Synopsis
Increased numbers of activated eosinophils in the blood and tissues that typically accompany hypereosinophilic disorders result from a variety of mechanisms. At one end of the process is augmented bone marrow production and egress, while at the other end of the process is enhanced selective tissue recruitment and survival. Accumulation alone is insufficient to lead to eosinophil-associated pathology, hence the importance of additional means for activation and release of eosinophil-derived mediators. Common themes in these events include participation of subsets of transcription factors, cytokines, chemokines, adhesion molecules and survival regulatory pathways. Besides an enhanced understanding of the contributions of these and other molecules to hypereosinophilic disorders achieved from mouse models and molecular strategies, exciting advancements translating these discoveries to the clinic have led to a flurry of new therapeutics specifically designed to target eosinophil-associated diseases. So far, this form of hypothesis testing in humans in vivo via pharmacology has generally supported the paradigms generated in vitro and in animal models, raising hopes and expectations that a spectrum of novel therapies may soon become available to help those with eosinophil-associated diseases.
Keywords: eosinophil, hematopoiesis, survival, apoptosis, adhesion, migration
Introduction
The paradigm that eosinophils play a significant pro-inflammatory and tissue damaging role in the pathogenesis of many eosinophil-associated diseases and hypereosinophilic syndromes continues to be supported by an ever-increasing number of definitive animal model and clinical studies. This includes recent evidence that eosinophils play a pivotal role in the development of tissue remodeling and fibrosis, in part through their potent elaboration of remodeling and fibrogenic growth factors 1-3. Studies using two different strains of eosinophil-deficient mice strongly support the concept that the eosinophil contributes to the pathophysiology of allergic diseases such as asthma 4, 5. As well, recent clinical trials using a humanized anti-IL-5 antibody (Mepolizumab™) to ablate eosinophils in the bone marrow, blood and tissues of patients show great promise for reversing eosinophilia and aspects of eosinophil-mediated tissue damage, remodeling and fibrosis in allergic diseases such as asthma 6, 7 and eosinophilic esophagitis (EE) 8, and the hypereosinophilic syndrome (HES) 9. This review addresses our current understanding of the mechanisms that regulate eosinophil lineage commitment and differentiation in the bone marrow, the development of blood and tissue eosinophilia, and their relationships to the pathogenesis and pathophysiology of hypereosinophilic disorders.
Cytokine regulation of eosinophilopoiesis in the bone marrow
Eosinophils differentiate in the bone marrow from stem cell-derived, CD34+ multipotential myeloid progenitors in response to a number of T cell-derived eosinophilopoietic cytokines and growth factors including IL-3, GM-CSF and IL-5. These cytokines affect the eosinophil lineage at three different levels including: (1) commitment, proliferation and differentiation of the hematopoietic progenitors, (2) priming, activation and survival in the blood and tissues for enhanced functional activities, and (3) recruitment and tissue localization (see below). Activated T cells are likely the primary source for IL-3, IL-5 and GM-CSF pertinent to eosinophil differentiation in the bone marrow and the development of reactive blood and tissue eosinophilia in allergic and parasitic diseases, and some hypereosinophilic syndromes, but other cell types including mast cells, macrophages, natural killer cells, endothelial cells, epithelial cells and stromal cells such as fibroblasts are also producers of these factors, e.g. GM-CSF. IL-5, produced primarily by activated Th2 cells 10 and mast cells 11, 12, regulates the production of eosinophils both in vitro from purified hematopoieitic progenitors, and in vivo 13-15. Both IL-3 and GM-CSF are pluripotent cytokines with activities on other hematopoietic lineages, whereas IL-5 is selective for the eosinophil lineage and plays a crucial role in driving committed eosinophil progenitor cell proliferation, terminal differentiation and post-mitotic activation 16. As a late-acting lineage-specific cytokine, IL-5 demonstrates maximum activity on an IL-5R+ eosinophil progenitor pool that is first expanded by earlier-acting multipotential cytokines including IL-3 and GM-CSF 16. However, IL-5 is both necessary and sufficient for eosinophil development to proceed 16, 17. The expression of the high-affinity receptor for IL-5 (IL-5R) is a prerequisite and very early lineage-specific event in the hematopoietic program for eosinophil development. Overexpression of IL-5 is observed in many eosinophil-associated diseases 18-20 and IL-5 transgenic mice develop profound eosinophilia 21, 22, indicating that IL-5 plays important roles in promoting the production and function of eosinophils in vivo. These early observations have been confirmed and expanded in studies of IL-5 deficient (gene knockout) mice 23, 24, which nevertheless produce basal levels of normal eosinophils in the bone marrow, but importantly, fail to develop blood and/or tissue eosinophilia, or lung damage, airways hyperreactivity and airways remodeling in murine allergic asthma models 24, 25, and do not develop blood or tissue eosinophilic responses to helminth parasites 23. The finding that IL-5 deficient (knockout) mice still generate basal levels of eosinophils in the bone marrow is consistent with the current paradigm that basal hematopoiesis occurs independently of the expression of lineage-specific cytokines such as erythropoietin (EPO), G-CSF, M-CSF, and IL-5 (the “inductive/instructive” model), and is instead regulated at the level of gene transcription through the combinatorial activities of hematopoietic-specific transcription factors that act to resolve lineage promiscuous gene expression patterns in early uncommitted hematopoietic progenitors 26-28 (see below).
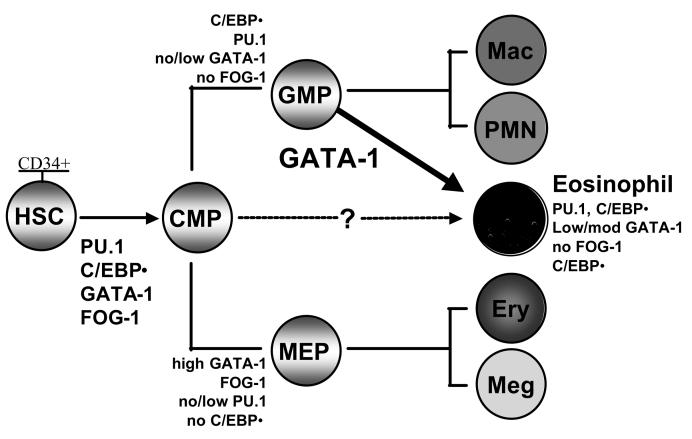
Transcriptional regulation of eosinophil lineage commitment and differentiation
Studies over the past 15 years of the mechanisms that regulate myeloid gene transcription, hematopoietic lineage-specification, and differentiation have provided novel insights into the roles of combinatorial networks of transcription factors in determining progenitor cell fate, including eosinophil lineage commitment and terminal differentiation. Current findings from avian, mouse and human studies suggest that a handful of transcription factors and their functional interactions are critical in influencing eosinophil lineage-specification, development and terminal differentiation 29. The combinatorial activities of GATA-1, C/EBPα (CCAAT enhancer-binding protein α) and the ets factor PU.1 are required for eosinophil development to proceed, with GATA-1 being the pivotal factor that determines whether granulocyte-macrophage progenitors will differentiate into eosinophils (requires GATA-1) or neutrophils or macrophages (absence of GATA-1) (Figure 1). In contrast, FOG-1 (friend of GATA-1), a co-activator of GATA-1 required for erythroid differentiation, functions as a co-repressor that antagonizes GATA-1 activity in eosinophil progenitors 30, and must therefore be down-regulated for eosinophil development to proceed in the bone marrow 31. Convincing data from mouse knockout studies have shown that eosinophils do not develop in GATA-1 null mice 32, and that transgenic deletion of a high-affinity palindromic double GATA site in the HS-2 region of the mouse GATA-1 promoter itself results in a lineage-specific block in eosinophil terminal differentiation 33, suggesting that double GATA sites may regulate eosinophil lineage-specific expression of GATA-1 itself. Consonant with this finding is the presence of similar high-affinity double GATA binding sites in the promoters of a number of hallmark eosinophil genes defining this lineage, including those encoding a number of the secondary granule proteins such as MBP1 and the IL-5-binding IL-5Rα subunit 34 and the eotaxin receptor CCR3 35. The antagonistic activities of GATA-1 and PU.1 have been functionally and mechanistically defined in erythroid versus myeloid differentiation 36, where they may serve to resolve progenitor cell lineage promiscuity as part of cell fate specification during hematopoietic development 26-28. In contrast, however, PU.1 and GATA-1 synergize in the eosinophil lineage for transcription of genes such as MBP1 34. The mechanism for PU.1-GATA-1 synergy may involve PU.1 enhancement rather than antagonism of GATA-1 DNA binding to unique double GATA sites present in a number of key eosinophil genes (Du J and Ackerman SJ, manuscript submitted). As with GATA-1 null mice, knockout of C/EBPα results in animals that are incapable of producing any granulocytes including eosinophils 37, and eosinophil terminal differentiation is likewise significantly impaired in PU.1 knockout mice 38(Du J and Ackerman SJ, manuscript in preparation).
Figure 1. Combinatorial transcription factor “codes” that specify eosinophil lineage commitment and terminal differentiation.
HSC, CD34+ hematopoietic stem cells; CMP, common myeloid progenitors; GMP granulocyte-macrophage progenitors; MEP, megakaryocyte/erythroid progenitors. GATA-1 is both necessary and sufficient to drive eosinophil development, and C/EBPε is required for eosinophil terminal differentiation. It remains unclear whether human eosinophils can also develop directly from CMP (Modified and updated from reference 29).
The current consensus regarding the combinatorial transcription factor code that selectively specifies and regulates eosinophilopoiesis as compared to the differentiation of other myeloid lineages is highlighted in Figure 1. Thus far, it appears that regulation of the relative levels and timing of expression of GATA-1, FOG-1, PU.1 and C/EBPα are necessary to generate eosinophils, such that the commitment and terminal differentiation of eosinophils from myeloid progenitors requires concomitant expression of C-EBPα, PU.1, a low to moderate level of GATA-1, with no expression of FOG-1 29. Once myeloid progenitors are committed to the eosinophil lineage, their terminal differentiation and functional maturation in the bone marrow has been shown to require the activity of another member of the C/EBP family of transcription factors, C/EBPε, that is expressed at highest levels during the promyelocyte to myelocyte transition. Studies of C/EBPε null (knockout) mice have shown that eosinophil (and neutrophil) terminal differentiation requires C/EBPε, as these mice lack terminally differentiated functionally mature granulocytes (both eosinophils and neutrophils) 39, 40. Similarly, patients with specific granule deficiency (SGD) have been shown to have a novel mutation in the C/EBPε gene that results in loss of function of this transcription factor, the consequence of which is a failure of both neutrophil and eosinophil terminal differentiation and functional maturation, including failed expression of important secondary granule protein genes in both granulocytes 41. Clearly, greater understanding of the combinatorial and functional interactions of the transcription factors that specify eosinophil lineage commitment, and regulate gene transcription and terminal differentiation is needed. Studies in this area may ultimately lead to the identification of novel targets for ablating eosinophil development in general in the bone marrow, or selectively knocking down eosinophil expression of key inflammatory mediators, such as the granule cationic proteins, or receptors such as CCR3 as therapeutic approaches to the treatment of eosinophil-mediated allergic diseases or hypereosinophilic syndromes.
Regulation of eosinophil differentiation by cytokines and exit from the bone marrow
Based on studies with anti-IL-5 antibody, it is now clear that IL-5 is critical for terminal eosinophil differentiation 42, 43. Indeed, one of the key terminal steps in eosinophil hematopoiesis involves surface expression of the IL-5 receptor (CD125/CD131) 44. Until this point, both eosinophils and basophils share maturation pathways. Remnants of these shared differentiation pathways persist even though their divergence is clear when examining their mature circulating counterparts. For example, circulating eosinophils continue to express low levels of the α chain of the high affinity IgE receptor (FcεRI) 45, 46 while basophils express low levels of major basic protein (MBP) 47, Charcot-Leyden crystal (CLC) protein (galectin-10) 48, and eosinophil-derived neurotoxin (EDN), eosinophil cationic protein (ECP) and eosinophil peroxidase (EPO) 49. Both cell types in the peripheral blood selectively express the chemokine receptor CCR3 50, but at least in mice, its expression on eosinophils in the bone marrow occurs late in differentiation and in conjunction with loss of expression of FIRE, an F4/80-related receptor expressed by mouse eosinophils with an as yet unknown human counterpart 51. Another terminal differentiation marker is Siglec-8 (and Siglec-F, the latter being its closest functional paralog in the mouse 52, 53), which is expressed more highly on peripheral blood eosinophils than in bone marrow eosinophils, and at much higher levels than on basophils 51, 54.
IL-5 deficient mice have markedly reduced numbers of bone marrow, tissue and circulating eosinophils 23, while the opposite is found in IL-5 transgenic mice 21, 22. However, the exact signals regulating egress from the bone marrow are not entirely understood. Studies in mice suggest that besides IL-5 55, CCR3 agonists, such as eotaxin-1 (CCL11), are important for eosinophils to leave the bone marrow 56. Infusion of a β2 integrin-blocking antibody prevented IL-5-mediated marrow release, while an antibody to α4 integrin enhanced release. Exactly how this occurs is not clear, but it is known that IL-5 and CCR3 agonists alter integrin function in a way that facilitates detachment from various counter-ligands as has been observed in vitro in models of endothelial cell adhesion and detachment under flow conditions 57-59. Separate studies in animals would suggest that eosinophil egress following allergen sensitization and challenge is partially T cell-dependent 60.
Eosinophil trafficking out of the circulation into tissues
Production of IL-5 and/or GM-CSF, as well as administration of these cytokines in humans, results in rapid and sustained peripheral blood eosinophilia 61, 62. Once in the circulation, eosinophils persist there for 18-24 hours before migrating to extravascular sites. This circulation time may be even longer in conditions associated with peripheral blood eosinophilia. Diseases associated with eosinophilia, such as hypereosinophilic syndromes, are frequently, but not always, associated with elevations in serum levels of IL-5 or GM-CSF 63-66. Whereas the bone marrow is the largest reservoir for eosinophil precursors and their differentiation, their predominant destination beyond the circulation, in normal humans, is the gastrointestinal tract. Based on animal studies, this homing occurs because of constitutive gut epithelial expression eotaxin-1 (CCL11) 67.
Levels of eosinophils in the circulation undergo diurnal variation, with highest levels in the evening and lowest levels in the early morning, in parallel with diurnal variations of endogenous cortisol levels 68. Studies with systemically administered adhesion molecule antagonists show that VLA-4 (CD49d/CD29), but not LFA-1 (CD11a/CD18), is involved in constitutive eosinophil trafficking because VLA-4 antibody administration resulted in about a doubling of circulating eosinophil and lymphocyte counts (both express VLA-4), whereas administration of antibodies to LFA-1 had no effect on circulating eosinophil counts 69, 70. The circulating eosinophil half-life under these conditions has not been specifically measured, but the presumption is that VLA-4 blockade interferes with constitutive homing of eosinophils into the gastrointestinal tract and perhaps other sites.
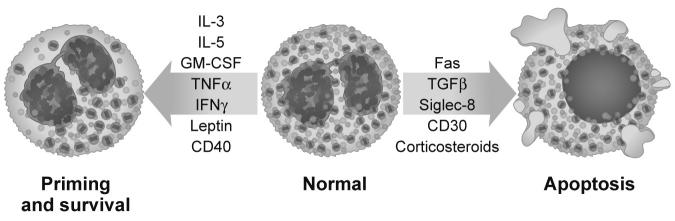
It is generally believed that once eosinophils leave the circulation and migrate into tissue sites, they do not recirculate, although data in animal models would suggest that eosinophils exogenously transferred to the lungs may traffic to regional lymph nodes and play a role in antigen presentation 71. Once in tissues, survival is dependent on local production of cytokines that prevent eosinophil apoptosis. Besides IL-5, other important locally produced survival factors include GM-CSF, and perhaps to a lesser degree IL-3, TNF-α, IFN-γ, leptin 72, CD40 engagement 73 and others 74 (Figure 2). Studies show that in the right local cytokine milieu, eosinophils and their precursors are capable of differentiating and surviving in tissues for several days 75-77. In addition, eosinophils that are obtained by bronchoalveolar lavage from asthmatics, or following segmental lung allergen challenge of allergic subjects, display prolonged survival for several days in culture even in the absence of added cytokines, whereas peripheral blood counterparts do not even survive 24 hours 78, 79. This is consistent with their having been exposed to survival-promoting cytokines in the inflamed lung, or having been induced to generate endogenous survival factors such as GM-CSF 80.
Figure 2. How eosinophils are influenced to make life or death decisions.
Stimuli that are known to promote eosinophil priming and survival are displayed, and are contrasted with those that facilitate eosinophil apoptosis. Also shown are some of the phenotypic characteristics that accompany the primed state compared to those seen in cells undergoing apoptosis. Art by Jacqueline Schaffer.
Based primarily on allergic inflammation models 81, selective recruitment of eosinophils to specific tissue sites is effectively regulated by unique patterns of cytokines that activate endothelial cells and induce tissue-resident cells to produce eosinophil-active chemokines and other chemoattractants to facilitate their preferential migration. In the category of the former, cytokines such as IL-4 and IL-13 selectively induce endothelial expression of VCAM-1 (CD106), therefore providing ligands for α4 integrin-mediated recruitment in a pathway that distinguishes eosinophil responses from neutrophil responses, since neutrophils lack α4 integrins 50, 82-84. Indeed, inhalation of the VLA-4 antagonist IVL745 had modest effects on late phase sputum eosinophilia in humans, but failed to alter the acute or late phase physiologic response 85. CCR3-active chemokines including the eotaxins (CCL11, CCL24, CCL26), RANTES (CCL5), MCP-4 (CCL13) and others, derived predominantly from tissue-resident cells such as keratinocytes in the skin and airway epithelium, further facilitate eosinophil adhesion and transendothelial migration 74. Taking a different approach, it was convincingly demonstrated that lung endothelial Gαi2 function, separate from its potential role on leukocytes, is required for eosinophil recruitment 86. Given the eosinophil expression of both VLA-4 and VLA-6 (CD49f/CD29), interactions with specific tissue matrix proteins, especially fibronectin and laminin respectively, may also be important in localization of these cells and promoting their autocrine GM-CSF-driven prolonged survival within tissues 87, 88. Both TNFα and IL-1 synergistically interact with IL-4 and IL-13 to augment VCAM-1 expression as well as ICAM-1 (CD54) expression 89. This is consistent with the important role of β2 integrins in mediating eosinophil recruitment responses 83, 90. Alpha-4 integrins can also contribute to rolling adhesion, but a more important component of eosinophil rolling adhesion is likely mediated through surface PSGL-1 (CD162) on the eosinophil interacting with endothelial P-selectin (CD62P) 91-93. Unlike its important role in neutrophil recruitment, E-selectin (CD62E) and L-selectin play little or no role in eosinophil recruitment responses 82, 94, 95. Nevertheless, a pan-selectin antagonist is in clinical trials, and despite a short half-life was capable of inhibiting allergen-induced late phase responses in the airways 96, 97. Whether late phase lung eosinophilia was affected was not determined. Additional chemoattractants implicated in selective eosinophil migration include complement fragments such as C5a, platelet-activating factor, as well as sulfidopeptide leukotrienes 98. Priming, a term used to describe the enhanced responsiveness of a cell to normally active stimuli, occurs in vitro following exposure to cytokines like GM-CSF and IL-5. In patients with eosinophilia, their eosinophils have a similar functionally primed phenotype, become “hypodense” on density gradients 99, 100, and develop a characteristic microscopic appearance with reduced or condensed granules (Figure 2) 101, along with enhanced surface expression of activation markers such as CD44 and CD69 102. These cells also demonstrate exaggerated adhesion and migration responses to virtually all of the stimuli mentioned above which can be a critical aspect of enhanced eosinophil trafficking seen in hypereosinophilic conditions 103.
For each of these recruitment pathways, there is some degree of tissue specificity regarding homing patterns. For example, constitutive expression of eotaxin as well as β7 integrins appear to be critical, if not absolutely necessary, for gut homing 104, while eotaxin-3 is markedly overexpressed in the esophagus in patients with eosinophilic esophagitis 105. Based on mouse studies, IL-5, IL-13, CD44 (a receptor for hylauronic acid) and CCR3-active chemokines are implicated in lung homing of eosinophils 106, 107, and injections of, or appearance following allergen challenge of, eotaxin-1 (CCL11), eotaxin-2 (CCL24), or RANTES (CCL5) is associated with eosinophil accumulation in the skin 108, 109. Blockade of LFA-1 had only modest effects on eosinophil accumulation in the human lung allergen challenge model 70, while an antibody to eotaxin appears to reduce nasal eosinophilia induced by allergen challenge 110, 111. Also of note is that cysteinyl leukotriene receptor antagonists reduce numbers of eosinophils in the airway and blood 112, and mice missing the LTB4 receptor have a profound decrease in lung eosinophils following allergen sensitization and challenge 113. Finally, eosinophils, along with basophils, mast cells and Th2 cells, express CRTh2, a high affinity receptor for prostaglandin D2 114, 115, while both Th2 cells and eosinophils express the H4 histamine receptor 116. The relative contribution of these pathways compared to others remains to be delineated when specific antagonists become available.
Activation of eosinophil degranulation and mediator release
In order for eosinophils to participate in local tissue pathobiology, more must occur besides their accumulation; indeed, activation of recruited eosinophils is felt to be a critical aspect of disease pathophysiology. For example, IL-5 transgenic mice have massively increased numbers of eosinophils in the circulation, spleen and other tissues, yet, without a second signal, these mice are relatively healthy. One of the major pathways by which eosinophils are activated is through cross-linking of surface immunoglobulin receptors, especially those involving IgA, and to a lesser degree IgG 98. Whereas there is general agreement that mouse eosinophils do not express FcεRI, this topic remains controversial for human eosinophils. The bulk of recent literature would suggest that human eosinophils express very low levels, if any, FcεRI on their surface, and if present clearly lack the β chain; so significant, direct activation of eosinophils via IgE remains unlikely 117-119. Stimulation of eosinophils leads to eosinophil granule protein release (e.g., ECP, EDN, EPO, MBP), superoxide generation and synthesis of leukotriene C4 98, 120, although the latter is difficult to induce with traditional eosinophil-activating stimuli. Eosinophils also produce platelet activating factor and a wide range of cytokines and chemokines, not the least of which include IL-1β and TGF-β, two key players in the eosinophil-mediated tissue remodeling and fibrosis seen in many eosinophil associated diseases 3, 121-123. While the quantities of cytokines and chemokines released per eosinophil versus other cells vary widely, GM-CSF is among the cytokines produced in greatest quantities by eosinophils 98, and as noted above, it functions in part in an autocrine fashion to prolong eosinophil survival once they are recruited into tissue inflammatory sites. Eosinophil activation in vitro by a number of agonists including IL-5, IFN-γ, sIgA and others has been shown to induce secretion of the granule cationic proteins (EPO, MBP1, EDN, ECP) and eosinophil-expressed cytokines (e.g. RANTES and IL-4) by a process termed piecemeal degranulation 124, 125 that involves differential mobilization and vesicular transport of these proteins 112, 126, 127. This is in contrast to secondary granule fusion and classical exocytosis, events rarely observed for eosinophils in inflammatory foci in tissues 128. Once secreted, the eosinophil granule cationic proteins have multiple potential pro-inflammatory activities that have been defined in vitro and in vivo including membrane, cell and tissue-damaging cytotoxicity 129, 130, the ability to selectively activate inflammatory cells, such as mast cells and basophils, to release inflammatory mediators (e.g. histamine) 131, potent blocking activity for inhibitory M2 muscarinic receptors in the airways in asthma models 132, as well as the ability to augment TGF-β primed fibroblast elaboration of the inflammatory and profibrotic IL-6 family of cytokines including IL-6 and IL-11 133, to name just a few. Thus, eosinophils: (1) come fully armed with pre-formed mediators of inflammation, tissue damage, remodeling, and fibrogenesis that are secreted at sites of eosinophilic inflammation in tissues in eosinophil-associated diseases such as the hypereosinophilic syndromes, and (2) have the capacity to generate newly formed protein (cytokines, chemokines) and lipid mediators (LTC4, PAF) of inflammation when primed and further activated during their recruitment from the bone marrow into the tissue in response to allergic and other stimuli 134.
Regulation of tissue eosinophil survival and activation
Once in tissues, if eosinophils do not encounter the appropriate survival milieu, the lack of exposure to such cytokines normally leads to their prompt apoptosis (Figure 2). Separate from this, however, are a number of pathways that actively, and to varying degrees, selectively induce eosinophil apoptosis. In humans, corticosteroids markedly and rapidly diminish numbers of circulating and tissue eosinophils, although the mechanisms responsible for this are complex and probably involve a combination of altered release from bone marrow, shortened circulation time, redistribution from the circulation into spleen and other organs, induction of apoptosis, and inhibition of cytokines and chemokines needed for eosinophil survival and recruitment 135-139. Besides steroids, other pro-apoptotic molecules for eosinophils include lidocaine 140, TGF-β 141, Siglec-8/Siglec-F 142, 143, Fas (CD95) 144 and CD30 145. While prior exposure to survival cytokines or priming conditions tends to improve resistance of the eosinophil to these death pathways, a unique situation is the Siglec-8 pathway, which is actually augmented by priming cytokines 142, 146. Other drugs used to reduce eosinophil numbers include hydroxyurea, although this drug is used to cause a more global inhibitory effect on hematopoiesis. Interferon-α and leukotriene synthesis or receptor blockers also reduce circulating eosinophil numbers 147. Recently, tyrosine kinase inhibitors such as imatinib mesylate (Gleevec™) have been shown to have profound effects on eosinophil numbers because a subset of individuals with hypereosinophilic syndrome have a deletion mutation on chromosome 4 resulting in the fusion of a gene with unknown function, namely FIP1L1, with the PDGFRα gene, resulting in a constitutively active tyrosine kinase 148. Thus, patients found to be FIP1L1-PDGFRα positive, either by fluorescence in situ hybridization or RT-PCR, are now treated with imatinib mesylate. Other constitutively active tyrosine kinases have been implicated in eosinophilic syndromes, including Type-2 fibroblast growth factor receptor-1 and PDGFRB 74.
Conclusions
The heterogeneity of hypereosinophilic syndromes, which ranges from patients with features of myeloproliferative disorders with cytogenetic abnormalities (e.g. FIP1L1-PDGFRα-positive chronic eosinophil leukemia, CEL), to patients with more benign clinical courses, such as episodic angioedema with eosinophilia, suggests that multiple disease processes are at play that regulate eosinophilopoiesis in the bone marrow, the recruitment of eosinophils to tissues, their survival, activation and secretion of inflammatory mediators, and pathophysiologic outcomes. Current research aimed at defining the causes of HES and mechanisms that regulate eosinophilia in these diseases, and the development of eosinophil-mediated end organ damage in eosinophil-associated diseases in general, should lead to more selective and improved therapies for hypereosinophilic syndromes. The therapeutic targets for these efforts currently include: 1) IL-5 and its high affinity receptor, 2) underlying T-cell clones (either immunocompetent or occult Tlymphoid malignancies) that elaborate eosinophilopoietins such as IL-5 or GM-CSF 63, 66, tissue or organ-specific dysfunctional elaboration of eosinophil-active chemoattractant factors such as eosinophil-selective chemokines (e.g. eotaxin-3 in the esophagus in eosinophilic esophagitis, EE) 105, 3) vascular endothelial adhesion molecules (VCAM/VLA-4), and 4) inhibitory receptors such as Siglec-8 142 and CD300a 149. The essential absence of end organ damage in some of the hypereosinophilic syndromes contrasts starkly with the morbidity (and mortality) associated with the development of endomyocardial fibrosis in HES. Because HES patients are clearly a heterogeneous group, clinical management based on current knowledge must be specifically tailored to the individual, with the overall goal of controlling the blood and tissue eosinophilia, and in particular, the eosinophil-mediated end organ damage 150. Current treatment options permit the control or eradication of eosinophilia and end organ damage in most HES patients 150, 151. The efficacy of imatinib mesylate (Gleevec™) in some patients with HES led to the identification of the FIP1L1-PDGFRα gene fusion that encodes a pathogenetically relevant and constitutively active tyrosine kinase 148. This seminal finding has lead to a reclassification of hypereosinophilias into better-defined clinical entities 150, 151, and has stimulated new research that may ultimately translate into improved clinical characterization and therapeutic options.
Finally, humanized anti-IL-5 antibody (Mepolizumab™) has recently shown clinical efficacy for controlling eosinophilia in HES in clinical trials 9, 42, 152, and looks highly promising for the treatment of a wide range of patients with FIP1L1-PDGFRA negative HES and possibly other eosinophilias 151, for example, eosinophilic gastrointestinal syndromes such as eosinophilic esophagitis, EE 8. Future research on HES and CEL should focus on the molecular basis of imatinib responsiveness in both FIP1L1-PDGFRα-positive and -negative patients, addressing how the constitutively activated FIP1L1-PDGFRα or other fusion or mutant activated kinases selectively lead to chronic hypereosinophilia and end organ damage. Studies of the effects of imatinib on the proliferation and terminal differentiation of bone marrow–derived eosinophil progenitors, and the survival and intracellular signaling pathways in eosinophils from imatinib-responsive patients may be particularly revealing in terms of the downstream targets of these novel kinases and the roles of eosinophil-active eosinophilopoietins and survival factors such as IL-5 and GM-CSF 153.
Acknowledgments
This work was supported in part by grants AI033043 (to SJA) and AI041472 (to BSB) from the National Institutes of Health. Dr. Bochner also received support as a Cosner Scholar in Translational Research from Johns Hopkins University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kay AB. The role of eosinophils in the pathogenesis of asthma. Trends Mol Med. 2005 Apr;11(4):148–152. doi: 10.1016/j.molmed.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Kay AB, Phipps S, Robinson DS. A role for eosinophils in airway remodelling in asthma. Trends Immunol. 2004 Sep;25(9):477–482. doi: 10.1016/j.it.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 3.Gomes I, Mathur SK, Espenshade BM, Mori Y, Varga J, Ackerman SJ. Eosinophilfibroblast interactions induce fibroblast IL-6 secretion and extracellular matrix gene expression: implications in fibrogenesis. J Allergy Clin Immunol. 2005 Oct;116(4):796–804. doi: 10.1016/j.jaci.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 4.Lee JJ, Dimina D, Macias MP, et al. Defining a link with asthma in mice congenitally deficient in eosinophils. Science. 2004 Sep 17;305(5691):1773–1776. doi: 10.1126/science.1099472. [DOI] [PubMed] [Google Scholar]
- 5.Humbles AA, Lloyd CM, McMillan SJ, et al. A critical role for eosinophils in allergic airways remodeling. Science. 2004 Sep 17;305(5691):1776–1779. doi: 10.1126/science.1100283. [DOI] [PubMed] [Google Scholar]
- 6.Phipps S, Flood-Page P, Menzies-Gow A, Ong YE, Kay AB. Intravenous anti-IL-5 monoclonal antibody reduces eosinophils and tenascin deposition in allergen-challenged human atopic skin. J Invest Dermatol. 2004 Jun;122(6):1406–1412. doi: 10.1111/j.0022-202X.2004.22619.x. [DOI] [PubMed] [Google Scholar]
- 7.Flood-Page P, Menzies-Gow A, Phipps S, et al. Anti-IL-5 treatment reduces deposition of ECM proteins in the bronchial subepithelial basement membrane of mild atopic asthmatics. J Clin Invest. 2003 Oct;112(7):1029–1036. doi: 10.1172/JCI17974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stein ML, Collins MH, Villanueva JM, et al. Anti-IL-5 (mepolizumab) therapy for eosinophilic esophagitis. J Allergy Clin Immunol. 2006 Dec;118(6):1312–1319. doi: 10.1016/j.jaci.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 9.Rothenberg ME, Gleich GJ, Roufosse FE, Rosenwasser LJ, Weller PF. Steroid-Sparing Effects of Anti-IL-5 Monoclonal Antibody (Mepolizumab) Therapy in Patients with HES: A Multicenter, Randomized, Double-Blind, Placebo-Controlled Trial. Blood (ASH Annual Meeting Abstracts) 2006;108(November):373. [Google Scholar]
- 10.Takatsu K, Tominaga A, Harada N, et al. T cell-replacing factor (TRF)/interleukin 5 (IL-5): molecular and functional properties. Immunol Rev. 1988;102:107–135. doi: 10.1111/j.1600-065x.1988.tb00743.x. [DOI] [PubMed] [Google Scholar]
- 11.Plaut M, Pierce JH, Watson CJ, Hanley-Hyde J, Nordan RP, Paul WE. Mast cell lines produce lymphokines in response to cross-linkage of Fc epsilon RI or to calcium ionophores. Nature. 1989;339(6219):64–67. doi: 10.1038/339064a0. [DOI] [PubMed] [Google Scholar]
- 12.Galli SJ, Gordon JR, Wershil BK, Elovic A, Wong DT, Weller PF. Mast cell and eosinophil cytokines in allergy and inflammation. In: Kay AB, Gleich GJ, editors. Eosinophils in Allergy and Inflammation. Vol. 2. Marcel Dekker; New York: 1994. pp. 255–280. [Google Scholar]
- 13.Palacios R, Karasuyama H, Rolink A. Ly1+ PRO-B lymphocyte clones. Phenotype, growth requirements and differentiation in vitro and in vivo. Embo J. 1987;6(12):3687–3693. doi: 10.1002/j.1460-2075.1987.tb02702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takatsu K, Yamaguchi N, Hitoshi Y, Sonoda E, Mita S, Tominaga A. Signal transduction through interleukin-5 receptors. Cold Spring Harb Symp Quant Biol. 1989;2:745–751. doi: 10.1101/sqb.1989.054.01.088. [DOI] [PubMed] [Google Scholar]
- 15.Yamaguchi Y, Suda T, Suda J, et al. Purified interleukin 5 supports the terminal differentiation and proliferation of murine eosinophilic precursors. J Exp Med. 1988;167(1):43–56. doi: 10.1084/jem.167.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanderson CJ. Eosinophil differentiation factor (interleukin-5) Immunol Ser. 1990;49:231–256. [PubMed] [Google Scholar]
- 17.Sanderson CJ. Control of eosinophilia. Int Arch Allergy Appl Immunol. 1991;94(14):122–126. doi: 10.1159/000235342. [DOI] [PubMed] [Google Scholar]
- 18.Owen WF, Rothenberg ME, Petersen J, et al. Interleukin 5 and phenotypically altered eosinophils in the blood of patients with the idiopathic hypereosinophilic syndrome. J Exp Med. 1989;170(1):343–348. doi: 10.1084/jem.170.1.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Owen W, Jr., Petersen J, Sheff DM, et al. Hypodense eosinophils and interleukin 5 activity in the blood of patients with the eosinophilia-myalgia syndrome. Proc Natl Acad Sci U S A. 1990;87(21):8647–8651. doi: 10.1073/pnas.87.21.8647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanderson CJ. Interleukin-5, eosinophils, and disease. Blood. 1992;79(12):3101–3109. [PubMed] [Google Scholar]
- 21.Dent LA, Strath M, Mellor AL, Sanderson CJ. Eosinophilia in transgenic mice expressing Interleukin-5. J. Exp. Med. 1990;172(5):1425–1431. doi: 10.1084/jem.172.5.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tominaga A, Takaki S, Koyama N, et al. Transgenic mice expressing a B-cell growth and differentiation factor gene (interleukin-5) develop eosinophilia and autoantibody production. J. Exp. Med. 1991;173(2):429–437. doi: 10.1084/jem.173.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kopf M, Brombacher F, Hodgkin PD, et al. IL-5-deficient mice have a developmental defect in CD5+ B-1 cells and lack eosinophilia but have normal antibody and cytotoxic T cell responses. Immunity. 1996 Jan;4(1):15–24. doi: 10.1016/s1074-7613(00)80294-0. [DOI] [PubMed] [Google Scholar]
- 24.Foster PS, Hogan SP, Ramsay AJ, Matthaei KI, Young IG. Interleukin 5 deficiency abolishes airways eosinophilia, airways hyperreactivity, and lung damage in mouse asthma model. J. Exp. Med. 1996;183:195–201. doi: 10.1084/jem.183.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cho JY, Miller M, Baek KJ, et al. Inhibition of airway remodeling in IL-5-deficient mice. J Clin Invest. 2004 Feb;113(4):551–560. doi: 10.1172/JCI19133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cantor AB, Orkin SH. Hematopoietic development: a balancing act. Curr Opin Genet Dev. 2001 Oct;11(5):513–519. doi: 10.1016/s0959-437x(00)00226-4. [DOI] [PubMed] [Google Scholar]
- 27.Miyamoto T, Akashi K. Lineage promiscuous expression of transcription factors in normal hematopoiesis. Int J Hematol. 2005 Jun;81(5):361–367. doi: 10.1532/ijh97.05003. [DOI] [PubMed] [Google Scholar]
- 28.Akashi K. Lineage promiscuity and plasticity in hematopoietic development. Ann N Y Acad Sci. 2005 Jun;1044:125–131. doi: 10.1196/annals.1349.016. [DOI] [PubMed] [Google Scholar]
- 29.McNagny K, Graf T. Making eosinophils through subtle shifts in transcription factor expression. J Exp Med. 2002 Jun 3;195(11):F43–47. doi: 10.1084/jem.20020636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamaguchi Y, Nishio H, Kishi K, Ackerman SJ, Suda T. C/EBPbeta and GATA-1 synergistically regulate activity of the eosinophil granule major basic protein promoter: implication for C/EBPbeta activity in eosinophil gene expression. Blood. 1999;94(4):1429–1439. [PubMed] [Google Scholar]
- 31.Querfurth E, Schuster M, Kulessa H, et al. Antagonism between C/EBPbeta and FOG in eosinophil lineage commitment of multipotent hematopoietic progenitors. Genes Dev. 2000;14(19):2515–2525. doi: 10.1101/gad.177200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hirasawa R, Shimizu R, Takahashi S, et al. Essential and instructive roles of GATA factors in eosinophil development. J Exp Med. 2002 Jun 3;195(11):1379–1386. doi: 10.1084/jem.20020170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu C, Cantor AB, Yang H, et al. Targeted deletion of a high-affinity GATA-binding site in the GATA-1 promoter leads to selective loss of the eosinophil lineage in vivo. J Exp Med. 2002;195(11):1387–1395. doi: 10.1084/jem.20020656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Du J, Stankiewicz MJ, Liu Y, et al. Novel combinatorial interactions of GATA-1, PU.1, and C/EBPepsilon isoforms regulate transcription of the gene encoding eosinophil granule major basic protein. J Biol Chem. 2002 Nov 8;277(45):43481–43494. doi: 10.1074/jbc.M204777200. [DOI] [PubMed] [Google Scholar]
- 35.Zimmermann N, Colyer JL, Koch LE, Rothenberg ME. Analysis of the CCR3 promoter reveals a regulatory region in exon 1 that binds GATA-1. BMC Immunol. 2005;6(1):7. doi: 10.1186/1471-2172-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stopka T, Amanatullah DF, Papetti M, Skoultchi AI. PU.1 inhibits the erythroid program by binding to GATA-1 on DNA and creating a repressive chromatin structure. Embo J. 2005 Nov 2;24(21):3712–3723. doi: 10.1038/sj.emboj.7600834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang DE, Zhang P, Wang ND, Hetherington CJ, Darlington GJ, Tenen DG. Absence of granulocyte colony-stimulating factor signaling and neutrophil development in CCAAT enhancer binding protein alpha- deficient mice. Proc Natl Acad Sci U S A. 1997;94(2):569–574. doi: 10.1073/pnas.94.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ackerman SJ, Du J, Xin F, et al. Eosinophilopoiesis: To be or not to be (an eosinophil)? That is the question: Transcriptional themes regulating eosinophil genes and development. Respir. Med. 2000;94:1135–1140. [Google Scholar]
- 39.Yamanaka R, Barlow C, Lekstrom-Himes J, et al. Impaired granulopoiesis, myelodysplasia, and early lethality in CCAAT/enhancer binding protein epsilon-deficient mice. Proc Natl Acad Sci U S A. 1997;94(24):13187–13192. doi: 10.1073/pnas.94.24.13187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamanaka R, Lekstrom-Himes J, Barlow C, Wynshaw-Boris A, Xanthopoulos KG. CCAAT/enhancer binding proteins are critical components of the transcriptional regulation of hematopoiesis (Review) Int J Mol Med. 1998;1(1):213–221. doi: 10.3892/ijmm.1.1.213. [DOI] [PubMed] [Google Scholar]
- 41.Rosenberg HF, Gallin JI. Neutrophil-specific granule deficiency includes eosinophils. Blood. 1993;82(1):268–273. [PubMed] [Google Scholar]
- 42.Klion AD, Law MA, Noel P, Kim YJ, Haverty TP, Nutman TB. Safety and efficacy of the monoclonal anti-interleukin-5 antibody SCH55700 in the treatment of patients with hypereosinophilic syndrome. Blood. 2004 Apr 15;103(8):2939–2941. doi: 10.1182/blood-2003-10-3620. [DOI] [PubMed] [Google Scholar]
- 43.Menzies-Gow A, Flood-Page P, Sehmi R, et al. Anti-IL-5 (mepolizumab) therapy induces bone marrow eosinophil maturational arrest and decreases eosinophil progenitors in the bronchial mucosa of atopic asthmatics. J Allergy Clin Immunol. 2003 Apr;111(4):714–719. doi: 10.1067/mai.2003.1382. [DOI] [PubMed] [Google Scholar]
- 44.Clutterbuck EJ, Hirst EMA, Sanderson CJ. Human interleukin-5 (IL-5) regulates the production of eosinophils in human bone marrow cultures: Comparison and interaction with IL-1, IL-3, IL-6, and GMCSF. Blood. 1989;73:1504–1512. [PubMed] [Google Scholar]
- 45.Seminario M-C, Saini SS, MacGlashan DW, Jr., Bochner BS. Intracellular expression and release of FcεRIα by human eosinophils. J. Immunol. 1999;162:6893–6900. [PubMed] [Google Scholar]
- 46.Smith SJ, Ying S, Meng Q, et al. Blood eosinophils from atopic donors express messenger RNA for the alpha, beta, and gamma subunits of the high-affinity IgE receptor (Fc epsilon RI) and intracellular, but not cell surface, alpha subunit protein. J Allergy Clin Immunol. 2000;105(2 Pt 1):309–317. doi: 10.1016/s0091-6749(00)90081-2. [DOI] [PubMed] [Google Scholar]
- 47.Ackerman SJ, Kephart GM, Habermann TM, Greipp PR, Gleich GJ. Localization of eosinophil granule major basic protein in human basophils. J. Exp. Med. 1983;158:946–961. doi: 10.1084/jem.158.3.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ackerman SJ, Weil GJ, Gleich GJ. Formation of Charcot-Leyden crystals by human basophils. J Exp Med. 1982;155(6):1597–1609. doi: 10.1084/jem.155.6.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abu-Ghazaleh RI, Dunnette SL, Loegering DA, et al. Eosinophil granule proteins in peripheral blood granulocytes. J Leukoc Biol. 1992 Dec;52(6):611–618. doi: 10.1002/jlb.52.6.611. [DOI] [PubMed] [Google Scholar]
- 50.Bochner BS, Schleimer RP. Mast cells, basophils, and eosinophils: distinct but overlapping pathways for recruitment. Immunol Rev. 2001 Feb;179:5–15. doi: 10.1034/j.1600-065x.2001.790101.x. [DOI] [PubMed] [Google Scholar]
- 51.Voehringer D, van Rooijen N, Locksley RM. Eosinophils develop in distinct stages and are recruited to peripheral sites by alternatively activated macrophages. J Leukoc Biol. 2007 Mar 5; doi: 10.1189/jlb.1106686. [DOI] [PubMed] [Google Scholar]
- 52.Aizawa H, Zimmermann N, Carrigan PE, Lee JJ, Rothenberg ME, Bochner BS. Molecular analysis of human Siglec-8 orthologs relevant to mouse eosinophils: identification of mouse orthologs of Siglec-5 (mSiglec-F) and Siglec-10 (mSiglec-G) Genomics. 2003 Nov;82(5):521–530. doi: 10.1016/s0888-7543(03)00171-x. [DOI] [PubMed] [Google Scholar]
- 53.Zhang JQ, Biedermann B, Nitschke L, Crocker PR. The murine inhibitory receptor mSiglec-E is expressed broadly on cells of the innate immune system whereas mSiglec-F is restricted to eosinophils. Eur J Immunol. 2004 Apr;34(4):1175–1184. doi: 10.1002/eji.200324723. [DOI] [PubMed] [Google Scholar]
- 54.Kikly KK, Bochner BS, Freeman S, et al. Identification of SAF-2, a novel siglec expressed on eosinophils, mast cells and basophils. J. Allergy Clin. Immunol. 2000;105:1093–1100. doi: 10.1067/mai.2000.107127. [DOI] [PubMed] [Google Scholar]
- 55.Palframan RT, Collins PD, Severs NJ, Rothery S, Williams TJ, Rankin SM. Mechanisms of acute eosinophil mobilization from the bone marrow stimulated by interleukin 5: The role of specific adhesion molecules and phosphatidylinositol 3-kinase. J. Exp. Med. 1998;188(9):1621–1632. doi: 10.1084/jem.188.9.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Palframan RT, Collins PD, Williams TJ, Rankin SM. Eotaxin induces a rapid release of eosinophils and their progenitors from the bone marrow. Blood. 1998 Apr 1;91(7):2240–2248. [PubMed] [Google Scholar]
- 57.Werfel S, Yednock T, Matsumoto K, Sterbinsky SA, Schleimer RP, Bochner BS. Functional regulation of β1 integrins and human eosinophils by divalent cations and cytokines. Am. J. Respir. Cell Mol. Biol. 1996;14:45–52. doi: 10.1165/ajrcmb.14.1.8534485. [DOI] [PubMed] [Google Scholar]
- 58.Weber C, Kitayama J, Springer TA. Differential regulation of β1 and β2 integrin avidity by chemoattractants in eosinophils. Proc. Natl. Acad. Sci. USA. 1996;93:10939–10944. doi: 10.1073/pnas.93.20.10939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tachimoto H, Burdick M, Hudson SA, Kikuchi M, Konstantopoulous K, Bochner BS. CCR3-active chemokines promote rapid detachment of eosinophils from VCAM-1 in vitro. J. Immunol. 2000;165:2748–2754. doi: 10.4049/jimmunol.165.5.2748. [DOI] [PubMed] [Google Scholar]
- 60.Minshall EM, Schleimer R, Cameron L, et al. Interleukin-5 expression in the bone marrow of sensitized Balb/c mice after allergen challenge. Am J Respir Crit Care Med. 1998 Sep;158(3):951–957. doi: 10.1164/ajrccm.158.3.9709114. [DOI] [PubMed] [Google Scholar]
- 61.Groopman JE, Mitsuyasu RT, DeLeo MJ, Oette DH, Golde DW. Effect of recombinant human granulocyte-macrophage colony-stimulating factor on myelopoiesis in the acquired immunodeficiency syndrome. N. Engl. J. Med. 1987;317:593–598. doi: 10.1056/NEJM198709033171003. [DOI] [PubMed] [Google Scholar]
- 62.Shi HZ, Xiao CQ, Zhong D, et al. Effect of inhaled interleukin-5 on airway hyperreactivity and eosinophilia in asthmatics. Am. J. Respir. Crit. Care Med. 1998;157(1):204–209. doi: 10.1164/ajrccm.157.1.9703027. [DOI] [PubMed] [Google Scholar]
- 63.Bochner BS, Friedman B, Krishnaswami G, Schleimer RP, Lichtenstein LM, Kroegel C. Episodic eosinophilia-myalgia-like syndrome in a patient without L-tryptophan use: association with eosinophil activation and increased serum levels of granulocyte-macrophage colony-stimulating factor. J Allergy Clin Immunol. 1991 Oct;88(4):629–636. doi: 10.1016/0091-6749(91)90157-j. [DOI] [PubMed] [Google Scholar]
- 64.Butterfield JH, Leiferman KM, Abrams J, et al. Elevated serum levels of interleukin-5 in patients with the syndrome of episodic angioedema and eosinophilia. Blood. 1992;79(3):688–692. [PubMed] [Google Scholar]
- 65.Fang J, Viksman MY, Ebisawa M, Bochner BS. Increased circulating levels of interleukin-5 in a case of steroid-resistant hypereosinophilic syndrome with ileal involvement. J. Allergy Clin. Immunol. 1994;94:129–131. doi: 10.1016/0091-6749(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 66.Simon HU, Plotz SG, Dummer R, Blaser K. Abnormal clones of T cells producing interleukin-5 in idiopathic eosinophilia. N. Engl. J. Med. 1999;341(15):1112–1120. doi: 10.1056/NEJM199910073411503. [DOI] [PubMed] [Google Scholar]
- 67.Mishra A, Hogan SP, Lee JJ, Foster PS, Rothenberg ME. Fundamental signals that regulate eosinophil homing to the gastrointestinal tract. J Clin Invest. 1999;103(12):1719–1727. doi: 10.1172/JCI6560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Winkel P, Statland BE, Saunders AM, Osborn H, Kupperman H. Within-day physiologic variation of leukocyte types in healthy subjects as assayed by two automated leukocyte differential analyzers. Am J Clin Pathol. 1981 May;75(5):693–700. doi: 10.1093/ajcp/75.5.693. [DOI] [PubMed] [Google Scholar]
- 69.Bochner BS. Adhesion molecules as therapeutic targets. Immunol Allergy Clin North Am. 2004 Nov;24(4):615–630. doi: 10.1016/j.iac.2004.06.003. vi. [DOI] [PubMed] [Google Scholar]
- 70.Gauvreau GM, Becker AB, Boulet LP, et al. The effects of an anti-CD11a mAb, efalizumab, on allergen-induced airway responses and airway inflammation in subjects with atopic asthma. J Allergy Clin Immunol. 2003 Aug;112(2):331–338. doi: 10.1067/mai.2003.1689. [DOI] [PubMed] [Google Scholar]
- 71.Shi HZ, Humbles A, Gerard C, Jin Z, Weller PF. Lymph node trafficking and antigen presentation by endobronchial eosinophils. J Clin Invest. 2000;105(7):945–953. doi: 10.1172/JCI8945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Conus S, Bruno A, Simon HU. Leptin is an eosinophil survival factor. J Allergy Clin Immunol. 2005 Dec;116(6):1228–1234. doi: 10.1016/j.jaci.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 73.Bureau F, Seumois G, Jaspar F, et al. CD40 engagement enhances eosinophil survival through induction of cellular inhibitor of apoptosis protein 2 expression: Possible involvement in allergic inflammation. J. Allergy Clin. Immunol. 2002;110:443–449. doi: 10.1067/mai.2002.126781. [DOI] [PubMed] [Google Scholar]
- 74.Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol. 2006;24:147–174. doi: 10.1146/annurev.immunol.24.021605.090720. [DOI] [PubMed] [Google Scholar]
- 75.Simon HU, Yousefi S, Schranz C, Schapowal A, Bachert C, Blaser K. Direct demonstration of delayed eosinophil apoptosis as a mechanism causing tissue eosinophilia. J. Immunol. 1997;158(8):3902–3908. [PubMed] [Google Scholar]
- 76.Cameron L, Christodoulopoulos P, Lavigne F, et al. Evidence for local eosinophil differentiation within allergic nasal mucosa: inhibition with soluble IL-5 receptor. J Immunol. 2000;164(3):1538–1545. doi: 10.4049/jimmunol.164.3.1538. [DOI] [PubMed] [Google Scholar]
- 77.Denburg JA, Keith PK. Systemic aspects of chronic rhinosinusitis. Immunol Allergy Clin North Am. 2004 Feb;24(1):87–102. doi: 10.1016/S0889-8561(03)00106-1. [DOI] [PubMed] [Google Scholar]
- 78.Broide DH, Lotz M, Cuomo AJ, Coburn DA, Federman EC, Wasserman SI. Cytokines in symptomatic asthma airways. J. Allergy Clin. Immunol. 1992;89(5):958–967. doi: 10.1016/0091-6749(92)90218-q. [DOI] [PubMed] [Google Scholar]
- 79.Kroegel C, Liu MC, Hubbard WM, Lichtenstein LM, Bochner BS. Blood and bronchoalveolar eosinophils in allergic subjects following segmental antigen challenge: surface phenotype, density heterogeneity, and prostanoid production. J. Allergy Clin. Immunol. 1994;93:725–734. doi: 10.1016/0091-6749(94)90252-6. [DOI] [PubMed] [Google Scholar]
- 80.Esnault S, Malter JS. GM-CSF regulation in eosinophils. Arch Immunol Ther Exp (Warsz) 2002;50(2):121–130. [PubMed] [Google Scholar]
- 81.Rosenberg HF, Phipps S, Foster PS. Eosinophil trafficking in allergy and asthma. J Allergy Clin Immunol. 2007 May 2; doi: 10.1016/j.jaci.2007.03.048. [DOI] [PubMed] [Google Scholar]
- 82.Fryer AD, Costello RW, Yost BL, et al. Antibody to VLA-4, but not to L-selectin, protects neuronal M2 muscarinic receptors in antigen-challenged guinea pig airways. J. Clin. Invest. 1997;99:2036–2044. doi: 10.1172/JCI119372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gonzalo JA, Lloyd CM, Kremer L, et al. Eosinophil recruitment to the lung in a murine model of allergic inflammation - the role of T cells, chemokines, and adhesion receptors. J. Clin. Invest. 1996;98(10):2332–2345. doi: 10.1172/JCI119045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gonzalo JA, Lloyd CM, Wen D, et al. The coordinated action of CC chemokines in the lung orchestrates allergic inflammation and airway hyperresponsiveness. J. Exp. Med. 1998;188(1):157–167. doi: 10.1084/jem.188.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Norris V, Choong L, Tran D, et al. Effect of IVL745, a VLA-4 antagonist, on allergen-induced bronchoconstriction in patients with asthma. J Allergy Clin Immunol. 2005 Oct;116(4):761–767. doi: 10.1016/j.jaci.2005.04.045. [DOI] [PubMed] [Google Scholar]
- 86.Pero RS, Borchers MT, Spicher K, et al. Galphai2-mediated signaling events in the endothelium are involved in controlling leukocyte extravasation. Proc Natl Acad Sci U S A. 2007 Mar 13;104(11):4371–4376. doi: 10.1073/pnas.0700185104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Anwar ARF, Moqbel R, Walsh GM, Kay AB, Wardlaw AJ. Adhesion to fibronectin prolongs eosinophil survival. J. Exp. Med. 1993;177(3):839–843. doi: 10.1084/jem.177.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Georas SN, McIntyre BW, Ebisawa M, Bednarczyk J, Schleimer RP, Bochner BS. Expression of a functional laminin receptor (α6β1, VLA-6) on human eosinophils. Blood. 1993;82:2872–2879. [PubMed] [Google Scholar]
- 89.Bochner BS. Cellular adhesion in inflammation. In: N.F. Adkinson J, Yunginger JW, Busse WW, Bochner BS, Holgate ST, Simons FER, editors. Allergy Principles and Practice. 6th Edition Mosby; St. Louis: 2003. pp. 117–134. [Google Scholar]
- 90.Broide DH, Sullivan S, Gifford T, Sriramarao P. Inhibition of pulmonary eosinophilia in P-selectin- and ICAM-1- deficient mice. Am. J. Respir. Cell Mol. Biol. 1998;18(2):218–225. doi: 10.1165/ajrcmb.18.2.2829. [DOI] [PubMed] [Google Scholar]
- 91.Bochner BS. Road signs guiding leukocytes along the inflammation superhighway. J. Allergy Clin. Immunol. 2000;106:817–828. doi: 10.1067/mai.2000.110813. [DOI] [PubMed] [Google Scholar]
- 92.Broide D, Sriramarao P. Eosinophil trafficking to sites of allergic inflammation. Immunol. Rev. 2001;179:163–172. doi: 10.1034/j.1600-065x.2001.790116.x. [DOI] [PubMed] [Google Scholar]
- 93.Wardlaw AJ. Molecular basis for selective eosinophil trafficking in asthma: A multistep paradigm. J Allergy Clin Immunol. 1999;104(5):917–926. doi: 10.1016/s0091-6749(99)70069-2. [DOI] [PubMed] [Google Scholar]
- 94.Bochner BS, Sterbinsky SA, Bickel CA, Werfel S, Wein M, Newman W. Differences between human eosinophils and neutrophils in the function and expression of sialic acid-containing counterligands for E-selectin. J. Immunol. 1994;152:774–782. [PubMed] [Google Scholar]
- 95.Broide DH, Miller M, Castaneda D, et al. Core 2 oligosaccharides mediate eosinophil and neutrophil peritoneal but not lung recruitment. Am. J. Physiol. Lung Cell Mol. Physiol. (in press) 2002;282:L259–L266. doi: 10.1152/ajplung.00214.2001. [DOI] [PubMed] [Google Scholar]
- 96.Beeh KM, Beier J, Meyer M, Buhl R, Zahlten R, Wolff G. Bimosiamose, an inhaled small-molecule pan-selectin antagonist, attenuates late asthmatic reactions following allergen challenge in mild asthmatics: a randomized, double-blind, placebo-controlled clinical cross-over-trial. Pulm Pharmacol Ther. 2006;19(4):233–241. doi: 10.1016/j.pupt.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 97.Meyer M, Beeh KM, Beier J, et al. Tolerability and pharmacokinetics of inhaled bimosiamose disodium in healthy males. Br J Clin Pharmacol. 2007 Apr;63(4):451–458. doi: 10.1111/j.1365-2125.2006.02775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kita H, Adolphson CR, Gleich GJ. Biology of eosinophils. In: N.F. Adkinson J, Yunginger JW, Busse WW, Bochner BS, Holgate ST, Simons FER, editors. Allergy Principles and Practice. 6th Edition Mosby; Philadelphia: 2003. pp. 305–332. [Google Scholar]
- 99.Prin L, Capron M, Tonnel AB, Bletry O, Capron A. Heterogeneity of human peripheral blood eosinophils: variability in cell density and cytotoxic ability in relation to the level and the origin of hypereosinophilia. Int Arch Allergy Appl Immunol. 1983;72(4):336–346. doi: 10.1159/000234893. [DOI] [PubMed] [Google Scholar]
- 100.Fukuda T, Dunnette SL, Reed CE, Ackerman SJ, Peters MS, Gleich GJ. Increased numbers of hypodense eosinophils in the blood of patients with bronchial asthma. Am Rev Respir Dis. 1985;132(5):981–985. doi: 10.1164/arrd.1985.132.5.981. [DOI] [PubMed] [Google Scholar]
- 101.Caulfield JP, Hein A, Rothenberg ME, et al. A morphometric study of normodense and hypodense human eosinophils that are derived in vivo and in vitro. Am J Pathol. 1990;137(1):27–41. [PMC free article] [PubMed] [Google Scholar]
- 102.Matsumoto K, Appiah-Pippim J, Schleimer RP, Bickel CA, Beck LA, Bochner BS. CD44 and CD69 represent different types of cell surface activation markers for human eosinophils. Am. J. Respir. Cell Mol. Biol. 1998;18:860–866. doi: 10.1165/ajrcmb.18.6.3159. [DOI] [PubMed] [Google Scholar]
- 103.Koenderman L, van der Bruggen T, Schweizer RC, et al. Eosinophil priming by cytokines: from cellular signal to in vivo modulation. Eur Respir J Suppl. 1996;22:119s–125s. [PubMed] [Google Scholar]
- 104.Brandt EB, Zimmermann N, Muntel EE, et al. The alpha4bbeta7-integrin is dynamically expressed on murine eosinophils and involved in eosinophil trafficking to the intestine. Clin Exp Allergy. 2006 Apr;36(4):543–553. doi: 10.1111/j.1365-2222.2006.02456.x. [DOI] [PubMed] [Google Scholar]
- 105.Blanchard C, Wang N, Stringer KF, et al. Eotaxin-3 and a uniquely conserved gene-expression profile in eosinophilic esophagitis. J Clin Invest. 2006 Feb;116(2):536–547. doi: 10.1172/JCI26679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Katoh S, Matsumoto N, Kawakita K, Tominaga A, Kincade PW, Matsukura S. A role for CD44 in an antigen-induced murine model of pulmonary eosinophilia. J Clin Invest. 2003 May;111(10):1563–1570. doi: 10.1172/JCI16583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zimmermann N, Hershey GK, Foster PS, Rothenberg ME. Chemokines in asthma: cooperative interaction between chemokines and IL-13. J Allergy Clin Immunol. 2003 Feb;111(2):227–242. doi: 10.1067/mai.2003.139. [DOI] [PubMed] [Google Scholar]
- 108.Menzies-Gow A, Ying S, Sabroe I, et al. Eotaxin (CCL11) and eotaxin-2 (CCL24) induce recruitment of eosinophils, basophils, neutrophils, and macrophages as well as features of early- and late-phase allergic reactions following cutaneous injection in human atopic and nonatopic volunteers. J Immunol. 2002 Sep 1;169(5):2712–2718. doi: 10.4049/jimmunol.169.5.2712. [DOI] [PubMed] [Google Scholar]
- 109.Beck LA, Dalke S, Leiferman KM, et al. Cutaneous injection of RANTES causes eosinophil recruitment: comparison of nonallergic and allergic human subjects. J Immunol. 1997 Sep 15;159(6):2962–2972. [PubMed] [Google Scholar]
- 110.Pereira S, Clark T, Darby Y, et al. Effects of anti-eotaxin monoclonal antibody CAT-213 on allergen-induced rhinitis. J Allergy Clin Immunol. 2003;111:S268. (abstr.) [Google Scholar]
- 111.Main S, Handy R, Wilton J, et al. A potent human anti-eotaxin1 antibody, CAT-213: isolation by phage display and in vitro and in vivo efficacy. J Pharmacol Exp Ther. 2006 Dec;319(3):1395–1404. doi: 10.1124/jpet.106.110734. [DOI] [PubMed] [Google Scholar]
- 112.Moqbel R, Lacy P. Exocytotic events in eosinophils and mast cells. Clin Exp Allergy. 1999 Aug;29(8):1017–1022. doi: 10.1046/j.1365-2222.1999.00633.x. [DOI] [PubMed] [Google Scholar]
- 113.Tager AM, Dufour JH, Goodarzi K, Bercury SD, von Andrian UH, Luster AD. BLTR mediates leukotriene B(4)-induced chemotaxis and adhesion and plays a dominant role in eosinophil accumulation in a murine model of peritonitis. J Exp Med. 2000 Aug 7;192(3):439–446. doi: 10.1084/jem.192.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nagata K, Hirai H, Tanaka K, et al. CRTH2, an orphan receptor of T-helper-2-cells, is expressed on basophils and eosinophils and responds to mast cell-derived factor(s) FEBS Lett. 1999;459(2):195–199. doi: 10.1016/s0014-5793(99)01251-x. [DOI] [PubMed] [Google Scholar]
- 115.Hirai H, Tanaka K, Yoshie O, et al. Prostaglandin D2 selectively induces chemotaxis in T helper type 2 cells, eosinophils, and basophils via seven-transmembrane receptor CRTH2. J. Exp. Med. 2001;193(2):255–261. doi: 10.1084/jem.193.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.O'Reilly M, Alpert R, Jenkinson S, et al. Identification of a histamine H4 receptor on human eosinophils--role in eosinophil chemotaxis. J Recept Signal Transduct Res. 2002 Feb-Nov;22(14):431–448. doi: 10.1081/rrs-120014612. [DOI] [PubMed] [Google Scholar]
- 117.Sihra BS, Kon OM, Grant JA, Kay AB. Expression of high-affinity IgE receptors (FcεRI) on peripheral blood basophils, monocytes, and eosinophils in atopic and nonatopic subjects: Relationship to total serum IgE concentrations. J. Allergy Clin. Immunol. 1997;99(5):699–706. doi: 10.1016/s0091-6749(97)70033-2. [DOI] [PubMed] [Google Scholar]
- 118.Kita H, Kaneko M, Bartemes KR, et al. Does IgE bind to and activate eosinophils from patients with allergy? J. Immunol. 1999;162:6901–6911. [PubMed] [Google Scholar]
- 119.Kayaba H, Dombrowicz D, Woerly G, Papin JP, Loiseau S, Capron M. Human eosinophils and human high affinity IgE receptor transgenic mouse eosinophils express low levels of high affinity IgE receptor, but release IL-10 upon receptor activation. J Immunol. 2001 Jul 15;167(2):995–1003. doi: 10.4049/jimmunol.167.2.995. [DOI] [PubMed] [Google Scholar]
- 120.Bandeira-Melo C, Weller PF. Eosinophils and cysteinyl leukotrienes. Prostaglandins Leukot Essent Fatty Acids. 2003 Aug-Sep;69(23):135–143. doi: 10.1016/s0952-3278(03)00074-7. [DOI] [PubMed] [Google Scholar]
- 121.Gharaee-Kermani M, Phan SH. The role of eosinophils in pulmonary fibrosis (Review) Int J Mol Med. 1998;1(1):43–53. [PubMed] [Google Scholar]
- 122.Levi-Schaffer F, Garbuzenko E, Rubin A, et al. Human eosinophils regulate human lung-and skin-derived fibroblast properties in vitro: a role for transforming growth factor beta (TGF-beta) Proc Natl Acad Sci U S A. 1999;96(17):9660–9665. doi: 10.1073/pnas.96.17.9660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Spry CJ. The pathogenesis of endomyocardial fibrosis: the role of the eosinophil. Springer Semin Immunopathol. 1989;11(4):471–477. doi: 10.1007/BF00201883. [DOI] [PubMed] [Google Scholar]
- 124.Dvorak AM, Ackerman SJ, Furitsu T, Estrella P, Letourneau L, Ishizaka T. Mature eosinophils stimulated to develop in human-cord blood mononuclear cell cultures supplemented with recombinant human interleukin-5. II. Vesicular transport of specific granule matrix peroxidase, a mechanism for effecting piecemeal degranulation. Am J Pathol. 1992;140(4):795–807. [PMC free article] [PubMed] [Google Scholar]
- 125.Melo RC, Perez SA, Spencer LA, Dvorak AM, Weller PF. Intragranular vesiculotubular compartments are involved in piecemeal degranulation by activated human eosinophils. Traffic. 2005 Oct;6(10):866–879. doi: 10.1111/j.1600-0854.2005.00322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Logan MR, Odemuyiwa SO, Moqbel R. Understanding exocytosis in immune and inflammatory cells: the molecular basis of mediator secretion. J Allergy Clin Immunol. 2003 May;111(5):923–932. [PubMed] [Google Scholar]
- 127.Moqbel R, Coughlin JJ. Differential secretion of cytokines. Sci STKE. 2006 Jun 6;2006(338):pe26. doi: 10.1126/stke.3382006pe26. [DOI] [PubMed] [Google Scholar]
- 128.Dvorak AM, Ackerman SJ, Weller PF. Subcellular morphology and biochemistry of eosinophils. In: Harris JR, editor. Blood Cell Biochemistry: Megakaryocytes, Platelets, Macrophages and Eosinophils. Vol. 2. Plenum Publishing Corporation; London: 1990. pp. 237–344. [Google Scholar]
- 129.Gleich GJ. Mechanisms of eosinophil-associated inflammation. J Allergy Clin Immunol. 2000 Apr;105(4):651–663. doi: 10.1067/mai.2000.105712. [DOI] [PubMed] [Google Scholar]
- 130.Martin LB, Kita H, Leiferman KM, Gleich GJ. Eosinophils in allergy: role in disease, degranulation, and cytokines. Int Arch Allergy Immunol. 1996 Mar;109(3):207–215. doi: 10.1159/000237239. [DOI] [PubMed] [Google Scholar]
- 131.Thomas LL, Page SM. Inflammatory cell activation by eosinophil granule proteins. Chem Immunol. 2000;76:99–117. doi: 10.1159/000058783. [DOI] [PubMed] [Google Scholar]
- 132.Jacoby DB, Costello RM, Fryer AD. Eosinophil recruitment to the airway nerves. J Allergy Clin Immunol. 2001 Feb;107(2):211–218. doi: 10.1067/mai.2001.112940. [DOI] [PubMed] [Google Scholar]
- 133.Rochester CL, Ackerman SJ, Zheng T, Elias JA. Eosinophil-fibroblast interactions. Granule major basic protein interacts with IL-1 and transforming growth factor-beta in the stimulation of lung fibroblast IL-6-type cytokine production. J Immunol. 1996;156(11):4449–4456. [PubMed] [Google Scholar]
- 134.Sedgwick JB, Calhoun WJ, Vrtis RF, Bates ME, McAllister PK, Busse WW. Comparison of airway and blood eosinophil function after in vivo antigen challenge. J Immunol. 1992;149(11):3710–3718. [PubMed] [Google Scholar]
- 135.Gleich GJ, Hunt LW, Bochner BS, Schleimer RP. Glucocorticoid effects on human eosinophils. In: Schleimer RP, Busse WW, O'Byrne P, editors. Inhaled glucocorticoids in asthma: mechanisms and clinical actions. Marcel Dekker, Inc.; New York: 1996. pp. 279–308. [Google Scholar]
- 136.Stellato C, Matsukura S, Fal A, et al. Differential regulation of epithelial-derived C-C chemokine expression by IL-4 and the glucocorticoid budesonide. J. Immunol. 1999;163(10):5624–5632. [PubMed] [Google Scholar]
- 137.Druilhe A, Letuve S, Pretolani M. Glucocorticoid-induced apoptosis in human eosinophils: mechanisms of action. Apoptosis. 2003 Oct;8(5):481–495. doi: 10.1023/a:1025590308147. [DOI] [PubMed] [Google Scholar]
- 138.Fan J, Heller NM, Gorospe M, Atasoy U, Stellato C. The role of post-transcriptional regulation in chemokine gene expression in inflammation and allergy. Eur Respir J. 2005 Nov;26(5):933–947. doi: 10.1183/09031936.05.00120204. [DOI] [PubMed] [Google Scholar]
- 139.Simon HU. Molecules involved in the regulation of eosinophil apoptosis. Chem Immunol Allergy. 2006;91:49–58. doi: 10.1159/000090229. [DOI] [PubMed] [Google Scholar]
- 140.Okada S, Hagan JB, Kato M, et al. Lidocaine and its analogues inhibit IL-5-mediated survival and activation of human eosinophils. J Immunol. 1998;160(8):4010–4017. [PubMed] [Google Scholar]
- 141.Alam R, Forsythe P, Stafford S, Fukuda Y. Transforming growth factor beta abrogates the effects of hematopoietins on eosinophils and induces their apoptosis. J Exp Med. 1994;179(3):1041–1045. doi: 10.1084/jem.179.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Nutku E, Aizawa H, Hudson SA, Bochner BS. Ligation of Siglec-8: a selective mechanism for induction of human eosinophil apoptosis. Blood. 2003 Jun 15;101(12):5014–5020. doi: 10.1182/blood-2002-10-3058. [DOI] [PubMed] [Google Scholar]
- 143.Zhang M, Angata T, Cho JY, Miller M, Broide DH, Varki A. Defining the in vivo function of Siglec-F, a CD33-related Siglec expressed on mouse eosinophils. Blood. 2007;109:4280–4287. doi: 10.1182/blood-2006-08-039255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Matsumoto K, Schleimer RP, Saito H, Iikura Y, Bochner BS. Induction of apoptosis in human eosinophils by anti-fas antibody treatment in vitro. Blood. 1995;86:1437–1443. [PubMed] [Google Scholar]
- 145.Matsumoto K, Terakawa M, Miura K, Fukuda S, Nakajima T, Saito H. Extremely rapid and intense induction of apoptosis in human eosinophils by anti-CD30 antibody treatment in vitro. J Immunol. 2004 Feb 15;172(4):2186–2193. doi: 10.4049/jimmunol.172.4.2186. [DOI] [PubMed] [Google Scholar]
- 146.von Gunten S, Vogel M, Schaub A, et al. Intravenous immunoglobulin preparations contain anti-Siglec-8 autoantibodies. J Allergy Clin Immunol. 2007 Apr;119(4):1005–1011. doi: 10.1016/j.jaci.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 147.Klion AD, Bochner BS, Gleich GJ, et al. Approaches to the treatment of hypereosinophilic syndromes: A workshop summary report. J. Allergy Clin. Immunol. 2006;117:1292–1302. doi: 10.1016/j.jaci.2006.02.042. [DOI] [PubMed] [Google Scholar]
- 148.Cools J, DeAngelo DJ, Gotlib J, et al. A tyrosine kinase created by fusion of the PDGFRA and FIP1L1 genes as a therapeutic target of imatinib in idiopathic hypereosinophilic syndrome. N Engl J Med. 2003 Mar 27;348(13):1201–1214. doi: 10.1056/NEJMoa025217. [DOI] [PubMed] [Google Scholar]
- 149.Munitz A, Bachelet I, Levi-Schaffer F. Reversal of airway inflammation and remodeling in asthma by a bispecific antibody fragment linking CCR3 to CD300a. J Allergy Clin Immunol. 2006 Nov;118(5):1082–1089. doi: 10.1016/j.jaci.2006.07.041. [DOI] [PubMed] [Google Scholar]
- 150.Ackerman SJ, Butterfield JH. Eosinophilia, Eosinophil-Associated Diseases and the Hypereosinophilic Syndromes. In: R. H, Benz EJ, Shattil SJ, et al., editors. Hematology, Basic Principles and Practice. 4th Edition Churchill Livingstone/W.B. Saunders; Philadelphia: 2004. pp. 763–786. [Google Scholar]
- 151.Klion AD, Bochner BS, Gleich GJ, et al. Approaches to the treatment of hypereosinophilic syndromes: a workshop summary report. J Allergy Clin Immunol. 2006 Jun;117(6):1292–1302. doi: 10.1016/j.jaci.2006.02.042. [DOI] [PubMed] [Google Scholar]
- 152.Klion AD, Rothenberg ME, Murray JJ, Singh A, Simon H-U. Safety and Tolerability of Anti-IL-5 Monoclonal Antibody (Mepolizumab) Therapy in Patients with HES: A Multicenter, Randomized, Double-Blind, Placebo-Controlled Trial. Blood (ASH Annual Meeting Abstracts) 2006;108(November):2694. [Google Scholar]
- 153.Yamada Y, Rothenberg ME, Lee AW, et al. The FIP1L1-PDGFRA fusion gene cooperates with IL-5 to induce murine hypereosinophilic syndrome (HES)/chronic eosinophilic leukemia (CEL)-like disease. Blood. 2006 May 15;107(10):4071–4079. doi: 10.1182/blood-2005-08-3153. [DOI] [PMC free article] [PubMed] [Google Scholar]