Abstract
Detachment of parenchymal cells from a solid matrix switches contextual cues from survival to death during anoikis. Marked shape changes accompany detachment and are thought to trigger cell death, although a working model to explain the coordination of attachment sensation, shape change, and cell fate is elusive. The constitutive form of the adapter Shc, p52Shc, confers survival properties, whereas the longer p66Shc signals death through association with cytochrome c. We find that cells that lack p66Shc display poorly formed focal adhesions and escape anoikis. However, reexpression of p66Shc restores anoikis through a mechanism requiring focal adhesion targeting and RhoA activation but not an intact cytochrome c–binding motif. This pathway stimulates the formation of focal adhesions and stress fibers in attached cells and tension-dependent cell death upon detachment. p66Shc may thus report attachment status to the cell by imposing a tension test across candidate anchorage points, with load failure indicating detachment.
Introduction
Parenchymal cells require integrin-dependent attachment to solid structures to survive. As a consequence of such anchorage dependence, most tissue cells start to undergo apoptotic death within hours of being forced into suspension in a fluid environment, a process termed anoikis (Frisch and Francis, 1994). Physiological anoikis occurs during the involution of mammary and prostate glands, whereas pathological loss of anoikis is thought to accompany the malignant cell's acquisition of metastatic capabilities.
Presently, anoikis is understood largely in terms of withdrawal of integrin-related outside-in survival signals. Thus, constitutive activation of the survival proteins focal adhesion kinase (FAK), Src, Akt, and Ras all bypass detachment-induced death (Frisch and Francis, 1994; Frisch et al., 1996; Martin et al., 2006). However, anoikis can be circumvented in the absence of Ras, phosphoinositide 3-kinase, or FAK activation (McFall et al., 2001; Díaz-Montero et al., 2006; Kang et al., 2007), suggesting that the activation of specific death pathways may be necessary in addition to the disabling of survival signals. Furthermore, integrin ligation without structural matrix rigidity is insufficient to prevent anoikis, indicating that integrins also control anoikis by sensing mechanical properties of the environment. Soluble matrix fragments or solid-phase RGD peptides bound to microbeads, e.g., ligate and cluster integrins, but neither prevent rounding nor increase the survival rate of floating endothelial cells (Re et al., 1994), which is consistent with observations that cell rounding by itself forces death upon attachment-dependent cells (Chen et al., 1997).
The shortcomings of a unidirectional outside-in signaling model to explain attachment sensation is consistent with the concept that cells initiate processes designed to gauge substrate stiffness in an “inside-outside-in” feedback loop (Discher et al., 2005). The molecular machinery responsible for such mechanosensation and its relationship to anchorage dependence have remained recondite; hematopoietic cells, for instance, lack such machinery, as they are both anchorage independent and insensitive to substrate stiffness (Discher et al., 2005). Interestingly, hematopoietic cells also repress expression of p66Shc, the long isoform of the integrin-associated adaptor Shc (Migliaccio et al., 1997). Whereas p52Shc is known to facilitate survival and proliferative signals, in large part through Ras recruitment and activation, p66Shc is best known as a proapoptotic protein. Here, we show that p66Shc permits activation of RhoA, which leads to tension-dependent death in floating cells. p66Shc may thus be important in the efferent limb of a mechanosensory loop reporting detachment.
Results and discussion
p66Shc mediates anoikis
p66Shc is poorly expressed in floating hematopoietic cell lines (Migliaccio et al., 1997), suggesting that either p66Shc may mediate adhesion or that its repression may be important for survival in suspension. We studied φNx-293 cells, as this epithelioid line forms adherent cultures yet is relatively resistant to death while floating. Indeed, these cells did not express p66Shc even when grown as adherent monolayers, and suspension over low attachment plates for 16–24 h did not increase cell death (Fig. 1 A). In preliminary studies, transient reexpression of p66Shc conferred sensitivity to anoikis in 293 cells. In contrast, overexpression of p52Shc had a minimal effect on cell death in attached or floating cells (Fig. 1 A). To avoid artifacts related to cell death from transfection itself, φNx-293 cells stably expressing Flag-p66Shc (293-p66 cells) were studied. In agreement with transient transfection studies, stable expression of p66Shc had no effect on cell death in adherent cells but caused death after detachment (Fig. 1 A). Cell death was accompanied by the activation of caspase 7, an executioner caspase activated during anoikis (Cardone et al., 1997; Bharadwaj et al., 2005), and the release of cytochrome c (Fig. S1, A and B, available at http://www.jcb.org/cgi/content/full/jcb.200706097/DC1). More specifically, selective induction and mitochondrial localization of Bim has been shown to comprise a characteristic hallmark of anoikis (Reginato et al., 2003). Accordingly, detachment of p66Shc-expressing cells caused a marked induction of mitochondria-associated Bim, without induction of Bax or Bid or suppression of Bcl-XL (Fig. 1 B).
Figure 1.
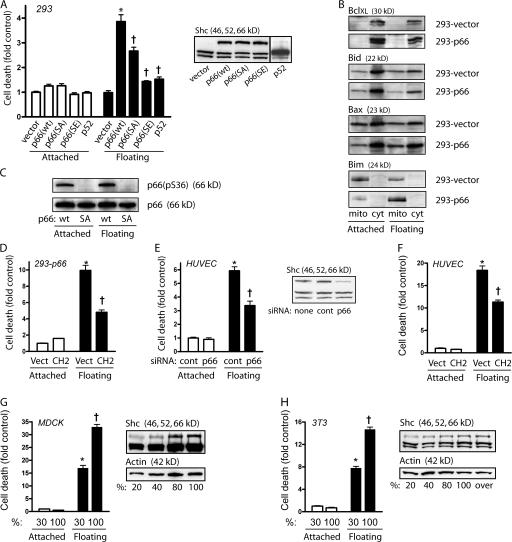
p66Shc confers anoikis. (A) 293 cells transiently transfected with pCINF-p52 or stably transfected with an empty vector, pCINF-p66 wt, pCINF-p66 (S36A), or p66 (S36E) were left adherent or suspended for 16 h in low-attachment plates. Nuclear DNA fragmentation was assessed and demonstrated increased cell death with detachment in cells expressing p66 (wt) or p66 (SA). (inset) Immunoblot for Shc showing p66Shc, p52Shc, and p46Shc expression. *, P < 0.001 compared with attached control; †, P < 0.001 compared with p66 (wt) floating. (B) Stable transfectants of empty vector (293-vector) or p66Shc (293-p66) were fractionated into mitochondrial or cytosolic content after 16 h of adherent or suspended culture and immunoblotted with the indicated antibodies. Bim was strongly induced and remained in the mitochondrial fraction with detachment. (C) 293 cells expressing either p66 wt or p66 (S36A) were immunoblotted after 16 h of suspension or adherence for phospho-S36 or total p66Shc. (D) Stable p66Shc cells were transiently transfected with either vector or the isolated Shc CH2 domain, and DNA fragmentation was measured after 16 h of adherence or suspension. (E) HUVEC were transfected with either control siRNA or siRNA against the CH2 domain of Shc, and DNA fragmentation was assessed 16 h after suspension. (inset) Immunoblot for Shc with decreased p66Shc expression. (F) HUVEC were transfected with a vector or CH2, and DNA fragmentation was measured after 16 h of suspension. (G) MDCK cells were plated at different densities and the next day left adherent or forced into suspension. DNA fragmentation at sparse and confluent states was assessed. (inset) Immunoblot for Shc at the indicated levels of confluence. (H) 3T3 cells were plated at different densities and anoikis was assessed as in G. (inset) Immunoblot for Shc at the indicated levels of confluence. (D–H) *, P < 0.001 compared with attached control; †, P < 0.001 compared with floating control. Error bars show mean ± SEM.
As the phosphorylation of Ser36 has been shown to be necessary to cause apoptosis in response to oxidative stress (Migliaccio et al., 1999; Pinton et al., 2007), we introduced either S36A or S36E mutations into p66Shc and again created stable 293 lines. Surprisingly, the S36A mutant was able to confer anoikis, albeit not to the same magnitude as wild-type (wt) p66Shc, whereas the phosphomimetic S36E mutation abrogated the anoikis-conferring property of p66Shc (Fig. 1 A), consistent instead with a possible protective effect of S36 phosphorylation against anoikis. Indeed, phosphorylation of Ser36 was present in adherent 293-p66 cells that did not undergo cell death and did not occur in 293-p66 (S36A) cells undergoing anoikis (Fig. 1 C). Thus, Ser36 phosphorylation was neither necessary nor sufficient for anoikis, suggesting a mechanism of cell death through p66Shc different from that previously described for other stimuli (Migliaccio et al., 1999; Pinton et al., 2007). As further confirmation of the effects of p66Shc and Ser36 on anoikis, we found that whereas vector-transfected 293 control cells were able to form colonies in soft agar, 293-p66 and 293-p66 (S36A) cells formed only abortive colonies (Fig. S1 C). In contrast, 293-p66 (S36E) cells, which escape anoikis, displayed prominent anchorage-independent growth.
Because p66Shc contains all the domains of the nonapoptotic p52Shc, it seemed unlikely that the former protein functioned simply as a dominant-negative version of p52Shc, unless the unique collagen homologous (CH) 2 N-terminal domain of p66Shc masked other Shc domains through intra- or transmolecular interactions. We found, however, that neither Flag-p66Shc nor the isolated Flag-CH2 domain coprecipitated with endogenous p66Shc, p52Shc, or the smaller p46Shc (unpublished data). Indeed, transient expression of the CH2 domain decreased rather than increased anoikis (Fig. 1 D), which is consistent with a unique role for p66Shc in causing anoikis.
We also studied nonimmortalized primary cells, human umbilical vein endothelial cells (HUVEC), based on their epithelioid behavior and marked sensitivity to anoikis. As expected, HUVEC expressed substantial levels of p66Shc and prominent detachment-induced cell death (Fig. 1 E) with the induction of Bim (Fig. S1 D). Knockdown of p66Shc significantly decreased but did not eliminate anoikis. Although this incomplete protection may be caused by the partial effect of siRNA, death pathways independent of p66Shc may exist. As with 293 cells, phosphorylation of Ser36 did not increase as HUVEC underwent anoikis (Fig. S1 E). Again supporting a specific role for p66Shc, transient expression of the isolated CH2 domain decreased anoikis by ∼33%, consistent with the transfection efficiency of HUVEC (Fig. 1 F).
Finally, sensitivity to anoikis is known to vary with the level of confluence in monolayers of nontransformed lines such as MDCK epithelial cells (Frisch and Francis, 1994). To investigate whether the endogenous regulation of p66Shc might account for anoikis susceptibility within a given cell line, we correlated levels of p66Shc with anoikis at sparse and confluent conditions. p66Shc progressively increased with cell density, paralleling the increase in anoikis seen in confluent cells (Fig. 1 G). In contrast, the increase in p66Shc in confluent cells did not cause cell death in attached MDCK cells. Notably, 3T3 cells, nontransformed fibroblastic cells known to undergo contact inhibition, also displayed increased p66Shc protein expression and sensitivity to anoikis upon reaching confluence (Fig. 1 H). Thus, in endothelial, epithelial, and mesenchymal cells, both externally manipulated and endogenously regulated levels of p66Shc determine sensitivity to anoikis.
Focal adhesion targeting by p66Shc is associated with anoikis
Although the Shc proteins are known to associate with integrins, specific integrin structures and binding mechanisms are not well understood. Focal adhesions are thought to function as predominant anchoring structures, so we investigated the targeting of GFP-fused p66Shc to native HUVEC focal adhesions. Using total internal reflection fluorescence (TIRF) microscopy to image the ventral cell surface, we found that p66-GFP targeted well-formed zyxin-positive focal adhesions in HUVEC (Fig. 2 B). In parallel, transient expression of p66-GFP in 293 cells, which lack endogenous p66Shc, conferred anoikis (Fig. 2 G). Focal adhesion targeting was preserved in p52Shc and did not occur with the isolated CH2 domain (Fig. 2, C and D). Association of Shc proteins with β1 integrins is known to occur indirectly through caveolin and Fyn through the Fyn Src homology (SH) 3 domain, presumably through proline-rich motifs contained within the p66Shc CH1 domain (Wary et al., 1998). Disruption of either of two polyproline motifs within the CH1 domain (P411S/P414S or P472S/P475S), however, had no effect on focal adhesion targeting (Fig. S2, A and B, available at http://www.jcb.org/cgi/content/full/jcb.200706097/DC1) and did not diminish p66Shc-related anoikis (Fig. 2 G). Furthermore, deletion of the entire CH1 domain neither altered focal adhesion targeting nor diminished anoikis (Figs. 2 G and S2 C). This latter deletion also argues against the possibility that p66Shc causes death by sequestering Grb2, which binds phosphotyrosine residues within the CH1 domain (Nicholson et al., 2001). Similarly, deletion of the C-terminal SH2 domain, known to bind growth factor receptors, N-cadherin, and the β4 integrin subunit (Dans et al., 2001; Ravichandran, 2001), did not abrogate association with focal adhesions and did not decrease anoikis (Figs. 2 G and S2 D).
Figure 2.
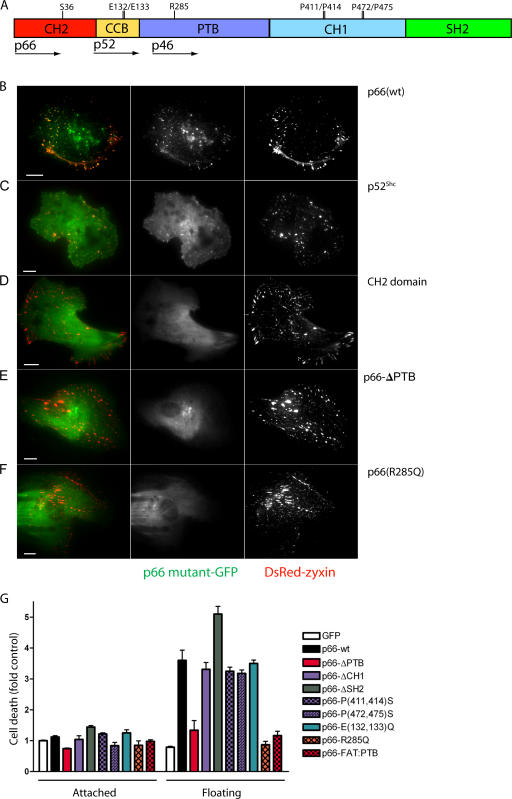
Focal adhesion targeting of p66Shc is associated with anoikis. (A) Schematic of Shc with start positions of p66Shc, p52Shc, and p46Shc shown. CH2, cytochrome c–binding (CCB), PTB, CH1, and SH2 domains are shown, with point mutations introduced. (B–F) HUVEC were cotransfected with GFP fusions of the indicated Shc mutations and DsRed-zyxin and imaged with TIRF microscopy. Focal adhesion targeting of p66Shc-GFP was lost with deletion of the PTB domain or R285Q mutation. Bars, 10 μm. (G) 293 cells were transfected with the indicated Shc-GFP mutants and DNA fragmentation was measured 16 h after suspension or adherent culture. Error bars show mean ± SEM.
Deletion of the phosphotyrosine-binding (PTB) domain, however, caused complete loss of both focal adhesion targeting and anoikis activity (Fig. 2, E and G). Structural studies of this domain have revealed independent binding surfaces for phosphotyrosine-containing peptides and acidic phospholipids. We found that p66Shc harboring a single R285Q mutation, which selectively disrupts the phosphotyrosine-anchoring pocket (Zhou et al., 1995), also delocalized p66Shc from focal adhesions and completely lacked anoikis activity (Fig. 2, F and G). However, replacing the p66Shc PTB domain with the focal adhesion targeting (FAT) domain of FAK, while restoring focal adhesion localization, did not restitute anoikis (Figs. 2 G and S2 E). Thus the specific molecular partner of p66Shc within focal adhesions may determine its ability to relay detachment signals. The lack of effect of the PTB/FAT domain swap also suggests that p66Shc does not simply compete with FAK for focal contact binding sites to diminish its survival. Indeed, p66Shc had little effect on FAK (Y397) phosphorylation in 293 cells (Fig. S2 G). FAK (Y397) phosphorylation was low in 293-vector and 293-p66 cells in both adherent and suspended states, suggesting that p66Shc does not induce cell death by decreasing FAK autophosphorylation during detachment.
Finally, p66Shc has recently been shown to translocate into the mitochondrial inner membrane, bind to cytochrome c directly, and undergo a redox cycle, causing oxidant-dependent permeability transition and apoptosis in adherent cells undergoing apoptosis (Giorgio et al., 2005). Disruption of cytochrome c binding through mutation of Glu132/Glu133 completely disables the apoptotic activity of p66Shc after oxidative stress or staurosporine (Giorgio et al., 2005; Pinton et al., 2007). We found, however, that p66 (E132Q and E133Q) remained localized to focal adhesions and retained its full ability to cause anoikis (Figs. 2 G and S2 F). Together with the persistence of anoikis in the S36A mutant, these data suggest a unique mechanism for p66Shc in mediating anoikis as opposed to other forms of apoptosis.
p66Shc increases RhoA activation, causing cell death upon detachment
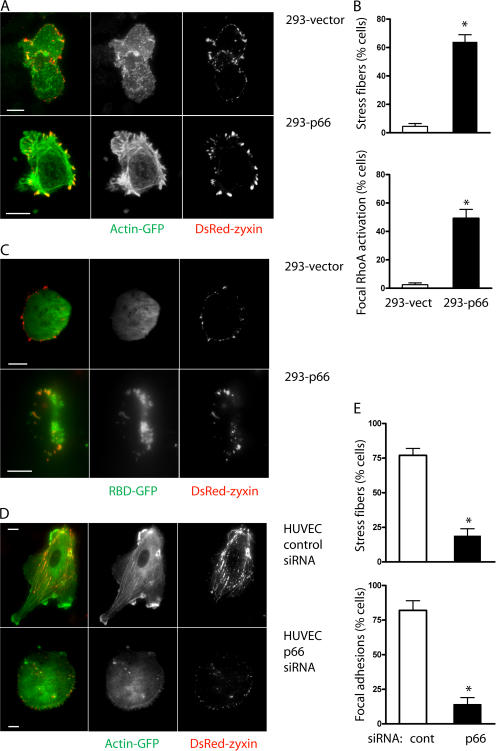
The targeting of p66Shc and p52Shc to focal adhesions is consistent with observations that cells lacking functional Shc proteins display adhesion defects and alterations in focal contact and actin fiber organization (Lai and Pawson, 2000), and that integrins that nucleate focal adhesions also permit Shc-dependent proliferative signals (Mettouchi et al., 2001). We questioned whether p66Shc specifically influenced activation of RhoA, known to drive focal adhesion formation (Nobes and Hall, 1995). Using the rhotekin rho-binding domain (RBD) fused to GFP (RBD-GFP) to report sites of RhoA activation, we again examined the ventral attachment surface of cells using TIRF microscopy. In control 293-vector cells, focal contacts identified by DsRed-zyxin were small and peripheral, often taking the appearance of focal complexes, and cells were rounded with little evidence of RBD-GFP accumulation and only occasional actin stress fibers (Fig. 3, A and B). In contrast, 293-p66 cells were more spread with typical focal adhesions and more abundant stress fibers present. Numerous accumulations of RBD-GFP indicated focal activation of RhoA at ventral sites corresponding to zyxin- containing contacts (Fig. 3, B and C). The influence of p66Shc on focal contact organization was also evident in HUVEC, which normally appear polarized with abundant focal adhesions and stress fibers. Knockdown of endogenous p66Shc caused a general loss of cell polarity with a substantial decrease in stress fibers and focal adhesions (Fig. 3, D and E).
Figure 3.
p66Shc increases focal adhesions and stress fibers. (A and B) 293-vector (top) or 293-p66 (bottom) cells were cotransfected with actin-GFP and DsRed-zyxin and imaged with TIRF microscopy. 293-p66 cells displayed more prominent actin stress fibers and more developed focal adhesions than 293-vector cells. (C) 293-vector (top) or 293-p66 (bottom) cells were cotransfected with DsRed-zyxin and RBD-GFP to mark sites of endogenous RhoA activation. Focal areas of RhoA activation were centered on DsRed-zyxin aggregates. (D and E) HUVEC were transfected with control or p66Shc siRNA, actin-GFP, and DsRed-zyxin. Knockdown of p66Shc generally diminished cell polarization and decreased actin stress fibers and focal adhesions. *, P < 0.001 compared with control. Error bars show mean ± SEM. Bars, 10 μm.
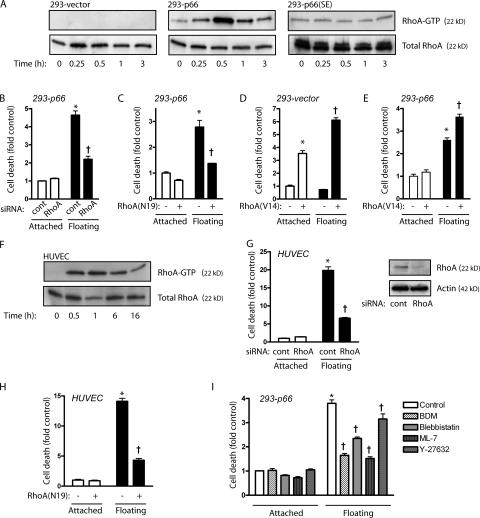
Upon detachment, 293-vector cells displayed no activation of RhoA, whereas detachment of 293-p66 cells caused robust RhoA activation within 30 min (Fig. 4 A). 293-p66 (S36E) cells, being defective in anoikis, also failed to activate RhoA upon detachment. Dominant-negative RhoA (N19) or knockdown of endogenous RhoA both decreased p66Shc-dependent anoikis, confirming a role for RhoA in detachment-induced death (Fig. 4, B and C). Conversely, active RhoA (V14) caused cell rounding (not depicted) and death in 293 cells lacking p66Shc even while attached, and further augmented cell death while floating (Fig. 4 D), suggesting a default decision of death by active RhoA in the absence of p66Shc. In support of this, RhoA (V14) did not increase cell death in attached 293-p66 cells, whereas it increased cell death in floating 293-p66 cells (Fig. 4 E). Thus p66Shc not only initiates RhoA activation after detachment but appears to restrain RhoA-induced death in attached cells, in essence reporting appropriate anchorage through RhoA context. Again examining HUVEC as primary culture cells sensitive to anoikis, we found similar robust RhoA activation within 30 min of detachment (Fig. 4 F). Antagonizing RhoA through either expression of RhoA (N19) or knockdown of endogenous RhoA suppressed anoikis, confirming a role for RhoA in this process in primary cells (Fig. 4, G and H).
Figure 4.
p66Shc increases RhoA activation, leading to anoikis. (A) 293-vector, 293-p66, or 293-p66 (S36E) cells were forced into suspension and harvested at the indicated times. RhoA activation was assessed by RBD pulldown. Expression of p66 (wt) but not p66 (S36E) increased RhoA activation within 30 min of suspension. 293-p66 cells were transfected with control or RhoA siRNA (B), or control or RhoA (N19; C), and DNA fragmentation was measured 16 h after suspension. 293-vector (D) or 293-p66 (E) cells were transfected with a vector or RhoA (V14), and DNA fragmentation was measured after 16 h in suspension. (F) HUVEC were suspended for the indicated times and RhoA activity was assessed by RBD pulldown. (G) HUVEC were transfected with control or RhoA siRNA, and DNA fragmentation was measured after 16 h of suspension. (inset) Immunoblot for RhoA. (H) HUVEC were infected with Ad-lacZ control virus or Ad-RhoA (N19), and DNA fragmentation was assessed 16 h after suspension. (I) 293-p66 cells were suspended for 8 h in the presence of 50 μM ML-7, 10 mM BDM, 1 μM (−)blebbistatin, or 10 μM Y-27632. *, P < 0.001 compared with adherent control; †, P < 0.001 compared with floating control. Error bars show mean ± SEM.
Because RhoA is known to increase tension across attachment sites with resultant focal adhesion and stress-fiber formation, we surmised that in floating cells such tension would be applied against unanchored points, allowing a mechanical readout for detachment. Accordingly, antagonists of actinomyosin contraction (myosin light chain kinase inhibitor ML-7; actin–myosin interaction inhibitor 2,3 butanedione monoxime [BDM]; and myosin II inhibitor (−)blebbistatin) decreased p66Shc-dependent anoikis (Fig. 4 I), indicating a role for the generation of cytoskeletal tension in detachment sensing. Surprisingly, the Rho kinase inhibitor Y-27632 had only minimal effects on anoikis, suggesting a larger contribution of non-Rho kinase effectors.
Conclusions
Shc is best known as a transforming protein, acting to facilitate growth factor–dependent Ras activation through Grb2/Sos recruitment. However, growth factor receptors can directly bind Grb2 without Shc, suggesting that Shc dictates an additional level of control for this and perhaps other pathways. One possibility consistent with its scaffolding function is that it may link distal signaling with other transmembrane complexes, thus assigning environmental context to GTPase signals. For example, Shc may modify VEGF-receptor signaling in response to cell–cell contact through its association with vascular endothelial–cadherin (Zanetti et al., 2002). In addition, Shc permits matrix-dependent EGF signaling to Ras and MAPKs (Moghal and Neel, 1998; Lai and Pawson, 2000) and, by association with specific integrins, allows Rac1-dependent cell cycle progression after attachment to extracellular matrix proteins (Mettouchi et al., 2001).
Our data suggest that p66Shc may follow this paradigm and report anchorage context through RhoA activation. Like Shc, RhoA is best known as a proliferative agent, at least in part working through Rho kinase–dependent increases in cytoskeletal tension (Pirone et al., 2006). Such RhoA-dependent tension is clearly coupled with the mechanical resistance offered by the cell's environment, as epithelial cells grown in increasingly stiff matrices acquire increasingly malignant phenotypes (Paszek et al., 2005). A similar RhoA-dependent test of matrix rigidity is used by mesenchymal stem cells to determine which differentiation program to initiate (McBeath et al., 2004; Engler et al., 2006). In the extreme case of detachment from a solid matrix, however, a liquid environment would be expected to provide no resistance to such tension, which would instead have to be borne entirely by internal struts such as microtubules. Notably, the anoikis-associated BH3-only protein Bim associates with microtubules and initiates apoptosis only upon its release from the cytoskeleton (Puthalakath et al., 1999). Although the mechanism by which unopposed tension causes death is as yet unclear, the situation may be similar to fibroblasts grown in a stressed collagen matrix, which undergo apoptosis when the matrix is mechanically unloaded despite continued attachment to collagen (Grinnell et al., 1999). Another comparable situation may occur in MDA-MD-231 breast cancer cells, in which overexpression of the motor protein tropomyosin-1 increases susceptibility to anoikis in a manner partially reversible by Y27632 (Bharadwaj et al., 2005).
Materials and methods
Plasmid construction
Human p66Shc was PCR cloned from a previously constructed HUVEC library and subcloned into pCINF containing an N-terminal Flag tag to create pCINF-p66. S36A and S36E mutations were subsequently introduced into pCINF-p66 by PCR mutagenesis. The isolated CH2 domain and the remaining p52Shc were subcloned into pCINF, and expression was assessed by immunoblot for the Flag tag. PCR strategies were used to subclone full-length p66Shc, p52Shc (residues 111–583 of p66Shc), and the CH2 domain (residues 1–110) onto the N terminus of EGFP (pEGFP-N3; CLONTECH Laboratories, Inc.). The P411S/P414S, P472S/P475S, E132Q/E133Q, and R285Q point mutations were each introduced into p66-GFP in single mutagenic PCR reactions. Starting with p66-GFP, domain deletions were accomplished by opposing PCR primers tailed with AgeI sites, resulting in deletions of the PTB (residues 119–317), CH1 (residues 318–485), and SH2 (residues 486–584) domains. The p66-ΔPTB plasmid was reopened with AgeI, and the FAT domain of FAK was ligated to create p66-PTB–FAT. The RBD of rhotekin was subcloned into pEGFP-N3 to form RBD-GFP. DsRed-zyxin was a gift from A. Huttenlocher (University of Wiconsin, Madison, WI) and actin-GFP was obtained from CLONTECH Laboratories, Inc. RhoA (N19) and RhoA (V14) were obtained from the University of Missouri at Rolla cDNA Resource Center and subcloned into pCI-neo (Promega). Adenovirus-harboring RhoA (N19) was a gift of C. Chen (University of Pennsylvania, Philadelphia, PA). New constructs were confirmed by sequencing.
Cell culture
φNx-293 cells were obtained from American Type Culture Collection with permission from G. Nolan (Stanford University, Stanford, CA). MDCK and NIH 3T3 cells were also obtained from American Type Culture Collection. Stable transfectants of 293 cells with pCINF, pCINF-p66, pCINF-p66 (S36A), or pCINF-p66 (S36E) were selected with G418. Single cell clones were tested for comparable expression of p66Shc between mutants and relative to p52Shc. Mixed clones, particularly of 293-p66, progressively lost expression of p66Shc and thus were not used. G418 was removed for at least two passages before anoikis studies. HUVEC (Clonetics) were used at passages four and five.
Reagents
The following antibodies were used: Shc, Bim, Bid, Bak, BclXL (BD Biosciences); p66Shc (pS36; Qbiogene); Bax, RhoA, cleaved caspase 7 (Cell Signaling Technology); cytochrome c (MitoSciences); FAK (Santa Cruz Biotechnology, Inc.); FAK (pY397; Zymed Laboratories); and actin (Millipore). ML-7, BDM, and (−)blebbistatin were obtained from Sigma-Aldrich, and Y-27632 was obtained from Calbiochem.
Cell death assays
Cells were plated on either cell culture–treated or low-attachment 24-well plates (Corning Inc.). DNA fragmentation was assessed using the cell death ELISA (Roche). Mitochondrial release of cytochrome c was assessed by digitonin permeabilization. Preliminary titration studies were performed in 293 cells to determine optimal digitonin concentrations for selective plasma membrane permeabilization. Cells were washed and permeabilized in 10 ng/ml digitonin in sucrose buffer on ice for 10 min. Mitochondria were harvested by centrifugation at 1,000 g for 5 min. Fractions were then immunoblotted for cytochrome c. Activated caspase 7 was assessed with antisera specific for cleaved caspase. Induction of BH domain proteins was assessed by fractionating cells into mitochondrial and cytosolic fractions and immunoblotting for BclXL, Bax, Bid, and Bim. Anchorage-independent growth was assessed by plating cells in 0.35% agar on a bed of 0.9% agar in DME with 10% fetal calf serum at a density of 2,000 cells/ml. Visible colonies were scored after 14 d.
Transfection and knockdown
HUVEC were electroporated with expression constructs 6–8 h after release from thymidine-induced G1 arrest as described previously (Wu et al., 2005). 293 cells were electroporated unsynchronized. Control cells were transfected with empty pCI-neo or pCINF. siRNA against the CH2 domain of p66Shc (nt 42–60), RhoA (nt 355–375), and the negative control luciferase were obtained from Dharmacon. Transfection of siRNA was accomplished with TransIT-TKO (Mirus). Adenoviral infection of HUVEC was performed at an MOI of 1:100, titrated to protein expression. Control cells were infected with the same titer of Ad-lacZ.
Microscopy
After transfection, cells were plated on fibronectin-coated chambered coverslips and observed live in full media without fixation 24–48 h later. TIRF microscopy was performed using a TE2000-U system (Nikon). Sequential red and green channels were acquired at 37°C through a 60×/NA 1.45 oil-immersion objective (Nikon) with a digital camera (Coolsnap ES; Roper) using Metamorph software (Molecular Devices). Morphological features were scored by examining all cells in at least five high-power fields per chamber in at least four chambers.
RhoA activity
RhoA GTP loading was assessed by a pulldown technique (Wu et al., 2005). The RBD of mouse rhotekin (residues 7–89) was previously ligated into pGEX-2TK (Wu et al., 2005), and recombinant proteins were purified with GSH-sepharose (GE Healthcare). Active GTP-loaded Rho proteins from the 10,000-g supernatant of cell lysate were pulled down and immunoblotted with antisera for RhoA.
Online supplemental material
Fig. S1 shows that p66Shc increases caspase 7 cleavage and cytochrome c release, decreases anchorage-independent growth in 293 cells, and increases Bim in suspended HUVEC. Fig. S2 shows that p66 (P411S and P414S), p66 (P472S and P475S), p66 (ΔCH1), p66 (ΔSH2), p66 (FAT-PTB), and p66 (E132Q and E133Q) display persistent focal adhesion targeting, and that p66Shc does not affect FAK (Y397) phosphorylation. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200706097/DC1.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (grants R01-HL067256, R01-HL61897, and R01-GMS067674) and the Veterans Administration.
Abbreviations used in this paper: BDM, 2,3 butanedione monoxime; CH, collagen homologous; FAK, focal adhesion kinase; FAT, focal adhesion targeting; HUVEC, human umbilical vein endothelial cells; PTB, phosphotyrosine-binding; RBD, rho-binding domain; SH, Src homology; TIRF, total internal reflection fluorescence; wt, wild type.
References
- Bharadwaj, S., R. Thanawala, G. Bon, R. Falcioni, and G.L. Prasad. 2005. Resensitization of breast cancer cells to anoikis by tropomyosin-1: role of Rho kinase-dependent cytoskeleton and adhesion. Oncogene. 24:8291–8303. [DOI] [PubMed] [Google Scholar]
- Cardone, M.H., G.S. Salvesen, C. Widmann, G. Johnson, and S.M. Frisch. 1997. The regulation of anoikis: MEKK-1 activation requires cleavage by caspases. Cell. 90:315–323. [DOI] [PubMed] [Google Scholar]
- Chen, C.S., M. Mrksich, S. Huang, G.M. Whitesides, and D.E. Ingber. 1997. Geometric control of cell life and death. Science. 276:1425–1428. [DOI] [PubMed] [Google Scholar]
- Dans, M., L. Gagnoux-Palacios, P. Blaikie, S. Klein, A. Mariotti, and F.G. Giancotti. 2001. Tyrosine phosphorylation of the beta 4 integrin cytoplasmic domain mediates Shc signaling to extracellular signal-regulated kinase and antagonizes formation of hemidesmosomes. J. Biol. Chem. 276:1494–1502. [DOI] [PubMed] [Google Scholar]
- Díaz-Montero, C.M., J.N. Wygant, and B.W. McIntyre. 2006. PI3-K/Akt-mediated anoikis resistance of human osteosarcoma cells requires Src activation. Eur. J. Cancer. 42:1491–1500. [DOI] [PubMed] [Google Scholar]
- Discher, D.E., P. Janmey, and Y.L. Wang. 2005. Tissue cells feel and respond to the stiffness of their substrate. Science. 310:1139–1143. [DOI] [PubMed] [Google Scholar]
- Engler, A.J., S. Sen, H.L. Sweeney, and D.E. Discher. 2006. Matrix elasticity directs stem cell lineage specification. Cell. 126:677–689. [DOI] [PubMed] [Google Scholar]
- Frisch, S.M., and H. Francis. 1994. Disruption of epithelial cell-matrix interactions induces apoptosis. J. Cell Biol. 124:619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch, S.M., K. Vuori, E. Ruoslahti, and P.Y. Chan-Hui. 1996. Control of adhesion-dependent cell survival by focal adhesion kinase. J. Cell Biol. 134:793–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgio, M., E. Migliaccio, F. Orsini, D. Paolucci, M. Moroni, C. Contursi, G. Pelliccia, L. Luzi, S. Minucci, M. Marcaccio, et al. 2005. Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell. 122:221–233. [DOI] [PubMed] [Google Scholar]
- Grinnell, F., M. Zhu, M.A. Carlson, and J.M. Abrams. 1999. Release of mechanical tension triggers apoptosis of human fibroblasts in a model of regressing granulation tissue. Exp. Cell Res. 248:608–619. [DOI] [PubMed] [Google Scholar]
- Kang, H.G., J.M. Jenabi, J. Zhang, N. Keshelava, H. Shimada, W.A. May, T. Ng, C.P. Reynolds, T.J. Triche, and P.H. Sorensen. 2007. E-cadherin cell-cell adhesion in ewing tumor cells mediates suppression of anoikis through activation of the ErbB4 tyrosine kinase. Cancer Res. 67:3094–3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai, K.M., and T. Pawson. 2000. The ShcA phosphotyrosine docking protein sensitizes cardiovascular signaling in the mouse embryo. Genes Dev. 14:1132–1145. [PMC free article] [PubMed] [Google Scholar]
- Martin, M.J., N. Melnyk, M. Pollard, M. Bowden, H. Leong, T.J. Podor, M. Gleave, and P.H. Sorensen. 2006. The insulin-like growth factor I receptor is required for Akt activation and suppression of anoikis in cells transformed by the ETV6-NTRK3 chimeric tyrosine kinase. Mol. Cell. Biol. 26:1754–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBeath, R., D.M. Pirone, C.M. Nelson, K. Bhadriraju, and C.S. Chen. 2004. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell. 6:483–495. [DOI] [PubMed] [Google Scholar]
- McFall, A., A. Ulku, Q.T. Lambert, A. Kusa, K. Rogers-Graham, and C.J. Der. 2001. Oncogenic Ras blocks anoikis by activation of a novel effector pathway independent of phosphatidylinositol 3-kinase. Mol. Cell. Biol. 21:5488–5499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettouchi, A., S. Klein, W. Guo, M. Lopez-Lago, E. Lemichez, J.K. Westwick, and F.G. Giancotti. 2001. Integrin-specific activation of Rac controls progression through the G(1) phase of the cell cycle. Mol. Cell. 8:115–127. [DOI] [PubMed] [Google Scholar]
- Migliaccio, E., S. Mele, A.E. Salcini, G. Pelicci, K.M. Lai, G. Superti-Furga, T. Pawson, P.P. Di Fiore, L. Lanfrancone, and P.G. Pelicci. 1997. Opposite effects of the p52shc/p46shc and p66shc splicing isoforms on the EGF receptor-MAP kinase-fos signalling pathway. EMBO J. 16:706–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio, E., M. Giorgio, S. Mele, G. Pelicci, P. Reboldi, P.P. Pandolfi, L. Lanfrancone, and P.G. Pelicci. 1999. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature. 402:309–313. [DOI] [PubMed] [Google Scholar]
- Moghal, N., and B.G. Neel. 1998. Integration of growth factor, extracellular matrix, and retinoid signals during bronchial epithelial cell differentiation. Mol. Cell. Biol. 18:6666–6678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson, P.R., S. Empereur, H.R. Glover, and S.M. Dilworth. 2001. ShcA tyrosine phosphorylation sites can replace ShcA binding in signalling by middle T-antigen. EMBO J. 20:6337–6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobes, C.D., and A. Hall. 1995. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 81:53–62. [DOI] [PubMed] [Google Scholar]
- Paszek, M.J., N. Zahir, K.R. Johnson, J.N. Lakins, G.I. Rozenberg, A. Gefen, C.A. Reinhart-King, S.S. Margulies, M. Dembo, D. Boettiger, et al. 2005. Tensional homeostasis and the malignant phenotype. Cancer Cell. 8:241–254. [DOI] [PubMed] [Google Scholar]
- Pinton, P., A. Rimessi, S. Marchi, F. Orsini, E. Migliaccio, M. Giorgio, C. Contursi, S. Minucci, F. Mantovani, M.R. Wieckowski, et al. 2007. Protein kinase C beta and prolyl isomerase 1 regulate mitochondrial effects of the life-span determinant p66Shc. Science. 315:659–663. [DOI] [PubMed] [Google Scholar]
- Pirone, D.M., W.F. Liu, S.A. Ruiz, L. Gao, S. Raghavan, C.A. Lemmon, L.H. Romer, and C.S. Chen. 2006. An inhibitory role for FAK in regulating proliferation: a link between limited adhesion and RhoA-ROCK signaling. J. Cell Biol. 174:277–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthalakath, H., D.C. Huang, L.A. O'Reilly, S.M. King, and A. Strasser. 1999. The proapoptotic activity of the Bcl-2 family member Bim is regulated by interaction with the dynein motor complex. Mol. Cell. 3:287–296. [DOI] [PubMed] [Google Scholar]
- Ravichandran, K.S. 2001. Signaling via Shc family adapter proteins. Oncogene. 20:6322–6330. [DOI] [PubMed] [Google Scholar]
- Re, F., A. Zanetti, M. Sironi, N. Polentarutti, L. Lanfrancone, E. Dejana, and F. Colotta. 1994. Inhibition of anchorage-dependent cell spreading triggers apoptosis in cultured human endothelial cells. J. Cell Biol. 127:537–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reginato, M.J., K.R. Mills, J.K. Paulus, D.K. Lynch, D.C. Sgroi, J. Debnath, S.K. Muthuswamy, and J.S. Brugge. 2003. Integrins and EGFR coordinately regulate the pro-apoptotic protein Bim to prevent anoikis. Nat. Cell Biol. 5:733–740. [DOI] [PubMed] [Google Scholar]
- Wary, K.K., A. Mariotti, C. Zurzolo, and F.G. Giancotti. 1998. A requirement for caveolin-1 and associated kinase Fyn in integrin signaling and anchorage-dependent cell growth. Cell. 94:625–634. [DOI] [PubMed] [Google Scholar]
- Wu, R.F., Y.C. Xu, Z. Ma, F.E. Nwariaku, G.A. Sarosi Jr., and L.S. Terada. 2005. Subcellular targeting of oxidants during endothelial cell migration. J. Cell Biol. 171:893–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanetti, A., M.G. Lampugnani, G. Balconi, F. Breviario, M. Corada, L. Lanfrancone, and E. Dejana. 2002. Vascular endothelial growth factor induces SHC association with vascular endothelial cadherin: a potential feedback mechanism to control vascular endothelial growth factor receptor-2 signaling. Arterioscler. Thromb. Vasc. Biol. 22:617–622. [DOI] [PubMed] [Google Scholar]
- Zhou, M.M., K.S. Ravichandran, E.F. Olejniczak, A.M. Petros, R.P. Meadows, M. Sattler, J.E. Harlan, W.S. Wade, S.J. Burakoff, and S.W. Fesik. 1995. Structure and ligand recognition of the phosphotyrosine binding domain of Shc. Nature. 378:584–592. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.