Abstract
Despite being a cell–matrix adhesion molecule, β4 integrin can prompt the multiplication of neoplastic cells dislodged from their substrates (anchorage-independent growth). However, the molecular events underlying this atypical behavior remain partly unexplored. We found that activation of the Met receptor for hepatocyte growth factor results in the tyrosine phosphorylation of β4, which is instrumental for integrin-mediated recruitment of the tyrosine phosphatase Shp2. Shp2 binding to β4 enhances the activation of Src, which, in turn, phosphorylates the multiadaptor Gab1 predominantly on consensus sites for Grb2 association, leading to privileged stimulation of the Ras–extracellular signal-regulated kinase (ERK) cascade. This signaling axis can be inhibited by small interfering RNA–mediated β4 depletion, by a β4 mutant unable to bind Shp2, and by pharmacological and genetic inhibition of Shp2 or Src. Preservation of the β4 docking sites for Shp2 as well as the integrity of Shp2, Src, or ERK activity are required for the β4-mediated induction of anchorage-independent growth. These results unravel a novel pathway whereby β4 directs tyrosine kinase–based signals toward adhesion-unrelated outcomes.
Introduction
The β4 integrin is a laminin receptor that mediates the stable adhesion of epithelial cells to the basal membrane (when incorporated in hemidesmosomes) and movement of carcinoma cells through the extracellular matrix (when redistributed at actin-rich protrusions; Mercurio and Rabinovitz, 2001; Litjens et al., 2006). These roles of β4 in modulating static or dynamic adhesion and in translating positional cues into different cellular fates are consistent with a conventional mechanochemical activity that has been traditionally ascribed to integrins. But, in addition to these properties, β4 is also endowed with an unorthodox function: it can be a positive regulator of the anchorage-independent growth of neoplastic cells (Zahir et al., 2003; Bertotti et al., 2005; Lipscomb et al., 2005). This function is counterintuitive given the established notion that transformed cells are able to grow and survive in the absence of adhesion and, thus, without demanding integrin-derived signals (Guo and Giancotti, 2004). Nonetheless, this atypical behavior could explain the frequently observed up-regulation of β4 in human carcinomas (Mercurio and Rabinovitz, 2001) and suggests that β4 overexpression could afford cells with a selective advantage for proliferation and survival in neoplastic contexts where tissue architecture and canonical cell–matrix interactions are compromised (Wilhelmsen et al., 2006).
A few modes of action have been proposed to explain this feature. In some instances, carcinoma cells may secrete abundant quantities of laminin-5, which can ligate β4 and sustain the expansion of nonadherent colonies through activation of a Rac–nuclear factor κB antiapoptotic pathway (Zahir et al., 2003). Alternatively, the expression of β4 is accompanied by the translational up-regulation of VEGF, which can act as a survival factor favoring colony formation (Lipscomb et al., 2005). Finally, we have recently demonstrated that β4 can promote anchorage-independent growth in response to activation of the Met receptor for hepatocyte growth factor (HGF; Bertotti et al., 2005). This activity does not rely on laminin engagement, as it is retained by a β4 truncated variant devoid of most of the extracellular domain and requires Met-dependent tyrosine phosphorylation of the β4 cytoplasmic domain. However, the signaling pathway regulating this laminin-independent activity remains to be elucidated. Using biochemical, pharmacological, and genetic approaches, we found that the Met-mediated phosphorylation of β4 initiates a previously unexplored transduction pathway that involves Shp2 recruitment, Src activation, and Gab1 phosphorylation and ultimately leads to dedicated stimulation of the MAPK/extracellular signal-regulated kinase (ERK) cascade. These results substantiate the crucial role of β4 in epithelial tumorigenesis and describe a novel integrin-dependent signaling pathway that could be exploited by growth factor receptors for the implementation of oncogenic responses.
Results
β4 integrin recruits the tyrosine phosphatase Shp2
We previously demonstrated that the β4 cytodomain can be tyrosine phosphorylated by Met. This event potentiates HGF-mediated cellular invasion through the stimulation of Ras and PI3K (Trusolino et al., 2001) and enhances anchorage-independent growth through still uncharacterized mechanisms (Bertotti et al., 2005). Although the signals implicated in Met/β4-dependent cell invasion also play a crucial role in cellular proliferation and are likely to contribute to β4-mediated anchorage-independent growth, the involvement of additional transduction pathways cannot be excluded.
To begin to explore new signaling circuits potentially controlled by β4 activity, we performed a progressive tyrosine/phenylalanine mutagenesis along the β4 tail and mapped three tyrosines (Tyr1257, Tyr1440, and Tyr1494) that, when sequentially mutated into phenylalanines, cause a gradual reduction in β4 phosphotyrosine content up to complete abrogation (Bertotti et al., 2005). Interestingly, an in silico analysis of the sequences surrounding these tyrosines revealed common structural features (Fig. 1 A): Tyr1257 and Tyr1494 are both located within immune T cell inhibitory motifs that have been characterized as canonical binding regions for protein and lipid phosphatases, including the SH2-containing tyrosine phosphatase Shp2; similarly, Tyr1440 is embedded in a degenerated consensus for Shp2. Based on these observations, we hypothesized that Tyr1257, Tyr1440, and Tyr1494 might be collectively involved in central signaling functions whose trait of union is multiple binding for Shp2.
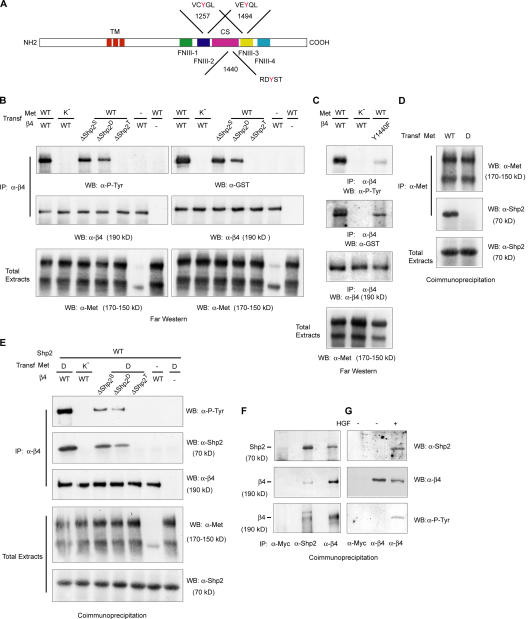
Figure 1.
Tyrosine-phosphorylated β4 interacts with Shp2. (A) Schematic representation of β4 structure. The cytoplasmic domain contains two pairs of type III fibronectin-like repeats (FNIII) separated by a connecting segment (CS). The consensus sequences for Shp2 and tyrosine positions are indicated. TM, transmembrane domain. (B) β4–Shp2 association by far-Western analysis. COS-7 cells were cotransfected with wild-type (WT) or kinase-dead (K−) Met and with wild-type β4 or β4 mutants bearing single (β4-ΔShp2S; Y1257F), double (β4-ΔShp2D; Y1257F and Y1494F), and triple (β4-ΔShp2T; Y1257F, Y1440F, and Y1494F) phenylalanine substitutions of the potential docking tyrosines for Shp2. Lysates from each experimental point were subdivided into two aliquots and immunoprecipitated (IP) in parallel with antibodies to β4; Western blots (WB) were probed with antiphosphotyrosine (P-Tyr) antibodies (left) or with a GST-Shp2 fusion protein followed by anti-GST antibodies (right). The blots were then reprobed with anti-β4 antibodies. A fraction of the lysates (1/50) was loaded to detect Met from total extracts. In COS-7 cells, exogenous Met is expressed as both the precursor (top band) and mature form (bottom band). The weak band observable in the sixth lane corresponds to endogenous Met. (C) A β4 mutant with a single phenylalanine permutation of Tyr1440 (β4-Y1440F) displays reduced but detectable tyrosine phosphorylation and can bind Shp2 in far-Western analysis. (D) Coimmunoprecipitation experiment showing that wild-type Met but not a Met mutant with phenylalanine substitutions of the two tyrosines of the multidocking site (MetD) associates with Shp2. (E) Coimmunoprecipitation of β4 and Shp2 from lysates of COS-7 cells overexpressing Shp2 and the indicated forms of Met and β4. (F and G) Coimmunoprecipitation of β4 and Shp2 in cells with the endogenous expression of both proteins. (F) Reciprocal coimmunoprecipitation experiment in GTL16 cells. Cell lysates were immunoprecipitated with antibodies to myc (negative control), Shp2 (positive control), or β4. Blots were probed with antibodies against Shp2 (top), β4 (middle), or phosphotyrosine (P-Tyr; bottom). Shp2 is present in β4 immunoprecipitates (top), and β4 is present in Shp2 immunoprecipitates (middle). Immunopurified β4 and Shp2-associated β4 appear to be constitutively tyrosine phosphorylated. (G) FG2 cells were either left untreated or were treated with 100 ng/ml HGF for 10 min. Lysates were immunoprecipitated with antibodies to myc or β4, and blots were probed as indicated. Shp2 can be recovered from β4 immunoprecipitates after HGF stimulation (top). Treatment with HGF induces the tyrosine phosphorylation of β4 (bottom).
To investigate this issue, we initially tested the potential association between β4 and Shp2 using an overexpression system (Fig. 1, B, C, and E). COS-7 cells were transiently transfected with the following: (1) the wild-type forms of Met and β4, a condition in which Met overexpression results in elevated tyrosine phosphorylation of the integrin; (2) wild-type β4 and a kinase-inactive variant of Met (MetK−), which is unable to phosphorylate the integrin; (3) wild-type Met and β4 mutants bearing single (β4-ΔShp2S), double (β4-ΔShp2D), or triple (β4-ΔShp2T) phenylalanine substitutions of the three critical tyrosines, with the consequent progressive reduction of β4 tyrosine phosphorylation levels (Fig. 1, B and D); and (4) wild-type β4 alone or wild-type Met alone as negative controls.
First, we examined direct interaction in vitro between β4 and Shp2 in far-Western analysis using a GST fusion protein of the Shp2 N-terminal SH2-containing domain (GST-Shp2). This probe recognized β4 immunoprecipitates when the integrin was fully phosphorylated by activated Met but not when Met-dependent phosphorylation was inhibited (Fig. 1 B). Association between GST-Shp2 and β4 was gradually weaker when using the single and double β4 mutants and was totally prevented upon expression of the β4 triple mutant (Fig. 1 B), indicating that all of the putative consensus sites for Shp2 are involved in this interaction. Notably, the efficacy of Shp2 binding paralleled the extent of β4 phosphorylation, suggesting that the tyrosines involved in Shp2 recruitment contain the bulk of the integrin substrate capacity for Met (Fig. 1 B). Tyrosine phosphorylation of β4 as well as Shp2 binding could not be observed in the absence of transfected Met (Fig. 1 B). Similarly, the transfection of Met alone was ineffective because COS-7 cells do not express endogenous α6β4 (Niessen et al., 1997). When Tyr1257 and Tyr1494 were mutated into phenylalanines, the overall phosphotyrosine content of β4 partially decreased, and β4–Shp2 interaction was less efficient; however, the complete abolition of β4 phosphorylation and Shp2 association was obtained only upon the phenylalanine permutation of Tyr1440 (Fig. 1 B). Thus, in principle, Tyr1440 could autonomously account for all of the observed binding events. To rule out this possibility, we repeated the far-Western experiment using a β4 mutant bearing a single phenylalanine substitution of Tyr1440 (β4-Y1440F). In COS-7 cells overexpressing Met and β4-Y1440F, the tyrosine phosphorylation of β4 and interaction between β4 and Shp2 were reduced but not abolished (Fig. 1 C). This indicates that Tyr1440 is necessary but not sufficient for Shp2 association and that Tyr1257 and Tyr1494 display a residual binding activity for the phosphatase.
Next, we analyzed association in vivo by coimmunoprecipitation experiments in COS-7 cells overexpressing Shp2 and the various forms of Met and β4. Met has been reported to associate with both Shp2 (Fixman et al., 1996) and β4 (Trusolino et al., 2001). Therefore, it may act as a bridging molecule between the integrin and the phosphatase. To exclude this possibility and assess a direct interaction between β4 and Shp2, we exploited a Met variant (MetD) that retains full catalytic activity (therefore, it is still able to phosphorylate β4) but contains phenylalanine substitutions of Tyr1349 and Tyr1356, which represent the docking residues for several signal transducers, including Shp2 (Ponzetto et al., 1994; Fixman et al., 1996; Furge et al., 2000). This mutant was validated for its inability to bind Shp2 (Fig. 1 D) and for its capacity to phosphorylate β4 (Fig. 1 E) upon transient transfection in COS-7 cells. Under these conditions, Shp2 could be efficiently recovered in β4 immunoprecipitates. Conversely, the interaction was abolished in the absence of β4 phosphorylation or in the presence of the β4 triple mutant displaying complete substitution of the critical tyrosines and was progressively diminished in the single and double mutants (Fig. 1 E). Again, the efficiency of the β4–Shp2 interaction paralleled the extent of β4 phosphorylation; no tyrosine phosphorylation of β4 nor association with Shp2 could be detected when β4 or Met were transfected individually (Fig. 1 E).
Finally, we assessed whether tyrosine-phosphorylated β4 can also recruit Shp2 in cells expressing endogenous proteins. Accordingly, we set up coimmunoprecipitation experiments in GTL16 cells, which express a constitutively active form of Met, and in FG2 cells, in which Met phosphorylation is inducible by HGF. Indeed, the association of Shp2 with β4 could be detected basally in GTL16 (Fig. 1 F) and only after ligand stimulation in FG2 cells (Fig. 1 G). The binding between tyrosine-phosphorylated β4 and Shp2 is specific, as no coimmunoprecipitation of Shp2 was observed with antibodies against an unrelated myc antigen (Fig. 1, F and G). As expected, β4 tyrosine phosphorylation was constitutive in GTL16 cells and was induced by HGF in FG2 cells (Fig. 1, F and G).
Association between β4 and Shp2 leads to increased phosphorylation of Gab1 through a Src-dependent mechanism
Translocation of Shp2 at the plasma membrane is essential for its function (Neel et al., 2003); therefore, the interaction of Shp2 with the intracellular domain of β4 is likely to stimulate Shp2-dependent transduction pathways. One of the best-documented activities of Shp2 relies on its ability to stimulate Src catalysis by controlling Csk recruitment (Ren et al., 2004; Zhang et al., 2004). This notion, together with the established observation that Src can promote anchorage-independent growth when aberrantly activated (Yeatman, 2004), prompted us to investigate whether the signaling contribution of β4 to this process could entail the stimulation of Src.
To explore this subject, we examined the activation of Src in MDA-MB-435 breast carcinoma cells, which do express Met but are devoid of β4, and their counterpart cells stably transfected with a human β4 cDNA (MDA-MB-435–β4 cells; Fig. 2 A). Under basal conditions, Src enzymatic function was already higher in β4 cells compared with mock cells, possibly as a result of residual Met-based β4 signaling or to adhesion-triggered Src stimulation as previously demonstrated (Dans et al., 2001; Gagnoux-Palacios et al., 2003). This priming favored the further activation of Src in response to HGF; indeed, ligand stimulation led to a rapid boost of Src activity in β4 cells, whereas the HGF-dependent activation of Src in mock cells displayed slower kinetics and weaker intensity (Fig. 2 B). To evaluate whether the stronger and accelerated activation of Src in β4-expressing cells is caused by the integrin ability to bind Shp2, we generated MDA-MB-435 transfectants expressing the β4 triple variant that is unable to bind Shp2 (hereafter referred to as β4ΔShp2). This mutant was exposed at the cell surface as efficiently as wild-type β4 together with the endogenous α6 subunit (Fig. 2 A). In line with our hypothesis, disruption of the association between β4 and Shp2 almost abolished the HGF-dependent activation of Src (Fig. 2 B). Interestingly, the β4ΔShp2 mutant affected the stimulation of Src more potently than the simple absence of β4, suggesting that this variant could not only behave as a signaling-dead molecule but also as a dominant repressor of other endogenous β4 signaling partners. Accordingly, β4 has been shown to impinge on signals emanating from the Ron tyrosine kinase receptor (Santoro et al., 2003), the EGF receptor (Mainiero et al., 1996; Rabinovitz et al., 1999), Erb-B2 (Gambaletta et al., 2000; Guo et al., 2006), and tetraspanins (Yang et al., 2004).
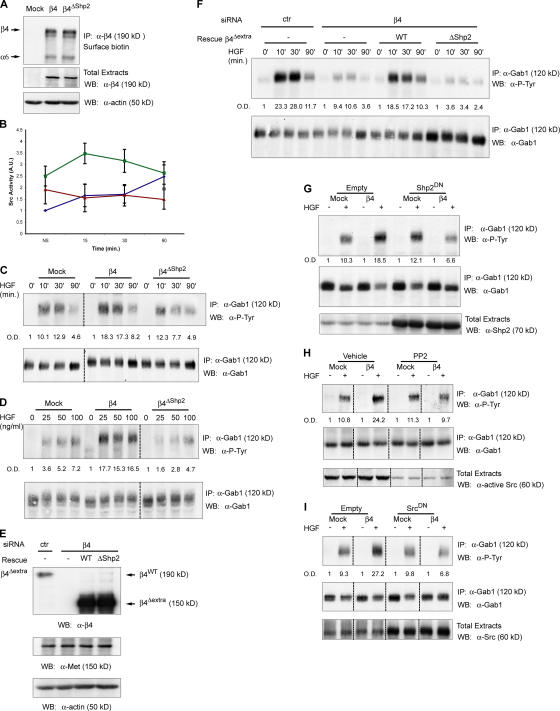
Figure 2.
Association between β4 and Shp2 leads to the increased phosphorylation of Gab1 through a Src-dependent mechanism. (A) Expression of β4 or the β4 mutant unable to bind Shp2 (β4ΔShp2) in MDA-MB-435 cells. Both molecules are exposed at the cell surface, as assessed by surface biotinylation (top), and are expressed at comparable levels, as shown in total cell extracts (middle). Equal loading was verified by probing the blot with antiactin antibodies (bottom). Surface biotinylation detects the entire α6β4 integrin complex. (B) In vitro kinase assay measuring Src activation upon stimulation with 100 ng/ml HGF at different time points in mock (blue), β4 (green), and β4ΔShp2 (red) MDA-MB-435 cells. Data are the means ± SEM (error bars) of five independent experiments performed in duplicate. (C) Differential Gab1 phosphorylation upon HGF stimulation for different times in mock, β4, and β4ΔShp2 cells. MDA-MB-435 transfectants were stimulated with 50 ng/ml HGF, and lysates were immunoprecipitated and probed as indicated. Dotted lines indicate the grouping of images from different parts of the same gel. Gab1 phosphorylation was quantitated by densitometric analysis (OD) relative to Gab1 protein content in immunoprecipitates. (D) Differential Gab1 phosphorylation upon stimulation with different doses of HGF for 30 min in mock, β4, and β4ΔShp2 cells. (E) Western blot analysis showing β4 expression in lysates from MDA-MB-231 cells infected with a scrambled (ctr) siRNA or with a β4-specific siRNA; β4 siRNA cells were reinfected with wild-type β4Δextra or with a ΔShp2 mutant. In all transfectants, Met expression was unaffected. (F) Differential Gab1 phosphorylation in the different MDA-MB-231 transfectants. (G–I) Inhibition of Shp2 and Src impairs the HGF-dependent phosphorylation of Gab1 in MDA-MB-435–β4 but not in mock cells. Cells were transiently transfected with a control vector (empty) or with catalytically inactive isoforms of either Shp2 (Shp2DN; G) or Src (SrcDN; I).The exogenous expression of Shp2 and Src is shown in the bottom panels (G and I). In H, cells were pretreated with 5 μM PP2 for 30 min. The PP2-mediated inhibition of Src was assessed with an antibody against active Src (bottom; H). HGF was given at 50 ng/ml for 30 min.
Next, we searched for a Src substrate that could be a potential target of this Met–β4–Shp2 signaling pathway. The Gab1 multiadaptor protein is the major Met substrate (Birchmeier et al., 2003) and can also be phosphorylated by Src (Chan et al., 2003). To verify whether the activation of Src in β4-expressing cells supports a more efficient phosphorylation of Gab1 in response to HGF, we analyzed Gab1 phosphorylation levels upon HGF stimulation in mock and β4 cells. In time-course experiments, the HGF-induced phosphorylation of Gab1 was more intense and durable in β4 than in mock cells (Fig. 2 C). The increased phosphorylation of Gab1 in β4 cells likely depends on Shp2 recruitment; indeed, the HGF treatment of cells expressing the β4ΔShp2 mutant led to a less efficient phosphorylation of Gab1 compared with cells expressing wild-type β4 (Fig. 2 C). Likewise, stimulation with increasing amounts of HGF produced a weak dose-dependent curve of Gab1 phosphorylation in mock cells. Conversely, Gab1 phosphorylation in β4 cells reached a plateau at a very low concentration of ligand, and the overall phosphorylation levels were more robust than in mock transfectants (Fig. 2 D). Again, the response of β4ΔShp2 cells was similar or even lower than that showed by mock cells (Fig. 2 D).
In a complementary approach, we abated β4 levels by lentiviral delivery of siRNA in MDA-MB-231 breast carcinoma cells, which endogenously synthesize both Met and the integrin (Fig. 2 E). We then reestablished β4 expression in β4-deficient cells using a siRNA-resistant variant that lacks most of the extracellular portion, including the siRNA target region, but retains the ability to transduce Met-dependent responses (β4Δextra; Trusolino et al., 2001; Bertotti et al., 2005). In addition to wild-type β4Δextra, which displays an intact cytoplasmic domain, we also expressed a β4Δextra mutant with phenylalanine substitutions of the tyrosines involved in Shp2 binding (Fig. 2 E). In all cases, Met expression was unaffected (Fig. 2 E). Consistent with that observed in MDA-MB-435 cells, β4 knockdown in MDA- MB-231 resulted in the reduced HGF-dependent phosphorylation of Gab1, whereas the rescue of β4 expression was accompanied by the restoration of higher Gab1 phosphorylation levels in the presence of a signaling-competent cytoplasmic portion but not when Shp2 recruitment was abolished (Fig. 2 F).
If Shp2 and Src specifically contribute to Gab1 phosphotyrosine content in a β4-dependent manner, the attenuation of Shp2 and/or Src activity should affect the HGF-triggered phosphorylation of Gab1 only in cells expressing β4. In fact, Shp2 inhibition by expression of a catalytically inactive dominant interfering isoform (Shp2DN) resulted in the decreased phosphorylation of Gab1 in MDA-MB-435–β4 cells but not in mock cells (Fig. 2 G). Similar results were obtained when mock and β4 cells were treated with the Src pharmacological inhibitor PP2 (Fig. 2 H) or upon transfection of a kinase-dead variant of Src (SrcDN; Fig. 2 I).
β4-dependent activation of Src leads to the selective association of Gab1 with Grb2
Gab1 is a scaffolding adaptor that associates with a variety of signal transducers after phosphorylation on multiple sites (Gu and Neel, 2003). We wondered whether the Src-dependent increase of Gab1 phosphorylation in β4 cells leads to a generic, quantitative enhancement of the overall Gab1 phosphotyrosine content or to a selective, qualitative phosphorylation of defined tyrosine residues with consequent specific binding to a given transducer.
To investigate this issue, we overexpressed Gab1 in the various MDA-MB-435 transfectants and assessed the ability of Gab1 to form supramolecular complexes with a representative panel of SH2-containing signaling molecules, including Grb2, Shc, PI3K, and Shp2 itself. Among these signaling effectors, Grb2 appeared to bind Gab1 more efficiently in β4 versus mock cells after HGF stimulation (Fig. 3 A). This binding was reduced in cells expressing the β4ΔShp2 mutant (Fig. 3 B); similarly, the PP2-mediated inhibition of Src, which decreases HGF-induced Gab1 phosphorylation only in β4 cells, impaired the association of Grb2 with Gab1 in the presence but not in the absence of β4 (Fig. 3 B).
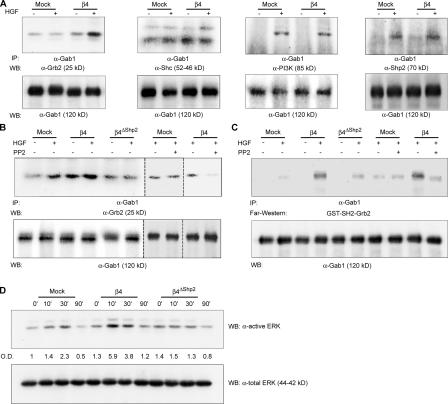
Figure 3.
β4-dependent activation of Src leads to the selective association of Gab1 with Grb2 and to the increased activation of ERK/MAPKs. (A) HGF stimulation results in the increased association of Gab1 with Grb2 in β4 cells. MDA-MB-435 mock and β4 cells were transiently transfected with Gab1 and stimulated with 100 ng/ml HGF for 30 min. Lysates were immunoprecipitated with antibodies to Gab1, and blots were probed as indicated. Densitometric analysis of the blots obtained in four experiments indicates that the HGF-dependent association of Grb2 with Gab1 in mock cells is increased 1.26-fold (± 0.48) compared with nonstimulated cells, whereas the HGF-dependent association of Gab1 with Grb2 in β4 cells is increased 4.7-fold (± 0.98; P < 0.01). (B) Abrogation of Shp2 binding or Src inhibition with PP2 impairs the coimmunoprecipitation of Gab1 with Grb2 in β4 cells. Dotted lines indicate the grouping of images from different parts of the same gel. (C) Far-Western analysis showing the association between immunoprecipitated Gab1 and GST-SH2-Grb2. In accordance with the coimmunoprecipitation data, HGF-dependent Gab1–Grb2 interaction is increased in β4 cells compared with mock and β4ΔShp2 transfectants. PP2-mediated inhibition of Src results in decreased Gab1–Grb2 interaction in β4 but not in mock cells. (D) HGF activates ERK/MAPKs longer and more efficiently in β4 cells than in mock or β4ΔShp2 cells. Cells were treated with 100 ng/ml HGF for the indicated times, and total lysates were probed on blots as indicated. Band intensities were quantitated by densitometric analysis with respect to total protein content.
Grb2 can bind tyrosine-phosphorylated Gab1 using the SH2 domain, but it also combines with Gab1 in a phosphorylation-independent manner using the SH3 domain (Holgado-Madruga et al., 1996). To verify that the increased association between Gab1 and Grb2 in β4 cells is in fact caused by the enhanced HGF-dependent phosphorylation of the Gab1 tyrosines responsible for SH2-mediated Grb2 binding, we performed a far-Western analysis using a GST fusion protein of the Grb2 SH2 domain (GST-SH2-Grb2). In accordance with the coimmunoprecipitation data, the interaction between GST-SH2-Grb2 and Gab1 after HGF stimulation was much more efficient in β4 cells. Again, binding was decreased in cells expressing the β4ΔShp2 mutant, and treatment with PP2 impaired Gab1–Grb2 association in β4 transfectants but not in mock cells (Fig. 3 C). Together, these results indicate that the Shp2-mediated activation of Src in β4 cells leads to the dedicated phosphorylation of Gab1 on tyrosine residues specifically responsible for Grb2 binding, a preferential association that can be abrogated by uncoupling β4 from Shp2 or by hindering Src function. Privileged interaction between Gab1 and Grb2 in β4 cells results in the increased stimulation of Ras-dependent effectors: indeed, the amplitude and persistence of ERK/MAPK activation after HGF stimulation were higher in β4 compared with mock and β4ΔShp2 transfectants (Fig. 3 D).
Shp2 and Src are required to promote β4-mediated anchorage-independent growth
To verify whether the aforementioned signaling pathway is in fact responsible for the ability of β4 to promote anchorage-independent growth, we performed soft agar assays on several different MDA-MB-435 transfectants (Figs. 2 E and 4 A). As expected, the ectopic expression of β4 considerably enhanced the basal and HGF-stimulated formation of suspended colonies compared with mock cells both in absolute numbers (Fig. 4 B, graph) and in size (Fig. 4 B, images). Analogous results were obtained upon transfection of the β4Δextra variant, further validating the role of this nonadhesive mutant as an efficient substitute of wild-type β4 in HGF-driven responses and confirming the notion that the Met-dependent signaling activity of β4 does not require laminin ligation (Fig. 4 B; Trusolino et al., 2001; Bertotti et al., 2005). In contrast, expression of the β4ΔShp2 signaling-dead mutant was less effective, indicating that the interaction between β4 and Shp2 is critical for this process (Fig. 4 B). Transfection of dominant-negative isoforms of Shp2 or Src in β4-expressing cells (Fig. 4 B) as well as treatment of β4 cells with the Src inhibitor PP2 or with the ERK inhibitor PD98059 (Fig. 4 C) potently impaired HGF-dependent clonogenic activity in soft agar. Together, these results reinforce the observation that Shp2, Src, and ERKs are central downstream transducers of β4-driven anchorage-independent growth.
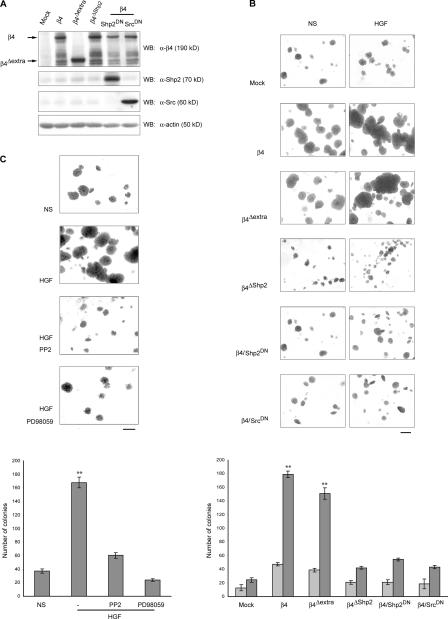
Figure 4.
β4 sustains HGF-induced anchorage-independent growth through the activation of Shp2, Src, and ERK. (A) Western blot analysis showing expression of the indicated cDNAs in MDA-MB-435 cells subjected to soft agar assays. Filters probed with anti-Shp2 and -Src antibodies were deliberately underexposed to better visualize the overexpression of exogenous proteins. (B) Representative images and quantitation of soft agar assays in MDA- MB-435 cells with ectopic expression of the indicated proteins in the absence (NS) or presence of 30 ng/ml HGF. Pictures show colony aspect at high magnification, whereas the graph reports colony numbers as counted at low magnification over the entire microscopic field. Light bars, no stimulation; dark bars, HGF. (C) Representative images and quantitation of soft agar assays in MDA-MB-435 cells expressing β4 that were either left untreated (NS) or were treated with HGF in the absence or presence of the Src inhibitor PP2 or the ERK inhibitor PD98059. Data are the means ± SEM (error bars) of two independent experiments performed in triplicate. **, P < 0.01 with respect to all other transfectants. Bars, 400 μm.
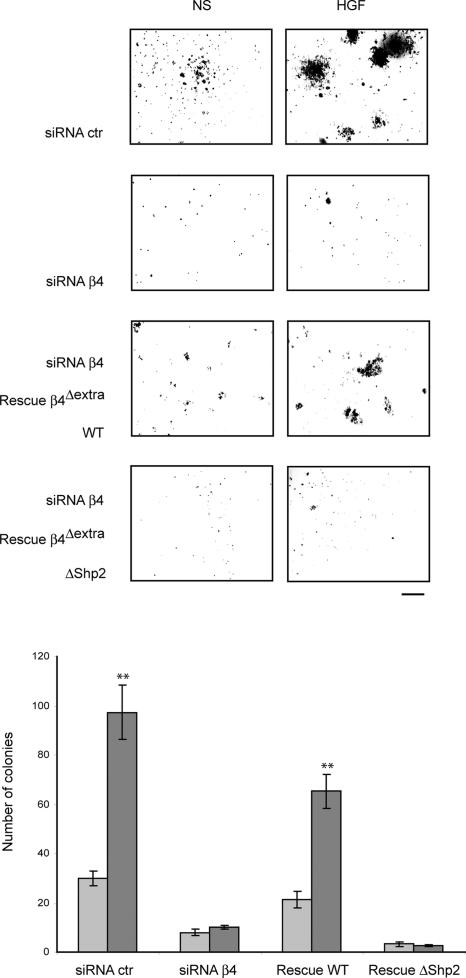
In a complementary way, siRNA-mediated reduction of β4 expression in MDA-MB-231 cells led to an almost complete abolition of basal and HGF-stimulated anchorage-independent growth. Colony-forming ability was partially reestablished upon the expression of the β4Δextra siRNA-resistant variant displaying a wild-type intracellular domain but not upon expression of the ΔShp2 mutant (Fig. 5).
Figure 5.
β4 sustains HGF-induced anchorage-independent growth through the recruitment of Shp2. Representative images and quantitation of soft agar assays in MDA-MB-231 cells displaying the artificial manipulation of β4 levels as shown in Fig. 2 E. Data are the means ± SEM (error bars) of two independent experiments performed in triplicate. **, P < 0.01 with respect to all other transfectants. WT, wild type. Bar, 400 μm.
Discussion
When a normal cell of epithelial origin happens to lose contact with the basement membrane, the surrounding stromal compartment impedes its multiplication and, in the meantime, stimulates its apoptotic elimination (Frisch and Screaton, 2001). To avoid this and to ensure successful metastatic dissemination, malignant elements undergo an adaptive process that relies mainly on two strategies. One is the abnormal and unpolarized secretion of basement membrane molecules, which aberrantly reconstitute the original histological niche, thus imparting surrogate proliferative and survival signals. Parallel (or alternative) to this is the hyperactivation of oncogenic pathways, which produce a global amplification of the cell signaling activity with consequent elusion of the external adhesive consensus (Hanahan and Weinberg, 2000). Whatever the mechanism used, the phenotypic outcome of this process is that neoplastic cells acquire the ability to grow in the absence of proper anchorage.
In this study, we show that the β4 integrin conspires with the tyrosine kinase Met for efficient execution of anchorage-independent growth by channeling Met signals toward activation of the Ras-ERK oncogenic cascade. This function relies on Met-triggered phosphorylation of the β4 cytoplasmic domain and on the ensuing recruitment of the tyrosine phosphatase Shp2 to the integrin tail. β4–Shp2 association results in the stimulation of Src, which, in turn, favors a more efficient phosphorylation of the Gab1 multiadaptor protein, mainly on tyrosines involved in Grb2 binding. Ultimately, privileged interaction between Gab1 and Grb2 leads to dedicated stimulation of the MAPK–ERK pathway.
Structural aspects of β4–Shp2 interaction
Association between β4 and Shp2 depends on the integrity of three tyrosines along the β4 cytoplasmic domain (Tyr1257, Tyr1440, and Tyr1494) that are inserted in canonical or degenerated motifs for the SH2 domain of Shp2 and, consequently, could act as docking sites for the phosphatase when phosphorylated. However, based on the crystal structure of a fragment of the β4 intracellular portion encompassing the first pair of type III fibronectin-like (FNIII) repeats (de Pereda et al., 1999), interaction between Shp2 and Tyr1257 appears to be more complicated because this tyrosine is part of the hydrophobic core of the second repeat (Fig. 1 A). Thus, it may not be easily accessible for phosphorylation because of conformational constraints.
This information is in disagreement with our findings and with a previous study showing the phosphorylation of Tyr1257 in response to the antibody-mediated ligation of β4 (Shaw, 2001). A possible explanation for this discrepancy is that although the aromatic ring of Tyr1257 is in fact involved in hydrophobic interactions with other residues and is not accessible to the solvent, the side chain is not completely buried in the FNIII core so that the OH group reaches the solvent-accessible surface of the protein. This kind of spatial organization seems to be permissive for phosphorylation; for example, the human Vaccinia H1-related phosphatase is phosphorylated by ZAP-70 at Tyr138 despite that only the OH group of the amino acid is partially exposed to the solvent (Alonso et al., 2003). Moreover, physical association with Met could affect the structural organization of β4 and induce a more relaxed conformation of the FNIII repeat, facilitating recognition by the kinase and transfer of the phosphate group.
In any case, our observation that the phenylalanine permutation of Tyr1257 results in reduction of the overall β4 phosphotyrosine content and in a less efficient association with Shp2 does not exclude the possibility that Tyr1257 is not a direct phosphorylation site, but it is necessary for the phosphorylation of other residues that, in turn, could be involved in Shp2 recruitment. This hypothesis is in agreement with the general notion that bindings between SH2 domains and the corresponding ligand motifs hardly occur in rigid structural environments such as the FNIII fold.
A novel signaling pathway connecting β4 integrin to ERK activation: biochemical and biological insights
The importance of the signaling axis explored here in β4-mediated anchorage-independent growth is underscored by the observations that a β4 mutant unable to bind Shp2 cannot sustain this process and that interference with Shp2, Src, or ERK impairs the biological activity of wild-type β4. Interestingly, Shp2 is known to act downstream from Met when physically associated with Gab1 to sustain the prolonged stimulation of ERKs (Maroun et al., 2000). However, the signaling intermediates propagating this activity remain elusive (Gu and Neel, 2003). Our findings confirm the crucial role of Shp2 as a positive regulator of Ras-dependent signals in response to HGF and provide an alternative pathway as well as several additional molecular actors that connect Met function to the enhancement of ERK activity.
The unorthodox observation that β4 contributes to the malignant phenotype by acting as a biochemical selector of tumorigenic signaling pathways unveils an unforeseen function that transcends the activity of β4 as an integrin and assimilates it to classical signal relay adapters endowed with oncogenic potential. Something similar has been described for β1A integrin, the most widely expressed spliced isoform of β1 integrins: in this case, interaction between β1A and the insulin-like growth factor-I tyrosine kinase receptor results in integrin-mediated recruitment of the downstream effector IRS1, which inhibits cell adhesion and stimulates insulin-like growth factor–dependent cellular proliferation and tumor growth (Goel et al., 2004).
Most of the signaling effectors along the transduction pathway discussed in this study are involved in human malignancies in vivo: Met, β4, and Src are often overexpressed (and coexpressed) in a vast number of human carcinomas (Mercurio and Rabinovitz, 2001; Trusolino and Comoglio, 2002; Ishizawar and Parsons, 2004), and Shp2 gain of function mutations have been described in some solid tumors and in various forms of leukemia (Bentires-Alj et al., 2004). Gab2, a close homologue of Gab1, is amplified and overexpressed in a fraction of human breast tumors and collaborates with the ErbB2 oncogene for activation of a Shp2–ERK pathway that drives mammary carcinogenesis (Bentires-Alj et al., 2006). Intriguingly, β4 has recently been shown to cooperate with ErbB2 in breast cancer onset (Guo et al., 2006). Finally, constitutively active Src transcriptionally up-regulates HGF in breast carcinoma cells, generating an autocrine loop that promotes cancer progression (Wojcik et al., 2006). Altogether, these findings suggest not only a functional role in vivo but also a cooperative effect and a potential epistatic relationship for these signals.
Finally, it is worth noting that the tyrosines involved in Shp2 binding represent biochemical hotspots for β4 signaling capacity also with respect to other transduction pathways: Tyr1257 is necessary for β4 phosphorylation in response to laminin ligation (Shaw, 2001), Tyr1440 is responsible for SH2-mediated interaction with the Grb2 upstream effector Shc (Dans et al., 2001), and Tyr1494 is involved in the adhesion-driven stimulation of PI3K (Shaw, 2001). It will be interesting to analyze whether these additional transducers can complement or substitute for the signaling axis identified in this study for fostering β4-mediated anchorage-independent growth and possibly tumorigenesis.
Met and β4 in cancer onset and progression
Accumulating evidence suggests that the aptitude of a neoplasm to disseminate at distant sites is already preordained by the protein repertoire that progenitor cells express relatively early in tumorigenesis. This indicates that the phenotype acquired en route by an evolving tumor cell can confer not only a selective advantage for cell replication (a condition necessary for formation of the primary tumor mass) but also, later during neoplastic progression, the tendency to metastasize (Bernards and Weinberg, 2002).
This line of thinking fits nicely with our findings on the role of β4 and Met in tumor evolution. We and others have demonstrated that both molecules, individually or in combination, are able to enhance the motility and survival of cancer cells as a prelude for neoplastic invasion and metastasis (Mercurio and Rabinovitz, 2001; Trusolino and Comoglio, 2002). Here, we show that the same molecules can also cooperate for anchorage-independent growth, which is the experimental and phenotypic hallmark of primary tumorigenesis. Together, these data allow us to draw a linear flowchart along which β4 and Met are causally involved first in the derailment of cell accretion and later in the promotion of metastatic spread.
This dual activity of β4 and Met on epithelial cells is paralleled by their common ability to induce a peculiar phase of the angiogenic process in endothelial cells. Specifically, both the inhibition of β4 signaling by an intracellularly truncated β4 mutant (Nikolopoulos et al., 2004) and the inhibition of Met function by a decoy receptor (Michieli et al., 2004) prevent branching of medium- and small-size vessels into microvessels during intratumor vascularization, suggesting that both molecules are selectively involved in the onset of the invasive step of pathological angiogenesis. At present, we do not know whether this vascular phenocopy is supported by a functional collaboration between β4 and Met. However, all of these observations highlight the versatility of the β4–Met system in promoting the various and sequential aspects of neoplastic progression.
Materials and methods
Materials, antibodies, and vectors
We used the following reagents: anti–human Met, anti-Shp2, anti-Src, antiactin, GST-Shp2, and GST-SH2-Grb2 (Santa Cruz Biotechnology, Inc.); anti-β4 integrin (BD Biosciences and Chemicon); anti-Gab1, anti-Shc, anti-PI3K, antiphosphotyrosine, and anti-GST (Upstate Biotechnology); anti-Grb2 (Transduction Laboratories); antiactive and total ERK/MAPK (Promega); antiactive Src (nonphospho-Tyr529; Biosource International); PP2 (Calbiochem); wild-type Shp2 and catalytically inactive C/S Shp2 in pJ3H vector (obtained from B.G. Neel, Harvard Medical School, Boston, MA); and wild-type Src and kinase-dead K/R Src in pCMV5 vector (obtained from J. Brugge, Harvard Medical School). The β4ΔShp2 construct containing phenylalanine mutations of Y1257, Y1440, and Y1494 was generated by PCR amplification of the BssHII–NotI fragment of a β4 template already containing the Y1257F and Y1494F substitutions (obtained from L.M. Shaw, Harvard Medical School). To create the β4 siRNA expression vector, oligonucleotides used by Chung et al. (2004) were annealed and ligated into pSUPER between the BglII and HindIII sites. BamHI- and XhoI-digested inserts were then subcloned into the pRLL5 lentiviral vector. A scrambled β4 oligonucleotide was used as a control. The constructs encoding for wild-type Met, kinase-inactive Met, wild-type β4, β4-1440F, and β4Δextra have been described previously (Trusolino et al., 2001). The β4 cDNA used in this study corresponds to PubMed accession no. AAC51632 and matches with the sequence originally cloned by Suzuki and Naitoh (1990).
Cell culture, transfection, and viral infection
COS-7, MDA-MB-435, and MDA-MB-231 cells were cultured in DME supplemented with 10% FBS (Invitrogen). The expression of exogenous proteins was obtained with LipofectAMINE- or LipofectAMINE 2000 (Invitrogen)–mediated transfection according to the manufacturer's protocol or with retroviral or lentiviral infection. Viral hybrid vectors were produced by the transient transfection of 293T cells. Viral supernatants were filtered through a 0.22-μm filter, and infections were performed in the presence of 4 μg/ml polybrene (Sigma-Aldrich).
Biochemical methods
For immunoprecipitations, 5 × 106 cells were lysed for 20 min at 4°C with 1 ml of a buffer containing 50 mM Hepes, pH 7.4, 5 mM EDTA, 2 mM EGTA, 150 mM NaCl, 10% glycerol, and 1% Triton X-100 in the presence of protease and phosphatase inhibitors. For β4–Shp2 coimmunoprecipitations in FG2 cells, 1% Brij58 was used instead of Triton X-100. Extracts were clarified at 12,000 rpm for 15 min, normalized with the BCA Protein Assay Reagent kit (Pierce Chemical Co.), and incubated with different mAbs for 2 h at 4°C. Immune complexes were collected with either protein G– or protein A–Sepharose, washed in lysis buffer in the presence of 1 M LiCl, and eluted. Total cellular proteins were extracted by solubilizing the cells in boiling SDS buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, and 1% SDS). Extracts were electrophoresed on SDS-polyacrylamide gels and transferred onto nitrocellulose membranes (Hybond; GE Healthcare). Nitrocellulose-bound antibodies were detected by the ECL system (GE Healthcare). Unless otherwise indicated, cells were stimulated with 50 ng/ml HGF for 30 min. PP2 was generally used at a 10-μM concentration and applied to cells for 30 min. In experiments aimed at analyzing the association of Gab1 with signal transducers and the HGF-dependent activation of ERKs, mock cells and cells expressing wild-type β4 or β4ΔShp2 were transiently transfected with a Gab1 cDNA.
For far-Western analysis, membranes were blocked for 1 h in TBS, 0.1% Tween-20, 5% BSA, and 3 μm glutathione and were incubated for 3 h with 1 μg/ml GST fusion protein previously adsorbed to 3 μM glutathione in 0.5% BSA. Filters were subsequently decorated with anti-GST antibody.
Src kinase assays were performed on Src immunoprecipitates using a commercial kit (Upstate Biotechnology) based on the phosphorylation of a specific substrate peptide (KVEKIGEGTYGVVYK) using the transfer of the γ-phosphate of γ-[32P]ATP by Src. The phosphorylated substrate was then separated from the residual γ-[32P]ATP using phosphocellulose paper and quantified with a scintillation counter.
Soft agar assay
3,000 cells were resuspended in complete medium containing 0.5% Seaplaque agar. Cells were seeded in 24-well plates containing a 1% agar underlay and supplemented twice a week with complete medium. In some experiments, MDA-MB-435–β4 cells were treated twice a week with 5 μM PP2 or 10 μM PD98059. Colonies were stained by the incorporation of tetrazolium salts 2 (for MDA-MB-435) or 3 wk (for MDA-MB-231) after seeding. Colonies were coded and scored in a blinded fashion by a second observer. Colony numbers were obtained using a phase-contrast light microscope (DMIL; Leica) fitted with a 32-grid eyepiece at a total magnification of 20×. Images were captured with ImageReady software (Adobe) using a microscope (DMIL; Leica) and a 20 × 0.30 objective (Leica) equipped with a digital camera (DFC320; Leica).
Statistical and densitometric analysis
Results are means ± SEM. Comparisons were made using the two-tailed t test. P-values <0.05 were considered to be statistically significant. Blot images were captured using a molecular imager (ChemiDoc XRS; Bio-Rad Laboratories). Densitometric analysis was performed with analysis software (Quantity One 1-D; Bio-Rad Laboratories) installed on the imager. Images were arranged and labeled using Illustrator (Adobe).
Acknowledgments
We are indebted to José M. de Pereda (University of Salamanca, Salamanca, Spain) for his helpful comments and discussion. We thank Leslie M. Shaw, Benjamin G. Neel, and Joan Brugge for reagents, Alessandra Crivellari and Francesco Galimi for help in some experiments, Raffaella Albano for technical assistance, Antonella Cignetto for secretarial assistance, and Catherine Tighe for editing the manuscript.
This work was supported by an Associazione Italiana per la Ricerca sul Cancro grant to P.M. Cornoglio and a Ministero dell'Istruzione dell'Università e della Ricerca (PRIN 2004) grant to L. Trusolino.
Abbreviations used in this paper: ERK, extracellular signal-regulated kinase; HGF, hepatocyte growth factor.
References
- Alonso, A., S. Rahmouni, S. Williams, M. van Stipdonk, L. Jaroszewski, A. Godzik, R.T. Abraham, S.P. Schoenberger, and T. Mustelin. 2003. Tyrosine phosphorylation of VHR phosphatase by ZAP-70. Nat. Immunol. 4:44–48. [DOI] [PubMed] [Google Scholar]
- Bentires-Alj, M., J.G. Paez, F.S. David, H. Keilhack, B. Halmos, K. Naoki, J.M. Maris, A. Richardson, A. Bardelli, D.J. Sugarbaker, et al. 2004. Activating mutations of the Noonan syndrome-associated SHP2/PTPN11 gene inhuman solid tumors and adult acute myelogenous leukemia. Cancer Res. 64:8816–8820. [DOI] [PubMed] [Google Scholar]
- Bentires-Alj, M., S.G. Gil, R. Chan, Z.C. Wang, Y. Wang, N. Imanaka, L.N. Harris, A. Richardson, B.G. Neel, and H. Gu. 2006. A role for the scaffolding adapter GAB2 in breast cancer. Nat. Med. 12:114–121. [DOI] [PubMed] [Google Scholar]
- Bernards, R., and R.A. Weinberg. 2002. A progression puzzle. Nature. 418:823. [DOI] [PubMed] [Google Scholar]
- Bertotti, A., P.M. Comoglio, and L. Trusolino. 2005. β4 integrin is a transforming molecule that unleashes Met tyrosine kinase tumorigenesis. Cancer Res. 65:10674–10679. [DOI] [PubMed] [Google Scholar]
- Birchmeier, C., W. Birchmeier, E. Gherardi, and G.F. Vande Woude. 2003. Met, metastasis, motility and more. Nat. Rev. Mol. Cell Biol. 4:915–925. [DOI] [PubMed] [Google Scholar]
- Chan, P.C., Y.L. Chen, C.H. Cheng, K.C. Yu, L.A. Cary, K.H. Shu, W.L. Ho, and H.C. Chen. 2003. Src phosphorylates Grb2-associated binder 1 upon hepatocyte growth factor stimulation. J. Biol. Chem. 278:44075–44082. [DOI] [PubMed] [Google Scholar]
- Chung, J., S.O. Yoon, E.A. Lipscomb, and A.M. Mercurio. 2004. The Met receptor and α6β4 integrin can function independently to promote carcinoma invasion. J. Biol. Chem. 279:32287–32293. [DOI] [PubMed] [Google Scholar]
- Dans, M., L. Gagnoux-Palacios, P. Blaikie, S. Klein, A. Mariotti, and F.G. Giancotti. 2001. Tyrosine phosphorylation of the β4 integrin cytoplasmic domain mediates Shc signaling to extracellular signal-regulated kinase and antagonizes formation of hemidesmosomes. J. Biol. Chem. 276:1494–1502. [DOI] [PubMed] [Google Scholar]
- de Pereda, J.M., G. Wiche, and R.C. Liddington. 1999. Crystal structure of a tandem pair of fibronectin type III domains from the cytoplasmic tail of integrin α6β4. EMBO J. 18:4087–4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fixman, E.D., T.M. Fournier, D.M. Kamikura, M.A. Naujokas, and M. Park. 1996. Pathways downstream of Shc and Grb2 are required for cell transformation by the tpr-Met oncoprotein. J. Biol. Chem. 271:13116–13122. [DOI] [PubMed] [Google Scholar]
- Frisch, S.M., and R.A. Screaton. 2001. Anoikis mechanisms. Curr. Opin. Cell Biol. 13:555–562. [DOI] [PubMed] [Google Scholar]
- Furge, K.A., Y.W. Zhang, and G.F. Vande-Woude. 2000. Met receptor tyrosine kinase: enhanced signaling through adapter proteins. Oncogene. 19:5582–5589. [DOI] [PubMed] [Google Scholar]
- Gagnoux-Palacios, L., M. Dans, W. van't Hof, A. Mariotti, A. Pepe, G. Meneguzzi, M.D. Resh, and F.G. Giancotti. 2003. Compartmentalization of integrin α6β4 signaling in lipid rafts. J. Cell Biol. 162:1189–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambaletta, D., A. Marchetti, L. Benedetti, A.M. Mercurio, A. Sacchi, and R. Falcioni. 2000. Cooperative signaling between α6β4 integrin and ErbB-2 receptor is required to promote phosphatidylinositol 3-kinase-dependent invasion. J. Biol. Chem. 275:10604–10610. [DOI] [PubMed] [Google Scholar]
- Goel, H.L., M. Fornaro, L. Moro, N. Teider, J.S. Rhim, M. King, and L. Languino. 2004. Selective modulation of type 1 insulin-like growth factor receptor signaling and functions by β1 integrins. J. Cell Biol. 166:407–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu, H., and B.G. Neel. 2003. The “Gab” in signal transduction. Trends Cell Biol. 13:122–130. [DOI] [PubMed] [Google Scholar]
- Guo, W., and F.G. Giancotti. 2004. Integrin signalling during tumour progression. Nat. Rev. Mol. Cell Biol. 5:816–826. [DOI] [PubMed] [Google Scholar]
- Guo, W., Y. Pylayeva, A. Pepe, T. Yoshioka, W.J. Muller, G. Inghirami, and F.G. Giancotti. 2006. β4 integrin amplifies ErbB2 signaling to promote mammary tumorigenesis. Cell. 126:489–502. [DOI] [PubMed] [Google Scholar]
- Hanahan, D., and R.A. Weinberg. 2000. The hallmarks of cancer. Cell. 100:57–70. [DOI] [PubMed] [Google Scholar]
- Holgado-Madruga, M., D.R. Emlet, D.K. Moscatello, A.K. Godwin, and A.J. Wong. 1996. A grb2-associated docking protein in EGF- and insulin-receptor signaling. Nature. 379:560–564. [DOI] [PubMed] [Google Scholar]
- Ishizawar, R., and S.J. Parsons. 2004. c-Src and cooperating partners in human cancer. Cancer Cell. 6:209–214. [DOI] [PubMed] [Google Scholar]
- Lipscomb, E.A., K.J. Simpson, S.R. Lyle, J.E. Ring, A.S. Dugan, and A.M. Mercurio. 2005. The α6β4 integrin maintains the survival of human breast carcinoma cells in vivo. Cancer Res. 65:10970–10976. [DOI] [PubMed] [Google Scholar]
- Litjens, S.H., J.M. de Pereda, and A. Sonnenberg. 2006. Current insights into the formation and breakdown of hemidesmosomes. Trends Cell Biol. 16:376–383. [DOI] [PubMed] [Google Scholar]
- Mainiero, F., A. Pepe, M. Yeon, Y. Ren, and F.G. Giancotti. 1996. The intracellular functions of α6β4 integrin are regulated by EGF. J. Cell Biol. 134:241–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroun, C.R., M.A. Naujokas, M. Holgado-Madruga, A.J. Wong, and M. Park. 2000. The tyrosine phosphatase SHP-2 is required for sustained activation of extracellular signal-regulated kinase and epithelial morphogenesis downstream from the met receptor tyrosine kinase. Mol. Cell. Biol. 20:8513–8525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercurio, A.M., and I. Rabinovitz. 2001. Towards a mechanistic understanding of tumor invasion - lessons from the α6β4 integrin. Semin. Cancer Biol. 11:129–141. [DOI] [PubMed] [Google Scholar]
- Michieli, P., M. Mazzone, C. Basilico, S. Cavassa, A. Sottile, L. Naldini, and P.M. Comoglio. 2004. Targeting the tumor and its microenvironment by a dual-function decoy Met receptor. Cancer Cell. 6:61–73. [DOI] [PubMed] [Google Scholar]
- Neel, B.G., H. Gu, and L. Pao. 2003. The “Shp”ing news: SH2 domain-containing tyrosine phosphatases in cell signalling. Trends Biochem. Sci. 28:284–293. [DOI] [PubMed] [Google Scholar]
- Niessen, C.M., E.H. Hulsman, L.C. Oomen, I. Kuikman, and A. Sonnenberg. 1997. A minimal region on the integrin β4 subunit that is critical to its localization in hemidesmosomes regulates the distribution of HD1/plectin in COS-7 cells. J. Cell Sci. 110:1705–1716. [DOI] [PubMed] [Google Scholar]
- Nikolopoulos, S.N., P. Blaikie, T. Yoshioka, W. Guo, and F.G. Giancotti. 2004. Integrin β4 signaling promotes tumor angiogenesis. Cancer Cell. 6:471–483. [DOI] [PubMed] [Google Scholar]
- Ponzetto, C., A. Bardelli, Z. Zhen, F. Maina, P. dalla Zonca, S. Giordano, A. Graziani, G. Panayotou, and P.M. Comoglio. 1994. A multifunctional docking site mediates signaling and transformation by the hepatocyte growth factor/scatter factor receptor family. Cell. 77:261–271. [DOI] [PubMed] [Google Scholar]
- Rabinovitz, I., A. Toker, and A.M. Mercurio. 1999. Protein kinase C-dependent mobilization of the α6β4 integrin from hemidesmosomes and its association with actin-rich cell protrusions drive the chemotactic migration of carcinoma cells. J. Cell Biol. 146:1147–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren, Y., S. Meng, L. Mei, Z.J. Zhao, R. Jove, and J. Wu. 2004. Roles of Gab1 and SHP2 in paxillin tyrosine dephosphorylation and Src activation in response to epidermal growth factor. J. Biol. Chem. 279:8497–8505. [DOI] [PubMed] [Google Scholar]
- Santoro, M.M., G. Gaudino, and P.C. Marchisio. 2003. The MSP receptor regulates α6β4 and α3β1 integrins via 14-3-3 proteins in keratinocyte migration. Dev. Cell. 5:257–271. [DOI] [PubMed] [Google Scholar]
- Shaw, L.M. 2001. Identification of insulin receptor substrate 1 (IRS-1) and IRS-2 as signaling intermediates in the α6β4 integrin-dependent activation of phosphoinositide 3-OH kinase and promotion of invasion. Mol. Cell. Biol. 21:5082–5093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki, S., and I. Naitoh. 1990. Amino acid sequence of a novel integrin β4 subunit and primary expression of the mRNA in epithelial cells. EMBO J. 9:757–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trusolino, L., and P.M. Comoglio. 2002. Scatter-factor and semaphorin receptors: cell signalling for invasive growth. Nat. Rev. Cancer. 2:289–300. [DOI] [PubMed] [Google Scholar]
- Trusolino, L., A. Bertotti, and P.M. Comoglio. 2001. A signaling adapter function for α6β4 integrin in the control of HGF-dependent invasive growth. Cell. 107:643–654. [DOI] [PubMed] [Google Scholar]
- Yang, X., O.V. Kovalenko, W. Tang, C. Claas, C.S. Stipp, and M.E. Hemler. 2004. Palmitoylation supports assembly and function of integrin-tetraspanin complexes. J. Cell Biol. 167:1231–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeatman, T.J. 2004. A renaissance for SRC. Nat. Rev. Cancer. 4:470–480. [DOI] [PubMed] [Google Scholar]
- Wilhelmsen, K., S.H. Litjens, and A. Sonnenberg. 2006. Multiple functions of the integrin α6β4 in epidermal homeostasis and tumorigenesis. Mol. Cell. Biol. 26:2877–2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcik, E.J., S. Sharifpoor, N.A. Miller, T.G. Wright, R. Watering, E.A. Tremblay, K. Swan, C.R. Mueller, and B.E. Elliott. 2006. A novel activating function of c-Src and Stat3 on HGF transcription in mammary carcinoma cells. Oncogene. 25:2773–2784. [DOI] [PubMed] [Google Scholar]
- Zahir, N., J.N. Lakins, A. Russell, W. Ming, C. Chatterjee, G.I. Rozenberg, M.P. Marinkovich, and V.M. Weaver. 2003. Autocrine laminin-5 ligates α6β4 integrin and activates RAC and NFkB to mediate anchorage-independent survival of mammary tumors. J. Cell Biol. 163:1397–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, S.Q., W. Yang, M.I. Kontaridis, T.G. Bivona, G. Wen, T. Araki, J. Luo, J.A. Thompson, B.L. Schraven, M.R. Philips, and B.G. Neel. 2004. Shp2 regulates SRC family kinase activity and Ras/Erk activation by controlling Csk recruitment. Mol. Cell. 13:341–355. [DOI] [PubMed] [Google Scholar]