Abstract
Glycosphingolipids are controlled by the spatial organization of their metabolism and by transport specificity. Using immunoelectron microscopy, we localize to the Golgi stack the glycosyltransferases that produce glucosylceramide (GlcCer), lactosylceramide (LacCer), and GM3. GlcCer is synthesized on the cytosolic side and must translocate across to the Golgi lumen for LacCer synthesis. However, only very little natural GlcCer translocates across the Golgi in vitro. As GlcCer reaches the cell surface when Golgi vesicular trafficking is inhibited, it must translocate across a post-Golgi membrane. Concanamycin, a vacuolar proton pump inhibitor, blocks translocation independently of multidrug transporters that are known to translocate short-chain GlcCer. Concanamycin did not reduce LacCer and GM3 synthesis. Thus, GlcCer destined for glycolipid synthesis follows a different pathway and transports back into the endoplasmic reticulum (ER) via the late Golgi protein FAPP2. FAPP2 knockdown strongly reduces GM3 synthesis. Overall, we show that newly synthesized GlcCer enters two pathways: one toward the noncytosolic surface of a post-Golgi membrane and one via the ER toward the Golgi lumen LacCer synthase.
Introduction
Sphingolipids comprise a relatively small but vital fraction of the mammalian membrane lipids (Holthuis et al., 2001). Sphingomyelin (SM) carries a phosphocholine headgroup on a ceramide backbone and occurs in every mammalian cell, just like glucosylceramide (GlcCer). GlcCer serves as the basis for a highly polymorphic set of complex glycosphingolipids (GSLs). The unique physicochemical properties of sphingolipids allow different modes of interaction with their environment. Sphingolipids are concentrated at the cell surface and endocytotic membranes, where their bulk presence provides the membranes with chemical and mechanical stability. In addition, sphingolipids have the tendency to cluster with cholesterol in an environment of glycerolipids. The roles of sphingolipids in protein sorting, signaling, and membrane deformation may therefore be explained by their ability to form lateral domains that specifically include or exclude membrane proteins. In addition, the broad diversity in glycosidic structure allows individual GSLs to interact specifically with proteins, including viral and bacterial pathogens like Shiga and cholera toxin. The mode of action of sphingolipids in cellular processes depends on their concentration in the various subcellular organelles and the trans-bilayer and lateral distribution in those membranes. How cells sense and control the sphingolipid concentration of their membranes is largely unknown, but the spatial organization of metabolism, action of translocators, and selectivity of transport are three important determinants that are intrinsically linked. Many but not all enzymes of sphingolipid metabolism have been identified, some of them only very recently: the SM synthase (SMS) family (Huitema et al., 2004; Yamaoka et al., 2004) and two nonlysosomal glucocerebrosidases (Yildiz et al., 2006; Boot et al., 2007; Hayashi et al., 2007). One contemporary challenge is to unravel how sphingolipid metabolism is organized and controlled in the cellular context.
Ceramides are synthesized in the ER by various ceramide synthases (Pewzner-Jung et al., 2006). Some cell types express the galactosylceramide (GalCer) synthase (GalCS), which acts on the lumenal side of the ER (Sprong et al., 1998, 2003). However, all other synthetic enzymes of ceramide-containing lipids are located in the Golgi, with the exception of SMS2 at the plasma membrane (Huitema et al., 2004). Newly synthesized ceramides are transported from the ER to the Golgi, where they are converted by the GlcCer synthase (GCS) and SMS1. An important question is how cells regulate the ceramide supply to these enzymes. A first clue has come from the finding that the synthesis of SM but not GlcCer depended on ceramide transport to the trans-Golgi by ceramide transport protein (CERT; Hanada et al., 2003), a pathway that is regulated via phosphoinositides, sterols, and CERT phosphorylation (Perry and Ridgway, 2006). However, both SMS1 and GCS have been localized biochemically to the cis-medial Golgi, whereas GCS has also been assigned to pre-Golgi and trans-Golgi membranes (Futerman et al., 1990; Jeckel et al., 1990, 1992; Futerman and Pagano, 1991; Kohyama-Koganeya et al., 2004). The current agreement is that GlcCer is synthesized on a cytosolic surface and translocates across the Golgi membrane for higher GSL synthesis in the late Golgi (Lannert et al., 1994, 1998).
However, the consensus picture is based on evidence obtained before the recent identification of early enzymes in sphingolipid metabolism and transport. In addition, the bulk of the lipid data has been collected using short-chain sphingolipid analogues by the lack of tools to study natural sphingolipids. Such short-chain analogues have much higher off rates from membranes and spontaneously exchange between membranes (Pagano et al., 1981). In addition, they are less well ordered in the membrane (Wang and Silvius, 2000). Therefore, we have addressed the following questions: (1) Where are the various enzymes situated along the Golgi stack? (2) Is natural GlcCer translocated across the Golgi membrane by multidrug transporters (van Meer et al., 2006)? (3) Is there a function for the glycolipid-binding proteins glycolipid transfer protein (GLTP) in the cytosol (West et al., 2004) and FAPP2 on the trans-Golgi (Godi et al., 2004) in GSL metabolism and transport (Godi et al., 2004; Malinina et al., 2004; Vieira et al., 2005, 2006)? Using a novel assay for the transmembrane translocation of natural GSLs, we report that GlcCer can reach the plasma membrane via nonvesicular transport and translocates to the cell surface. In contrast to the short-chain lipid analogues, natural GSLs are not translocated by the multidrug transporters but by a novel mechanism that, in turn, does not recognize short-chain lipids. In addition, we have uncovered a pathway used by most GlcCer to reach the Golgi lumen. It involves transport from the Golgi to the ER by the glycolipid-binding protein FAPP2.
Results
Sphingolipid synthesis concentrates in the late Golgi and depends on CERT
Because the intra-Golgi localization of SMS1 and GCS is a matter of controversy (see Introduction), we first addressed their intracellular localization by immunofluorescence and immuno-EM (IEM). Attempts to localize the endogenous enzymes of mammalian cells with specific antibodies have failed so far, most likely because of their low expression levels. As an alternative, we ectopically expressed epitope-tagged constructs in HeLa cells and determined their cellular distribution using fluorescence microscopy (Fig. 1 and Fig. S1 A, available at http://www.jcb.org/cgi/content/full/jcb.200704091/DC1) and EM (Fig. 2). In the confocal fluorescence microscope, both GCS and SMS1 displayed a virtually identical staining pattern with endogenous GM130, a cis-Golgi marker (Nakamura et al., 1995), in transiently transfected HeLa cells (Fig. 1). Neither SMS1 nor GCS was found in the nuclear envelope and ER. To determine the intra-Golgi localization of GCS and SMS1, HeLa cell lines stably expressing these enzymes were analyzed by IEM. Golgi stacks containing five cisternae were used for statistical analysis, and endogenous GM130 was used as a cis-Golgi reference for every image. The intra-Golgi distribution of a protein was determined by counting all gold particles over a Golgi stack (n = 20 per protein), after which the number of gold particles found over a specific cisterna was expressed as a percentage of total gold (Fig. 2 B). Indeed, GM130 was restricted mostly to the cis-most cisterna (81%), with only a little label in the next cisterna (Fig. 2, A and B). In contrast to GM130, labeling for GCS was found in all five cisternae but with higher concentrations in cisternae 3–5 (medial–trans). SMS1 was mostly present at the trans-side of the Golgi and clearly peaked in the fourth cisterna. Quantitation showed that GCS and SMS1 had a substantially differential distribution: almost 50% of GCS localized to the first three cisternae, whereas only 33% of SMS1 was found there. Notably, SMS1 and GM130, which showed almost complete overlap by fluorescence microscopy, displayed different intra-Golgi distributions by IEM: GM130 was concentrated at the cis-side, and SMS1 was concentrated at the trans-side of the Golgi.
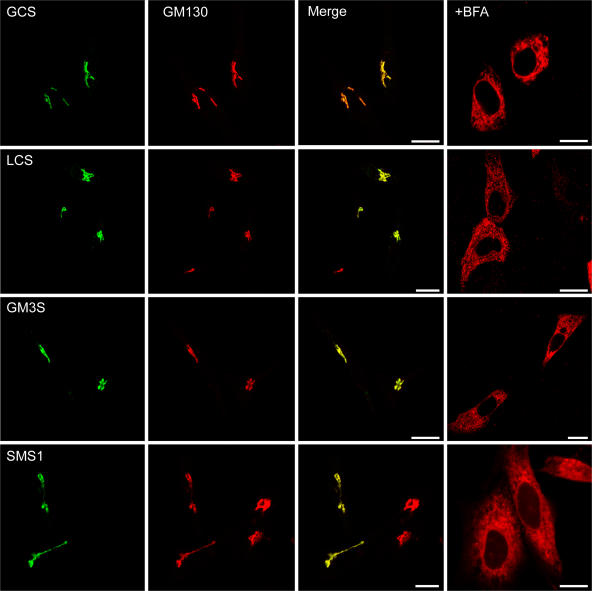
Figure 1.
Cellular localization of enzymes in Golgi sphingolipid synthesis by confocal fluorescence microscopy. HeLa cells were transiently transfected with the epitope-tagged enzymes (Table I). After 18 h, cells were fixed, permeabilized, and labeled with rabbit antibodies against the HA or V5 epitopes and with mouse antibody against GM130, a cis-Golgi matrix protein. Cells were counterstained with FITC-labeled anti–rabbit (left) and Texas red–labeled anti–mouse (GM130) antisera. Overlapping distributions appear as yellow in the merged images. After brefeldin A (BFA) treatment for 0.5 h, the glycosyltransferases were labeled with the specific antibodies followed by Texas red–labeled secondary antibodies. BFA fuses the Golgi stack to the ER. GCS, GlcCer synthase; LCS, LacCer synthase; GM3S, GM3 synthase; SMS1, SM synthase 1. For GCS, the transfected cells displayed 6.4× the activity of wild-type cells. Bars, 10 μm.
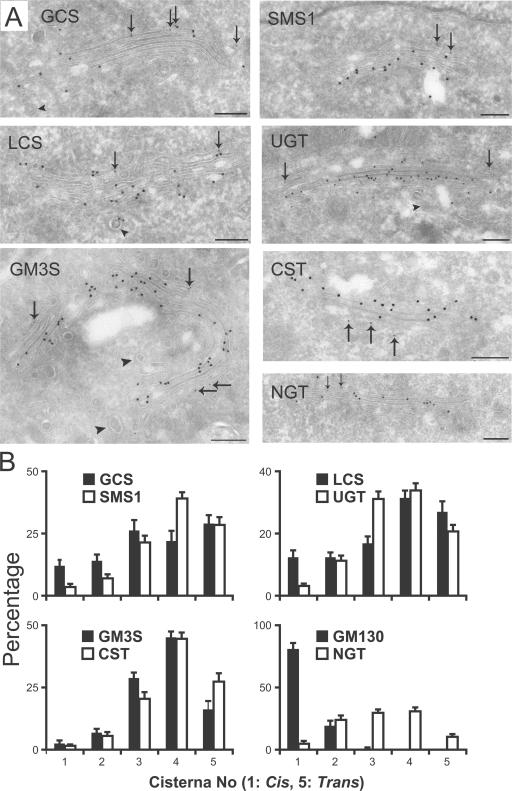
Figure 2.
Cellular localization of enzymes in Golgi sphingolipid synthesis by IEM. (A) HeLa cells stably transfected with the epitope-tagged enzymes (Table I) were double labeled for GM130 (10 nm protein A–gold; indicated by arrows) as a cis-Golgi marker and the specific enzyme (15 nm protein A–gold). Only a little labeling was present outside the Golgi in all cases, and this was similar to the signal found on untransfected cells. Clathrin coats, which are indicated by arrowheads, were often present on vesicles or tubules close to the TGN. CST, CMP–sialic acid transporter (SLC35A1); NGT, UDP-N-acetylglucosamine transporter (SLC35A3); UGT, UDP-Gal transporter (SLC35A2). (B) For each enzyme, the gold labeling was quantified in Golgi stacks that contained five cisternae. The cisterna labeled with the cis-Golgi reference GM130 was denoted as 1. The number of gold particles per cisterna was expressed as a percentage of the total gold particles within that Golgi stack: mean percentage ± SEM (error bars; n = 20). Bars, 200 nm.
Lactosylceramide (LacCer) synthesis not only depends on the presence of GlcCer but also on localization of the LacCer synthase (LCS) and the UDP-Gal transporter (UGT). LCS was found in all cisternae but peaked in cisternae 4 and 5. The location of the UGT was more restricted to the medial–trans-Golgi and peaked in cisternae 3 and 4. LacCer is further converted to GM3 (sialyllactosylceramide) by the transfer of sialic acid from CMP–sialic acid. The GM3 synthase (GM3S) and the CMP–sialic acid transporter (CST) localized exclusively to the Golgi stack, predominantly to the trans side (Fig. 2). Although a 100-fold overexpression of a Golgi-resident protein had no effect on the distribution of this protein within the HeLa Golgi (Rabouille et al., 1995), overexpression of a tagged version of a protein could lead to mislocalization. As one internal control, we therefore determined the localization of the UDP-GlcNAc transporter (NGT), which provides the medial-Golgi GlcNAc transferase with its substrate. It localized to the three medial cisternae with only little labeling in the first and last Golgi cisternae (Fig. 2, A and B). Collectively, the data indicate that the enzymes synthesizing LacCer and GM3 show a preferential medial–trans-localization, like SMS1, whereas the GCS is more evenly distributed over the Golgi stack.
Translocation of short-chain but not natural GlcCer across the Golgi membrane
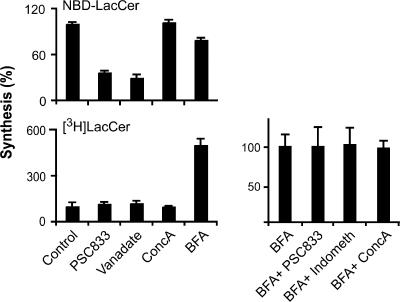
Because GlcCer is synthesized at the cytosolic aspect of the Golgi (see Introduction), we next addressed the question of whether GlcCer directly flips to the lumenal side, where it can be used for complex GSL synthesis. Exogenously added N-6-NBD-aminohexanoyl (C6-NBD)–GlcCer was readily converted to C6-NBD–LacCer in a postnuclear supernatant (PNS) of HeLa or CHO cells (Fig. 3; Burger et al., 1996; Lannert et al., 1998; Sprong et al., 2003). The translocation was 70% reduced by PSC833, an inhibitor of the ABC transporter ABCB1, a translocator for C6-NBD–GlcCer (van Helvoort et al., 1996), and vanadate, which blocks the nucleotide-binding domain of ABC transporters. To test whether natural GlcCer translocates across the Golgi membrane, we monitored the conversion of exogenous [3H]GlcCer to LacCer after insertion into the cytosolic leaflet of membranes in the HeLa PNS by the small cytosolic GLTP (Fig. S2, A and B; available at http://www.jcb.org/cgi/content/full/jcb.200704091/DC1). However, only a little [3H]LacCer synthesis was observed unless the Golgi had been fused to the ER by brefeldin A (BFA) before the experiment, in which case the synthesis of [3H]LacCer but not C6-NBD–LacCer increased fivefold (Fig. 3). The translocation of [3H]GlcCer was not inhibited by PSC833 or indomethacin, inhibitors of the ABC transporters ABCB1 and ABCC1, nor by concanamycin A, a vacuolar proton pump inhibitor. ABCB1 and -C1 translocate short-chain GlcCer (van Helvoort et al., 1996; van Meer et al., 2006), but the translocation of natural GlcCer across the ER–Golgi membrane was independent of these multidrug transporters.
Figure 3.
Natural GlcCer translocates less efficiently across the Golgi than across the ER membrane. PNSs were prepared from HeLa cells that had been (mock) treated with 1 μg/ml BFA. Equal volumes of PNS were incubated with or without 5 μM PSC833, 20 μM indomethacin (indometh), 1 mM vanadate, or 5 nM concanamycin A (ConcA) for 10 min at 37°C followed by a 1-h incubation with 2 mM UDP-Gal and 1 μM C6-NBD–GlcCer complexed to BSA or 66 kBq/ml [3H]GlcCer solubilized with recombinant GLTP. Each panel represents an independent set of experiments (n = 3). Lipids were extracted, analyzed, and expressed as the percentage of LacCer synthesized in the control. Error bars represent SEM.
Transport of simple but not complex GSLs continues with BFA
To see whether GlcCer could translocate toward the outer leaflet of the plasma membrane, from where it can reach the site of LacCer synthesis (Trinchera et al., 1990b; Sprong et al., 2001), we labeled the newly synthesized lipids with [14C]palmitate and, after various times, extracted the GSLs from the cell surface by GLTP. Based on previous experience, the assay was optimized starting from a 1,000-fold higher GLTP concentration than that used for the delivery of GlcCer to PNS membranes in Fig. 3, and the standard assay conditions were defined as 45 min at 37°C with 1.5 mg/ml GLTP (Fig. S2, C and D). The addition of acceptor liposomes to the medium inhibited rather than stimulated GlcCer extraction by GLTP (Fig. S2 E), most likely because GLTP interacted with the liposomes even if they contained no GSLs, thereby lowering the free GLTP concentration. Under these conditions, GLTP extracted 30–50% of the radiolabeled GlcCer and 20–70% of the GM3 from various cell types in 45 min but <0.5% of the SM and glycerophospholipids (Fig. 4, A–C). To check whether the phospholipid distribution across the membrane was affected by the extraction of the GSLs, exposure on the cell surface of inner leaflet phosphatidylserine was measured using FITC-labeled annexin V. No substantial annexin binding was observed in the GLTP-treated cells as compared with a positive control (Fig. 4 D). BFA induces fusion between the Golgi and ER and blocks vesicular traffic from the merged ER–Golgi to the plasma membrane. In mouse fibroblast (MF) cells, BFA did not affect GM3 synthesis (Fig. S4, available at http://www.jcb.org/cgi/content/full/jcb.200704091/DC1) but fully blocked its transport (Fig. 4 A), which is in line with the relocation of GM3S to the ER and the absence of a nonvesicular pathway for GM3 transport (Warnock et al., 1994). The same was observed for GalCer sulfate (SGalCer) in the oligodendrocytic D6P2T cells (Fig. 4 C). In contrast, GlcCer transport persisted in the presence of BFA. GalCer (and galactosyldiacylglycerol), which is synthesized in the ER lumen of CHO cells transfected with GalCS and in the ER lumen of D6P2T cells, behaved identically to GlcCer.
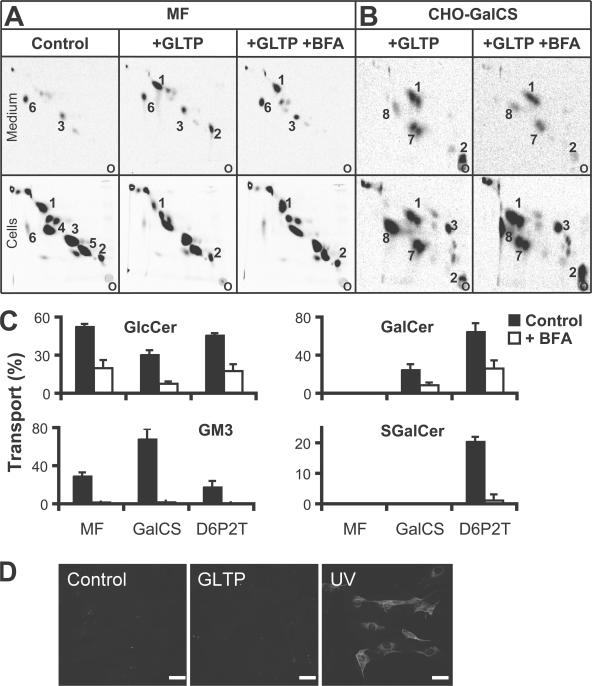
Figure 4.
BFA completely blocks transport to the surface of complex glycolipids but not of GlcCer and GalCer. (A) 2D TLC radiogram of a typical experiment in which MF cells pretreated ± 1 μg/ml BFA and labeled with [14C]palmitate for 1.5 h were incubated for 45 min at 37°C with 1.5 mg/ml GLTP. The lipids were extracted from cells and medium, separated by 2D TLC, and analyzed by phosphorimaging (see Materials and methods). The marked lipids are (1) GlcCer, (2) GM3, (3) phosphatidylcholine, (4) phosphatidylethanolamine, (5) SM, and (6) precursor [14C]palmitate. The origin is marked (o). First dimension, NH4OH solvent. (B) CHO-GalCS cells were labeled with [14C]galactose for 1.5 h ±1 μg/ml BFA, and the transport of GlcCer, GalCer, and GM3 was assessed in a subsequent 45-min incubation with 1.5 mg/ml GLTP (medium), again ±BFA. Lipid numbering as in A, plus (7) GalCer and (8) galactosyldiacylglycerol. First dimension, NH4OH solvent; borate plates. (C) MF, CHO-GalCS, and D6P2T cells were treated as in A and B. Lipid transport as a percentage of that lipid found in the medium was calculated from at least three independent experiments for each lipid, each in triplicate. HeLa cells synthesized Gb3 instead of GM3, which could not be tested because its synthesis is interrupted by BFA (Fig. S4, available at http://www.jcb.org/cgi/content/full/jcb.200704091/DC1). Error bars represent SEM. (D) MF cells were incubated with or without 1.5 mg/ml GLTP for 45 min at 37°C, fixed, stained with FITC-conjugated annexin V, and analyzed by fluorescence microscopy. As a positive control, MF cells were forced into apoptosis by overnight growth in serum-free medium, exposure to 200 J/m2 UV light, and incubation in regular medium for 2 h at 37°C. The pictures were taken using identical settings. Bars, 10 μm.
Transfer proteins GLTP and FAPP2
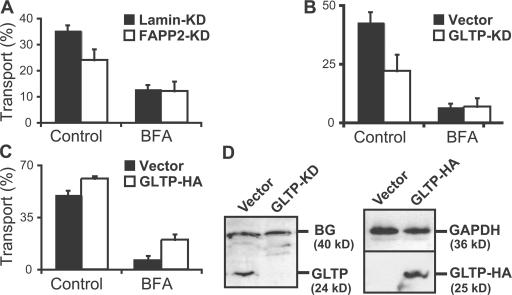
The fact that GlcCer and GM3 were synthesized in the same compartment in the presence of BFA (Fig. 1) and that GlcCer but not GM3 reached the cell surface under these conditions implied that GlcCer had reached the plasma membrane in the absence of vesicular traffic. Because natural GlcCer, in contrast to short-chain C6-NBD–GlcCer, does not readily exchange through the cytosol, we tested whether transport in the presence of BFA was mediated by a transfer protein. FAPP2, a protein that shares the glycolipid-binding domain with GLTP, has been found to be ubiquitously expressed (Godi et al., 2004). However, the knockdown of FAPP2 in MEB4 cells had no effect on the transport of newly synthesized GlcCer to the cell surface in the presence of BFA (Fig. 5 A). Likewise, a knockdown of GLTP in D6P2T cells did not reduce the transport of GlcCer (or GalCer; not depicted) to the plasma membrane in the presence of BFA (Fig. 5 B), indicating that transport from the merged ER–Golgi to the plasma membrane in the presence of BFA was not mediated by these proteins or that they are redundant. On the other hand, GLTP overexpression stimulated the transport of GlcCer (and GalCer) in the presence of BFA twofold, showing that GLTP in vivo is able to fulfill this function (Fig. 5 C). Unexpectedly, FAPP2 knockdown in MEB4 cells reduced transport to the plasma membrane by 30% in the absence of BFA, and the knockdown of GLTP in D6P2T cells reduced the transport of GlcCer (and also of GalCer; unpublished data) by 30–50%, implying that >30–50% of the GlcCer that reached the cell under normal conditions had been transported by FAPP2 or GLTP followed by translocation across a post-Golgi membrane. We did not succeed in making a double knockdown for FAPP2 and GLTP, possibly because this is a lethal combination. Transport of GM3 in MEB4 cells was not reduced by FAPP2 knockdown. As before, it was effectively blocked by BFA (unpublished data).
Figure 5.
Effect of the expression levels of FAPP2 and GLTP on GSL transport to the cell surface. (A) MEB4 cells stably expressing RNAi plasmids against FAPP2 or lamin were preincubated with or without 1 μg/ml BFA for 0.5 h and labeled with [14C]palmitic acid for 1.5 h. [14C]GSLs were extracted from the cell surface during an additional 45-min incubation with 1.5 mg/ml GLTP in the medium. The lipids in the cells and media combined with the washes were analyzed and quantified. Transport is expressed as the percentage of GlcCer recovered in the medium. (B and C) D6P2T cells stably expressing RNAi plasmids against GLTP or empty vector (B) or stably transfected with GLTP (C) were treated with BFA and labeled with [14C]galactose, and transport of [14C]GlcCer to the cell surface was measured as in A. Error bars represent SEM. (D) Western blot analysis from D6P2T clones expressing either RNAi plasmids (B) against GLTP and empty vector as control or pCDNA3.1 plasmids with GLTP-HA (C) using a rabbit antiserum against mouse GLTP. A background band at ∼40 kD (BG) and a blot against glyceraldehyde-3 phosphate dehydrogenase (GAPDH) were used as loading controls.
In contrast to C6-NBD–GlcCer, natural GlcCer is not translocated by ABCB1 or -C1 but by a novel mechanism sensitive to concanamycin A
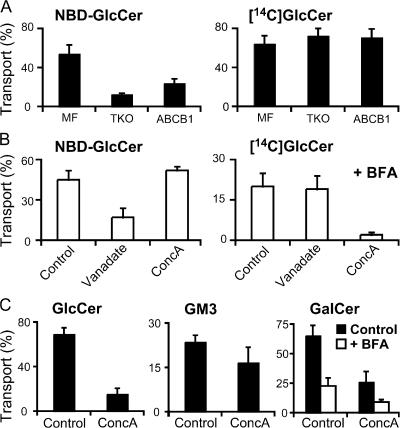
We have previously reported that a variety of short-chain GlcCer analogues, including C6-NBD–GlcCer, was transported to the cell surface by the multidrug transporters ABCB1 and -C1 (van Meer et al., 2006). Therefore, we addressed whether the transport of natural GlcCer to the cell surface in the presence of BFA (Fig. 5) was mediated by these ABC transporters by using an MF cell line derived from the Abcb1a/Abcb1b/Abcc1 triple knockout (TKO) mouse (Wijnholds et al., 2000) vs. TKO cells stably transfected with human ABCB1 (MDR1). The transport of C6-NBD–GlcCer was strongly reduced in TKO cells (down to zero with BFA) and was partially restored by transfection with ABCB1 (Fig. 6 A), demonstrating that the translocation of C6-NBD–GlcCer across the plasma membrane of MF cells was fully the result of Abcb1 and Abcc1. In line with this, the C6-NBD–GlcCer translocation in MF cells was reduced by specific inhibitors (PSC833 for Abcb1a/b) or general inhibitors (glibenclamide and vanadate) of ABC transporters (Fig. 6 B). In strong contrast, knockout of the multidrug transporters had no effect on the transport of natural GlcCer either in the absence or presence (unpublished data) of BFA. Transfection of the TKO cells with ABCB1 did not increase the translocation of natural GlcCer, nor was there a substantial effect of the various inhibitors, with one exception: concanamycin A, a specific inhibitor of the vacuolar proton ATPase (Bowman et al., 2006), essentially abolished the translocation of natural GlcCer without effect on C6-NBD–GlcCer (Fig. 6 B). Also, in the absence of BFA, GlcCer transport to the cell surface was virtually abolished by concanamycin A (Fig. 6 C), which cannot be explained by an inhibition of vesicular traffic as indicated by the mild reduction in GM3 transport. Concanamycin had no effect on the synthesis of GM3 from newly synthesized GlcCer (unpublished data).
Figure 6.
In contrast to C6-NBD–GlcCer, endogenous GlcCer is translocated by a mechanism that does not depend on multidrug transporters but is blocked by concanamycin A. (A) MF cells, TKO cells, an MF line with a triple knockout for the multidrug transporters Abcb1a, -b1b, and -c1, and TKO cells transfected with human ABCB1 were incubated with C6-NBD–ceramide for 1 h or [14C]palmitic acid for 1.5 h at 37°C. Labeled GlcCer was extracted from the cell surface by BSA and GLTP and quantitatively analyzed as described in Materials and methods. The same experiment was performed in the presence of BFA (not depicted). (B) MF cells were preincubated with 1 μg/ml BFA ± 1 mM vanadate or 5 nM concanamycin A (ConcA) for 0.5 h at 37°C and incubated with C6-NBD–ceramide with BSA or [14C]palmitate followed by GLTP, all in the presence of the inhibitors. 5 μM PSC833, 20 μM indomethacin, and 50 μM glibenclamide gave similar results to vanadate, both +BFA and –BFA. (C) MF cells (GlcCer and GM3) or D6P2T cells (GalCer) were preincubated with or without 1 μg/ml BFA and 5 nM concanamycin A for 0.5 h at 37°C followed by incubations with [14C]palmitate and GLTP, all in the presence of the inhibitors. Labeled GlcCer was extracted from the cell surface and quantified as in A. Transport was calculated as the percentage of the specific lipid that was recovered in the medium. Error bars represent SEM.
GlcCer readily reaches the lumen of the ER in vivo
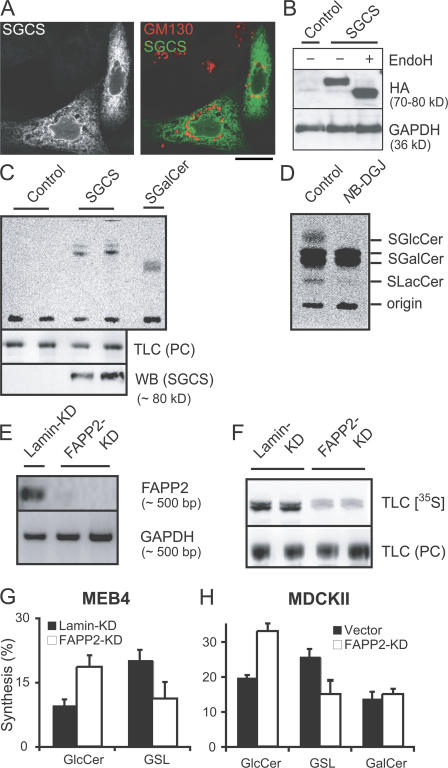
Because concanamycin did not affect the rate of GM3 synthesis, GlcCer destined for GM3 synthesis must have followed a different transport pathway independent of the post-Golgi translocation that was inhibited by concanamycin. Therefore, we tested the possibility that this GlcCer reached the site of LacCer synthesis in the lumen of the Golgi via the ER. To test whether GlcCer is present in the ER lumen, we introduced an enzyme into the ER lumen, the SGalCer synthase, that transfers a sulfate from 3′-phosphoadenosine 5′-phosphosulfate (PAPS) to GalCer and GlcCer (Gasa et al., 1990) but that normally acts in the trans-Golgi in conjunction with the PAPS transporter (Farrer et al., 1995). A chimera (SGCS [chimeric protein consisting of an HA-tagged PAPS transporter and SGalCer synthase]) was constructed of the SGalCer synthase to an HA-tagged PAPS transporter. In MEB4 cells, SGCS showed a diffuse reticular staining and clear staining of the nuclear envelope. The patterns of SGCS and the cis-Golgi marker GM130 were mutually exclusive (Fig. 7 A). In accordance with its location in the ER, SGCS remained sensitive to EndoH digestion (Fig. 7 B), implying the presence of high mannose N-glycans. The molecular mechanism retaining the SGCS in the ER has remained unclear. It may contain a previously hidden ER retention signal, or maybe the complex is partially unfolded. When SGCS-transfected MEB4 cells were labeled with [35S]H2SO4, one major radioactive band appeared on TLC plates that ran faster than SGalCer (Fig. 7 C). When GlcCer and [35S]PAPS were added to a PNS of MEB4 cells transfected with the SGCS construct, a lipid product was made that ran above SGalCer. Synthesis depended on GlcCer, on PAPS, and on the transfection. A lipid with the same mobility on TLC was found in D6P2T cells. The band disappeared when the cells had been incubated with the GCS inhibitor N-butyldeoxygalactonojirimycin, identifying the unknown lipid as SGlcCer (Fig. 7 D and Fig. S3 A, available at http://www.jcb.org/cgi/content/full/jcb.200704091/DC1). In addition, it was absent after a GCS knockdown but not an LCS knockdown (Fig. S3 B), which efficiently inhibited the synthesis of LacCer (not depicted). Finally, similar amounts of the [35S]lipid were synthesized in SGCS-transfected CHO and mutant CHO-lec8 cells (Fig. S3 C), which have threefold reduced LacCer levels (Sprong et al., 2003). Thus, GlcCer reached the lumen of the ER, where it was converted to SGlcCer by SGCS.
Figure 7.
Transport of newly synthesized GlcCer to the lumen of the ER and its conversion to GM3 are greatly reduced by knocking down FAPP2. (A) MEB4 cells were transiently transfected with a construct containing the sulfo-GalCS and the PAPS transporter (SGCS). After 18 h, the cells were fixed and labeled with rabbit antibodies against the HA tag of SGCS and with mouse antibodies against GM130 followed by FITC (left) and Texas red secondary antibodies (right). (B) Cells (mock) transfected with SGCS were washed and lysed in 50 mM sodium citrate, pH 5.5, 2 mM EDTA, 1 mM 2-mercaptoethanol, 0.1% SDS, and protease inhibitors. Half of the transfected sample received endoglycosidase H (EndoH), and all samples were incubated for 6 h at 30°C. Proteins were resolved on 7.5% SDS-PAGE followed by Western blot detection with rabbit anti-HA antibodies. The housekeeping enzyme glyceraldehyde-3 phosphatase dehydrogenase (GAPDH) served as a loading control. (C) MEB4 cells (mock) transfected with SGCS were incubated with 600 kBq [35S]H2SO4/105 cells. After 24 h, the lipids were extracted, separated by 1D TLC, and stained with iodine to visualize the bulk lipid phosphatidylcholine (PC) as a loading control. [35S]lipids were visualized by phosphorimager screens. Western blot analysis with anti-HA antibodies was performed on parallel dishes. (D) D6P2T cells were labeled overnight with [35S]H2SO4 in the presence or absence of 40 μM of the GCS inhibitor N-butyldeoxygalactonojirimycin (NB-DGJ), after which the lipids were analyzed by TLC and phosphorimaging. The more hydrophilic spot was most likely SLacCer. (E) Semiquantitative RT-PCR on RNAs from MEB4 clones stably expressing RNAi plasmids against PCR fragments of FAPP2 or lamin as a control to detect expression levels of FAPP2 and GAPDH as a control. (F) MEB4 cells stably expressing RNAi plasmids against FAPP2 or lamin were transiently transfected with SGCS. After 18 h, cells were labeled with 3,600 kBq/ml [35S]H2SO4 for 8 h. The lipids were analyzed as in C. Parallel dishes had an equal expression of SGCS as checked by Western blotting against HA (not depicted). (G and H) MEB4 cells stably expressing RNAi plasmids against FAPP2 or lamin and MDCKII cells infected with empty virus (control) or with retrovirus-mediated RNAi against FAPP2 were labeled overnight with 72 kBq/ml [14C]galactose. Lipids were extracted and separated by 2D TLC, and radiolabeled lipids were quantified using a phosphorimager as described in Materials and methods. GSL, LacCer plus GM3 (MEB4) and a mix of complex GSLs (MDCKII). Values are expressed as a percentage of total [14C]lipids and are the mean of three experiments in triplicate. Error bars represent SEM. Bar, 10 μm.
Role of FAPP2 in GlcCer transport to the ER
Selective GlcCer transport from the cytosolic surface of the Golgi back to the ER would be most easily explained by the activity of a cytosolic transfer protein followed by a translocation across the ER membrane. Therefore, we tested the involvement of the glycolipid-binding protein FAPP2 in this pathway. When lamin and FAPP2 knockdown cells were transfected with SGCS and labeled with [35S]H2SO4, SGlcCer synthesis in the FAPP2 knockdown cells was only 30% of that of the control (Fig. 7, D–F). In MEB4 cells, FAPP2 knockdown resulted in a twofold decrease in the synthesis of GM3 with a concomitant accumulation of GlcCer (Fig. 7 G). Similar results (Fig. 7 H) were obtained in MDCKII cells, in which FAPP2 expression was reduced by retrovirus-mediated RNAi (Vieira et al., 2005). GalCer levels in these cells were normal, indicating that ceramide levels in the ER were unaffected. FAPP2 specifically affected the conversion of GlcCer to GM3 via LacCer. These experiments show that FAPP2, which binds to the Golgi (Fig. S1 A) via PI4P (Godi et al., 2004), plays an important role in the transport of GlcCer to the sulfation site in the ER and to the site of LacCer and GM3 synthesis in the lumen of the Golgi. Concanamycin A did not affect GlcCer translocation across the ER membrane (Fig. 3), nor did it affect GM3 synthesis from newly synthesized GlcCer (not depicted), showing that ER translocation occurred by a different mechanism and that the pathways of newly synthesized GlcCer to the Golgi lumen and to the plasma membrane are independent.
Discussion
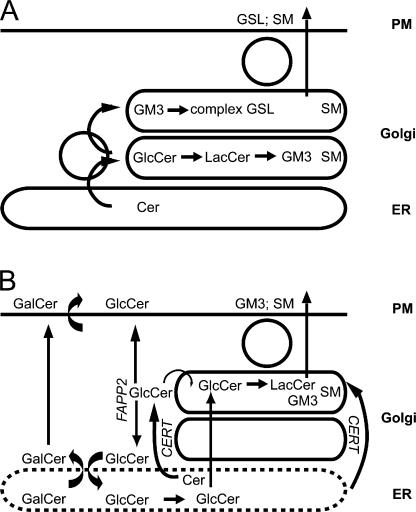
Until recently, sphingolipid assembly in the Golgi was thought to be organized according to the same simple linear model as glycoprotein processing (Fig. 8 A). Because it is not water soluble, ceramide synthesized in the ER would be transported by the secretory pathway to the cis-Golgi, where it would be converted to SM by SMS (Futerman et al., 1990; Jeckel et al., 1990) and to GlcCer by GCS (Futerman and Pagano, 1991; Jeckel et al., 1992). Subsequently, GlcCer would encounter the enzymes of complex GSL synthesis in an ordered array along the Golgi stack (Trinchera et al., 1990a). The finding that GlcCer is synthesized on the cytosolic surface but converted to LacCer in the lumen forced the questions whether and how GlcCer crosses the Golgi membrane. This seemed solved by (1) the finding that short-chain GlcCer analogues were able to cross the Golgi membrane (Lannert et al., 1994, 1998; Burger et al., 1996), (2) the identification of the multidrug transporters ABCB1 and -C1 as floppases for these molecules (van Meer et al., 2006), and (3) a correlation between ABCB1 activity and complex GSL synthesis in living cells (De Rosa et al., 2004). However, the mechanism of SM synthesis is much more intricate in that SMS receives its substrate via the cytosolic transfer protein CERT, which binds to ER and trans-Golgi and is regulated at various levels via phosphorylation (Perry and Ridgway, 2006; Fugmann et al., 2007; Kumagai et al., 2007).
Figure 8.
Synthesis and transport of GSLs. (A) In the traditional model, ceramide moves to the cis-Golgi via vesicular transport and is converted to SM and GlcCer. GlcCer is further processed by transferases in the trans-Golgi and TGN. (B) In the updated model, most ceramide is transported to the trans-Golgi by CERT, SM is produced in the lumen, and GlcCer is produced on the cytosolic surface. Most GlcCer is transported back to the ER via FAPP2, whereas another fraction reaches the cytosolic surface of the plasma membrane (or endosome), where it is translocated. The ER GlcCer (our assays do not strictly exclude the possibility that this is actually the cis-Golgi) enters the secretory pathway and is converted to LacCer and GM3 in the trans-Golgi followed by further modification in the TGN, depending on cell type. It remains unclear whether any GlcCer translocates directly across the Golgi membrane.
Also, the synthesis and processing of GlcCer are more sophisticated than suspected (Fig. 8 B): (1) The GCS is concentrated in the trans-Golgi, not in the cis-Golgi, and its activity was inhibited more than twofold upon CERT knockdown. (2) In contrast to short-chain GlcCer, natural GlcCer did not flop efficiently across the Golgi membrane and was not a substrate for the multidrug transporter ABCB1. (3) Instead, newly synthesized GlcCer reached the outside of the plasma membrane by a nonvesicular transport pathway and was translocated via a mechanism that was inhibited by concanamycin A, an inhibitor of the vacuolar ATPase, suggesting the involvement of a proton gradient. (4) Finally, most GlcCer reached the LCS in the Golgi lumen via the ER, with a role for the trans-Golgi glycolipid-binding protein FAPP2 in shuttling GlcCer to the ER. This suggests that GlcCer may play a role in transport and sorting events at the ER. In addition, FAPP2 appears to regulate complex GSL synthesis. The finding that multiple pathways remove GlcCer from the cytosolic surface of the trans-Golgi suggests the possibility that GlcCer exerts a physiological function at that location.
Intra-Golgi organization of sphingolipid metabolism
The finding that SM synthesis depends on the ER/trans-Golgi protein CERT for its ceramide supply suggested that the Golgi SMS is located in the trans-Golgi (Munro, 2003). Indeed, in HeLa cells, transfected SMS1 peaked in the fourth cisterna out of five (Fig. 2). Like Hanada et al. (2003), we observed that CERT knockdown also affected GCS synthesis from [14C]serine, suggesting that [14C]serine-derived ceramide is transported by CERT to the GCS in the trans-Golgi. However, these authors reported that GlcCer synthesis from [3H]sphingosine was independent of CERT, suggesting the existence of different ceramide pools and the preferential delivery of [3H]sphingosine-derived ceramide by vesicular transport to cis–medial-Golgi GCS. A bimodal distribution of GCS and not SMS had been observed before by cell fractionation (Futerman and Pagano, 1991; Jeckel et al., 1992) and is now corroborated by IEM of the transfected GCS (Fig. 2). In contrast to the Drosophila melanogaster GCS (Kohyama-Koganeya et al., 2004), mammalian GCS was not in the ER. In the Golgi lumen, GlcCer is converted to LacCer, which, in most cells, is efficiently sialylated to GM3. The responsible enzymes, LCS and GM3S, have been assigned to the Golgi stack from the fact that they were still able to use newly synthesized GlcCer and LacCer after BFA treatment (van Meer, 1993). Cell fractionation assigned LCS to the trans-Golgi (Lannert et al., 1998) and GM3S to the cis-Golgi (Trinchera et al., 1990a), but GM3 synthesis was found highest in the trans-Golgi (Lannert et al., 1998; Allende et al., 2000). IEM located both enzymes and their nucleotide sugar transporters to the trans-Golgi, peaking in cisterna 4 (Fig. 2), whereas NGT, the transporter for UDP-GlcNAc into the medial-Golgi, was more evenly distributed. Biochemical experiments have indicated that LCS and GM3S form a molecular complex (Giraudo and Maccioni, 2003), explaining the efficient utilization of LacCer by GM3S.
Translocation of GlcCer toward the noncytosolic surface
After synthesis, GlcCer must cross a membrane to reach the LCS in the Golgi lumen. When exogenous GlcCer analogues with shortened fatty tails were added to the Golgi, they were converted to LacCer (Lannert et al., 1994, 1998; Burger et al., 1996), a process sensitive to the ABCB1 inhibitor PSC833 (Fig. 3). This suggests translocation by Abcb1, which also translocates newly synthesized C6-NBD–GlcCer across plasma membranes (Fig. 6, A and B; van Helvoort et al., 1996). In contrast, LacCer synthesis from natural GlcCer and translocation of newly synthesized natural GlcCer across the plasma membrane were insensitive to PSC833 (Fig. 6 B). The latter translocation was sensitive to concanamycin, which suggests the involvement of a pH gradient. Because endosomal recycling still occurs under BFA (Lippincott-Schwartz et al., 1991), the translocation most likely occurred in the endosomes. How the low pH affects the transmembrane translocation of GlcCer is presently under investigation. Although concanamycin has some inhibitory effect on vesicular traffic (Fig. 6 C; Weisz, 2003), this does not explain the abolition of cell surface appearance under non-BFA conditions. One other potential translocator for GlcCer identified in skin, ABCA12 (Lefevre et al., 2003), is not expressed in the cell lines used in this study.
The synthesis of LacCer from exogenous GlcCer in a PNS was greatly stimulated by BFA (Fig. 3). This suggests that GlcCer translocation across the trans-Golgi membrane was limiting and that GlcCer efficiently flopped across the membrane of the ER–Golgi compartment, which, for 75%, consists of ER membrane (but is not the native ER membrane). Also, GalCer (and galactosyldiacylglycerol) appears to flip across the ER–Golgi, but in the opposite direction: after synthesis on the lumenal side of the ER–Golgi in the presence of BFA, GalCer still reached the cell surface (Fig. 4, B and C). In the absence of vesicular traffic from this organelle to the plasma membrane (as indicated by the lack of transport of GM3, SGalCer, and proteins; Fig. S5, available at http://www.jcb.org/cgi/content/full/jcb.200704091/DC1), this transport must have involved translocation toward the cytosolic surface of the ER membrane, monomeric transport through the cytosol to the cytoplasmic side of the plasma membrane, and outward translocation across the plasma membrane. There are several studies in the literature that have addressed lipid flipping in the ER, and every lipid tested so far readily moves across the ER membrane in both directions by an energy-independent mechanism (Papadopulos et al., 2007). We now find that this event is insensitive to inhibition of the multidrug transporters (Fig. 3 B).
Cytosolic GlcCer transport and ER lipid rafts
Under BFA, when vesicular transport (measured as the appearance of GM3 and SGalCer) was inhibited, natural GlcCer still reached the cell surface (Fig. 4 C). This shows that GlcCer synthesized in the ER–Golgi reached the cytosolic surface of the plasma membrane or an endosomal compartment (Warnock et al., 1994). Surprisingly, neither a knockdown of FAPP2 nor of GLTP, the two known cytosolic proteins with a glycolipid-binding domain, had an inhibitory effect, suggesting the involvement of a third protein or an independent mechanism like membrane contact sites (Levine and Loewen, 2006). The knockdowns of these proteins did have an inhibitory effect in the absence of BFA, in which virtually all GlcCer arriving at the cell surface was translocated by the concanamycin-sensitive non-ER mechanism. This suggests that under normal conditions, GlcCer destined for the cell surface is transported by FAPP2 or GLTP from the cytosolic surface of the Golgi to the post-Golgi membrane, where subsequent translocation occurs. The observation that the GlcCer transport remaining under BFA conditions was insensitive to FAPP2 or GLTP knockdown suggests that either there is a redundancy between these proteins or that, possibly, ultrastructural connections between the Golgi and post-Golgi membranes are involved that are broken by BFA (Lippincott-Schwartz et al., 1991). After the translocation of GlcCer to the noncytoplasmic leaflet of the post-Golgi membrane, part of this GlcCer might recycle to the Golgi, where it would be converted to LacCer (Trinchera et al., 1990b; Sprong et al., 2001), but this pathway may not be very active considering the lack of effect of concanamycin on GM3 synthesis (unpublished data).
The finding that GlcCer reached the ER lumen as measured via an assay involving enzymatic modification suggested the involvement of one of the two known cytosolic GlcCer-binding proteins. Because MEB4 cells have low levels of GLTP (not depicted), we knocked down FAPP2, a protein with a GLTP domain that is recruited to the trans-Golgi and TGN by PI4P (Fig. S1 A; Godi et al., 2004). The FAPP2 knockdown dramatically reduced GlcCer transport to the ER lumen (Fig. 7 F). FAPP2 may transport GlcCer as a monomer via its GlcCer-binding domain, which is analogous to ceramide transfer by CERT. Presently, we cannot exclude the possibility that FAPP2 is involved in the generation of retrograde vesicles that, on their cytosolic surface, are enriched in GlcCer. FAPP2 knockdown inhibited GM3 synthesis by 50% (Fig. 7, G and H), suggesting that under normal conditions, at least half of the GlcCer used for GM3 synthesis passes through the ER. It would be delivered to the cytosolic ER surface by FAPP2, flip across the ER, and would be concentrated into ER–Golgi transport vesicles. This lateral concentration in the ER membrane may be very similar to the proposed formation of lipid rafts in the Golgi (van Meer, 1993). Localization of the Drosophila GCS to the ER (Kohyama-Koganeya et al., 2004) has the same implications. Also, GalCer, which is synthesized on the lumenal surface of the ER of some cell types, may contribute to such a mechanism. The occurrence of lipid rafts in the ER (defined by detergent insolubility) has been reported for yeast (Bagnat et al., 2000) and mammalian cells, where they were GlcCer dependent (Smith et al., 2006). They were suggested to play a role in protein sorting and toxin retrotranslocation across the ER membrane, respectively. The effects of FAPP2 knockdown on sphingolipid-mediated events at the TGN and apical plasma membrane of MDCK cells (Vieira et al., 2005, 2006) may be caused by the inhibition by FAPP2 of the GlcCer flux through the ER or by its inhibitory effect on complex GSL synthesis (Fig. 7 H).
Nearly all GlcCer that reached the cell surface followed the concanamycin-sensitive pathway (Fig. 6 C). Concanamycin did not inhibit transport across the ER–Golgi (Fig. 3), which suggests that the GlcCer that enters the Golgi via the ER is converted to higher GLSs and does not reach the cell surface as GlcCer. Thus, no GlcCer would pass through the trans-Golgi lumen to the plasma membrane, in contrast to GalCer, which is not further glycosylated. This may explain why oligodendrocytes synthesize very little SGlcCer as compared with SGalCer (Fig. 7 D).
Physiological functions of GlcCer
Apart from being the precursor for the complex GSLs and playing a role in membrane organization as part of lipid rafts on the lumenal surface of cellular membranes, GlcCer may exert specific functions at one other location that is unusual for GSLs, the cytosolic surface of the Golgi and the ER membrane. GlcCer is synthesized on the cytosolic surface of the Golgi, from where it does not simply disappear by translocation across the Golgi membrane. From independent work, we have suggested a function for GlcCer on the cytosolic surface of the Golgi in sorting membrane proteins to the melanosomes (Sprong et al., 2001), which we have now identified as a stimulation of the vacuolar proton pump (unpublished data). Relevant issues are the sidedness of GlcCer in the ER and the location and mechanism by which it flops to the lumenal side of the membrane. In addition, the function of the recently discovered GlcCer degrading ER and cytosolic β-glucosidases (Yildiz et al., 2006; Boot et al., 2007; Hayashi et al., 2007), the absence of which results in raised GlcCer levels, remains to be resolved. The fact that the GCS occurs in most eukaryotes, even in 50% of all fungi (Saito et al., 2006), suggests that eukaryotes use GlcCer for basic functions that we are about to unravel, but it is likely that evolution has overlaid the basic principles by many levels of regulation. This may explain why these basic principles of GlcCer action have remained obscure to date.
Materials and methods
Materials
BSA, fraction V, and other chemicals were purchased from Sigma-Aldrich unless indicated otherwise and were used in the highest purity available. Silica TLC plates were obtained from Merck, organic solvents were purchased from Riedel de Haën, and cell culture media, reagents, and FCS were obtained from PAA. Cell culture plastics were purchased from Costar. Tran[35S]label (>37 TBq/mmol), [35S]H2SO4 (74 MBq/mmol), D-[1-14C]galactose (1.8 GBq/mmol), [U-14C]palmitic acid (18 GBq/mmol), [9,10(n)-3H]palmitic acid (1.5 TBq/mmol), and L-[3-14C]serine (1.9 GBq/mmol) were obtained from GE Healthcare and MP Biomedicals. Lipids and lipid standards were obtained from Avanti Polar Lipids, Inc. C6-NBD–fatty acid was purchased from Invitrogen. C6-NBD– and [3H]palmitoyl-GlcCer were synthesized as described in supplemental Materials and methods (available at http://www.jcb.org/cgi/content/full/jcb.200704091/DC1). EST clones were purchased from RZPD. Rabbit antisera against the V5 and HA epitope (Y11) were obtained from Sigma-Aldrich and Santa Cruz Biotechnology, Inc., respectively. The rabbit peptide antibody against mouse GLTP was a gift from K. Aikawa-Kojima (Ochanomizu University, Tokyo, Japan). The rabbit serum against GM130 was a gift from E. Sztul (University of Alabama, Birmingham, AL). Mouse anti-V5 antibody was purchased from Invitrogen, mouse mAb 16B12 anti-HA was obtained from BabCO, mouse anti-GM130 antibodies were obtained from BD Biosciences, and mouse anti–glyceraldehyde-3 phosphate dehydrogenase was purchased from Applied Biosystems. Fluorescent secondary goat antibodies were obtained from Jackson ImmunoResearch Laboratories, Inc. HRP-conjugated secondary goat anti–rabbit IgG was purchased from DakoCytomation. Recombinant GLTP was produced as described in supplemental Materials and methods.
DNA constructs
Plasmids are described in Table I. ORFs were amplified by PCR using cDNA clones as a template. PCR products were gel purified and cloned into mammalian expression vectors containing a sequence encoding for a triple HA tag (3*YPYDVPDYA) at the 5′ or 3′ end or a single HA or V5 tag (GKPIPNPLLGLDST) at the 3′ end. SGCS is a chimeric construct containing a triple HA tag, the rat PAPS transporter, a double myc tag as spacer (2*EQKLISEEDL), and the mouse SGalCer synthase. Primers containing RNAi sequences of FAPP2 and lamin were inserted between the BglII and HindIII sides of the RNAi plasmid pKoen (Deneka et al., 2003). All synthetic constructs were verified by restriction analysis and dye termination sequencing of both strands.
Table I.
cDNAs and constructs used in this study
| Protein | Accession no. | Plasmid | Species | Tag (N/C) | Origin |
|---|---|---|---|---|---|
| CST | NM006416 | pCDNA3.1h | Human | HA (C) | Ashikov et al., 2005 |
| FAPP2 | NM032639 | pCDNA3.1h | Human | HA (C) | RZPD |
| GCS | NM003358 | pCDNA3.1h | Human | HA3b (C) | Sprong et al., 1998 |
| GLTPa | NM175799 | pCDNA3.1h | Bovine | HA (C) | West et al., 2004 |
| GLTPa | NM175799 | pQE9 | Bovine | HIS6 (N) | West et al., 2004 |
| GM3S | AF119416 | pNHA3 | Mouse | HA3 (N) | Giraudo and Maccioni, 2003 |
| LCS | AF097158 | pNHA3 | Mouse | HA3 (N) | Giraudo and Maccioni, 2003 |
| NGT | NM012243 | pCDNA3.1 | Human | V5 (C) | Ashikov et al., 2005 |
| SGCSc |
NM199111, NM016922 |
pNHA3 | Rat, mouse | HA3 (N) | RZPD |
| SMS1a | NM147156 | pCDNA3.1 | Human | V5 (C) | Huitema et al., 2004 |
| UGTa | NM005660 | pMKIT-neo | Human | HA (C) | Miura et al., 1996 |
| MDR1a | NM000927 | pRc/RSV | Human | NA | van Helvoort et al., 1996 |
| CERT | NM023420 | pKoen | Mouse | RNAi | Synthetic |
| FAPP2 | BC052360 | pKoen | Mouse | RNAi | Synthetic |
| GCS | NM011673 | pKoen | Mouse | RNAi | Synthetic |
| Lamin | NM001002011 | pKoen | Mouse | RNAi | Synthetic |
| LCS | AF097158 | pKoen | Mouse | RNAi | Synthetic |
NA, not applicable. GenBank/EMBL/DDBJ accession nos. are given in the second column.
The construction of this plasmid has been described previously (see Origin column).
Triple HA tag.
Chimeric construct; see Materials and methods.
Cell culture, transfection, and RNAi
HeLa, CHO, and CHO-lec8 cells were purchased from American Type Culture Collection. D6P2T cells were a gift from S. Pfeiffer (University of Connecticut Medical School, Farmington, CT; Bansal and Pfeiffer, 1987). CHO-GalCS cells have been described previously (as CHO-CGalT; van der Bijl et al., 1996). MEB4 cells were purchased from the Institute of Physical and Chemical Research Cell Bank. The MF and TKO lines (Wijnholds et al., 2000; De Rosa et al., 2004) were obtained from P. Borst (Netherlands Cancer Institute, Amsterdam, Netherlands). MDCK strain II cells infected with recombinant retroviruses carrying short hairpin RNA against FAPP2 have been described previously (Vieira et al., 2005). All cells were grown in DME, stable glutamine, 4.5 g/liter glucose, and 10% FCS at 37°C with 5% CO2. Stable transfectants were grown in the presence of 200 U/ml hygromycin B or 0.6 mg/ml geneticin (G418). Cells were transfected using ∼1 μl LipofectAMINE 2000 and ∼0.3 μg DNA per squared centimeter of cells. For transient protein expression, the cells were used 1 d after transfection. For the generation of stable transfectants, the cells were trypsinized 24 h after transfection and divided over four 15-cm dishes in culture medium containing hygromycin B or G418. Medium was refreshed weekly, and, after ∼3 wk, individual colonies were selected for green fluorescence in the nucleus (Deneka et al., 2003) and trypsinized using a metal cylinder.
Quantitative IEM and confocal laser-scanning microscopy
Stably transfected HeLa cells were fixed with 2% PFA + 0.2% glutaraldehyde in 0.1 M phosphate buffer, pH 7.4, and incubated for 4 h at room temperature followed by an overnight incubation at 4°C. After fixation, cells were rinsed with PBS and with PBS containing 0.02 M glycine, scraped, and pelleted in 12% gelatin. Small blocks of the embedded cell pellets were infiltrated overnight with 2.3 M sucrose, mounted on aluminum pins, and frozen in liquid nitrogen. Ultrathin cryosections were cut at −120°C, picked up with 1% methylcellulose and 1.2 M sucrose, thawed, and collected on grids. After washing with PBS containing 0.02 M glycine, sections were double labeled as described previously (Slot et al., 1991) with antibodies against the tags (mouse) and the cis-Golgi marker GM130 (rabbit). For sub-Golgi localization of the tagged enzymes, immunogold particles were quantified per cisterna. For each enzyme, 20 Golgi stacks were selected on double-labeled sections based on the following criteria: clear visibility of five cisternae per Golgi, which we denoted as cisterna 1–5 (Martinez-Menarguez et al., 2001), the presence of at least five gold particles for the specific tag, and the presence of GM130 label as a cis-Golgi reference. The cisterna containing the GM130 marker was located at one end of each Golgi stack and was denoted as cisterna 1. EM pictures of the selected Golgi stacks were made at a magnification of 30,000× or 40,000×. The number of gold particles for the specific tag was counted per cisterna and expressed as a percentage of the total gold particles within that Golgi stack. The results for each enzyme are expressed as the mean percentage ± SEM (n = 20) of immunogold label for each cisterna. The reliability of the sample size was determined by accumulating data (for a given enzyme) until mean percentages remained stable within 10% of the end value for the last added five Golgi stacks. 200–500 gold particles were localized for each protein. The significance of the peak value of the gold particle percentage in a given cisterna was determined by performing paired t tests between cisternae with the lowest and highest percentage of gold label (P ≤ 0.05). Confocal laser-scanning microscopy was performed as described in supplemental Materials and methods.
Transport incubations on intact cells
24-well dishes with ∼2 × 105 MF, CHO-GalCS, or D6P2T cells were preincubated with various drugs (1 μg/ml BFA, 5 μM PSC833, 50 μM glibenclamide, and 1 mM vanadate) for 0.5 h and metabolically labeled in the presence of inhibitors with radioactive precursors or C6-NBD–ceramide at 37°C. For natural lipids and proteins, cells were labeled with 24 kBq/ml [1-14C]palmitate, 15 kBq/ml [3-14C]serine, 3,600 kBq/ml [35S]H2SO4, or 72 kBq/ml [14C]galactose in culture medium for 1.5 h at 37°C and 5% CO2. Serine labeling was performed in MEMα because of its lower level of serine. The medium was replaced by HBSS with 20 mM Hepes, pH 7.4 (HBSS′), containing purified GLTP to extract GSLs from the cell surface at 37°C. After varying times and GLTP concentrations (see Results; typically 45 min with 1.5 mg/ml GLTP ± BFA), the medium was removed, and the cells were washed twice with 0.5 ml HBSS′. Wash buffers and medium were pooled and centrifuged for 5 min at 800 g to remove detached cells. The lipids were extracted from medium and cells as described in Lipid analysis. Transport is calculated as [14C]- or [35S]lipid in the medium as the percentage of the total amount of that lipid in medium plus cells. Effects of drugs or RNAi treatments on synthesis were determined by measuring the sphingolipid of interest against an internal standard like [14C]phosphatidylserine in triplicate. For C6-NBD–GlcCer labeling, cells were incubated for 1 h with 1 μM C6-NBD–ceramide in HBSS′ + 1% BSA (wt/vol) to back-exchange newly synthesized C6-NBD–lipids appearing at the surface at 37°C. The buffer was replaced by HBSS′ + 1% BSA and incubated for 0.5 h on ice. The lipids were extracted from the combined label and washing buffers and from the cells as described in the Lipid analysis section. Transport is expressed as C6-NBD–lipid in the medium as the percentage of total C6-NBD–lipid. Measurements were performed in triplicate. For protein transport analysis, cells were incubated with 800 kBq/ml [35S]amino acids in culture medium for 135 min. Cells and media were analyzed as described in the SDS-PAGE and Western blotting section. Transport was calculated as the percentage of control.
Golgi translocation assay measuring [3H]LacCer synthesis
15-cm2 dishes with ∼1.5 × 107 HeLa cells were preincubated in the presence or absence of 1 μg/ml BFA in culture medium for 0.5 h at 37°C in 5% CO2. Cells were then washed twice with PBS and scraped in homogenizing buffer (120 mM K+-glutamate, 15 mM KCl, 5 mM NaCl, 0.8 mM CaCl2, 5 mM MnCl2, 2 mM MgCl2, 1.6 mM EGTA, 20 mM Hepes/KOH, pH 7.2, 1 μg/ml apoprotein, 1 μg/ml leupeptin, 1 μg/ml pepstatin, 5 μg/ml antipain, and 1 mM benzamidine). Cells were centrifuged at 300 g for 5 min at 4°C, taken up in 800 μl of homogenizing buffer, and broken up by passing 10 times through a homogenizer (0.016 ball size; European Molecular Biology Laboratory). Nuclei and unbroken cells were removed from PNS by spinning for 10 min at 300 g at 4°C. Soluble GLTP–[3H]GlcCer complexes were prepared as follows: 0.4 MBq [3H]GlcCer in 10 μl ethanol were injected into 1,200 μl of homogenizing buffer containing 12 μg GLTP. The mix was incubated for 20 min at 37°C and spun down for 5 min at 20,000 g to remove aggregates. 100 μl PNS (0.25 mg/ml) was incubated for 1 h at 37°C in the presence or absence of BFA, with 100 μl GLTP–[3H]GlcCer complexes, 2 mM UDP-Gal, and 2 mM ATP in a volume of 500 μl. Lipids were extracted as described in the next section, and LacCer synthesis was measured as the percentage of control.
Lipid analysis
Lipids were extracted and applied to TLC plates, which, when used to separate GalCer from GlcCer, had been dipped in 2.5% wt/vol boric acid in MeOH and dried (all as described previously; van der Bijl et al., 1996). Lipids were generally separated by 2D TLC using in the first dimension either CHCl3/MeOH/25% vol/vol NH4OH/water (65:35:4:4 vol/vol) or CHCl3/MeOH/0.2% aqueous CaCl2 (55:45:10 vol/vol) with the acidic solvent CHCl3/MeOH/acetone/HAc/water (50:20:10:10:5 vol/vol) for the second dimension. C6-NBD–lipids were analyzed by 1D TLC in the acidic running solvent. 1D TLC plates of [35S]H2SO4-labeled cells and of PNS were developed in the CaCl2 mixture. Radiolabeled spots were detected by exposure of phosphorimaging screens and read-out on a Personal FX phosphorimager. TLC plates with fluorescent lipids were directly developed using a phosphorimager (STORM 860; Molecular Dynamics). Spots were identified by comparison with standards and quantified using Quantity One software (Bio-Rad Laboratories).
SDS-PAGE and Western blotting
Cells were washed three times with PBS and were resuspended in protein sample buffer (200 mM Tris-HCl, pH 6.8, 3% wt/vol SDS, 12% vol/vol glycerol, 1 mM EDTA, 0.003% wt/vol bromophenol blue, and 50 mM DTT). Media were centrifuged at 3,000 g to remove cell debris, and one-third volume of 4× protein sample buffer was added. Samples containing SGCS were incubated for 10 min at room temperature and for 0.5 h at 50°C. All other samples were heated for 5 min at 95°C and resolved by SDS-PAGE on 7.5% minigels. Radioactive gels were dried and analyzed by fluorography or a Personal FX phosphorimager (Bio-Rad Laboratories) using Quantity One software (Bio-Rad Laboratories). For Western blotting, nitrocellulose transfers were blocked for 1.5 h in PBS, 5% Protifar (Nutricia), and 0.2% Tween 20 (Blotto). Primary antibody incubations were performed for 1 h in Blotto. Detection was performed with HRP-conjugated secondary antibodies using enhanced chemiluminescence (GE Healthcare).
Online supplemental material
Fig. S1 shows that FAPP2 is localized in the perinuclear region of HeLa cells, where it partially colocalizes with the medial Golgi marker mannosidase II, whereas GLTP distributes all over the cytosol except for the nucleus. Knocking down CERT resulted in an 80% reduction in the synthesis of SM and a 70% reduced synthesis of GlcCer and GM3, which is in line with a trans-Golgi location of a large fraction of GCS. Fig. S2 shows the purified recombinant GLTP as a single band in a Coomassie-stained gel. The protein extracted C10-pyrene-GlcCer but not SM from membranes. Extraction of radiolabeled GlcCer from fibroblasts was concentration and time dependent, whereas exogenous liposomes inhibited the extraction. Fig. S3 shows that the product of the ER sulfotransferase construct SGCS was not SLacCer. Product synthesis was inhibited by the GCS inhibitor N-butyldeoxygalactonojirimycin and by GCS knockdown but not by knocking down LCS. In addition, there was no reduced synthesis of the compound in Lec8 cells, which synthesize far less LacCer because they lack the galactose importer in their ER and Golgi. Fig. S4 shows that BFA does not inhibit GM3 synthesis but does inhibit the synthesis of Gb3 in HeLa cells. Fig. S5 shows that BFA fully inhibited protein secretion in melanocytes but that FAPP2 knockdown had no effect on protein secretion. Supplemental Materials and methods provides information about the synthesis of sphingolipids containing fluorescent or radiolabeled fatty acids, the purification of GLTP, and confocal laser-scanning microscopy. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200704091/DC1.
Acknowledgments
We are grateful to Kyoko Aikawa-Kojima, Hans Bakker, Hugo Maccioni, Monika Suchanek, Piet Borst, and Elizabeth Sztul for their generosity in providing reagents and for helpful discussions. We also thank René Scriwanek and Marc van Peski for excellent photographic work. We thank our colleagues for critical reading of the manuscript and for helpful discussions.
This work was supported by grant 902-23-197 from ZonMW with financial support from the Netherlands Organization for Scientific Research, European Council (grant MRTN-CT-2004-5330 Flippases), Mizutani Foundation for Glycoscience (GvM), Academy of Finland, the Sigrid Jusélius and Magnus Ehrnrooth Foundations, Liv och Hälsa r.f., and Åbo Akademi University (grant to P. Mattjus).
Note added in proof. While a revised version of this manuscript was under consideration, a related paper was published (D'Angelo, G., E. Polishchuk, G. Di Tullio, M. Santoro, A. Di Campli, A. Godi, G. West, J. Bielawski, C.C. Chuang, A.C. van der Spoel, et al. 2007. Nature. 449:62–67).
D. Halter and S. Neumann contributed equally to this paper.
D. Halter's present address is Dept. of Bio-Molecular Engineering, Philips Research Laboratories Eindhoven, 5656 AA Eindhoven, Netherlands.
J. Wolthoorn's present address is TNO Quality of Life, Analytical Research Department, 3700 AJ Zeist, Netherlands.
O.V. Vieira's present address is Centro de Neurociencias e Biologia Celular, Universidade de Coimbra, 3000 Coimbra, Portugal.
H. Sprong's present address is Laboratory for Zoonoses and Environmental Microbiology, National Institute of Public Health and Environment (RIVM), 3720 BA Bilthoven, Netherlands.
Abbreviations used in this paper: BFA, brefeldin A; C6-NBD, N-6-NBD-aminohexanoyl; CERT, ceramide transport protein; CST, CMP–sialic acid transporter; GalCer, galactosylceramide; GalCS, GalCer synthase; GCS, GlcCer synthase; GlcCer, glucosylceramide; GLTP, glycolipid transfer protein; GM3S, GM3 synthase; GSL, glycosphingolipid; IEM, immuno-EM; LacCer, lactosylceramide; LCS, LacCer synthase; MF, mouse fibroblast; PAPS, 3′-phosphoadenosine 5′-phosphosulfate; PNS, postnuclear supernatant; SGalCer, GalCer sulfate; SM, sphingomyelin; SMS, SM synthase; TKO, triple knockout; UGT, UDP-Gal transporter.
References
- Allende, M.L., J. Li, D.S. Darling, C.A. Worth, and W.W. Young Jr. 2000. Evidence supporting a late Golgi location for lactosylceramide to ganglioside GM3 conversion. Glycobiology. 10:1025–1032. [DOI] [PubMed] [Google Scholar]
- Ashikov, A., F. Routier, J. Fuhlrott, Y. Helmus, M. Wild, R. Gerardy-Schahn, and H. Bakker. 2005. The human solute carrier gene SLC35B4 encodes a bifunctional nucleotide sugar transporter with specificity for UDP-xylose and UDP-N-acetylglucosamine. J. Biol. Chem. 280:27230–27235. [DOI] [PubMed] [Google Scholar]
- Bagnat, M., S. Keranen, A. Shevchenko, and K. Simons. 2000. Lipid rafts function in biosynthetic delivery of proteins to the cell surface in yeast. Proc. Natl. Acad. Sci. USA. 97:3254–3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal, R., and S.E. Pfeiffer. 1987. Regulated galactolipid synthesis and cell surface expression in Schwann cell line D6P2T. J. Neurochem. 49:1902–1911. [DOI] [PubMed] [Google Scholar]
- Boot, R.G., M. Verhoek, W. Donker-Koopman, A. Strijland, J. van Marle, H.S. Overkleeft, T. Wennekes, and J.M. Aerts. 2007. Identification of the non-lysosomal glucosylceramidase as beta-glucosidase 2. J. Biol. Chem. 282:1305–1312. [DOI] [PubMed] [Google Scholar]
- Bowman, B.J., M.E. McCall, R. Baertsch, and E.J. Bowman. 2006. A model for the proteolipid ring and bafilomycin/concanamycin-binding site in the vacuolar ATPase of Neurospora crassa. J. Biol. Chem. 281:31885–31893. [DOI] [PubMed] [Google Scholar]
- Burger, K.N., P. van der Bijl, and G. van Meer. 1996. Topology of sphingolipid galactosyltransferases in ER and Golgi: transbilayer movement of monohexosyl sphingolipids is required for higher glycosphingolipid biosynthesis. J. Cell Biol. 133:15–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rosa, M.F., D. Sillence, C. Ackerley, and C. Lingwood. 2004. Role of multiple drug resistance protein 1 in neutral but not acidic glycosphingolipid biosynthesis. J. Biol. Chem. 279:7867–7876. [DOI] [PubMed] [Google Scholar]
- Deneka, M., M. Neeft, I. Popa, M. van Oort, H. Sprong, V. Oorschot, J. Klumperman, P. Schu, and P. van der Sluijs. 2003. Rabaptin-5alpha/rabaptin-4 serves as a linker between rab4 and gamma(1)-adaptin in membrane recycling from endosomes. EMBO J. 22:2645–2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrer, R.G., M.P. Warden, and R.H. Quarles. 1995. Effects of brefeldin A on galactosphingolipid synthesis in an immortalized Schwann cell line: evidence for different intracellular locations of galactosylceramide sulfotransferase and ceramide galactosyltransferase activities. J. Neurochem. 65:1865–1873. [DOI] [PubMed] [Google Scholar]
- Fugmann, T., A. Hausser, P. Schoffler, S. Schmid, K. Pfizenmaier, and M.A. Olayioye. 2007. Regulation of secretory transport by protein kinase D-mediated phosphorylation of the ceramide transfer protein. J. Cell Biol. 178:15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futerman, A.H., and R.E. Pagano. 1991. Determination of the intracellular sites and topology of glucosylceramide synthesis in rat liver. Biochem. J. 280:295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futerman, A.H., B. Stieger, A.L. Hubbard, and R.E. Pagano. 1990. Sphingomyelin synthesis in rat liver occurs predominantly at the cis and medial cisternae of the Golgi apparatus. J. Biol. Chem. 265:8650–8657. [PubMed] [Google Scholar]
- Gasa, S., M.T. Casl, A. Makita, N. Sakakibara, T. Koyanagi, and T. Atsuta. 1990. Presence and characterization of glycolipid sulfotransferase in human cancer serum. Eur. J. Biochem. 189:301–306. [DOI] [PubMed] [Google Scholar]
- Giraudo, C.G., and H.J. Maccioni. 2003. Ganglioside glycosyltransferases organize in distinct multienzyme complexes in CHO-K1 cells. J. Biol. Chem. 278:40262–40271. [DOI] [PubMed] [Google Scholar]
- Godi, A., A. Di Campli, A. Konstantakopoulos, G. Di Tullio, D.R. Alessi, G.S. Kular, T. Daniele, P. Marra, J.M. Lucocq, and M.A. De Matteis. 2004. FAPPs control Golgi-to-cell-surface membrane traffic by binding to ARF and PtdIns(4)P. Nat. Cell Biol. 6:393–404. [DOI] [PubMed] [Google Scholar]
- Hanada, K., K. Kumagai, S. Yasuda, Y. Miura, M. Kawano, M. Fukasawa, and M. Nishijima. 2003. Molecular machinery for non-vesicular trafficking of ceramide. Nature. 426:803–809. [DOI] [PubMed] [Google Scholar]
- Hayashi, Y., N. Okino, Y. Kakuta, T. Shiknai, M. Tani, H. Narimatsu, and M. Ito. 2007. Klotho-related protein is a novel cytosolic neutral beta-glycosylceramidase. J. Biol. Chem. 10.1074/jbc.M700832200. [DOI] [PubMed]
- Holthuis, J.C., T. Pomorski, R.J. Raggers, H. Sprong, and G. van Meer. 2001. The organizing potential of sphingolipids in intracellular membrane transport. Physiol. Rev. 81:1689–1723. [DOI] [PubMed] [Google Scholar]
- Huitema, K., J. Van Den Dikkenberg, J.F. Brouwers, and J.C. Holthuis. 2004. Identification of a family of animal sphingomyelin synthases. EMBO J. 23:33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeckel, D., A. Karrenbauer, R. Birk, R.R. Schmidt, and F. Wieland. 1990. Sphingomyelin is synthesized in the cis Golgi. FEBS Lett. 261:155–157. [DOI] [PubMed] [Google Scholar]
- Jeckel, D., A. Karrenbauer, K.N. Burger, G. van Meer, and F. Wieland. 1992. Glucosylceramide is synthesized at the cytosolic surface of various Golgi subfractions. J. Cell Biol. 117:259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohyama-Koganeya, A., T. Sasamura, E. Oshima, E. Suzuki, S. Nishihara, R. Ueda, and Y. Hirabayashi. 2004. Drosophila glucosylceramide synthase: a negative regulator of cell death mediated by proapoptotic factors. J. Biol. Chem. 279:35995–36002. [DOI] [PubMed] [Google Scholar]
- Kumagai, K., M. Kawano, F. Shinkai-Ouchi, M. Nishijima, and K. Hanada. 2007. Interorganelle trafficking of ceramide is regulated by phosphorylation-dependent cooperativity between the PH and START domains of CERT. J. Biol. Chem. 282:17758–17766. [DOI] [PubMed] [Google Scholar]
- Lannert, H., C. Bunning, D. Jeckel, and F.T. Wieland. 1994. Lactosylceramide is synthesized in the lumen of the Golgi apparatus. FEBS Lett. 342:91–96. [DOI] [PubMed] [Google Scholar]
- Lannert, H., K. Gorgas, I. Meißner, F.T. Wieland, and D. Jeckel. 1998. Functional organization of the Golgi apparatus in glycosphingolipid biosynthesis. Lactosylceramide and subsequent glycosphingolipids are formed in the lumen of the late Golgi. J. Biol. Chem. 273:2939–2946. [DOI] [PubMed] [Google Scholar]
- Lefevre, C., S. Audebert, F. Jobard, B. Bouadjar, H. Lakhdar, O. Boughdene-Stambouli, C. Blanchet-Bardon, R. Heilig, M. Foglio, J. Weissenbach, et al. 2003. Mutations in the transporter ABCA12 are associated with lamellar ichthyosis type 2. Hum. Mol. Genet. 12:2369–2378. [DOI] [PubMed] [Google Scholar]
- Levine, T., and C. Loewen. 2006. Inter-organelle membrane contact sites: through a glass, darkly. Curr. Opin. Cell Biol. 18:371–378. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz, J., L. Yuan, C. Tipper, M. Amherdt, L. Orci, and R.D. Klausner. 1991. Brefeldin A's effects on endosomes, lysosomes, and the TGN suggest a general mechanism for regulating organelle structure and membrane traffic. Cell. 67:601–616. [DOI] [PubMed] [Google Scholar]
- Malinina, L., M.L. Malakhova, A. Teplov, R.E. Brown, and D.J. Patel. 2004. Structural basis for glycosphingolipid transfer specificity. Nature. 430:1048–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Menarguez, J.A., R. Prekeris, V.M. Oorschot, R. Scheller, J.W. Slot, H.J. Geuze, and J. Klumperman. 2001. Peri-Golgi vesicles contain retrograde but not anterograde proteins consistent with the cisternal progression model of intra-Golgi transport. J. Cell Biol. 155:1213–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura, N., N. Ishida, M. Hoshino, M. Yamauchi, T. Hara, D. Ayusawa, and M. Kawakita. 1996. Human UDP-galactose translocator: molecular cloning of a complementary DNA that complements the genetic defect of a mutant cell line deficient in UDP-galactose translocator. J. Biochem. (Tokyo). 120:236–241. [DOI] [PubMed] [Google Scholar]
- Munro, S. 2003. Cell biology: earthworms and lipid couriers. Nature. 426:775–776. [DOI] [PubMed] [Google Scholar]
- Nakamura, N., C. Rabouille, R. Watson, T. Nilsson, N. Hui, P. Slusarewicz, T.E. Kreis, and G. Warren. 1995. Characterization of a cis-Golgi matrix protein, GM130. J. Cell Biol. 131:1715–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano, R.E., O.C. Martin, A.J. Schroit, and D.K. Struck. 1981. Formation of asymmetric phospholipid membranes via spontaneous transfer of fluorescent lipid analogues between vesicle populations. Biochemistry. 20:4920–4927. [DOI] [PubMed] [Google Scholar]
- Papadopulos, A., S. Vehring, I. Lopez-Montero, L. Kutschenko, M. Stockl, P.F. Devaux, M. Kozlov, T. Pomorski, and A. Herrmann. 2007. Flippase activity detected with unlabeled lipids by shape changes of giant unilamellar vesicles. J. Biol. Chem. 282:15559–15568. [DOI] [PubMed] [Google Scholar]
- Perry, R.J., and N.D. Ridgway. 2006. Oxysterol-binding protein and vesicle-associated membrane protein-associated protein are required for sterol-dependent activation of the ceramide transport protein. Mol. Biol. Cell. 17:2604–2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pewzner-Jung, Y., S. Ben-Dor, and A.H. Futerman. 2006. When do Lasses (longevity assurance genes) become CerS (ceramide synthases)? Insights into the regulation of ceramide synthesis. J. Biol. Chem. 281:25001–25005. [DOI] [PubMed] [Google Scholar]
- Rabouille, C., N. Hui, F. Hunte, R. Kieckbusch, E.G. Berger, G. Warren, and T. Nilsson. 1995. Mapping the distribution of Golgi enzymes involved in the construction of complex oligosaccharides. J. Cell Sci. 108:1617–1627. [DOI] [PubMed] [Google Scholar]
- Saito, K., N. Takakuwa, M. Ohnishi, and Y. Oda. 2006. Presence of glucosylceramide in yeast and its relation to alkali tolerance of yeast. Appl. Microbiol. Biotechnol. 71:515–521. [DOI] [PubMed] [Google Scholar]
- Slot, J.W., H.J. Geuze, S. Gigengack, G.E. Lienhard, and D.E. James. 1991. Immuno-localization of the insulin regulatable glucose transporter in brown adipose tissue of the rat. J. Cell Biol. 113:123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, D.C., D.J. Sillence, T. Falguieres, R.M. Jarvis, L. Johannes, J.M. Lord, F.M. Platt, and L.M. Roberts. 2006. The association of Shiga-like toxin with detergent-resistant membranes is modulated by glucosylceramide and is an essential requirement in the endoplasmic reticulum for a cytotoxic effect. Mol. Biol. Cell. 17:1375–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprong, H., B. Kruithof, R. Leijendekker, J.W. Slot, G. van Meer, and P. van der Sluijs. 1998. UDP-galactose:ceramide galactosyltransferase is a class I integral membrane protein of the endoplasmic reticulum. J. Biol. Chem. 273:25880–25888. [DOI] [PubMed] [Google Scholar]
- Sprong, H., S. Degroote, T. Claessens, J. van Drunen, V. Oorschot, B.H. Westerink, Y. Hirabayashi, J. Klumperman, P. van der Sluijs, and G. van Meer. 2001. Glycosphingolipids are required for sorting melanosomal proteins in the Golgi complex. J. Cell Biol. 155:369–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprong, H., S. Degroote, T. Nilsson, M. Kawakita, N. Ishida, P. van der Sluijs, and G. van Meer. 2003. Association of the Golgi UDP-galactose transporter with UDP-galactose:ceramide galactosyltransferase allows UDP-galactose import in the endoplasmic reticulum. Mol. Biol. Cell. 14:3482–3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchera, M., B. Pirovano, and R. Ghidoni. 1990. a. Sub-Golgi distribution in rat liver of CMP-NeuAc GM3- and CMP-NeuAc:GT1b alpha 2—-8sialyltransferases and comparison with the distribution of the other glycosyltransferase activities involved in ganglioside biosynthesis. J. Biol. Chem. 265:18242–18247. [PubMed] [Google Scholar]
- Trinchera, M., R. Ghidoni, S. Sonnino, and G. Tettamanti. 1990. b. Recycling of glucosylceramide and sphingosine for the biosynthesis of gangliosides and sphingomyelin in rat liver. Biochem. J. 270:815–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Bijl, P., G.J. Strous, M. Lopes-Cardozo, J. Thomas-Oates, and G. van Meer. 1996. Synthesis of non-hydroxy-galactosylceramides and galactosyldiglycerides by hydroxy-ceramide galactosyltransferase. Biochem. J. 317:589–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Helvoort, A., A.J. Smith, H. Sprong, I. Fritzsche, A.H. Schinkel, P. Borst, and G. van Meer. 1996. MDR1 P-glycoprotein is a lipid translocase of broad specificity, while MDR3 P-glycoprotein specifically translocates phosphatidylcholine. Cell. 87:507–517. [DOI] [PubMed] [Google Scholar]
- van Meer, G. 1993. Transport and sorting of membrane lipids. Curr. Opin. Cell Biol. 5:661–673. [DOI] [PubMed] [Google Scholar]
- van Meer, G., D. Halter, H. Sprong, P. Somerharju, and M.R. Egmond. 2006. ABC lipid transporters: extruders, flippases, or flopless activators? FEBS Lett. 580:1171–1177. [DOI] [PubMed] [Google Scholar]
- Vieira, O.V., P. Verkade, A. Manninen, and K. Simons. 2005. FAPP2 is involved in the transport of apical cargo in polarized MDCK cells. J. Cell Biol. 170:521–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira, O.V., K. Gaus, P. Verkade, J. Fullekrug, W.L. Vaz, and K. Simons. 2006. FAPP2, cilium formation, and compartmentalization of the apical membrane in polarized Madin-Darby canine kidney (MDCK) cells. Proc. Natl. Acad. Sci. USA. 103:18556–18561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, T.Y., and J.R. Silvius. 2000. Different sphingolipids show differential partitioning into sphingolipid/cholesterol-rich domains in lipid bilayers. Biophys. J. 79:1478–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnock, D.E., M.S. Lutz, W.A. Blackburn, W.W. Young Jr., and J.U. Baenziger. 1994. Transport of newly synthesized glucosylceramide to the plasma membrane by a non-Golgi pathway. Proc. Natl. Acad. Sci. USA. 91:2708–2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz, O.A. 2003. Acidification and protein traffic. Int. Rev. Cytol. 226:259–319. [DOI] [PubMed] [Google Scholar]
- West, G., Y. Nymalm, T.T. Airenne, H. Kidron, P. Mattjus, and T.T. Salminen. 2004. Crystallization and X-ray analysis of bovine glycolipid transfer protein. Acta Crystallogr. D Biol. Crystallogr. 60:703–705. [DOI] [PubMed] [Google Scholar]
- Wijnholds, J., E.C. de Lange, G.L. Scheffer, D.J. van den Berg, C.A. Mol, M. van der Valk, A.H. Schinkel, R.J. Scheper, D.D. Breimer, and P. Borst. 2000. Multidrug resistance protein 1 protects the choroid plexus epithelium and contributes to the blood-cerebrospinal fluid barrier. J. Clin. Invest. 105:279–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaoka, S., M. Miyaji, T. Kitano, H. Umehara, and T. Okazaki. 2004. Expression cloning of a human cDNA restoring sphingomyelin synthesis and cell growth in sphingomyelin synthase-defective lymphoid cells. J. Biol. Chem. 279:18688–18693. [DOI] [PubMed] [Google Scholar]
- Yildiz, Y., H. Matern, B. Thompson, J.C. Allegood, R.L. Warren, D.M. Ramirez, R.E. Hammer, F.K. Hamra, S. Matern, and D.W. Russell. 2006. Mutation of beta-glucosidase 2 causes glycolipid storage disease and impaired male fertility. J. Clin. Invest. 116:2985–2994. [DOI] [PMC free article] [PubMed] [Google Scholar]