Abstract
Ca2+ channel β subunits determine the transport and physiological properties of high voltage–activated Ca2+ channel complexes. Our analysis of the distribution of the Cavβ subunit family members in hippocampal neurons correlates their synaptic distribution with their involvement in transmitter release. We find that exogenously expressed Cavβ4b and Cavβ2a subunits distribute in clusters and localize to synapses, whereas Cavβ1b and Cavβ3 are homogenously distributed. According to their localization, Cavβ2a and Cavβ4b subunits modulate the synaptic plasticity of autaptic hippocampal neurons (i.e., Cavβ2a induces depression, whereas Cavβ4b induces paired-pulse facilitation [PPF] followed by synaptic depression during longer stimuli trains). The induction of PPF by Cavβ4b correlates with a reduction in the release probability and cooperativity of the transmitter release. These results suggest that Cavβ subunits determine the gating properties of the presynaptic Ca2+ channels within the presynaptic terminal in a subunit-specific manner and may be involved in organization of the Ca2+ channel relative to the release machinery.
Introduction
High voltage–activated Ca2+ channels in neurons consist of several subunits, a pore-forming α1 subunit (Cavα1), and several auxiliary subunits, including α2δ and β (Cavβ; Catterall, 2000). Cavβ subunits are involved in transport of the pore-forming α1 subunit to the plasma membrane (Dolphin, 2003; Herlitze et al., 2003). Cavβ subunits shield an ER retention signal on the α1 subunit, thereby guiding the pore-forming subunit to the target membrane (Bichet et al., 2000).
Cavβ subunits also determine the biophysical properties of the Ca2+ channel. The effects of the Cavβ subunit family members on the biophysical properties are complex. Four family members have been described (Cavβ1–4). P/Q-type channels assembled with Cavβ1b and β3 subunits in heterologous expression systems are fast inactivating in comparison with Cavβ4- and β2-assembled channels (Stea et al., 1994; Fellin et al., 2004; Luvisetto et al., 2004). Cavβ2 has the most dramatic effects on the channel properties, causing the channel to inactivate very slowly. In addition, the Cavβ2 subunit is unique because this subunit can be attached to the plasma membrane via its palmitoylated N-terminal protein domain (Chien et al., 1998).
Several studies also suggest that at least certain Cavβ subunit family members can target and function independently of the Cavα1 subunits at the plasma membrane and other intracellular structures such as the nucleus. For example, these subunits may be involved in gene transcription (Hibino et al., 2003) and the regulation of Ca2+ oscillations and insulin secretion (Berggren et al., 2004).
Recently, the crystal structures of the Cavβ core domains and the interaction domain between Cavβ and Cavα1 have been determined (Chen et al., 2004; Opatowsky et al., 2004; Van Petegem et al., 2004). These studies revealed that the Cavβ subunits belong to the membrane-associated guanylate kinase family containing Src homology type 3 and guanylate kinase domains (Hanlon et al., 1999; Richards et al., 2004; Rousset et al., 2005). A mutagenesis study of the Src homology type 3 and guanylate kinase domains showed that these domains regulate the inactivation of these Ca2+ channels (McGee et al., 2004) but also suggested that Cavβ subunits are involved in scaffolding and in the precise localization of Ca2+ channel complexes to defined subcellular domains. Indeed, deletion of the nonconserved N and C termini of the Cavβ4b subunit results in a loss of synaptic localization and presynaptic function (Wittemann et al., 2000). In addition, the isolated N terminus of Cavβ4a is capable of interacting with proteins of the vesicle release machinery (Vendel et al., 2006).
All Cavβ subunits are expressed in the brain. Their subcellular distribution within neurons reveals that they are localized to neuronal cell bodies and dendrites. In addition, Cavβ has been suggested to be localized to synaptic terminals (Herlitze and Mark, 2005). However, its precise function for determining synaptic transmission and, in particular, synaptic plasticity is unclear. Therefore, the goal of this study is to analyze the distribution of endogenously and exogenously expressed Cavβ subunits in hippocampal neurons and to correlate their distribution with their effects on synaptic transmission. Our results suggest that Cavβ2a and Cavβ4b subunits are targeted to presynaptic terminals, where they determine whether synapses facilitate or depress.
Results
Distribution of endogenous Cavβ subunits in hippocampal neurons
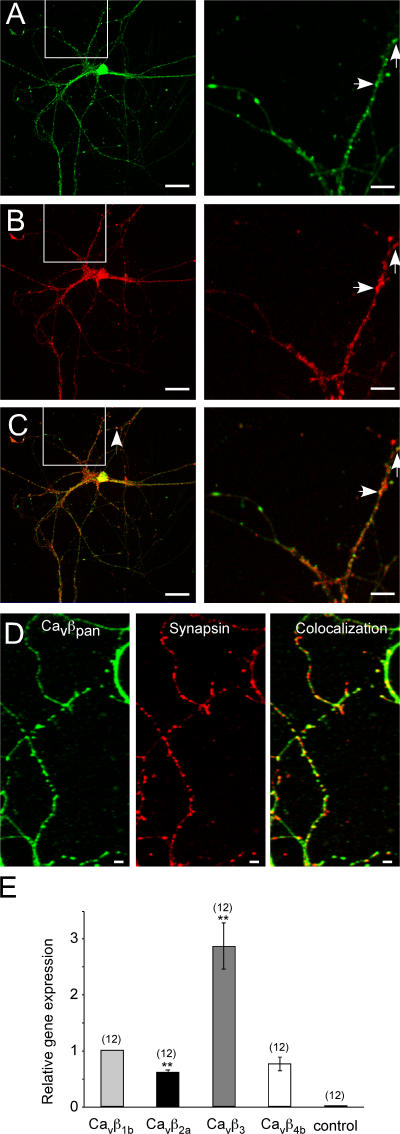
We first investigated whether hippocampal neurons in culture express endogenous Cavβ subunits, as would be predicted by the presence of the endogenous high voltage–activated Ca2+ channels (Reid et al., 1998; Wittemann et al., 2000). We produced a peptide-derived antibody, which recognizes all β-subunit family members (pan-β antibody). As indicated in Fig. 1 A, the antibody recognized specifically Cavβ subunits in hippocampal neurons, as demonstrated by antagonistic action of the epitope peptide (not depicted). Many, but not all, of the puncta colocalize with the synaptic markers synaptobrevin 2 (Fig. 1, A–C) and synapsin 1 (Fig. 1 D). The subunits are expressed throughout the neuron with high and uniform staining detected in the soma and proximal dendrites, with more clustered distribution in synaptic areas. We next analyzed whether we could detect Cavβ subunit–specific mRNAs in these neurons and whether we could see quantitative differences among the four different Cavβ mRNAs. As a positive control, we used 18S RNA. Real-time PCR revealed the highest mRNA levels for the Cavβ3 subunits and lower mRNA levels for Cavβ1,2,4 (Cavβ1 ≥ Cavβ4 ≥ Cavβ2; Fig. 1 E). The results indicate that all four Cavβ subunits are expressed in hippocampal neurons in culture, which localize to the soma and to synapses.
Figure 1.
Endogenous distribution and expression of Cavβ subunits in cultured hippocampal neurons. (A–C) (A, green) Confocal pictures of the endogenous Cavβ subunits detected with a pan-β antibody reveal punctate staining. (B, red) Hippocampal neurons were stained with an anti–synaptobrevin-II antibody and visualized with an AlexaFluor546-coupled secondary antibody. (C) Overlay of A and B demonstrates that the endogenous Cavβ subunits are partially colocalized with the synaptic vesicle marker synaptobrevin-II. (right) Boxed areas show that several pan-staining puncta are colocalized with synaptobrevin-II (arrows). Magnification of the indicated areas from the neuron shown on the left. (D) Cavβ subunits colocalize with the synaptic marker synapsin-I. Confocal images of the endogenous Cavβ subunits in hippocampal neurons visualized with the pan-β antibody (left), synapsin-I visualized with an anti–synapsin-I antibody (middle), and overlay of the two pictures (right) reveal that endogenous Cavβ subunits partially colocalize with the presynaptic marker synapsin-I. (E) Endogenous Cavβ subunit mRNAs are expressed at different levels in cultured hippocampal neurons. The mRNA expression levels of Cavβ2a, Cavβ3, and Cavβ4b were normalized to the mRNA level of the Cavβ1b subunit. The bar graph shows that the Cavβ3 mRNA level was approximately three times higher than Cavβ1b, whereas the Cavβ2a expression level was ∼50% lower (n = 12; **, P < 0.01) in comparison with Cavβ1b. The mRNA expression level for Cavβ4b was not different from that of Cavβ1b. Error bars represent SEM. Bars (A–C), 25 μm; (D) 5 μm.
Distribution of exogenously expressed Cavβ subunits in hippocampal neurons
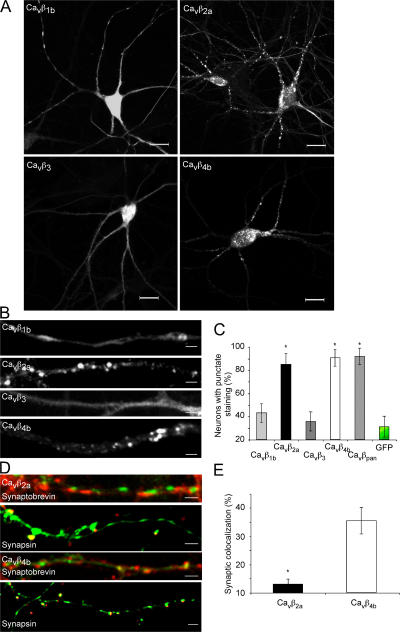
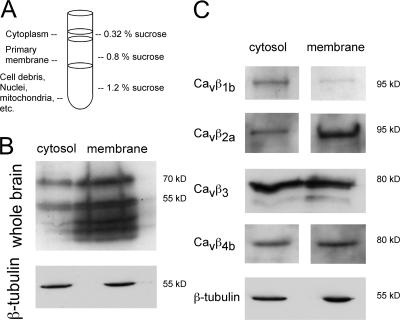
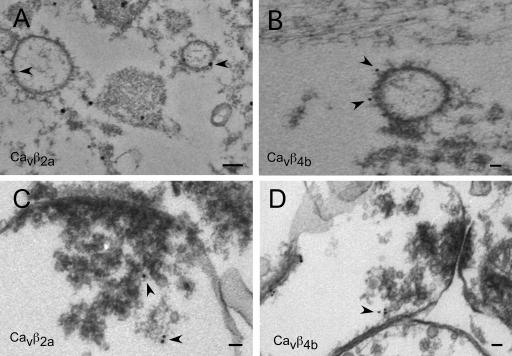
We next analyzed whether the exogenous expression of the Cavβ members resembles the endogenous distribution of the Cavβ subunits as determined in Fig. 1 and whether Cavβ subunits can target to synaptic sites when expressed alone in neurons (Fig. 2). We found that the Cavβ1b and Cavβ3 subunits reveal a more homogenous distribution, whereas the Cavβ2a and Cavβ4b subunits are highly clustered (Fig. 2, A–C). When cells expressing these exogenous subunits were immunostained with the synaptic marker synaptobrevin-II or synapsin-1, we found that Cavβ4b subunits revealed a higher degree of colocalization with synaptic markers than Cavβ2a. Association of the Cavβ subunits with the Cavα1 subunits predicts that both proteins should be distributed in cytoplasmic as well as membrane regions, which we confirmed by Western blots from cytosolic and membrane fractions of whole rat brain using the pan-Cavβ antibody (Fig. 3, A and B). Exogenous expression of the Cavβ subunits revealed a similar distribution, with subtype-specific enrichment within either the cytoplasmic or the membrane fraction (Fig. 3 C). Cavβ2a subunits are highly enriched in the membrane fraction, whereas Cavβ1b was mostly concentrated in the cytoplasm (Fig. 3 C). Cavβ3 and Cavβ4 subunits were equally distributed in both fractions (Fig. 3 C). Because Ca2+ channel Cavβ2a and Cavβ4b subunits reveal a mainly punctuate distribution within the neurons, we wanted to know whether we can detect Cavβ subunits in presynaptic terminals on vesicles or vesicular structures (Fig. 4). The high expression levels of the GFP-tagged subunits allowed us to study their localization by immunoelectron microscopy. As a negative control, we used the untagged GFP overexpressed in hippocampal neurons. As shown in Fig. 4, Cavβ2a and Cavβ4b subunits were detected on vesicular structures (Fig. 4, A and B) and close to presynaptic terminals (Fig. 4, C and D). We also observed that both Cavβ2a and Cavβ4b were attached to the plasma membrane (Fig. 4 D). In contrast, GFP was found only in the nucleus and outside of the nucleus but was not associated with vesicles or transported to the presynapse (unpublished data). The results suggest that both Cavβ2a and Cavβ4b subunits are transported to synaptic sites and to the plasma membrane, where they most likely associate with the Cavα1 subunits to form channel complexes.
Figure 2.
Exogenously expressed Cavβ1-4 subunits distribute in different patterns in hippocampal neurons and colocalize to various degrees with presynaptic marker proteins. (A) Fluorescence pattern of neurons from low density hippocampal cultures infected with the indicated GFP-tagged Cavβ subunit (i.e., Cavβ1–4) reveal either a punctate (Cavβ2a and Cavβ4b) or a more diffuse, cytosolic staining (Cavβ1b and Cavβ3). (B) Increased magnification of hippocampal neurites reveal that Cavβ2a and Cavβ4b are clustered, whereas Cavβ1b and Cavβ3 are diffusely distributed. (C) The bar graph indicates that neurons overexpressing Cavβ2a and Cavβ4b mainly reveal punctate staining similar to the endogenous distribution of Cavβ subunits, whereas the majority of neurons infected with Cavβ1b and Cavβ3 or GFP alone do not reveal punctate staining (n = 110–153 for each subunit; *, P < 0.01 compared with GFP). Quantification of the percentage of transfected neurons showing that punctate staining was performed by generating 11–13 randomly chosen fields within each group of neurons analyzed. In each field, the number of total infected neurons was counted, and the percentage of those showing punctate patterns was calculated. The percentages in each group were then averaged. n is equal to the total number of infected neurons counted. (D and E) Cavβ2a and Cavβ4b reveal differences in their percentages to colocalize with presynaptic marker proteins. (D) Cultured hippocampal neurons were infected with GFP-tagged Cavβ2a and Cavβ4b and costained with synaptobrevin-II or synapsin-I (red). Representative confocal images of hippocampal neurites reveal that the fluorescent puncta in Cavβ2a- and Cavβ4b (green)-expressing neurons reveal a different colocalization percentage with the presynaptic marker (red). (E) Quantification of the GFP puncta containing either Cavβ2a or Cavβ4b that colocalize with synaptobrevin-II. The percentage of synaptic colocalization is given as the number of Cavβ GFP puncta, which colocalized with the number of synaptobrevin-II puncta relative to the amount of GFP puncta within the region of interest analyzed (n = 20–21; *, P < 0.01). Error bars represent SEM. Bars (A), 15 μm; (B and D) 2 μm.
Figure 3.
Cavβ subunits are found in cytoplasmic and membrane fractions in hippocampal neurons. (A and B) Rat whole brains (postnatal day 0–3) were homogenized and fractionated in a discontinuous sucrose gradient. Primary membrane and cytosolic fractions were taken for Western blot analysis. (B) When immunoblotted with the pan-β antibody, the endogenous Cavβ subunits were mainly located in the membrane fraction but also found in the cytosolic fraction. (C) Exogenously expressed Cavβ subunits revealed a subunit-specific distribution between the cytosolic and membrane fraction. 13–16 h after infection with Cavβ subunits, 14-d in vitro hippocampal neurons were harvested, and cell extracts were blotted with anti-GFP antibodies.
Figure 4.
Immunoelectron microscopy reveals that Cavβ2a and Cavβ4b subunits are associated with membranes and vesicular structures and are targeted to presynaptic terminals in hippocampal neurons. (A and B) Immunoelectron microscopy pictures of 14-d in vitro autaptic neurons exogenously expressing GFP-tagged Cavβ2a (A) or GFP-tagged Cavβ4b (B) subunits. In neurons expressing Cavβ2a and Cavβ4b subunits, gold particles were found attached or close to vesicular structures (arrowheads). (C and D) In adult hippocampal slices, exogenously expressing GFP-tagged Cavβ2a (C) and Cavβ4b subunits (D) were found in presynaptic terminals close to synaptic vesicles and attached to the cell membranes (arrowheads). Bars, 50 nm.
Effect of Cavβ subunits on Ca2+ channel currents in HEK293 cells and hippocampal neurons
Cavβ subunits determine the biophysical properties of the Ca2+ channel. When expressed with the P/Q-type channel in Xenopus laevis oocytes or HEK293 cells, Cavβ subunits determine the time course of inactivation in a subunit-specific manner. Cavβ1b- and Cavβ3-assembled channels inactivate rapidly, whereas Cavβ2a- assembled channels inactivate slowly (Stea et al., 1994). Cavβ4b-assembled channels inactivate with a time course that lies between Cavβ1b,3 and Cavβ2a. The gating properties of the presynaptic Ca2+ channels determine Ca2+ influx into the presynaptic terminal and, therefore, determine transmitter release and synaptic plasticity, such as facilitation and depression.
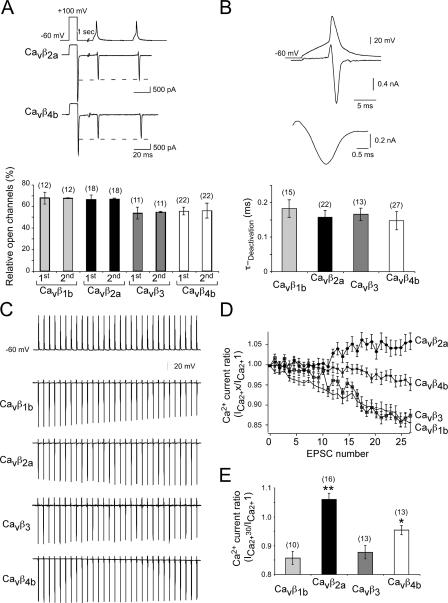
We wanted to know how P/Q-type channels assembled with different Cavβ subunits open and closed during action potential (AP) waveforms, which we obtained from cultured hippocampal neurons. We expressed Cavα12.1 subunits together with the Cavα2δ and the various Cavβ subunits in HEK293 cells and applied 30 APs to analyze how many channels would be opened during AP trains. To determine the proportion of open channels, we used the following protocol. Based on the voltage dependence of the activation of P/Q-type channels, we applied a 10-ms depolarizing test pulse to a test potential in which ∼100% of channels within the cells were open (Herlitze et al., 1996, 1997, 2001; Mark et al., 2000). This value is given by the amplitude of the tail current. We then compared the tail current elicited by the AP to the tail current elicited by the 10-ms depolarization to +100 mV. We were interested in three values. We wanted to know whether activation with the AP waveforms would reveal differences in the opening of the channels when assembled with different Cavβ subunits. The results indicated that the AP opens between 55 and 65% of the channels. No considerable differences were observed between channels assembled with the different Cavβ subunits (Fig. 5, A and B).
Figure 5.
Hippocampal AP waveform protocols detect differences in the amount of open P/Q-type channels assembled with the different Cavβ subunits during long 20-Hz stimulations but not for paired pulses. (A) HEK293 cells expressing Cavα12.1, Cavα2δ, and one of the four Cavβ1b, Cavβ2a, Cavβ3, and Cavβ4b subunits were held at −60 mV, and Ca2+ currents were elicited by a 20-Hz AP train 1 s after a prepulse to 100 mV for 10 ms. This prepulse was given to open ∼100% of the Ca2+ channels expressed in the cell (top). The tail current elicited by the prepulse was used to relate the tail current elicited by the AP to gain an understanding about the percentage of channels opened by the AP. The example whole cell currents (top) and the bar graph (bottom) indicate that approximately the same amount of channels were opened by the first AP and the second AP for the Cavβ1–4-assembled P/Q-type channels analyzed. (B) Increased time resolution of the underlying current elicited by the AP. The deactivation time of the tail currents can be fitted with a single exponential. Only currents were included and analyzed in the experiments described in A–E, which reveal fast deactivation kinetics and no change in the deactivation kinetics between the first and the last tail current elicited. (C) Examples of P/Q-type channel currents assembled with Cavβ1–4 subunits during a 20-Hz 30-pulse AP waveform train. (D) Relative ICa2+ ratio for the P/Q-type channel currents assembled with Cavβ1–4 subunits. The tail current amplitudes were related to the tail current elicited by the first AP. (E) Comparison of the relative amplitude of the tail currents elicited by the first and 30th AP during the 20-Hz AP train reveals that currents through P/Q-type channels assembled with Cavβ1b and Cavβ3 subunits are relatively smaller than currents through P/Q-type channels assembled with Cavβ2a and Cavβ4b. Error bars represent SEM. *, P < 0.05; **, P < 0.01.
We next compared the ratio between the amount of channels opened by the first and the second AP (Fig. 5 A). By comparing this value, we gain information on differences on the influx of Ca2+ through Ca2+ channels into the presynaptic terminal, which may determine whether synapses facilitate or depress during paired pulses. No differences were detected between channels assembled with different Cavβ subunits. We next analyzed whether a 20-Hz train of 30 APs leads to a decrease in channel opening, as would be expected from the inactivation of Ca2+ channels during long, constant depolarizations (Stea et al., 1994; Herlitze et al., 1997). When comparing the proportion of channels opened by the first AP relative to the amount of channels opened by the 30th AP, we found that currents mediated by Cavβ1b- and Cavβ3-assembled channels are reduced by 10–15% (Fig. 5, D and E). In contrast, currents mediated by Cavβ4b- assembled channels are reduced by 2% (Fig. 5, D and E), and currents mediated by Cavβ2a-assembled channels increased by 5% (Fig. 5, D and E). Thus, P/Q-type channels assembled with different β subunits reveal substantial differences in the amount of channel opening during long AP trains.
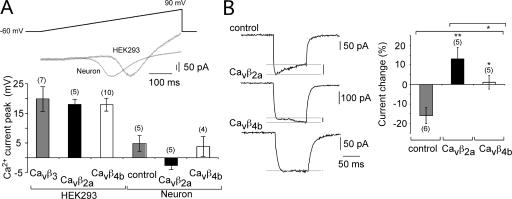
It has been shown that the biophysical properties of P/Q-type channels depends on the cellular environment in which the pore-forming Cavα1 subunit is expressed (Tottene et al., 2002). We found that the maximal current elicited by a 500-ms-long voltage ramp is shifted to more negative potentials (around 20 mV) in neurons expressing non–L-type channels in comparison with HEK293 cells expressing P/Q-type channels encoded by the Cavα12.1, Cavα2δ, and Cavβ subunits (Fig. 6 A). Therefore, Cavβ subunit–mediated effects on presynaptic Ca2+ channel (non–L type) inactivation may be shielded in neurons by, for example, neuronal-specific channel-interacting proteins. To show that the Cavβ subunits (i.e., Cavβ2a and Cavβ4b) also change the biophysical properties of non–L-type channels in hippocampal neurons, we analyzed the Ca2+ channel inactivation of somatic neuronal non–L-type channels. As shown in Fig. 6 B, the exogenous expression of Cavβ2a and Cavβ4b subunits reduce non–L-type channel inactivation in a subunit-specific manner. Cavβ2a subunit expression leads to an increase in the non–L-type current during a 100-ms test pulse from −60 to 0 mV, whereas neuronal non–L-type currents in the presence of Cavβ4b subunits do not change in size (Fig. 6 B).
Figure 6.
Cavβ subunits expressed in hippocampal neurons change the biophysical properties of the endogenous non–L-type channels. (A) The activation of non–L-type channels in hippocampal neurons is shifted to more negative potentials when compared with P/Q-type channels exogenously expressed in HEK293 cells. (top) Example current traces (IV curve) of non–L-type currents from hippocampal neurons in comparison with currents through P/Q-type channels (Cavα12.1, Cavα2δ, and Cavβ4b) expressed in HEK293 cells elicited by 500-ms voltage ramps from −60 to 90 mV. (bottom) Diagram of the voltage at which the peak current appears during the voltage ramp for P/Q-type channels expressed in HEK293 cells and non–L-type currents from hippocampal neurons in the presence or absence of Cavβ2a and Cavβ4b subunits. (B) The inactivation properties of non–L-type channels in hippocampal neurons are changed in the presence of Cavβ2a and Cavβ4b subunits. (left) Example traces of non–L-type currents elicited by a voltage pulse from −60 to 0 mV reveals that in the presence of Cavβ2a and Cavβ4b subunits, inactivation is slowed. (right) Diagram of the current change (percentage) within the 100-ms current trace. The current at the beginning of the test pulse (10 ms) is compared with the current at the end of the test pulse (95 ms). Error bars represent SEM. *, P < 0.05; **, P < 0.01.
Effects of Cavβ subunits on synaptic transmission
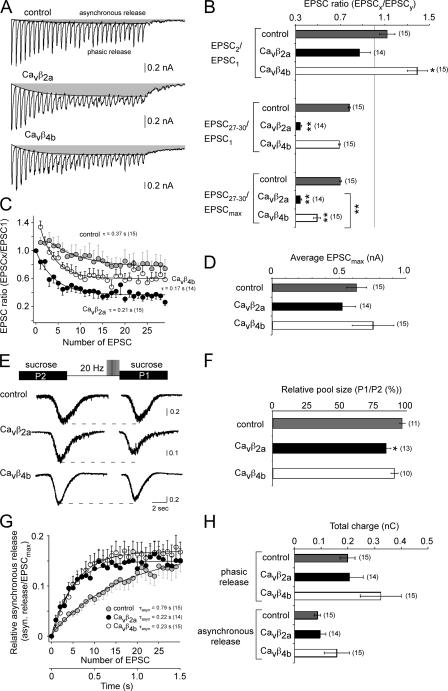
Our results on the recombinant P/Q-type channels and endogenous neuronal Ca2+ channels suggest that Ca2+ influx into the presynaptic terminal should be altered during long 20-Hz AP trains but not for paired-pulse responses. We analyzed the effect of the Cavβ subunits on paired-pulse facilitation (PPF) by comparing the first and second excitatory postsynaptic current (EPSC; defined as the paired-pulse ratio [PPR]) and analyzed the effect on synaptic depression by comparing the first and last EPSCs (averaged 27–30 EPSCs) within a 20-Hz stimulation protocol when 30 pulses were elicited in 4 mM of extracellular Ca2+ (Fig. 7, A and C). Because we did not observe effects on synaptic transmission when Cavβ1b and Cavβ3 subunits were expressed in our initial studies (unpublished data), we only analyzed Cavβ4b and Cavβ2a subunit effects on synaptic transmission in the following experiments. According to our results regarding the effects of Cavβ subunits on the inactivation properties of Cav2 channels, we found that Cavβ2a subunits did not change the PPR as expected from the aforementioned biophysical analysis. However, Cavβ4b subunits increased the PPR, leading to facilitation (Fig. 7, A and C).
Figure 7.
Cavβ subunit–specific determination of facilitation and depression in autaptic hippocampal neurons. (A and B) EPSC recordings of autaptic hippocampal neurons exogenously expressing Cavβ2a and Cavβ4b subunits in 4 mM Ca2+. (A) Representative autaptic EPSC traces from noninfected and Cavβ2a and Cavβ4b subunit–infected neurons reveal that in the presence of Cavβ2a and Cavβ4b subunits, synaptic depression is increased during long 20-Hz stimulations. Gray areas represent the asynchronous release. (B) Bar graph of the quantified EPSC ratios of Cavβ2a and Cavβ4b subunit–infected and noninfected neurons. The EPSC amplitudes of the first and second EPSC (top), the first and the mean from the 27–30th EPSC (middle), and the largest EPSC during a train and the mean from the 27–30th EPSC (bottom). (C) Relative EPSCs for Cavβ2a- and Cavβ4b-infected and noninfected neurons. The EPSC amplitudes during the 20-Hz pulse train were related to the EPSC elicited by the first pulse. The decline in EPSC amplitude could be fitted with a single exponential starting from the largest EPSC within the pulse train. (D) Bar graph of the mean maximal EPSC amplitude of Cavβ2a and Cavβ4b subunit–infected and noninfected neurons during a 20-Hz pulse train. (E and F) Comparison of the RRP before and after 30 2-ms-long pulses to 10 mV (20-Hz stimulation). (E) Example traces of sucrose responses before (P2) and after the 20-Hz stimulation (P1). (F) Relative pool size for Cavβ2a- and Cavβ4b-infected and noninfected neurons. The relative pool size was determined by the ratio between the sucrose response after the 20-Hz stimulation (P1) and the sucrose response before the stimulation (P2). (G) Time course of asynchronous release during a 20-Hz pulse train. The charge of the largest EPSC (EPSCmax) within the 20-Hz train was compared with the charge of the asynchronous release for each EPSC. (H) Bar graph of the quantified total charge during a 20-Hz stimulation protocol for the phasic release and asynchronous release for Cavβ2a- and Cavβ4b-infected and noninfected neurons. The mean values of the time course of depression (C) and the asynchronous release (G) were fitted with a single exponential, and the time constants for each fit and the number of experiments (given in parentheses) are given in the diagrams. Error bars represent SEM. * P, < 0.05; **, P < 0.01.
We next analyzed whether the Cavβ4b and Cavβ2a subunits influence synaptic transmission during longer AP trains. The biophysical analysis predicts that in the presence of Cavβ4b and Cavβ2a subunits, Ca2+ influx into the presynaptic terminal should be increased as a result of the noninactivating properties of the presynaptic Ca2+ channels in comparison with Cavβ1b and Cavβ3 subunits. The increased Ca2+ influx may cause more vesicle depletion (depression) and may influence asynchronous transmitter release, which has been shown to be proportional to the residual [Ca2+]i (Atluri and Regehr, 1998). Analysis of the synaptic responses during 30 20-Hz AP trains revealed that Cavβ4b- and Cavβ2a-expressing neurons show larger depression in comparison with wild-type neurons (Fig. 7, A–C) Note that the amount of depression is related to the largest EPSC compared with the minimal EPSC at the end of the stimulus train. The largest EPSC in noninfected neurons and Cavβ4b-expressing neurons is the EPSC elicited by the second pulse. Therefore, depression is significantly larger for Cavβ2a (0.34 ± 0.01; n = 14) as well as for Cavβ4b (0.49 ± 0.03; n = 15) in comparison with noninfected neurons (0.7 ± 0.01; n = 15). To determine whether Cavβ4b- and Cavβ2a-expressing neurons reveal more vesicle depletion during AP trains, we compared the readily releasable pool size before and after 20-Hz train stimulations. As shown in Fig. 7 (E and F), the pool size is substantially reduced in Cavβ2a-expressing neurons (12 ± 3.5%) and is slightly reduced in Cavβ4b-expressing neurons (9 ± 2.7%) in comparison with control neurons (3 ± 2.2%). However, the Cavβ4b effects were not substantial. To further verify that Cavβ4b- and Cavβ2a-expressing neurons may increase the Ca2+ influx into the presynaptic terminal, we analyzed the asynchronous release. We found that onset of the asynchronous release was much faster and the amount of asynchronous relative to the phasic release at the beginning of the AP train was increased in Cavβ4b- and Cavβ2a- expressing neurons in comparison with control neurons (Fig. 7 G). Although the total amount of phasic and asynchronous release (Fig. 7 H) as well as the mean EPSC amplitude (Fig. 7 D) were slightly increased in Cavβ4b-expressing neurons in comparison with control and Cavβ2a-expressing neurons, the differences were not substantial.
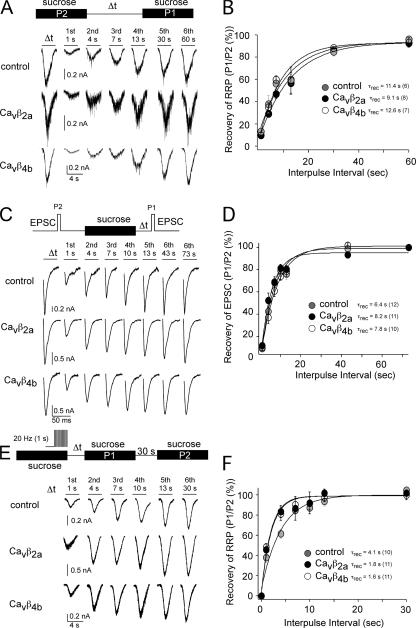
These aforementioned results support the idea that during AP trains, the Ca2+ influx into the presynaptic terminal is larger in the presence of Cavβ4b and Cavβ2a subunits. A larger Ca2+ influx into the presynaptic terminal during AP trains in Cavβ4b and Cavβ2a subunit–expressing neurons should also result in faster vesicle recycling (Stevens and Wesseling, 1998). To test this hypothesis, we repeated the experiments described in Stevens and Wesseling (1998). We first analyzed the recovery of the readily releasable vesicle pool (RRP) after RRP depletion without 20-Hz stimulation trains applied during depletion. No differences were found for the recovery of the RRP regardless of whether Cavβ4b or Cavβ2a subunits were expressed in the neurons (Fig. 8, A and B). Also, recovery of the EPSC after RRP depletion was not different between neurons expressing or not expressing Cavβ4b and Cavβ2a subunits (Fig. 8, C and D), suggesting that exogenously expressed Cavβ4b and Cavβ2a subunits most likely do not interfere with the vesicle recycling. We next analyzed the RRP recovery after 20-Hz stimulation trains were applied during the initial sucrose application (Fig. 8 E). We confirmed the observation described by Stevens and Wesseling (1998) that the RRP recovery for all neurons analyzed (regardless of whether Cavβ subunits were expressed or not) was accelerated by the 20-Hz stimulus train (Fig. 8, E and F). Interestingly, RRP recovery was faster in Cavβ4b and Cavβ2a subunit–expressing neurons in comparison with control neurons (τrec without 20-Hz train stimulation: control = 11.4 s, Cavβ2a = 9.1 s, and Cavβ4b = 12.6 s; τrec after 20-Hz train stimulation: control = 4.1 s, Cavβ2a = 1.8 s, and Cavβ4b = 1.6 s), suggesting again that Ca2+ influx into the presynaptic terminal is increased during 20-Hz stimulation trains in Cavβ4b and Cavβ2a subunit–expressing neurons.
Figure 8.
Cavβ2a and Cavβ4b subunits expressed in hippocampal neurons accelerate the recovery of the readily releasable pool when trains of APs have been evoked previously. To evaluate whether Cavβ2a and Cavβ4b subunits affect vesicle recycling, we analyzed the recovery of the RRP and the EPSC after RRP depletion. The RRP was measured by applying hypertonic solution (500 mM sucrose) for 4 s. (A) Example traces of the time-dependent RRP recovery. (B) Time course of the recovery of the RRP. There were no substantial differences in the time course of RRP recovery between Cavβ2a- and Cavβ4b-expressing and nonexpressing neurons. (C) Example traces of the EPSC recovery after RRP depletion. (D) Time course of the recovery of the EPSC after RRP depletion. EPSCs were elicited by a 2-ms test pulse to 10 mV. (E) Example traces of the time-dependent RRP recovery when 20 stimulus trains have been evoked for 1 s at the end of the first sucrose application. (F) Time course of the recovery of the RRP reveals that in the presence of Cavβ2a and Cavβ4b, the RRP recovery is faster. The relative recovery of the RRP and the EPSC was calculated by comparing the sucrose or EPSC response after the initial sucrose response to the initial (first) response. For RRP recovery in E and F, the sucrose response after the first depletion (P1) was compared with the sucrose response 30 s after the second sucrose response (P2). The interpulse intervals are given in A, C, and E. The mean values of the recovery of the RRPs and EPSCs shown in B, D, and F were fitted with a single exponential, and the time constant for each fit are given in the diagram. The numbers of experiments are given in parentheses. Error bars represent SEM.
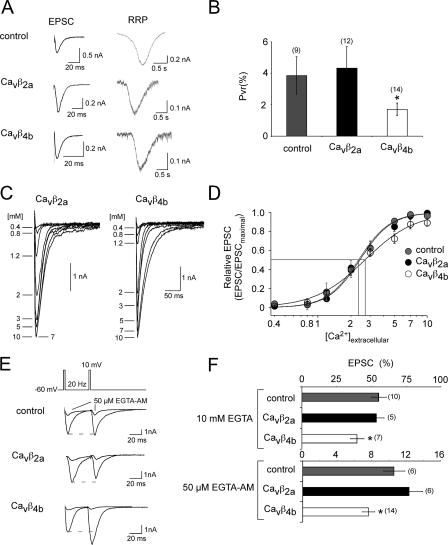
Although the exogenous expression of Cavβ subunits determines the synaptic responses during long AP trains according to the biophysical properties of the assembled presynaptic Ca2+ channels, the induced facilitation by Cavβ4b during paired pulses cannot be explained by the biophysical properties of presynaptic Ca2+ channel assembled with the Cavβ4b subunit. However, this may suggest that in the presence of Cavβ4b subunit, the Ca2+ dependence of the vesicle release is altered, which may result in a reduced release probability (Thomson, 2000). Therefore, we compared the release probability of noninfected and Cavβ2a- as well as Cavβ4b-expressing neurons. The release probability can be examined by comparing the RRP with the number of vesicles elicited by a single AP. The RRP size is determined by application of a hypertonic sucrose solution (Rosenmund and Stevens, 1996). As shown in Fig. 9, we found that the release probability in the presence of Cavβ4b was reduced in comparison with noninfected and Cavβ2a-expressing neurons. No differences in the mean RRP and EPSC size were detected between the neurons expressing different Cavβ subunits, probably because only a small number of cells were analyzed.
Figure 9.
Cavβ4b subunits expressed in hippocampal neurons reduce the synaptic release probability, change the Ca2+-dependent transmitter release, and are more sensitive to EGTA. (A, left) Representative EPSC traces evoked by 2-ms depolarizing pulses from −60 to 10 mV are shown for noninfected and Cavβ2a- and Cavβ4b-infected neurons. (right) Representative traces of the hypertonically mediated release of quanta from the same neuron shown on the left upon the application of 500 mM sucrose for 4 s. (B) Probability of synaptic vesicle release was evaluated by calculating the ratio of release evoked by the AP to that evoked by hypertonic sucrose. In autaptic neurons infected with Cavβ4b, the vesicular release probability is significantly reduced compared with noninfected or Cavβ2a subunit–infected neurons. (C) Representative EPSC traces elicited by the application of increasing extracellular Ca2+ concentrations of autaptic hippocampal neurons expressing Cavβ4b subunits. (D) Dose-response curve of the EPSC amplitude by increasing extracellular Ca2+ concentrations. The Ca2+-dependent EPSC responses of noninfected, Cavβ4b-, or Cavβ2a-infected neurons were free fitted according to the Hill equation (EPSC = EPSCmaximal/(1 + (EC50/[Ca2+]o)Hill coefficient). The EPSCs were then normalized to the maximal EPSC given by each fit. The mean normalized EPSCs for the given Ca2+ concentrations are shown. The curves again were fitted according to the Hill equation. The Hill coefficients are 2.7 ± 0.4 for control and Cavβ2a-infected neurons and 1.9 ± 0.4 for Cavβ4b-infected neurons. (E) Representative EPSC traces before and after a 50-μM EGTA-AM application evoked by two 2-ms depolarizing pulses from −60 to 10 mV within 50 ms are shown for noninfected and Cavβ2a- and Cavβ4b-infected neurons. (F) Bar graph of the remaining EPSC amplitude after 10 mM EGTA was applied intracellularly for 20 min (top) and after 50 μM EGTA-AM was applied extracellularly for 15 min (bottom). Error bars represent SEM. *, P < 0.05.
The relationship between the Ca2+ influx into the presynaptic terminal and the vesicle release is approximately given by the following equation: vesicle release ∝ [Ca2+]Hill coefficient, where the Hill coefficient is defined as the Ca2+ cooperativity. The Ca2+ cooperativity in many synapses is high (three to four), indicating that a small change in Ca2+ influx can result in drastic changes in transmitter release. Thus, in our experiments, the Hill coefficient gives an indirect measure of the Ca2+ influx through presynaptic Ca2+ channels relative to the transmitter release. This means that a change in the number, localization, or organization of the presynaptic Ca2+ channels most likely results in a change in Ca2+ dependence of the transmitter release. Interestingly, in the presence of the Cavβ4b subunits, the Ca2+-dependent transmitter release dose-response curve became more shallow, with a small change in the half maximal [Ca2+] concentration (EC50) when compared with wild-type neurons or neurons exogenously expressing Cavβ2a subunits (Fig. 9). Because the Cavβ4b subunit particularly changed the cooperativity of the transmitter release, this result may suggest that Cavβ4b is involved in organization of the Ca2+ channel domains necessary for efficient vesicle release. For example, Cavβ4b-assembled channels may be further apart from the release machinery. If this is the case, synaptic transmission in Cavβ4b-expressing neurons should be more sensitive to the slow Ca2+ buffer EGTA. Indeed, we found that when 10 mM EGTA was applied intracellularly or 50 μM EGTA-AM was applied extracellularly, the EPSC amplitude was substantially more reduced in Cavβ4b-expressing neurons to 60 and 93%, whereas in Cavβ2a-expressing neurons and control neurons, the EPSC amplitude was reduced only by 50 and 87–89% (Fig. 9, E and F).
Discussion
In this study, we investigated the targeting and function of Cavβ subunits in hippocampal neurons. We found that Cavβ2a and Cavβ4b are sufficiently targeted to synaptic sites, where they influence synaptic transmission during long AP trains according to the biophysical properties that these subunits induce in the presynaptic Ca2+ channel. During paired pulses, Cavβ4b subunits also altered the Ca2+ dependence of transmitter release. The physiological consequences and implications of the findings are discussed below.
Targeting of Cavβ subunits to the plasma membrane and synaptic terminals
We show that Cavβ2a and Cavβ4b are targeted to synaptic sites and colocalize with synaptic markers. All Cavβ subunits (exogenously and endogenously expressed) are found to various degrees in cytoplasmic and membrane fractions, as suggested by an overexpression study of Cavβ subunits in HEK293 cells (Chien et al., 1998). In particular, Cavβ2a subunits are associated with the membrane fraction, as predicted from their N-terminal located palmitoylation site (Dolphin, 2003; Herlitze et al., 2003). This is in agreement with previous studies performed in HEK293 cells in which palmitoylated Cavβ2a subunits reach the plasma membrane independently of the Cavα1 subunit (Chien et al., 1998; Bogdanov et al., 2000). Cavβ2a subunits could also be found on vesicular structures, supporting the view that they most likely are associated with Cavα1 subunits, where they are transported as preassembled channel complexes to synaptic sites (Ahmari et al., 2000; Shapira et al., 2003). Our studies for Cavβ1b and Cavβ3 reveal that these subunits, when expressed alone, distribute more homogenously in neurons and do not substantially influence the synaptic parameters analyzed. The reason for this could be that Cavβ1b and Cavβ3 are not sufficiently transported to the presynaptic terminals as suggested by Maximov and Bezprozvanny (2002). On the other hand, because Cavβ3 is the main mRNA detected in hippocampal neurons, most synaptic Ca2+ channels could be assembled with Cavβ3 subunits. Therefore, the biophysical properties of the presynaptic Ca2+ channels would not be affected by either Cavβ1b and Cavβ3, because the biophysical differences of channels assembled with these subunits are small.
Cavβ subunits may determine synaptic plasticity during longer AP trains as a result of the effects on the inactivation properties of the presynaptic Ca2+ channel complexes
Cavβ in particular determines the time course of inactivation of high voltage–activated Ca2+ channels. How P/Q-type channels assembled with different Cavβ subunits behave when AP waveforms derived from hippocampal neurons are used as command potentials has not been studied before. Interestingly, we did not detect substantial differences in the opening of the channels for the first two APs, which would determine the Ca2+ influx into the presynaptic terminal during paired pulses underlying short-term synaptic plasticity, but found that Cavβ1b- and Cavβ3-assembled channels exhibited substantial differences in the proportion of channels open after 30 APs or longer trains when compared with the Cavβ2a- and Cavβ4b-assembled channels (20 Hz; Fig. 5 D). We have to point out that the determination of the biophysical properties of the P/Q-type channel in HEK293 cells cannot directly be compared with the effects these subunits have on the native presynaptic Ca2+ channels. For example, Tottene et al. (2002) showed that the maximal current amplitude (when the peak current was analyzed with voltage step protocols) of the pore-forming human Cavα12.1 subunit expressed in neurons from Cavα12.1 knockout mice was shifted by −20 mV when compared with the same channel subunit coexpressed with Cavα2bδ and Cavβ2e in HEK293 cells. A similar shift in the maximal current amplitude was seen in our experiments when we compared the voltage ramps of rat Cavα12.1-, Cavα2δ-, and Cavβ2a,4b-assembled channels in HEK293 cells with the non–L-type currents elicited by voltage ramps in noninfected or Cavβ subunit–infected neurons. This indicates that non–L-type currents in neurons differ in their biophysical properties probably because of cell type–specific interacting proteins and variations as well as combinations of splice variants contributing to the non–L-type current. The differences in channel opening and, therefore, Ca2+ influx correlate well with the observed effects Cavβ subunits have on synaptic depression, asynchronous release, and activity-dependent RRP recovery.
Synaptic depression can be achieved via various cellular mechanisms. Therefore, an increase in Ca2+ influx leading to faster vesicle depletion is only one possibility (Zucker and Regehr, 2002). Synaptic depression can also be independent of vesicle depletion. For example, a decrease in presynaptic Ca2+ influx into the calyx of Held is the major cause of synaptic depression at this synapse type (Xu and Wu, 2005). In addition, a reduction in the AP amplitude during high repetitive firing (>20 Hz) has been correlated with a reduction in the transmitter release (Brody and Yue, 2000). Because we did not observe any change in the AP amplitude when we elicited and measured 20-Hz AP trains in the presence or absence of Cavβ2a and Cavβ4b subunits, a decline in AP amplitude is most likely not involved in the depression effects observed (Fig. S1, available at http://www.jcb.org/cgi/content/full/jcb.200702072/DC1). Because our synaptic terminals are too small to directly record the Ca2+ influx, we cannot exclude the possibility that the presynaptic Ca2+ influx into the terminal is reduced. However, the decrease in channel inactivation, particularly for Cavβ2a subunit–assembled channels, correlated with the faster RRP recovery and faster onset of asynchronous release does not agree with this mechanism but rather suggests a larger Ca2+ influx into the presynaptic terminal.
Cavβ4b subunits change the cooperativity of transmitter release
Exogenous expression of Cavβ4b subunits induced PPF. PPF occurs at low release probability synapses during high frequency stimulation and is associated with a restricted Ca2+ influx during the first AP accompanied by a build up in presynaptic Ca2+ concentration and, thus, an increase in the synaptic release probability once the second AP reaches the presynaptic terminal (Thomson, 2000; Zucker and Regehr, 2002). To analyze whether the increase in PPR in the presence of Cavβ4b subunits could account for a reduction in channel opening caused by Cavβ4b, we examined the possibility of detecting differences in the amount of channels opened by a hippocampal AP. We could not detect substantial differences between the Cavβ-assembled channels during the paired-pulse protocol used. To provide an explanation for the facilitation behavior of Cavβ4b-expressing synapses, we analyzed several parameters, including Ca2+ dependence of the transmitter release, the effect of the expression of Cavβ subunits on the contribution of N- and P/Q-type channels to synaptic transmission, and somatic non–L-type currents. We found that the expression of Cavβ4b changes the shape of the Ca2+ response curve, which is most likely not correlated with a change in the ratio between the P/Q- or N-type channel or a Cavβ4b channel–specific effect on the terminal (Fig. S2, available at http://www.jcb.org/cgi/content/full/jcb.200702072/DC1). This result suggests that in the presence of Cavβ4b subunits at the presynaptic terminal, the cooperativity of the Ca2+- dependent transmitter release is changed. Recently, the cooperativity of the transmitter release was determined using the same rat hippocampal autapse system. The cooperativity was estimated to around 3 (Reid et al., 1998), a value which we determined and confirmed in our study of wild-type and Cavβ2a-expressing neurons. The authors did not find a difference in the contribution between N- or P/Q-type channels. This is important to note because Cavβ4 could preferentially assemble with one or the other channel type. For example, the preferential assembly with N- or P/Q-type channels would have important implications for the synaptic transmission at the calyx of Held, where N-type channels are suggested to be further apart from the release site than P/Q-type channels (Wu et al., 1999). The change in cooperativity in the presence of Cavβ4b subunits may suggest that the coupling between the Ca2+ channels and the release machinery is affected or that the Ca2+ channels are more distant from the release site. The idea is supported by our finding that Cavβ4b-expressing neurons are more sensitive to the slow Ca2+ chelator EGTA. This is an important finding given the recent observation that the N terminus of the Cavβ4a subunit can bind synaptotagmin and the microtubule- associated protein 1A (Vendel et al., 2006). This raises the possibility that the Cavβ4a subunit is creating a Cavβ subunit–specific anchor between the Ca2+ channel and the synaptic release machinery (Weiss, 2006), whereas the Cavβ4b subunit would not. Therefore, Cavβ4b subunit–assembled channels might be further apart from the release machinery or may change the placement of the readily releasing vesicle next to the Ca2+ channels, which may cause the change in the Ca2+ response curve. In fact, it has been suggested recently that recruitment and placement of the synaptic vesicles to sites where Ca2+ channels cluster are important for rapid neurotransmitter release (Wadel et al., 2007).
Physiological consequences of neuronal Ca2+ channels assembled with different Cavβ subunits
Cavβ subunit–specific effects on synaptic transmitter release (i.e., facilitation and/or depression) will arise if a certain subunit is abundant in a neuronal circuit or synapse. For example, in the thalamus, a brain region that is critical for seizure activity, high expression levels of Cavβ4 subunits are found, whereas Cavβ1–3 subunits seem to be absent or at a lower abundance (Tanaka et al., 1995; Burgess and Noebels, 1999). Loss of Cavβ4 subunit function results in absence seizure epilepsy correlated with a reduced excitatory synaptic transmission in the thalamus (Caddick et al., 1999). Cavβ2 subunits have been suggested to play a crucial role for Cav1.4 function at the ribbon synapse of the outer plexiform layer of the retina, where these channels mediate glutamate release, whereas the role of Cavβ2 within the brain is poorly understood. Because Cavβ subunits are targets of protein phosphorylation and regulate the trafficking of the Ca2+ channels (Dolphin, 2003; Herlitze et al., 2003), it can be expected that activity-dependent trafficking of specific Cavβ subtypes in and out of synaptic terminals may occur as an important mechanism for the regulation of synaptic plasticity within a presynaptic terminal.
Materials and methods
Cell culture
Microisland and continental cultures of hippocampal neurons were prepared according to a modified version of published procedures (Bekkers and Stevens, 1991). In brief, hippocampal CA1-CA3 neurons from newborn rats (postnatal day 0–3) were enzymatically dissociated in 2 U/ml DME plus papain (Worthington) for 60 min at 37°C. Dissociated neurons were either plated onto astrocyte-covered poly-d-lysine/collagen (Sigma- Aldrich)-treated microislands that were prepared 3–5 d before plating (autaptic cultures) or were plated onto poly-d-lysine/collagen-treated coverslips that were placed invertly over astrocyte feeder cells (continental cultures). Neuronal cultures were grown in Neurobasal-A media (Invitrogen) supplemented with 4% B-27 (Invitrogen) and 2 mM Glutamax (Invitrogen) for 12–15 d.
Immunocytochemistry and imaging
Continental hippocampal cultures were prepared as described in the previous section and were infected with GFP-tagged Cavβ subunits. 12–18 h after infection, neurons were fixed with 4% PFA and permeabilized with 0.2% Triton X-100 in PBS. Anti–synaptobrevin-II (SYSY) and antisynapsin (Invitrogen) antibodies were used to label the synaptic markers. Neurons were incubated with the primary antibody overnight at 4°C, washed, and incubated with AlexaFluor568-conjugated secondary antibody (Invitrogen) for 30 min at room temperature. Cells were embedded in Prolong Gold Antifade (Invitrogen). Images were acquired with a confocal microscope (LSM 510; Carl Zeiss MicroImaging, Inc.) mounted on an inverted microscope (Axiovert 200M; Carl Zeiss MicroImaging, Inc.). Images were acquired with a 63× oil plan Apo NA 1.4 objective at room temperature, processed with the built-in LSM 510 software (version 3.5; Carl Zeiss MicroImaging, Inc.), and analyzed by using VOLOCITY software (Improvision).
Pan-β antibody
Polyclonal anti–pan-β antibody was raised by Harlan Bioproduct for Science according to a published procedure (Vance et al., 1998). In short, a highly conserved peptide sequence presented in all β subunits (CESYTSRPSDSDVSLEEDRE) was synthesized, and a standard 112-d protocol was used for polyclonal antibody production (Harlan Bioproduct for Science). The specificity of the product was documented with Western blots using rat brain homogenate as well as homogenates of HEK293 cells expressing Cavβ1b, Cavβ2, Cavβ3, or Cavβ4 subunit and resulted only in bands with desired molecular weights.
Electrophysiology and analysis
For HEK293 cell recordings, HEK293 cells (tsA201 cells) were transfected with the Ca2+ channel subunits Cavα12.1 and Cavα2δ, with Cavβ1b, Cavβ2a, Cavβ3, or Cavβ4b, and with GFP to identify positively transfected cells (molar ratio of 2:1:1:0.25). Whole cell recordings were performed as described previously (Li et al., 2005). For EPSC recordings, only dots containing a single neuron forming excitatory synapses (autapses) were used using an EPC-9 amplifier (HEKA). Recordings were performed at room temperature.
For EPSC measurements as well as for recordings of Ca2+ currents in HEK293 cells, the extracellular recording solution contained 172 mM NaCl, 2.4 mM KCl, 10 mM Hepes, 10 mM glucose, 4 mM CaCl2, and 4 MgCl2, pH 7.3; the internal solution contained 145 mM potassium gluconate, 15 mM Hepes, 1 mM potassium-EGTA, 4 mM Na-ATP, and 0.4 mM Na-GTP, pH 7.3. For EGTA experiments (Fig. 9, E and F), the internal solution contained 10 mM potassium EGTA (Sigma-Aldrich), or 50 μM EGTA-AM (Invitrogen) was applied 15 min before recording to the extracellular recording solution. Currents were elicited by a 2-ms-long test pulse to 10 mV and recorded and analyzed as described previously (Wittemann et al., 2000). For recordings using various extracellular Ca2+ concentrations (extracellular [Ca2+]o), solutions containing different extracellular [Ca2+]o were applied directly onto the recorded neurons by using a fast-flow perfusion system (ALA Scientific). Non–L-type channel recordings in cultured hippocampal neurons were performed as previously described (Li et al., 2005; Han et al., 2006). The internal recording solution contained 120 mM N-methyl-d-glucamine, 20 mM tetraethylammonium-Cl−, 10 mM Hepes, 1 mM CaCl2, 14 mM phosphocreatine (Tris), 4 mM Mg-ATP, 0.3 mM Na2GTP, and 11 mM EGTA, pH 7.2, with methanesulfonic acid. The external solution contained 145 mM tetraethylammonium, 10 mM Hepes, 10 mM CaCl2, and 15 mM glucose, pH 7.4, with methanesulfonic acid. In addition, 1 μM tetrodotoxin (Sigma-Aldrich) and 5 μM nimodipine (Sigma-Aldrich) were added to the external solution to block voltage-dependent Na+ channels and L-type Ca2+ channels. Non–L-type currents were elicited by 500-ms voltage clamp ramps from –60 to 90 mV with 1-min intervals and by 100-ms-long voltage pulses from −60 to 0 mV (Fig. 6 B). Here, capacitative and tail currents were subtracted after the experiment. The sizes of RRPs were measured according to published procedures (Rosenmund and Stevens, 1996; Han et al., 2006). In short, 500 mM sucrose was applied directly onto the recorded autaptic neurons for 4 s by using a fast-flow perfusion system (ALA Scientific). The EPSC and RRP charge was calculated by integrating the currents elicited by the single AP or the sucrose application.
The asynchronous and phasic release was calculated as described in Otsu et al. (2004). In brief, we estimated the phasic release by integrating the EPSC after each pulse within the 20-Hz stimulation protocol after subtraction of a baseline value measured 1 ms before each test pulse. The asynchronous release was calculated by subtracting the phasic release from the total integrated current for each EPSC. The holding current was subtracted before integration in every experiment. Statistical significance throughout the experiments was evaluated with analysis of variance using Igor Pro software (Wavemetrics). Standard errors are mean ± SEM.
Quantitative real-time PCR
107 cells of acutely dissociated hippocampal neurons were plated on poly-d-lysine–collagen–coated plates for continental culture as described in the Cell culture section. The total RNA was subtracted from 14-d in vitro–cultured neurons with the RNeasy Mini kit (QIAGEN) and purified with on-column DNase digestion using the RNase-Free DNase Set (QIAGEN). For RT-PCR, 1 μg RNA was used for reverse transcription with the Advantage RT-for-PCR kit (BD Biosciences) to generate 100 μl cDNA, and 3 μl of the final RT product was used for real-time PCR of each Cavβ subunit. Real-time PCR quantification was performed on the iCycler Iq Detection System (Bio-Rad Laboratories) with CYBR green assay (Bio-Rad Laboratories). The DNA fragments of Cavβ1b, Cavβ2a, Cavβ3, and Cavβ4b were amplified from cDNA with the following primer pairs: Cavβ1b forward (GGCTGTGAGGTTGGTTTCAT) and Cavβ1b backward (TGTCACCTGACTTGCTGGAG); Cavβ2 forward (CATGAGACCAGTGGTGTTGG) and Cavβ2 backward (CAGGGAGATGTCAGCAGTGA); Cavβ3 forward (CAGGTTTGATGGCAGGATCT) and Cavβ3 backward (GTGTCAGCATCCAACACCAC); Cavβ4 forward (GAGAGCGAAGTCCAAACCTG) and Cavβ4 backward (TCACCAGCCTTCCTATCCAC); and 18S forward (AAACGGCTACCACATCCAAG) and 18S backward (CCTCCAATGGATCCTCGTTA).
The specificity of RT-PCR products was documented with gel electrophoresis and resulted in a single product with desired length. The melt curve analysis showed that each primer pair had a single product- specific melting temperature. All primer pairs have at least 95% of PCR efficiency, as reported from the slopes of the standard curves generated by iQ software (version 3.1; Bio-Rad Laboratories). The PCR reactions used a modified two-step profile with initial denaturation for 3 min at 95°C, 40 cycles of 95°C for 15 s, and at 57°C for 25 s. Relative gene expression data were analyzed with the 2-ΔΔCT method (Livak and Schmittgen, 2001).
Electron microscopy
For immunoelectron microscopy of the cultured hippocampal neurons, 14-d in vitro neurons were infected with GFP-tagged Cavβ2a or Cavβ4b subunits with the Semliki Forest virus (SFV) expression system for 12 h before fixing with 4% PFA in 1× PBS for 20 min at 4°C. Cells were washed with 1× PBS containing 0.05% (vol/vol) Triton X-100, blocked with 10% goat serum (Invitrogen), and incubated with polyclonal rabbit anti-GFP antibody (Invitrogen) at 4°C overnight. The neurons were then rinsed five times with PBS/0.05% Triton X-100 for 3 min and incubated with goat anti–rabbit IgG conjugated with 10-nm gold particles (Electron Microscopy Sciences) for 2 h at room temperature on a shaker. After rinsing, neurons were fixed with 2% glutaraldehyde and 4% PFA in 0.1 M cacodylate buffer at 4°C overnight. After postfixing with 1% osmium tetroxide and staining with 1% uranyl acetate, neurons were dehydrated through an ethanol series from 50 to 100% ethanol and were transferred to propylene oxide, infiltrated with Embed 812 (Electron Microscopy Sciences) for 12 h, and hardened for 24 h at 60°C. Coverslips were removed, and 60-nm sections were cut on an ultramicrotome (Ultracut E; Reichert-Jung. The slices were recovered on Formvar-coated single slot copper grids and examined in a electronic microscope (JEM-1200EX; JEOL) at 80 kV.
For the brain slice immunoelectron microscopy, 100-nm-thick adult rat brain slices were prepared on a vibrating blade microtome (VT 1000S; Leica) and immediately infected with GFP-tagged Cavβ2a or Cavβ4b subunits with the SFV expression system for 12 h in an incubator with 5% CO2 at 37°C. The expression of the subunits was verified by the GFP fluorescent signals before the slices were fixed with 4% PFA in 1× PBS at 4°C overnight. The slices were rinsed with 1× PBS containing 0.05% (vol/vol) Triton X-100 five times for 3 min, blocked with 10% goat serum (Invitrogen), and incubated with a polyclonal rabbit anti-GFP antibody (Invitrogen) overnight at 4°C. Procedures and conditions for the second antibody, postfixation, and embedding, etc., were the same as for cultured neurons.
cDNAs and virus production
Rat Cavβ1b, Cavβ2a, Cavβ3, and Cavβ4b were gifts from T. Snutch (University of British Columbia, Vancouver, Canada) and E. Perez-Reyes (University of Virginia, Charlottesville, VA). They were cloned in frame into pEGFP-C1–3 vectors (CLONTECH Laboratories, Inc.) and then into the Semliki forest virus vector pSFV1 (Life Technologies) for virus production. Thus, the GFP tag is located on the N terminus of the Cavβ subunits.
Membrane fractionation
About 8 × 106 hippocampal neurons were cultured on four collagen/poly-d-lysine–coated 100-mm culture dishes for 14 d and infected with GFP-tagged Cavβ1b, Cavβ2a, Cavβ3, and Cavβ4b carrying virus for 13–16 h. Infected or noninfected cells were scraped in 0.32 M sucrose-TBS (0.15 M NaCl and 0.05 Tris, pH 7.4) containing 1× Complete Mini protease inhibitor (Roche) and were homogenized for 50 strokes with Dounce tissue grinder (Wheaton Millville) before promptly being loaded on top of freshly prepared 0.8 M/1.2 M sucrose-TBS gradient for centrifugation. Centrifugation was performed in a J-2-21 M/E ultracentrifuge (Beckman Coulter) at 3 × 104 rpm with a SW25.1 rotor for 45 min at 4°C. Equal volumes of the cytosol and membrane fractions were used for Western blots, which were performed according to standard procedures (Mark et al., 1995).
Online supplemental material
Fig. S1 shows that the AP amplitude during 20-Hz stimulations is not reduced in noninfected or Cavβ2a and Cavβ 4b subunits expressing hippocampal neurons. Fig. S2 shows that Cavβ subunits expressed in hippocampal neurons do not change the relative contribution of N- and P/Q-type channels to non–L-type currents and EPSCs. Fig. S3 shows that the N terminus of Cavβ4b interferes with synaptic transmitter release in hippocampal neurons. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200702072/DC1.
Supplementary Material
Acknowledgments
We would like to thank Dr. L. Landmesser for reading the manuscript, Drs. L. Landmesser, R. Miller, and A. Malouf for helpful discussions, and Dr. R. Miller for help with the immunoelectron microscopy.
This work was supported by National Institutes of Health grants NS0447752 and NS42623 to S. Herlitze.
Abbreviations used in this paper: AP, action potential; EPSC, excitatory postsynaptic current; PPF, paired-pulse facilitation; PPR, paired-pulse ratio; RRP, readily releasable vesicle pool; SFV, Semliki Forest virus.
References
- Ahmari, S.E., J. Buchanan, and S.J. Smith. 2000. Assembly of presynaptic active zones from cytoplasmic transport packets. Nat. Neurosci. 3:445–451. [DOI] [PubMed] [Google Scholar]
- Atluri, P.P., and W.G. Regehr. 1998. Delayed release of neurotransmitter from cerebellar granule cells. J. Neurosci. 18:8214–8227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekkers, J.M., and C.F. Stevens. 1991. Excitatory and inhibitory autaptic currents in isolated hippocampal neurons maintained in cell culture. Proc. Natl. Acad. Sci. USA. 88:7834–7838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berggren, P.O., S.N. Yang, M. Murakami, A.M. Efanov, S. Uhles, M. Kohler, T. Moede, A. Fernstrom, I.B. Appelskog, C.A. Aspinwall, et al. 2004. Removal of Ca2+ channel beta3 subunit enhances Ca2+ oscillation frequency and insulin exocytosis. Cell. 119:273–284. [DOI] [PubMed] [Google Scholar]
- Bichet, D., V. Cornet, S. Geib, E. Carlier, S. Volsen, T. Hoshi, Y. Mori, and M. De Waard. 2000. The I-II loop of the Ca2+ channel alpha1 subunit contains an endoplasmic reticulum retention signal antagonized by the beta subunit. Neuron. 25:177–190. [DOI] [PubMed] [Google Scholar]
- Bogdanov, Y., N.L. Brice, C. Canti, K.M. Page, M. Li, S.G. Volsen, and A.C. Dolphin. 2000. Acidic motif responsible for plasma membrane association of the voltage-dependent calcium channel beta1b subunit. Eur. J. Neurosci. 12:894–902. [DOI] [PubMed] [Google Scholar]
- Brody, D.L., and D.T. Yue. 2000. Release-independent short-term synaptic depression in cultured hippocampal neurons. J. Neurosci. 20:2480–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess, D.L., and J.L. Noebels. 1999. Voltage-dependent calcium channel mutations in neurological disease. Ann. NY Acad. Sci. 868:199–212. [DOI] [PubMed] [Google Scholar]
- Caddick, S.J., C. Wang, C.F. Fletcher, N.A. Jenkins, N.G. Copeland, and D.A. Hosford. 1999. Excitatory but not inhibitory synaptic transmission is reduced in lethargic (Cacnb4(lh)) and tottering (Cacna1atg) mouse thalami. J. Neurophysiol. 81:2066–2074. [DOI] [PubMed] [Google Scholar]
- Catterall, W.A. 2000. Structure and regulation of voltage-gated Ca2+ channels. Annu. Rev. Cell Dev. Biol. 16:521–555. [DOI] [PubMed] [Google Scholar]
- Chen, Y.H., M.H. Li, Y. Zhang, L.L. He, Y. Yamada, A. Fitzmaurice, Y. Shen, H. Zhang, L. Tong, and J. Yang. 2004. Structural basis of the alpha1-beta subunit interaction of voltage-gated Ca2+ channels. Nature. 429:675–680. [DOI] [PubMed] [Google Scholar]
- Chien, A.J., T. Gao, E. Perez-Reyes, and M.M. Hosey. 1998. Membrane targeting of L-type calcium channels. Role of palmitoylation in the subcellular localization of the beta2a subunit. J. Biol. Chem. 273:23590–23597. [DOI] [PubMed] [Google Scholar]
- Dolphin, A.C. 2003. Beta subunits of voltage-gated calcium channels. J. Bioenerg. Biomembr. 35:599–620. [DOI] [PubMed] [Google Scholar]
- Fellin, T., S. Luvisetto, M. Spagnolo, and D. Pietrobon. 2004. Modal gating of human CaV2.1 (P/Q-type) calcium channels: II. the b mode and reversible uncoupling of inactivation. J. Gen. Physiol. 124:463–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, J., M.D. Mark, X. Li, M. Xie, S. Waka, J. Rettig, and S. Herlitze. 2006. RGS2 determines short-term synaptic plasticity in hippocampal neurons by regulating Gi/o mediated inhibition of presynaptic Ca2+ channels. Neuron. 51:575–586. [DOI] [PubMed] [Google Scholar]
- Hanlon, M.R., N.S. Berrow, A.C. Dolphin, and B.A. Wallace. 1999. Modelling of a voltage-dependent Ca2+ channel beta subunit as a basis for understanding its functional properties. FEBS Lett. 445:366–370. [DOI] [PubMed] [Google Scholar]
- Herlitze, S., and M.D. Mark. 2005. Distribution and targeting mechanisms of voltage activated Ca2+ channels. In Voltage-Gated Calcium Channels. G.W. Zamponi, editor. Kluwer Academic/Plenum Publishing Corp., New York. 113–40.
- Herlitze, S., D.E. Garcia, K. Mackie, B. Hille, T. Scheuer, and W.A. Catterall. 1996. Modulation of Ca2+ channels by G-protein beta gamma subunits. Nature. 380:258–262. [DOI] [PubMed] [Google Scholar]
- Herlitze, S., G.H. Hockerman, T. Scheuer, and W.A. Catterall. 1997. Molecular determinants of inactivation and G protein modulation in the intracellular loop connecting domains I and II of the calcium channel alpha1A subunit. Proc. Natl. Acad. Sci. USA. 94:1512–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlitze, S., H. Zhong, T. Scheuer, and W.A. Catterall. 2001. An allosteric mechanism for modulation of Ca2+ channels by G proteins, voltage-dependent facilitation, protein kinase C, and Cavb subunits. Proc. Natl. Acad. Sci. USA. 98:4699–4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlitze, S., M. Xie, J. Jan, A. Hümmer, K.V. Melnik-Martinez, R.L. Moreno, and M.D. Mark. 2003. Targeting mechanisms of high voltage-activated Ca2+ channels. J. Bioenerg. Biomembr. 35:621–637. [DOI] [PubMed] [Google Scholar]
- Hibino, H., R. Pironkova, O. Onwumere, M. Rousset, P. Charnet, A.J. Hudspeth, and F. Lesage. 2003. Direct interaction with a nuclear protein and regulation of gene silencing by a variant of the Ca2+-channel beta 4 subunit. Proc. Natl. Acad. Sci. USA. 100:307–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X., A. Hummer, J. Han, M. Xie, K. Melnik-Martinez, R.L. Moreno, M. Buck, M.D. Mark, and S. Herlitze. 2005. G protein beta2 subunit-derived peptides for inhibition and induction of G protein pathways. Examination of voltage-gated Ca2+ and G protein inwardly rectifying K+ channels. J. Biol. Chem. 280:23945–23959. [DOI] [PubMed] [Google Scholar]
- Livak, K.J., and T.D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. [DOI] [PubMed] [Google Scholar]
- Luvisetto, S., T. Fellin, M. Spagnolo, B. Hivert, P.F. Brust, M.M. Harpold, K.A. Stauderman, M.E. Williams, and D. Pietrobon. 2004. Modal gating of human CaV2.1 (P/Q-type) calcium channels: I. The slow and the fast gating modes and their modulation by β subunits. J. Gen. Physiol. 124:445–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark, M.D., Y. Liu, S.T. Wong, T.R. Hinds, and D.R. Storm. 1995. Stimulation of neurite outgrowth in PC12 cells by EGF and KCl depolarization: a Ca(2+)-independent phenomenon. J. Cell Biol. 130:701–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark, M.D., S. Wittemann, and S. Herlitze. 2000. G protein modulation of recombinant P/Q-type calcium channels by regulators of G protein signalling proteins. J. Physiol. 528:65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maximov, A., and I. Bezprozvanny. 2002. Synaptic targeting of N-type calcium channels in hippocampal neurons. J. Neurosci. 22:6939–6952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee, A.W., D.A. Nunziato, J.M. Maltez, K.E. Prehoda, G.S. Pitt, and D.S. Bredt. 2004. Calcium channel function regulated by the SH3-GK module in beta subunits. Neuron. 42:89–99. [DOI] [PubMed] [Google Scholar]
- Opatowsky, Y., C.C. Chen, K.P. Campbell, and J.A. Hirsch. 2004. Structural analysis of the voltage-dependent calcium channel beta subunit functional core and its complex with the alpha 1 interaction domain. Neuron. 42:387–399. [DOI] [PubMed] [Google Scholar]
- Otsu, Y., V. Shahrezaei, B. Li, L.A. Raymond, K.R. Delaney, and T.H. Murphy. 2004. Competition between phasic and asynchronous release for recovered synaptic vesicles at developing hippocampal autaptic synapses. J. Neurosci. 24:420–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid, C.A., J.M. Bekkers, and J.D. Clements. 1998. N- and P/Q-type Ca2+ channels mediate transmitter release with a similar cooperativity at rat hippocampal autapses. J. Neurosci. 18:2849–2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards, M.W., A.J. Butcher, and A.C. Dolphin. 2004. Ca2+ channel beta-subunits: structural insights AID our understanding. Trends Pharmacol. Sci. 25:626–632. [DOI] [PubMed] [Google Scholar]
- Rosenmund, C., and C.F. Stevens. 1996. Definition of the readily releasable pool of vesicles at hippocampal synapses. Neuron. 16:1197–1207. [DOI] [PubMed] [Google Scholar]
- Rousset, M., T. Cens, and P. Charnet. 2005. Alone at last! New functions for Ca2+ channel beta subunits? Sci. STKE. 10.1126/stke.2752005pe11. [DOI] [PubMed]
- Shapira, M., R.G. Zhai, T. Dresbach, T. Bresler, V.I. Torres, E.D. Gundelfinger, N.E. Ziv, and C.C. Garner. 2003. Unitary assembly of presynaptic active zones from Piccolo-Bassoon transport vesicles. Neuron. 38:237–252. [DOI] [PubMed] [Google Scholar]
- Stea, A., W.J. Tomlinson, T.W. Soong, E. Bourinet, S.J. Dubel, S.R. Vincent, and T.P. Snutch. 1994. Localization and functional properties of a rat brain alpha 1A calcium channel reflect similarities to neuronal Q- and P-type channels. Proc. Natl. Acad. Sci. USA. 91:10576–10580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens, C.F., and J.F. Wesseling. 1998. Activity-dependent modulation of the rate at which synaptic vesicles become available to undergo exocytosis. Neuron. 21:415–424. [DOI] [PubMed] [Google Scholar]
- Tanaka, O., H. Sakagami, and H. Kondo. 1995. Localization of mRNAs of voltage-dependent Ca(2+)-channels: four subtypes of alpha 1- and beta-subunits in developing and mature rat brain. Brain Res. Mol. Brain Res. 30:1–16. [DOI] [PubMed] [Google Scholar]
- Thomson, A.M. 2000. Facilitation, augmentation and potentiation at central synapses. Trends Neurosci. 23:305–312. [DOI] [PubMed] [Google Scholar]
- Tottene, A., T. Fellin, S. Pagnutti, S. Luvisetto, J. Striessnig, C. Fletcher, and D. Pietrobon. 2002. Familial hemiplegic migraine mutations increase Ca(2+) influx through single human CaV2.1 channels and decrease maximal CaV2.1 current density in neurons. Proc. Natl. Acad. Sci. USA. 99:13284–13289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Petegem, F., K.A. Clark, F.C. Chatelain, and D.L. Minor Jr. 2004. Structure of a complex between a voltage-gated calcium channel beta-subunit and an alpha-subunit domain. Nature. 429:671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance, C.L., C.M. Begg, W.L. Lee, H. Haase, T.D. Copeland, and M.W. McEnery. 1998. Differential expression and association of calcium channel alpha1B and beta subunits during rat brain ontogeny. J. Biol. Chem. 273:14495–14502. [DOI] [PubMed] [Google Scholar]
- Vendel, A.C., M.D. Terry, A.R. Striegel, N.M. Iverson, V. Leuranguer, C.D. Rithner, B.A. Lyons, G.E. Pickard, S.A. Tobet, and W.A. Horne. 2006. Alternative splicing of the voltage-gated Ca2+ channel beta4 subunit creates a uniquely folded N-terminal protein binding domain with cell-specific expression in the cerebellar cortex. J. Neurosci. 26:2635–2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadel, K., E. Neher, and T. Sakaba. 2007. The coupling between synaptic vesicles and Ca(2+) channels determines fast neurotransmitter release. Neuron. 53:563–575. [DOI] [PubMed] [Google Scholar]
- Weiss, N. 2006. The calcium channel beta4a subunit: a scaffolding protein between voltage-gated calcium channel and presynaptic vesicle-release machinery? J. Neurosci. 26:6117–6118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittemann, S., M.D. Mark, J. Rettig, and S. Herlitze. 2000. Synaptic localization and presynaptic function of calcium channel beta 4-subunits in cultured hippocampal neurons. J. Biol. Chem. 275:37807–37814. [DOI] [PubMed] [Google Scholar]
- Wu, L.G., R.E. Westenbroek, J.G.G. Borst, W.A. Catterall, and B. Sakmann. 1999. Calcium channel types with distinct presynaptic localization couple differentially to transmitter release in single calyx-type synapses. J. Neurosci. 19:726–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, J., and L.G. Wu. 2005. The decrease in the presynaptic calcium current is a major cause of short-term depression at a calyx-type synapse. Neuron. 46:633–645. [DOI] [PubMed] [Google Scholar]
- Zucker, R.S., and W.G. Regehr. 2002. Short-term synaptic plasticity. Annu. Rev. Physiol. 64:355–405. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.