Abstract
The cloning of the genes encoding cancer antigens has opened new possibilities for the treatment of patients with cancer. In this study, immunodominant peptides from the gp100 melanoma-associated antigen were identified, and a synthetic peptide, designed to increase binding to HLA-A2 molecules, was used as a cancer vaccine to treat patients with metastatic melanoma. On the basis of immunologic assays, 91% of patients could be successfully immunized with this synthetic peptide, and 13 of 31 patients (42%) receiving the peptide vaccine plus IL-2 had objective cancer responses, and four additional patients had mixed or minor responses. Synthetic peptide vaccines based on the genes encoding cancer antigens hold promise for the development of novel cancer immunotherapies.
The identification of the genes encoding melanoma-associated antigens has opened new possibilities for the immunotherapy of patients with cancer1,2. To identify melanoma antigens involved in tumor rejection in humans, we utilized tumor-infiltrating lymphocytes (TILs) grown from metastatic melanoma nodules and adoptively transferred to the autologous melanoma patient3,4. Those TILs associated with in vivo tumor regression were used to screen tumor-derived cDNA libraries to identify the antigens they recognized. Two antigens, MART-1 and gp100, whose recognition was restricted by HLA-A2*0201 (HLA-A2) were initially identified, and both were shown to be nonmutated differentiation antigens expressed by cells of melanocytic lineage including melanomas, normal melanocytes and pigmented retinal cells, but not in other normal tissues or non-melanoma tumors5,6. By screening large numbers of peptides from these two molecules conforming to known HLA-A2 binding motifs, a single, immunodominant, nine amino acid peptide was identified in the MART-1 molecule, and five different epitopes recognized by TILs were identified in the gp100 molecule7,8.
The MART-1 epitope, m27–35, and two gp100 epitopes, g209–217 and g280–288, bound to the HLA-A2 molecule with intermediate affinity and did not have optimal amino acids at one of the known MHC-binding anchor residues. We thus studied a large number of synthetic peptides in which single- and double-amino acid substitutions were introduced at HLA-A2 binding positions9. A modified g209–217 peptide (referred to as g209-2M), in which a methionine replaced the natural threonine at position 2, bound to the HLA-A2 molecule with greater affinity than the unmodified peptide and was shown to have an increased ability to generate melanoma reactive cytotoxic T lymphocytes (CTLs) in vitro when used for sensitization of peripheral blood mononuclear cells (PBMCs) from patients by using techniques previously described10–14. Because these in vitro studies suggested a relation between immunogenicity and the MHC binding affinity of the peptide from this nonmutated self protein, we have now conducted a clinical study in which HLA-A2+ patients with metastatic melanoma were immunized with the synthetic peptide, g209-2M. A variety of immunization strategies using peptides have been evaluated in animal models15–24. For the present clinical trial, we selected a method of immunization involving emulsification of peptide in incomplete Freund's adjuvant (IFA) injected every 3 weeks, because of the success of this schedule in the induction of cell-mediated immune responses in mice16. In our previous trials using unmodified peptides, we saw no evidence of a dose response between 0.1 mg and 10 mg, and we therefore selected the intermediate dose of 1 mg for the current trial13,14.
Few previous studies of the immunization of melanoma patients with melanoma peptides have been performed. Hu et al. treated patients with a MAGE-1 peptide pulsed onto antigen-presenting cells and presented evidence of immunization in lymphocytes obtained from tumor nodules or at the vaccination site, although it required three in vitro restimulations to detect this response25. Jaeger et al. injected nine melanoma patients with a combination of peptides derived from the MART-1, tyrosinase, and gp100 proteins injected intradermally weekly for 4 weeks26,27. Following vaccination, in vitro evidence of immunization against peptides could be detected in the PBMCs of three of six patients against the MART-1 peptide, two of six patients against the tyrosinase peptide and in none of six patients receiving the gp100 peptide. An initial in vitro stimulation and one restimulation with peptide were required for detecting these activities. Marchand et al. injected a MAGE-3 peptide in 14 patients with melanoma, and although two showed a partial tumor regression, lymphocytes reactive with the peptide could not be detected in these patients after immunization28. We previously immunized 23 patients with an immunodominant peptide from the MART-1 antigen and could detect an increase in antipeptide precursors in PBMCs, but three repetitive in vitro stimulations were required for detecting this reactivity13.
As shown in the present clinical study of patients immunized with the modified g209-2M peptide, PBMCs obtained from these patients after, but not before, immunization exhibited a high degree of reactivity against the native g209–217 peptide, as well as against HLA-A2* melanoma cells. The administration of the g209-2M peptide, along with adjuvant IL-2, mediated tumor regression in 42% of patients with metastatic melanoma.
Clinical characteristics of the patients
Nine patients received the g209–218 peptide in IFA, 11 received the g209-2M peptide in IFA, and 31 received the g209-2M peptide in IFA plus systemic IL-2. Eighty-three percent of patients were between the ages of 31 and 60, and all had good performance status (ECOG 0 or 1). All patients had extensive metastatic disease, and many were heavily pretreated. All had undergone prior surgery, and many had received prior chemotherapy, radiotherapy or immunotherapy sometimes including IL-2; 82% of patients had received two or more different treatments.
Specificity of the immunologic assay
The assessment of immune reactivity of PBMCs was based on the ability to generate specific antipeptide and antitumor reactivity following in vitro exposure of PBMCs to the immunizing peptide (see Methods). The in vitro sensitization assay was highly specific. As seen in a characteristic experiment shown in Table 1, PBMCs before immunization exhibited no in vitro sensitization against peptide after 11 days or incubation with the g209–217 or the g209-2M peptide, but developed specific anti-influenza reactivity after incubation with the control flu peptide. However, post-immunization PBMCs obtained from the patient following two injections of the g209-2M peptide in IFA could be specifically sensitized in vitro to the native g209–217 peptide as well as to the flu peptide. Reactivity of these in vitro sensitized cultures was also seen against two HLA-A2+ melanomas (501-mel and SK23-mel), but not against two HLA-A2− melanomas (624.28-mel and 888-mel).
Table 1.
Specificity of reactivity against g209–217 peptide and HLA-A2+ melanomas
| No. of immunizations | In vitro sensitization with peptide* | Stimulator (pg IFN-γ/ml) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| T2 | T2(280) | T2(flu) | T2(209) | 501 mel (A2+) | SK23 mel (A2+) | 624.28 mel (A2−) | 888 mel (A2−) | ||
| None | 209-2M | 135 | 102 | 230 | 146 | 195 | 180 | 89 | 115 |
| 209 | 118 | 90 | 238 | 84 | 148 | 231 | 162 | 261 | |
| flu | 178 | 123 | 35,570† | 124 | 290 | 284 | 172 | 282 | |
| 2 | 209-2M | 86 | 74 | 61 | 24,150 | 72,780 | 43,250 | 124 | 33 |
| 209 | 86 | 62 | 165 | 9,890 | 25,710 | 19,480 | 104 | 98 | |
| flu | 121 | 118 | 36,460 | 132 | 313 | 448 | 85 | 362 | |
Day 11 after culture with 1 µg/ml peptide; PBMCs were tested for IFN-γ release after culture with tumor or T2 cells pulsed with peptide.
In this and subsequent tables values greater that 100 pg IFN-γ/ml and at least twice that of all controls are underlined.
Immunization with the g209–217 peptide in IFA
The reactivity of PBMCs from eight of the nine patients immunized with the native g209–217 peptide in IFA before and after two immunizations is shown in Table 2 (sufficient lymphocytes were not obtained from patient 5). Only two of eight patients showed reproducible evidence of immunization to the native g209–217 peptide (patients 4 and 6), and one of these patients (patient 6) exhibited a low level of immune reactivity against the g209–217 peptide before immunization.
Table 2.
Reactivity of PBMCs from patients immunized with g209–217 peptide in IFA
| Before immunization | After immunization | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Assay stimulator*(pg IFN-γ/ml) | |||||||||
| Patient | Expt.† | T2 | T2(280) | T2(209) | T2-(209-2M) | T2 | T2(280) | T2(209) | T2(209-2M) |
| 1 | 1 | 85 | 94 | 77 | 82 | 61 | 68 | 69 | 89 |
| 2 | 36 | 30 | 47 | 43 | 22 | 36 | 152 | 72 | |
| 3 | 128 | 142 | 88 | ND | 52 | 45 | 72 | ND | |
| 2 | 1 | 264 | 234 | 221 | 276 | 514 | 362 | 341 | 381 |
| 2 | 246 | 167 | 179 | ND | 113 | 83 | 61 | ND | |
| 3 | 1 | 291 | 253 | 273 | 362 | 107 | 113 | 97 | 128 |
| 2 | 49 | 33 | 41 | ND | 51 | 35 | 138 | ND | |
| 4 | 1 | 60 | 52 | 74 | 46 | 114 | 90 | 283 | 414 |
| 2 | 74 | 74 | 56 | ND | 76 | 143 | 814 | ND | |
| 6 | 1 | 69 | 45 | 392 | 516 | 152 | 150 | 4355 | 5282 |
| 2 | 15 | 19 | 931 | ND | 26 | 21 | 4379 | ND | |
| 7 | 1 | 15 | 13 | 11 | ND | 91 | 56 | 43 | ND |
| 8 | 1 | 85 | 69 | 78 | ND | 84 | 97 | 91 | ND |
| 9 | 1 | 126 | 122 | 134 | 151 | 105 | 103 | 142 | 176 |
| 2 | 67 | 85 | 104 | ND | 46 | 68 | 38 | ND | |
PBMCs incubated with g209-2M peptide for 11 to 13 days before assay against T2 cells alone or pulsed with 1 µg/ml peptide.
All patients tested after two immunizations. Patient 5 not tested because insufficient PBMCs were available.
Immunization with the modified 209-2M peptide in IFA
Peripheral blood mononuclear cells from all 11 patients before immunization with the modified 209-2M peptide in IFA failed to show reactivity to peptide-pulsed T2 cells (Table 3). However, following immunization with the modified 209-2M peptide in IFA, 10 of 11 patients showed a consistently high level of immunization against the native g209–217 peptide, but not against the control g280–288 peptide. All patients were tested after two immunizations except patient 1 who was tested after one immunization. Only patient 3 consistently failed to show any evidence of reactivity following immunization. Multiple independent experiments were performed on separate aliquots of PBMCs, and the results of replicate consecutive experiments on these 11 patients are shown in Table 3.
Table 3.
Reactivity of PBMCs from patients immunized with 209-2M peptide in IFA
| Before immunization | After immunization* | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Assay stimulator†(pg IFN-γ/ml) | |||||||||
| Patient | Expt. | T2 | T2(280) | T2(209) | T2-(209-2M) | T2 | T2(280) | T2(209) | T2(209-2M) |
| 1 | 1 | 21 | 22 | 12 | 20 | 42 | 37 | 6,897 | 57,060 |
| 2 | 1 | 58 | 56 | 66 | 50 | 54 | 48 | 1,851 | 4,012 |
| 2 | 1 | 4 | 2 | ND‡ | 30 | 22 | >1,000 | ND | |
| 3 | 1 | 33 | 26 | 33 | 35 | 46 | 53 | 56 | 54 |
| 2 | 145 | 127 | 124 | ND | 49 | 36 | 46 | ND | |
| 4 | 1 | 133 | 123 | 184 | 213 | 40 | 35 | 2,631 | 6,086 |
| 2 | 50 | 36 | 41 | 43 | 45 | 52 | 1,618 | 3,751 | |
| 3 | 351 | 299 | 501 | 470 | 86 | 825 | 944 | 1,057 | |
| 5 | 1 | 28 | 30 | 21 | 24 | 41 | 35 | 4,366 | 6,402 |
| 2 | 154 | 156 | 152 | 142 | 128 | 129 | 295 | 323 | |
| 3 | 29 | 18 | 37 | 32 | 27 | 22 | 856 | 1,126 | |
| 6 | 1 | 38 | 28 | 24 | 31 | 44 | 47 | 152 | 671 |
| 2 | 154 | 166 | 210 | 153 | 128 | 72 | 662 | 887 | |
| 3 | 96 | 61 | 117 | 127 | 22 | 14 | 2,374 | 5,407 | |
| 7 | 1 | 44 | 66 | 72 | 82 | 104 | 81 | 4,424 | 5,411 |
| 2 | 10 | 8 | 13 | 16 | 197 | 224 | 1,293 | 1,583 | |
| 3 | 127 | 105 | ND | 120 | 61 | 67 | 1,244 | ND | |
| 8 | 1 | ND | ND | ND | ND | 17 | 25 | 845 | ND |
| 2 | 1553 | 562 | 719 | ND | 79 | 78 | 2,326 | ND | |
| 3 | ND | ND | ND | ND | 43 | ND | 1,768 | ND | |
| 9 | 1 | 345 | 337 | 355 | ND | 209 | 183 | 2,253 | ND |
| 2 | 13 | 13 | 10 | ND | 229 | 262 | 1,550 | ND | |
| 3 | 1434 | 816 | 513 | ND | 495 | 517 | 2,408 | ND | |
| 10 | 1 | 247 | 283 | 413 | ND | 29 | 39 | 1,271 | ND |
| 2 | 135 | 102 | 146 | ND | 86 | 74 | 24,150 | ND | |
| 3 | 117 | 147 | 150 | ND | 6 | 9 | 39,690 | ND | |
| 11 | 1 | 53 | 53 | 56 | ND | 65 | 71 | 154 | ND |
| 2 | 46 | 50 | 47 | ND | 29 | 39 | 63 | ND | |
| 3 | ND | ND | ND | ND | 87 | 83 | 205 | ND | |
Patient 1 after one immunization; all other patients after two immunizations.
Day 11 to 13 after culture with 209-2M peptide, PBMCs were tested fro IFN-γ release following 24-h incubation with peptide-pulsed T2 cells.
Not done.
To test the reactivity of PBMCs against tumor cells, PBMCs obtained from patients before and after immunization with g209-2M in IFA were sensitized in vitro against the g209-2M peptide and tested against T2 cells pulsed with the native peptides, as well as against HLA-A2+ and HLA-A2− melanoma cells (Table 4). In this representative experiment, all five patients showed significant post immunization reactivity against 1 µof the native g209–217 peptide pulsed onto T2 cells. Post-immunization PBMCs from four of the five patients exhibited reactivity against T2 cells pulsed with 10−2 µM native peptide, and one patient recognized T2 cells pulsed with 10−4 µM peptide. Post-immunization PBMCs from four of the patients recognized both HLA-A2+, but not the two HLA-A2− melanomas. The recognition of peptide-pulsed T2 cells as determined by the level of specific interferon-γ (IFN-γ) secretion correlated directly with the recognition of melanoma cells in 41 independent cultures from multiple patients (P < 0.001). Thus, cultures reactive with the native peptide also were capable of reacting with HLA-A2+ melanoma lines, although reactivity against the melanomas was often less than that against the T2 cells pulsed with peptide. Current studies of in vivo and in vitro immunization are aimed at generating T-cell receptors with the high affinity required to react strongly with the low concentrations of peptide present on melanomas.
Table 4.
Reactivity against tumor cells of PBMCs from patients before and after immunization with 209-2M peptide in IFA
| Patient | No. of Immunizations | Stimulator*(pg IFN-γ/ml) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| T2(280) | T2(209) | T2(A2+) | 501-mel (A2+) | SK23-mel (A2−) | 888-mel (A2−) | 624.28-mel | |||
| 7 | 0 | 169 | 175 | 220 | 28 | 72 | 84 | 51 | |
| 2 | 209 | 243 | 2,555 | 1,211 | 2,037 | 98 | 60 | ||
| 8 | 0 | 528 | 691 | 729 | 70 | 640 | 933 | 806 | |
| 4 | 202 | 284 | 13,600 | 11,580 | 14,720 | 408 | 489 | ||
| 9 | 0 | 13 | 13 | 10 | ND | ND | ND | ND | |
| 4 | 229 | 590 | 3,987 | 676 | 889 | 291 | 235 | ||
| 10 | 0 | 117 | 147 | 150 | 19 | 90 | 39 | 42 | |
| 4 | 15 | 18 | 24,040 | 23,860 | 21,580 | 2 | 4 | ||
| 11 | 0 | 46 | 50 | 47 | 11 | 39 | 14 | 17 | |
| 4 | 29 | 30 | 106 | 5 | 43 | 4 | 10 | ||
PBMCs incubated with g209-2M peptide for 13 days and then tested for reactivity to tumors and to T2 cells pulsed with 1 µM of the g209–217 peptide on the central g280–288 peptide.
† Not done
Although we had previously attempted to measure precursor frequencies against the immunodominant peptides using PBMCs obtained before immunization, we were never able to do so successfully because of the very low frequency of reactive cells. Limiting dilution assays were thus performed on pre- and post-immunization PBMCs from patients 2, 5 and 7, who received two, two and four immunizations with the g209-2M peptide, respectively. Preimmunization precursor frequencies against the native g209–217 peptide were less than 1/30,000, whereas, after immunization with g209-2M peptide, the precursor frequency against the native peptide was 1/5,900, 1/2,800 and 1/3,300, respectively. These experiments thus demonstrated that patients immunized with the g209-2M synthetic peptide in IFA consistently developed high levels of circulating immune precursors reactive with the native g209–217 peptide and with tumor.
Immunization with the 209-2M peptide in IFA plus interleukin-2
Nineteen patients with metastatic melanoma received the g209-2M peptide in IFA followed either the next day (14 patients) or beginning 5 days later (5 patients) with a single cycle of 720,000 IU/kg IL-2 intravenously every 8 hours to tolerance. After two peptide immunizations plus IL-2, only 3 of these 19 patients (16%) developed immune reactivity in circulating PBMCs against the g209–217 peptide, as measured in the cytokine-release assay compared with 10 of 11 (91%) patients who received 209-2M peptide in IFA without IL-2 (P < 0.0001).
Twelve additional patients received g209-2M peptide in IFA alone [or as part of other studies with granulocyte-macrophage colony-stimulating factor (GM-CSF) or IL-12] for at least two cycles before moving to therapy with the g209-2M peptide in IFA plus IL-2. Precursors in these patients that developed as a result of their initial immunization with peptide without IL-2 continued to be detected in PBMCs following the administration of peptide plus IL-2 (data not shown).
Clinical response in patients receiving the peptide vaccines
One of nine patients who received the g209–217 peptide in IFA experienced an objective cancer regression that lasted 4 months. None of the 11 patients who received the g209-2M peptide in IFA experienced an objective cancer regression, although 3 patients exhibited mixed responses with complete or partial regression of some lesions.
Of the 19 patients that received the g209-2M peptide in IFA concomitantly with IL-2,8 patients (42%) exhibited an objective cancer regression. Three additional patients experienced mixed responses, and three others experienced stable disease, two of whom subsequently underwent complete resection of their metastatic sites. Of the 12 additional patients who received at least two cycles of g209-2M peptide in IFA without IL-2 followed subsequently by g209-2M peptide in IFA plus IL-2, we saw that 5 (42%) experienced objective cancer regression and one additional patient had a mixed response. Objective regression was seen of metastases in the brain, lung, liver, lymph nodes, muscle, skin and subcutaneous tissues. Details of the patients who experienced clinical responses are shown in Table 5 and Fig. 1.
Table 5.
Characteristics of patient exhibiting an objective response
| Patient | Age (yr)/Sex | Site of tumor | Type | Response Duration (mo) |
|---|---|---|---|---|
| a | 48/M | Lung | CR | 6 |
| b | 51/M | Lung | PR | 6 |
| Subcutaneous | ||||
| c | 44/M | Lymph node | PR | 2+ |
| Lung | ||||
| Subcutaneous | ||||
| Cutaneous | ||||
| d | 45/F | Lymph node | PR | 4 |
| Bone | ||||
| Subcutaneous | ||||
| e | 42/F | Cutaneous | PR | 7+ |
| Subcutaneous | ||||
| Liver | ||||
| f | 41/F | Lung | PR | 5+ |
| g | 39/M | Lung | PR | 6 |
| h | 22/F | Lung hilum | PR | 5 |
| i | 48/M | Cutaneous | PR | 6 |
| j | 43/M | Subcutaneous | PR | 5+ |
| k | 42/F | Lung | PR | 2 |
| Lymph node | ||||
| Liver | ||||
| Brain | ||||
| l | 47/F | Lung | PR | 5+ |
| Lymph node | ||||
| m | 59/M | Subcutaneous | PR | 3+ |
Fig. 1.
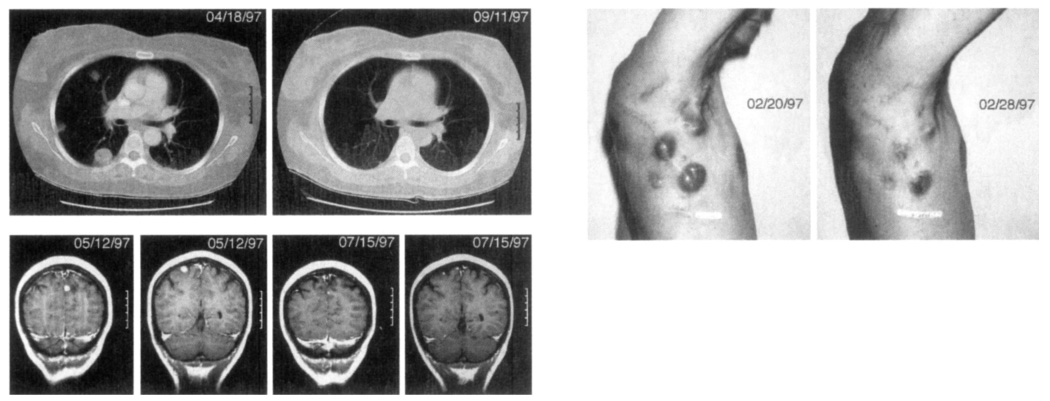
Cancer regression in patients receiving immunization with 209-2M peptide in IFA plus IL-2. top left, Regression of multiple lung metastases in a 47-year-old female (patient I in Table 5). bottom left, Regression of two brain metastases in a 42-year-old female (patient k). This patient had four brain metastases all of which underwent significant regression, above, Regression of multiple cutaneous and subcutaneous metastases in a 44-year-old male (patient c). Significant regression was seen by 8 days after starting treatment.
Except for mild, transient erythema and induration at the injection site seen in some patients, there were no side effects associated with peptide injection in IFA, and all were treated as outpatients. One of the 31 patients receiving peptide plus IL-2 died of aspiration pneumonia in the midst of repeat treatment after attaining an ongoing partial response. Other patients treated with IL-2 experienced the usual side effects associated with its administration and returned to baseline within days after stopping IL-2 administration. Despite the generation of immune responses to the nonmutated "self" sequences, no ocular or aural autoimmune manifestations were seen in any patient.
Thus, in both groups of patients receiving the g209-2M peptide plus IL-2, 42% of patients experienced an objective cancer regression, often of bulky metastatic disease (Table 5, Fig. 1). In our prior experience with the use of this regimen of high-dose bolus IL-2 alone in 134 consecutive patients with melanoma, a response rate of 17% was seen29. In 62 melanoma patients receiving this regimen of high-dose IL-2 at our institution in other studies not involving peptide immunization, but during the same time interval as the study reported here, the response rate was 15%. Although the dangers of comparison with retrospective or simultaneous nonrandomized patient groups are well appreciated, the 42% objective response rate we have seen in patients receiving the g209-2M peptide plus IL-2 is substantially higher than what we or others have seen using IL-2 alone.
Discussion
The development of effective means for immunizing patients against their growing cancer is a major goal of studies in human tumor immunology. A variety of shared tumor-associated antigens present on melanomas represent possible candidates for such immunization1,2. The gp100 antigen was selected for evaluation because of its recognition by TILs, whose adoptive transfer was highly associated with tumor regression in patients with melanoma8. In prior phase I studies, patients were immunized with escalating doses of one of the immunodominant gp100 peptides (g154–162, g209–217, g280–288). The immunologic reactivity of patients before and after immunization was evaluated by in vitro sensitization of PBMCs to the peptide pulsed onto antigen-presenting cells10–14. Patients receiving the g209–217 and g280–288 peptides, but not the g154–162 peptide, developed specific immune precursors, although multiple in vitro sensitizations were necessary to elicit reproducible immune responses14. In in vitro studies, a synthetic modification of the g209–217 peptide (called 209-2M), which exhibited increased binding to HLA-A2 molecules, had an increased ability to generate melanoma-reactive CTLs after multiple stimulation of the PBMCs of HLA-A2+ patients9. Because of these findings, we conducted the current study in which patients were immunized with this modified peptide.
The present study makes two major points. It represents the first time that a self-peptide (in this case a synthetic modification designed to increase MHC binding) has provided a consistent and powerful means of immunizing patients to generate lymphocyte precursors against growing tumor. In addition, immunization with this peptide plus IL-2 appears to provide significantly higher cancer regression rates than those seen with either agent alone.
In the present study, 2 of 8 patients that received the native g209–217 peptide, compared with 10 of 11 patients immunized with the modified g209-2M peptide, developed highly reproducible reactivity against the native g209–217 peptide and against melanoma cells (P = 0.006). Of importance was the ability to detect this reactivity after a single exposure of PBMCs to peptide without the need for any restimulation in vitro. Thus, in contrast to our prior studies with unmodified peptides18–19 and the reported studies mentioned above, the degree of immunization was substantially higher using the g209-2M peptide than had previously been seen. Significant reactivity to the native g209–217 peptide was seen within 4 days after exposure to peptide in vitro and continued for at least 18 days in culture (data not shown).
Further evidence for the strong immunization against the native g209–217 peptide came from studies of the precursor frequencies present in circulating PBMCs. In our prior studies and those of others, it has rarely been possible to measure precursor frequencies against melanoma antigens because of their very low frequency30–32. In the present series, precursor frequencies against the native peptide were less than 1 in 30,000 (the lower limit of detection in this assay) PBMCs before immunization compared with frequencies between 1 in 2800 and 1 in 5900 after immunization. In these patients, therefore, precursor frequencies were of the same magnitude often seen against viral or allogeneic antigens33,34.
The mechanism by which immunization with the g209-2M modified peptide in IFA resulted in high levels of circulating cellular immunity against the native peptide, as well as against melanoma cells, is unclear. The emulsification of peptide in adjuvant is thought to provide prolonged exposure to antigen and to activate nonspecific inflammatory cells and possible recruitment of antigen-presenting cells to the site of immunization. Nonspecific recruitment of helper cells at the sites of vaccination or at regional draining sites may provide the necessary help to stimulate immune reactions. It is known, however, that specifically reactive CD8+ cells can be generated following immunization with MHC class I-restricted peptides in the absence of CD4+ cells22,35. The decrease in circulating precursor cells when IL-2 was administered with peptide may be due to activation of these cells and traffic to the tumor site, and attempts to reisolate these cells from the tumor are in progress.
Despite the induction of high levels of these tumor-reactive cells following immunization with the g209-2M peptide in IFA alone, none of the 11 patients in this study experienced an objective tumor response, although several patients had mixed responses with shrinkage of some lesions. Several possibilities exist to explain this paradox, although it is likely that tumor cells do not contain the appropriate costimulatory or adhesion molecules required to activate the resting precursor cells that circulate in peripheral blood as a result of immunization. Peripheral anergy may thus result from contact with antigen in the absence of costimulation. An additional source of helper function may be required. In experimental systems, provision of helper epitopes, as well as cytokines such as IL-2 or IL-12, have been shown to increase the immunizing capacity of MHC class I-reactive antigens20,21,23,36–38.
Although the 42% response rate seen in the present study using the peptide vaccine plus IL-2 appears higher than the 17% response rate seen in prior studies using IL-2 alone29, randomized trials to evaluate the efficacy of g209-2M peptide immunization plus IL-2 are required. Several modifications to attempt to improve upon these results are in progress. We have recently initiated a clinical trial in which patients with melanoma are immunized simultaneously with four separate peptides (g209-2M, g280-9V, MART-1:27–35 and tyrosinase:369–377) from three different melanoma antigens (gp100, MART-1 and tyrosinase), all recognized by TIL cells associated with tumor rejection. In another trial, PBMCs from patients after immunization with g209-2M in IFA are being activated by antigen and IL-2 in vitro and used for adoptive transfer to the autologous tumor-bearing patient.
This study demonstrates that it is possible, in patients with metastatic cancer, to increase significantly the number of lymphocyte precursors reactive with normal nonmutated differentiation proteins such as gp100 by immunization with synthetic high binding peptides in IFA and to mediate tumor regression. Many cancers such as those that occur in the breast, prostate and ovary express proteins unique to, or overexpressed by, the tissue of origin of the tumor, and these differentiation proteins may also be suitable targets for immunotherapy.
Methods
Peptides
Each of the peptides utilized in this study was prepared under Good Manufacturing Practice (GMP) conditions by Multiple Peptide Systems (San Diego, CA): g209–217, ITQVPFSV; g209-2M, IMQVPFSV; g280–288, YLEPGPVTA. The identity of each of the peptides was confirmed by mass spectral analysis. The peptides were >98% pure as assessed by high-pressure liquid chromatography analysis and had an endotoxin level less than 0.1 endotoxin units per milliliter. Each of the peptides was supplied as a white powder soluble in water.
Cultured cell lines
The melanoma cell lines 501 -mel (HLA-A2+), 624-38-mel (HLA-A2+), 888-mel (HLA-A2−), 624-28-mel (HLA-A2−) were established in the Surgery Branch, NCI. The melanoma line SK23-mel (HLA-A2+) was supplied by T. Boon at the Ludwig Institute for Cancer Research, Brussels, Belgium. These tumor lines and the T2 cell line (HLA-A2+, TAP-deficient T-B cell hybrid) were maintained in continuous culture in medium consisting of RPMI 1640 supplemented with 10% fetal bovine serum, 100 units/ml penicillin, 100 µg/ml streptomycin and 50 µg/ml gentamycin.
Clinical protocol
All patients had histologically confirmed metastatic melanoma and underwent a complete clinical evaluation including measurements and X-rays of all evaluable tumor sites. All patients were confirmed to be HLA-A*0201+ by using high-resolution nested sequence polymerase chain reaction subtyping, and all signed an informed consent before treatment. No patient had received any treatment in the prior month nor were they receiving any immunosuppressive drugs including steroids. Before treatment, patients underwent a leukapheresis. PBMCs were isolated by Ficoll-Hypaque separation and were cryopreserved at 108 cells/vial and stored at −180 °C.
Three sequential clinical trials were performed. In the first and second trials, either the native g209–217 peptide (9 patients) or the modified g209-2M peptide (11 patients) at 1.5 mg in 1.5 ml was mixed with an equal volume of incomplete Freund's adjuvant (IFA; Montanide ISA-51, Seppic, France) and spun vigorously in a vortex mixer for 12 min to form an emulsion. Two aliquots of 1 ml each were injected into the subcutaneous tissue of the anterior thigh (total peptide injection of 1 mg). All treatments were performed in an outpatient clinic. Patients received from two to six immunizations at 3-week intervals and were leukapheresed to cryopreserve PBMCs 3 weeks after every other immunization, and clinical assessment of tumor status was performed at these times as well.
In the third trial (31 patients), IL-2 (Cetus-Oncology Division, Chiron Corp, Emeryville, CA) at a dose of 720,000 IU/kg (corresponding to 120,000 Cetus units/kg) was administered as an intravenous bolus over 15 min starting either 1 or 5 days after the peptide injection. Recombinant IL-2 was provided as a lyophilized powder and was reconstituted with 1.2 ml of sterile water per vial. Vials also contained 5% mannitol and approximately 120 µg of SDS per mg of IL-2. The IL-2 was diluted in 50 ml of normal saline containing 5% human serum albumin for infusion. Patients received IL-2 every 8 hours until grade 3 or 4 toxicity was reached that could not be easily reversed by standard supportive measures. After two cycles of peptide plus IL-2, occasional cycles involved peptide alone if additional time for recovery from IL-2 side effects were required. IL-2 was routinely administered on a general surgical ward, although some patients were transferred to an intensive care unit for monitoring or for the administration of vasopressors. All patients received concomitant medications, including acetaminophen (650 mg every 4 h), indomethacin (50 mg every 8 h), and ranitidine (150 mg every 12 h), to prevent some of the side effects associated with IL-2 administration.
Evaluation of response to treatment
After two and four peptide injections, all known sites of disease were evaluated. If patients showed evidence of stable or regressing disease, additional treatments were administered and similar criteria were used to decide on subsequent courses of treatment. If patients had progression of disease, no further therapy was administered as part of this protocol.
A response was considered complete if all measurable tumor disappeared. A partial response was defined as a 50% or greater decrease of the sum of the products of the longest perpendicular diameters of all lesions, lasting at least 1 month and without increase of any tumor or the appearance of any new tumor.
In vitro assessment of anti-g209–217 peptide and melanoma-specific reactivity
Cryopreserved PBMCs were thawed into complete medium (CM) consisting of Iscove's modified DMEM with 25 mM HEPES buffer, 10% heat-inactivated human AB serum, 2 mM l-glutamine, 100 units/ml penicillin and 100 µg/ml streptomycin (Biofluids, Rockville, MD; Sigma, St. Louis, MO; Pel-Freez, Brown Deer, WI). Cells were washed once and resuspended at 1.5 × 106 cells/ml in 2 ml containing peptide. Two days later recombinant IL-2 (Chiron Corp.) was added to the cultures. On day 5, CM (1 ml) was withdrawn and replaced with fresh CM containing IL-2. CM (1 ml) was replaced whenever the medium became acidic. To determine the optimal conditions for assessing PBMC reactivity to the immunizing peptides in vitro, experiments were conducted using PBMCs obtained from patients following immunization. A variety of peptide concentrations ranging from 0.01 to 10 µM were evaluated, as were harvest times from 4 to 18 days and IL-2 concentrations from 30 IU to 300 IU per ml in the culture. On the basis of these optimization experiments (data not shown) in vitro assays were conducted using 3 × 106 PBMCs in 2 mls of medium incubated with 1 to 2 µM peptide with 300 IU/ml IL-2 added on day 2 and harvested between days 11 and 13 after initiation of the culture. The harvested cells were washed once in HBSS and 105 cells were added in 0.1 ml to wells of flat-bottom 96-well plates. Stimulator cells consisting of 105 T2 cells pulsed with peptide were added in 0.1 ml. Alternatively, 105 melanoma cells were added as stimulators. To pulse T2 cells with peptide, either 10−4 µM, 10−2 µM or 1 µM peptide was incubated with 6 × 106 T2 cells in 3 ml for 3 h at 37 °C with intermittent mixing. The cells were then washed once with HBSS before addition to the responder cells. The cultures were incubated for 18 to 24 h at 37 °C in 5% CO2. IFN-γ release into the supernatant was measured using a standard ELISA assay.
To determine precursor frequencies, limiting dilution assays were performed by adding varying numbers of lymphocytes to flat-bottom 96-well plates in 24 replicate wells along with 105 irradiated autologous PBMCs and 1 µM g209-2M peptide. IL-2 (300 IU/ml) was added to each well on day 2, and medium was changed on day 5 and whenever the medium became acidic. After 10 to 14 days, the cells were replica plated and stimulated with T2 cells alone or T2 cells pulsed with native g209–217 peptide. After overnight incubation, the supernatant was harvested and tested for IFN-γ release using a standard ELISA assay. The fraction of positive wells (>2 times IFN-γ release compared with wells stimulated with unpulsed T2 cells) was used to calculate precursor frequency using standard Poisson analysis.
References
- 1.Rosenberg SA. The development of new cancer therapies based on the molecular identification of cancer regression antigens. Cancer J. Sci. Am. 1995;1:89–100. [PubMed] [Google Scholar]
- 2.Boon T. Tumor antigens recognized by cytolytic T lymphocytes: Present perspectives for specific immunotherapy. Int. J. Cancer. 1993;54:177–180. doi: 10.1002/ijc.2910540202. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg SA, et al. Use of tumor infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma: Preliminary report. N. Engl. J. Med. 1988;319:1676–1680. doi: 10.1056/NEJM198812223192527. [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg SA, et al. Treatment of patients with metastatic melanoma using autologous tumor-infiltrating lymphocytes and interleukin-2. J. Natl. Cancer Inst. 1994;86:1159–1166. doi: 10.1093/jnci/86.15.1159. [DOI] [PubMed] [Google Scholar]
- 5.Kawakami Y, et al. Identification of a human melanoma antigen recognized by tumor infiltrating lymphocytes associated with in vivo tumor rejection. Proc. Natl. Acad. Sci. USA. 1994;91:6458–6462. doi: 10.1073/pnas.91.14.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawakami Y, et al. Cloning of the gene coding for a shared human melanoma antigen recognized by autologous T cells infiltrating into tumor. Proc. Natl. Acad. Sci. USA. 1994;91:3515–3519. doi: 10.1073/pnas.91.9.3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawakami Y, et al. Identification of the immunodominant peptides of the MART-1 human melanoma antigen recognized by the majority of HLA-A2 restricted tumor infiltrating lymphocytes. J. Exp. Med. 1994;180:347–352. doi: 10.1084/jem.180.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawakami Y, et al. Recognition of multiple epitopes in the human melanoma antigen gp100 by tumor infiltrating T-lymphocytes associated with in vivo tumor regression. J. Immunol. 1995;154:3461–3968. [PubMed] [Google Scholar]
- 9.Parkhurst MR, et al. Improved induction of melanoma reactive CTLs with peptides from the melanoma antigen gp100 modified at HLA-A*0210 binding residues. J. Immunol. 1996;157:2537–2548. [PubMed] [Google Scholar]
- 10.Rivoltini L, et al. Induction of tumor-reactive CTLs from peripheral blood and tumor-infiltrating lymphocytes of melanoma patients by in vitro stimulation with an immunodominant peptide of the human melanoma antigen MART-1. J. Immunol. 1995;54:2257–2265. [PubMed] [Google Scholar]
- 11.Salgaller ML, et al. Recognition of multiple epitopes in the human melanoma antigen gp100 by peripheral blood lymphocytes stimulated in vitro with synthetic peptides. Cancer Res. 1995;55:4972–4979. [PubMed] [Google Scholar]
- 12.Marincola FM, Rivoltini L, Salgaller ML, Player M, Rosenberg SA. Differential anti-MART-1/MelanA CTL activity in peripheral blood of HLA-A2 melanoma patients in comparison to healthy donors: Evidence for in vivo priming by tumor cells. J. Immunother. 1996;19:266–277. doi: 10.1097/00002371-199607000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Cormier JN, et al. Enhancement of cellular immunity in melanoma patients immunized with a peptide from MART-1/Melan A. Cancer J. Sci. Am. USA. 1996;3:37–44. [PMC free article] [PubMed] [Google Scholar]
- 14.Salgaller ML, Marincola FM, Cormier JN, Rosenberg SA. Immunization against epitopes in the human melanoma antigen gp100 following patient immunization with synthetic peptides. Cancer Res. 1996;56:4749–4757. [PubMed] [Google Scholar]
- 15.Aichele P, Hengartner H, Zinkergnagel RM, Schulz M. Antiviral cytotoxic T cell response induced by in vivo priming with a free synthetic peptide. J. Exp. Med. 1990;171:815–1820. doi: 10.1084/jem.171.5.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kast WM, et al. Protection against lethal sendai virus infection by in vivo priming of virus-specific cytotoxic T lymphocytes with a free synthetic peptide. Proc. Natl. Acad. Sci. USA. 1991;88:2283–2287. doi: 10.1073/pnas.88.6.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Celluzzi CM, Mayorodomo JI, Storkus WJ, Lotze MT, Falo LD. Peptide-pulsed dendritic cells induced antigen-specific, CTL-mediated protective tumor immunity. J. Exp. Med. 1996;183:283–287. doi: 10.1084/jem.183.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mandelboim O, et al. CTL induction by a tumour-associated antigen octapeptide derived from a murine lung carcinoma. Nature. 1994;369:67–71. doi: 10.1038/369067a0. [DOI] [PubMed] [Google Scholar]
- 19.Deres K, Schild H, Wiesmuller KH, Jung C, Rammensee H-G. In vivo priming of virus-specific cytotoxic T lymphocytes with synthetic lipopetide vaccine. Nature. 1989;342:561–564. doi: 10.1038/342561a0. [DOI] [PubMed] [Google Scholar]
- 20.Lasarte J-J, Sarobe P, Gullon A, Prieto J, Borras-Cuesta F. Induction of cytotoxic T lymphocytes in mice against the principal neutralizing domain of HIV-1 by immunization with an engineered T-cytotoxic-T-Helper synthetic peptide construct. Cell Immunol. 1992;141:211–218. doi: 10.1016/0008-8749(92)90140-k. [DOI] [PubMed] [Google Scholar]
- 21.Shirai M, et al. Helper-cytotoxic T lymphocyte (CTL) determinant linkage required for priming of anti-HIV CD8+ CTL in vivo with peptide vaccine constructs. J. Immunol. 1994;152:549–556. [PubMed] [Google Scholar]
- 22.Minev BR, Restifo NP, McFarland BJ, Spiess PJ, Rosenberg SA. Insertion signal sequence fused to minimal peptides elicits specific CD8+ T-cell responses and prolongs survival of thymoma-bearing mice. Cancer Res. 1994;54:4155–4161. [PMC free article] [PubMed] [Google Scholar]
- 23.Noguchi Y, Richards EC, Chen YT, Old LJ. Influence of interleukin-12 on p53 peptide vaccination against established Meth A sarcoma. Proc. Natl. Acad. Sci. USA. 1995;92:2219–2223. doi: 10.1073/pnas.92.6.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kyburz D, et al. T cell immunity after a viral infection versus T cell tolerance induced by soluble viral peptides. Eur. J. Immunol. 1993;23:1956–1962. doi: 10.1002/eji.1830230834. [DOI] [PubMed] [Google Scholar]
- 25.Hu X, et al. Enhancement of cytolytic T lymphocyte precursor frequency in melanoma patients following immunization with the MAGE-1 peptide loaded antigen presenting cell-based vaccine. Cancer Res. 1996;56:2479–2483. [PubMed] [Google Scholar]
- 26.Jaeger E, et al. Generation of cytotoxic T-cell responses with synthetic melanoma-associated peptides in vivo: Implications for tumor vaccines with melanoma-associated antigens. Int. J. Cancer. 1996;66:162–169. doi: 10.1002/(SICI)1097-0215(19960410)66:2<162::AID-IJC4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 27.Jaeger E, et al. Granulocyte-macrophage-colony-stimulating factor enhances immune responses to melanoma-associated peptides in vivo. Int. J. Cancer. 1996;67:54–62. doi: 10.1002/(SICI)1097-0215(19960703)67:1<54::AID-IJC11>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 28.Marchand M, et al. Tumor regression responses in melanoma patients treated with a peptide encoded by gene MAGE-3 [Letter to the Editor] Int. J. Cancer. 1995;63:883–885. doi: 10.1002/ijc.2910630622. [DOI] [PubMed] [Google Scholar]
- 29.Rosenberg SA, et al. Treatment of 283 consecutive patients with metastatic melanoma or renal cell cancer using high-dose bolus interleukin-2. JAMA. 1994;271:907–913. [PubMed] [Google Scholar]
- 30.Coulie P, et al. Precursor frequency analysis of human cytolytic T lymphocytes directed against autologous melanoma cells. Int. J. Cancer. 1992;50:289–297. doi: 10.1002/ijc.2910500220. [DOI] [PubMed] [Google Scholar]
- 31.Mazzocchi A, et al. Frequency of cytotoxic T lymphocyte precursors (CTLp) interacting with autologous tumor via the T-cell receptor: Limiting dilution analysis of specific CTLp in peripheral blood and tumor-invaded lymph nodes of melanoma patients. Int. J. Cancer. 1994;58:330–339. doi: 10.1002/ijc.2910580304. [DOI] [PubMed] [Google Scholar]
- 32.Herr W, Wolfel T, Heike M, Meyer zun Buschenfelde K-H, Knuth A. Frequency analysis of tumor-reactive cytotoxic T lymphocytes in peripheral blood of a melanoma patient vaccinated with autologous tumor cells. Cancer Immunol. Immunother. 1994;39:93–99. doi: 10.1007/BF01525314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharrock CEM, Kaminski E, Man S. Limiting dilution analysis of human T cells: A useful clinical tool. Immunol. Today. 1990;11:281–286. doi: 10.1016/0167-5699(90)90113-n. [DOI] [PubMed] [Google Scholar]
- 34.Gomez IBA, Gomard E, Levy JP. Limiting-dilution analysis of the HLA restriction of anti-Epstein-Barr virus-specific cytolytic T lymphocytes. Clin. Exp. Immunol. 1991;84:501–507. [PMC free article] [PubMed] [Google Scholar]
- 35.Vasilakos JP, Michael JG. Herpes simplex virus class I-restricted peptide induces cytotoxic T lymphocytes in vivo independent of CD4+ T cells. J. Immunol. 1993;150:2346–2355. [PubMed] [Google Scholar]
- 36.Bronte V, et al. IL-2 enhances the function of recombinant proxvirus- based vaccines in the treatment of established pulmonary metastases. J. Immunol. 1995;154:4282–5292. [PMC free article] [PubMed] [Google Scholar]
- 37.Irvine KR, Roa RB, Rosenberg SA, Restifo NP. Cytokine enhancement of DNA immunization leads to treatment of established pulmonary metastases. J. Immunol. 1996;156:238–245. [PMC free article] [PubMed] [Google Scholar]
- 38.Rao JB, et al. Interleukin-12 is an effective adjuvant to recombinant vaccinia virus based tumor vaccines. J. Immunol. 1996;156:3357–3365. [PMC free article] [PubMed] [Google Scholar]


