stage to avoid embryonic lethality (Hobert et al., 1999). The resulting unc-97(RNAi) adult animals were then stained with anti– UNC-97. As shown in Fig. 4 B, some muscle cells have normally localized UNC-97, whereas other muscle cells show reduced levels of UNC-97 that is poorly organized. Significantly, in the cells showing reduced UNC-97, UNC-98 is aggregated and MHC A is mislocalized (Fig. 4 B). This suggests that the interaction of UNC-98 with UNC-97 allows its attachment to anchored focal adhesion structures. UNC-98, when properly localized at the base of the M-lines, via its interaction with UNC-97, recruits MHC A to the center of the A-band (the M-line). This interpretation is supported by the following data. When the N terminus of UNC-98, the portion of UNC-98 that has been shown not to bind UNC-97 (Mercer et al., 2003), is overexpressed in a wild-type background, it is diffuse within the myofibril and unable to correctly localize to focal adhesion structures. However, UNC-97 is normally localized to the dense bodies and M-lines (Fig. 3 B).
The results are consistent with a model in which UNC-98 acts as a molecular bridge between UNC-97 under the muscle cell membrane and MHC A at the M-line (Fig. 5). Previous studies suggest that myofibril assembly is directed by signals arising from outside the muscle cell. This was first demonstrated by showing that weak alleles of unc-52 (later shown to encode an ECM protein) show retardation of myofibril assembly (Mackenzie et al., 1978). The assembly process begins with the localization of UNC-52 (perlecan) in the ECM and PAT-2 and -3 (integrins) in the muscle cell membrane, clustering at the bases of future M-lines and dense bodies (Hresko et al., 1994; Williams and Waterston, 1994). This is believed to be followed by an association of the cytoplasmic tail of PAT-3 (β-integrin) with a complex of proteins that includes UNC-97 (PINCH). Previously, it was shown that the first two LIM domains of UNC-97 interact with the four C2H2 Zn fingers of UNC-98 and that UNC-98 is localized to M-lines (Mercer et al., 2003). In this study, our data indicate that the N-terminal portion of UNC-98 interacts specifically with the C-terminal tails of MHC A, but not MHC B (Fig. 1). This result is consistent with the fact that in C. elegans body wall muscle M-line proteins are likely to be specifically associated with MHC A, but not MHC B, as MHC A is localized to the middle portion of thick filaments (Miller et al., 1983). Supporting evidence for an interaction between UNC-98 and MHC A was provided by showing that UNC-98 interacts with purified myosin in vitro and copurifies with thick filaments (Fig. 2). By using antibodies to probe loss-of-function mutants and RNAi animals, it was shown that the localization of UNC-98 and MHC A are dependent on each other and on UNC-97 (Figs. 3 and 4).
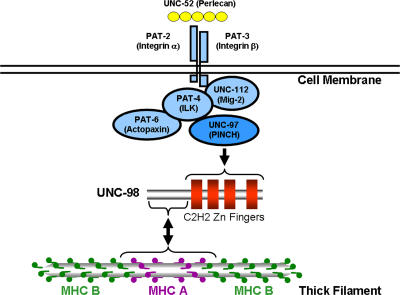
Figure 5.
UNC-98 as a molecular bridge between integrin-associated proteins and thick filaments at the M-line. In C. elegans body wall muscle, the myofibrils are closely apposed to the muscle cell membrane, and both the dense bodies (Z-disks) and M-lines are attached to the muscle cell membrane. At the base of both these focal adhesion–like structures are clustered UNC-52 (perlecan) in the ECM and the integrins in the muscle cell membrane. Associated with the cytoplasmic tail of PAT-3 is a complex of four proteins, including UNC-97 (PINCH). At the M-line, UNC-97 interacts with the four C2H2 Zn fingers of UNC-98, whereas the N terminus of UNC-98 interacts with the C-terminal portion of MHC A, but not MHC B. In nematode thick filaments, these MHCs are differentially localized, with MHC A in the middle and MHC B in the polar regions. An arrow points from UNC-97 to UNC-98 because, when the level of UNC-97 is reduced, UNC-98 is mislocalized. The arrow points in both directions between UNC-98 and MHC A because, in the absence of MHC A, UNC-98 is found in large aggregates. When UNC-98 levels are reduced, MHC A is not localized in its normal sharply defined pattern.
Another model for the data is that UNC-98 has a signaling function, shuttling between the integrin-associated complex near the cell membrane and the thick filaments in the A-band. Using standard immunoprecipitation buffers, UNC-98 is poorly solubilized from whole worms (unpublished data). This suggests that if a shuttling or non–thick filament–attached fraction were present, it is at low quantities.
There are two possibilities as to why the unc-98 mutation does not result in a more severe disorganization of MHC A. First, the unc-98 allele used, although the most severe allele of the three unc-98 alleles, is not a molecular null (Mercer et al., 2003) and some, albeit truncated UNC-98 protein, can be seen by immunoblot (Fig. S1 A). Even by RNAi for unc-98, the phenotype is not more severe than any of the unc-98 mutant alleles, and a substantial amount of UNC-98 protein can be found by Western blot (unpublished data). Second, the pathway we have revealed in which UNC-98 links integrin complexes to thick filaments may be only one of several pathways that link the plasma membrane to thick filaments. For example, UNC-97 (PINCH) may interact with proteins other than UNC-98 that directly interact with myosin. Indeed, UNC-96, whose mutant phenotype is very similar to that of UNC-98 and is localized to M-lines and copurifies with thick filaments (Zengel and Epstein, 1980; Mercer et al., 2006), is linked to UNC-97 through two novel LIM domain proteins (unpublished data). Additionally, other members of the integrin-associated complex (UNC-112, PAT-4, and PAT-6) may also interact with proteins that link to thick filaments. Finally, the thick filaments of peripheral myofibrils may be linked to the muscle cell membrane through other proteins, such as dystrophin, spectrin, and vinculin. In mammalian skeletal muscle, these three proteins have been localized to M-lines of peripheral myofibrils (Porter et al., 1992).
Linkage of thick filaments to integrin adhesion complexes at the M-line likely plays a role in transmission of contractile forces across the cell membrane to the ECM. Although an obvious vertebrate homologue of UNC-98 cannot be discerned, given its membership in a very large Zn finger protein family, it is expected that functional homologues of UNC-98 do exist. It is proposed that in vertebrate muscle, a similar mechanism of linkage between integrins and myosin thick filaments occurs at the M-lines of peripheral myofibrils.
Materials and methods
C. elegans strains
The following strains were used in this study: wild-type N2; GB246, unc-98(sf19); N2; sfEx12[unc-98∷GFP construct D; rol-6]; N2; sfEx25[unc-98∷GFP construct E] (Mercer et al., 2003); RW1596, myo-3(st386); stEx30[Pmyo-3∷GFP; rol-6(su1006)] (Campagnola et al., 2002); NL2099, rrf-3(pk1462) (Simmer et al., 2002); HE130, unc-98(su130); and HE110, unc-97(su110) (Zengel and Epstein, 1980). RW1596 was provided by P. Hoppe (Western Michigan University, Kalamazoo, MI) and R. Waterston (University of Washington, Seattle, WA). NL2099, HE130, and HE110 were obtained from the Caenorhabditis Genetics Center.
Yeast two hybrid
Strain PJ69-4A containing pGDBU-C1 (James et al., 1996) with a cDNA insert (cDNA library provided by R. Barstead, Oklahoma Medical Research Foundation, Oklahoma City, OK) for expression of aa 1–112 of UNC-98 was used for screening (named pGDBU98-4c). Four million yeast colonies were screened, and interactors were identified as previously described (Mackinnon et al., 2002). Of 759 colonies activating the HIS3 reporter, 94 activated the ADE2 reporter. These positive clones were retransformed into pGDBU98-4c, confirming 33 positives, which were sequenced. Preys were designed using pGAD-C1 (James et al., 1996) to express MHC A (2), aa 1636–1937; (3), aa 1636–1870; (4), aa 1938–1969; (5), aa 1871–1969; and (6), aa 1771–1969 (Fig. 1 B); MHC B (aa 1632–1963); MHC C (aa 1639–1947); and MHC D (aa 1630–1938).
Protein and antibody purification
UNC-98 aa 1–112 and aa 1–310 were expressed using pET-24a, and UNC-97 (aa 146–201; the least conserved LIM domain) was expressed using both pET-24a and pGEX-6p-1 (GE Healthcare). The plasmids were transformed into E. coli BL21 (DE3)-RIL (Stratagene) and induced, and the proteins were purified as described previously (Mercer et al., 2003, 2006). Rabbits were immunized with 97-LIM3 (Spring Valley Laboratories, Inc.) to obtain Benian-16 antiserum. 97LIM-3∷GST and aa 1–112 of UNC-98 were induced and used to affinity purify Benian-16 and EU131 (Mercer et al., 2003), generating APBenian-16 and NAPEU131.
Myosin ELISAs
Total myosin II from wild-type C. elegans was prepared as described by Epstein et al. (1974) and MacLeod et al. (1977) except that the final step used a HiPrep 16/60 Sephacryl S-300 column. Fractions containing myosin were combined, and the concentration was determined. The ELISA was performed using the procedures described in Mercer et al. (2006) with the following alterations: plates were coated with 100 μl of myosin at 50 μM, incubated in 100 μl UNC-98 aa 1–112 or aa 1–310 (in 50 mM Tris, pH 7.5) at 0–1 μM, and reacted with 75 μl of anti–UNC-98 (APEU131) at 1:1,000.
Western blots
75 μg of wild-type, unc-98(sf19), and unc-98(su130) extracts and 50 μg of wild-type and unc-97(su110) extracts were separated and transblotted. The UNC-98 blot was exposed to antibodies affinity purified with full-length UNC-98 (APEU131) at 1:300 and aa 1–112 of UNC-98 (NAPEU131) at 1:1,000. The UNC-97 blot was exposed to anti–UNC-97 (APBenian-16) at 1:200. The proteins were visualized with HRP-conjugated secondary antibodies (1:10,000) and ECL (GE Healthcare).
Thick filament purification
Thick filaments from wild-type animals were purified as previously described (Epstein et al., 1988; Deitiker and Epstein, 1993). Proteins from each step of the procedure were separated on a 4–15% SDS-PAGE gel and transblotted. The blot was exposed to anti–UNC-98 (NAPEU131) at 1:200 or anti–UNC-97 (APBenian-16) at 1:100. The supernatant from the 5,000-g spin was fractionated by a 19–38% sucrose gradient. Fractions collected from the bottom of the gradient were loaded onto duplicate SDS-PAGE gels and transblotted. One blot was exposed to anti-actin (C4) at 1:2,500 (MP Biomedicals), anti-paramyosin (5–23) at 1:1,200, and anti-MHC B (5–8) 1:5,000 (Miller et al., 1983); the other was exposed to anti–UNC-98 (NAPEU131) at 1:100. Proteins were visualized as described above.
unc-97 RNAi
Embryos from rrf-3(pk1462) animals were suspended in S medium overnight to synchronize L1 larvae (Sulston and Hodgkin, 1988). L1 worms were fed bacteria (Kamath and Ahringer, 2003) expressing double-stranded RNA targeting unc-97 (Ahringer clone F14D12.2; Geneservice Ltd) until they reached young adulthood and were fixed.
Immunostaining
Wild-type animals were costained with anti–UNC-97 (APBenian-16; 1:100) and either anti–α-actinin (MH35) at 1:200 (Francis and Waterston, 1985) or anti–UNC-89 (MH42) at 1:200 (Benian et al., 1996) using the procedures of Nonet et al. (1993). Alexa 488– and Alexa 647–conjugated secondary antibodies (Invitrogen) were used at 1:200. Images were captured with a confocal microscopy system (Radiance 2100; Bio-Rad Laboratories, Inc.) and displayed using LaserSharp2000 software. Using procedures described in Benian et al. (1996), N2, unc-98(sf19), myo-3(st386); stEx30[Pmyo-3∷GFP; rol-6(su1006)], N2; sfEx12[unc-98∷GFP construct D; rol-6], unc-97 RNAi animals, and N2; sfEx25[unc-98∷GFP construct E] animals were stained using anti–MHC A (5–6) at 1:400 (Miller et al., 1983), anti–UNC-98 (APEU131) at 1:200 (Mercer et al., 2003), and anti–UNC-97 (APBenian-16) at 1:100. FITC and Cy-3–conjugated secondary antibodies (Jackson ImmunoResearch Laboratories) were used at 1:400. Images were captured with a deconvolution microscopy system described in Mercer et al. (2003) and processed using Photoshop (Adobe).
Online supplemental material
Table S1 is a summary of prey clones recovered from a yeast two- hybrid screen using the N terminus of UNC-98 as bait. Fig. S1 shows verification of the specificity of UNC-98 antibodies and demonstration that UNC-98, but not UNC-97, copurifies with thick filaments. Fig. S2 shows that anti–UNC-97 antibodies recognize a protein of ∼40 kD that localizes to M-lines and dense bodies of wild-type muscle. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200608043/DC1.
Supplementary Material
Acknowledgments
We thank Pam Hoppe and Bob Waterston for worm strain RW1596 and Bob Barstead for RB2, a random primed C. elegans cDNA library. We thank Dan Kalman and Krishna Bhat for the use of their deconvolution and confocal microscope systems. We also thank Denise Flaherty and Maureen Powers for advice on methods of protein purification, ELISA, and microscopy. Some strains used in this work were provided by the Caenorhabditis Genetics Center, which is supported by the National Center for Research Resources of the National Institutes of Health.
This work was supported by grants from the National Institutes of Health to G.M. Benian (AR051466 and AR052133), a predoctoral fellowship from the American Heart Association Southeast Affiliate to R.K. Miller (0415274B), and by grants from the National Institutes of Health (AR0500051) and the Muscular Dystrophy Association to H.F. Epstein.
Abbreviation used in this paper: MHC, myosin heavy chain.
References
- Benian, G.M., T.L. Tinley, X. Tang, and M. Borodovsky. 1996. The C. elegans gene unc-89, required for muscle M-line assembly, encodes a giant modular protein composed of Ig and signal transduction domains. J. Cell Biol. 132:835–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campagnola, P.J., A.C. Millard, M. Terasaki, P.E. Hoppe, C.J. Malone, and W.A. Mohler. 2002. Three-dimensional high-resolution second-harmonic generation imaging of endogenous structural proteins in biological tissues. Biophys. J. 82:493–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitiker, P.R., and H.F. Epstein. 1993. Thick filament substructures in C. elegans: evidence for two populations of paramyosin. J. Cell Biol. 123:303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibb, N.J., I.N. Maruyama, M. Krause, and J. Karn. 1989. Sequence analysis of the complete C. elegans myosin heavy chain gene family. J. Mol. Biol. 205:603–613. [DOI] [PubMed] [Google Scholar]
- Epstein, H.F., R.H. Waterston, and S. Brenner. 1974. A mutant affecting the heavy chain of myosin in C. elegans. J. Mol. Biol. 90:291–300. [DOI] [PubMed] [Google Scholar]
- Epstein, H.F., G.C. Berliner, D.L. Casey, and I. Ortiz. 1988. Purified thick filaments form the nematode C. elegans: evidence for multiple proteins associated with core structures. J. Cell Biol. 106:1985–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ervasti, J.M. 2003. Costameres: the Achilles' heel of Herculean muscle. J. Biol. Chem. 278:13591–13594. [DOI] [PubMed] [Google Scholar]
- Francis, G.R., and R.H. Waterston. 1985. Muscle organization in C. elegans: localization of proteins implicated in thin filament attachment and I-band organization. J. Cell Biol. 101:1532–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobert, O., D.G. Moerman, K.A. Clark, M.C. Beckerle, and G. Ruvkun. 1999. A conserved LIM protein that affects muscular adherens junction integrity and mechanosensory function in C. elegans. J. Cell Biol. 144:45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppe, P.E., and R.H. Waterston. 1996. Hydrophobicity variations along the surface of the coiled-coil rod may mediate striated muscle myosin assembly in C. elegans. J. Cell Biol. 135:371–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hresko, M.C., B.D. Williams, and R.H. Waterston. 1994. Assembly of body wall muscle and muscle cell attachment structures in C. elegans. J. Cell Biol. 124:491–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James, P., J. Halladay, and E.A. Craig. 1996. Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics. 144:1425–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath, R.S., and J. Ahringer. 2003. Genome-wide RNAi screening in C. elegans. Methods. 30:313–321. [DOI] [PubMed] [Google Scholar]
- Mackenzie, J.M., R.L. Garcea, J.M. Zengel, and H.F. Epstein. 1978. Muscle development in C. elegans mutants exhibiting retarded sarcomere construction. Cell. 15:751–762. [DOI] [PubMed] [Google Scholar]
- Mackinnon, A.C., H. Qadota, K.R. Norman, D.G. Moerman, and B.D. Williams. 2002. C. elegans PAT-4 /ILK functions as an adaptor protein within integrin adhesion complexes. Curr. Biol. 12:787–797. [DOI] [PubMed] [Google Scholar]
- MacLeod, A.R., R.H. Waterston, R.M. Fishpool, and S. Brenner. 1977. Identification of the structural gene for a myosin heavy-chain in C. elegans. J. Mol. Biol. 114:133–140. [DOI] [PubMed] [Google Scholar]
- McDonald, K.A., M. Lakonishok, and A.F. Horwitz. 1995. αv and α3 integrin subunits are associated with myofibrils during myofibrillogenesis. J. Cell Sci. 108:2573–2581. [DOI] [PubMed] [Google Scholar]
- Mercer, K.B., D.B. Flaherty, R.K. Miller, H. Qadota, T.L. Tinley, D.G. Moerman, and G.M. Benian. 2003. C. elegans UNC-98, a C2H2 Zn finger protein is a novel partner of UNC-97/PINCH in muscle adhesion complexes. Mol. Biol. Cell. 14:2492–2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer, K.B., R.K. Miller, T.L. Tinley, S. Sheth, H. Qadota, and G.M. Benian. 2006. C. elegans UNC-96 is a new component of M-lines that interacts with UNC-98 and paramyosin and is required in adult muscle for assembly and/or maintenance of thick filaments. Mol. Biol. Cell. 17:3832–3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, D.M., I. Ortiz, G.C. Berliner, and H.F. Epstein. 1983. Differential localization of two myosins within nematode thick filaments. Cell. 34:477–490. [DOI] [PubMed] [Google Scholar]
- Moerman, D.G., and B.D. Williams. 2006. Sarcomere assembly in C. elegans muscle. WormBook, editor. The C. elegans Research Community, WormBook, /10.1895/wormbook.1.81.1, http://www.wormbook.org. [DOI] [PMC free article] [PubMed]
- Nonet, M.L., K. Grundahl, B.J. Meyer, and J.B. Rand. 1993. Synaptic function is impaired but not eliminated in C. elegans mutants lacking synaptotagmin. Cell. 73:1291–1305. [DOI] [PubMed] [Google Scholar]
- Porter, G.A., G.M. Dmytrenko, J.C. Winkelmann, and R.J. Bloch. 1992. Dystrophin colocalizes with β-spectrin in distinct subsarcolemmal domains in mammalian skeletal muscle. J. Cell Biol. 117:997–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samarel, A.M. 2005. Costameres, focal adhesions, and cardiomyocyte mechanotransduction. Am. J. Physiol. Heart Circ. Physiol. 289:H2291–H2301. [DOI] [PubMed] [Google Scholar]
- Schachat, F., H.E. Harris, and H.F. Epstein. 1977. Two homogeneous myosins in body wall muscle of C. elegans. Cell. 10:721–728. [DOI] [PubMed] [Google Scholar]
- Schriefer, L.A., and R.H. Waterston. 1989. Phosphorylation of the N-terminal region of C. elegans paramyosin. J. Mol. Biol. 207:451–454. [DOI] [PubMed] [Google Scholar]
- Simmer, F., M. Tijsterman, S. Parrish, S.P. Koushika, M.L. Nonet, A. Fire, J. Ahringer, and R.H.A. Plasterk. 2002. Loss of the putative RNA-directed RNA polymerase RRF-3 makes C. elegans hypersensitive to RNAi. Curr. Biol. 12:1317–1319. [DOI] [PubMed] [Google Scholar]
- Sulston, J., and J. Hodgkin. 1988. Methods. In The Nematode C. elegans. W.B. Wood, editor. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York. 587–606.
- Waterston, R.H. 1989. The minor myosin heavy chain, MHC A, of C. elegans is necessary for the initiation of thick filament assembly. EMBO J. 8:3429–3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterston, R.H., D.G. Moerman, D.L. Baillie, and T.R. Lane. 1982. Mutations affecting myosin heavy chain accumulation and function in the nematode C. elegans. In Disorders of the motor unit. D.M. Schotland, editor. John Wiley, New York. 747–760.
- Williams, B.D., and R.H. Waterston. 1994. Genes critical for muscle development and function in C. elegans identified through lethal mutations. J. Cell Biol. 124:475–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zengel, J.M., and H.F. Epstein. 1980. Identification of genetic elements associated with muscle structure in C. elegans. Cell Motil. 1:73–97. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.