Abstract
Purpose
A strain of Salmonella typhimurium (VNP20009), attenuated by chromosomal deletion of the purI and msbB genes, was found to target to tumor and inhibit tumor growth in mice. These findings led to the present phase I study of the intravenous infusion of VNP20009 to patients with metastatic cancer.
Patients and Methods
In cohorts consisting of three to six patients, 24 patients with metastatic melanoma and one patient with metastatic renal cell carcinoma received 30-minute intravenous bolus infusions containing 106 to 109 cfu/m² of VNP20009. Patients were evaluated for dose-related toxicities, selective replication within tumors, and antitumor effects.
Results
The maximum-tolerated dose was 3 × 108 cfu/m². Dose-limiting toxicity was observed in patients receiving 1 × 109 cfu/m², which included thrombocytopenia, anemia, persistent bacteremia, hyperbilirubinemia, diarrhea, vomiting, nausea, elevated alkaline phosphatase, and hypophosphatemia. VNP20009 induced a dose-related increase in the circulation of proinflammatory cytokines, such as interleukin (IL)-1β, tumor necrosis factor alpha, IL-6, and IL-12. Focal tumor colonization was observed in two patients receiving 1 × 109 cfu/m² and in one patient receiving 3 × 108 cfu/m². None of the patients experienced objective tumor regression, including those patients with colonized tumors.
Conclusion
The VNP20009 strain of Salmonella typhimurium can be safely administered to patients, and at the highest tolerated dose, some tumor colonization was observed. No antitumor effects were seen, and additional studies are required to reduce dose-related toxicity and improve tumor localization.
Attenuated Salmonella strains have recently been developed that possess antitumor activity in mice and are capable of both selective amplification within tumors and expression of effector genes encoding therapeutic proteins.1,2 Anecdotal reports of Salmonella bacteria selectively infecting the solid tumors of cancer patients3–8 raised the possibility that these bacteria could be used as a vehicle to target human tumors in vivo.
To increase safety and reduce toxicity, Salmonella typhimurium was attenuated by chromosomal deletion of the purI and msbB genes. The purI deletion created a requirement for an external source of adenine,1 whereas the deletion of the msbB gene reduced the toxicity associated with lipopoly-saccharide (LPS) by preventing the addition of a terminal myristyl group to the lipid A domain.9 The msbB mutation in Salmonella resulted in lower toxicity in mice by reducing the induction of proinflammatory cytokines and nitric oxide synthase.9 The attenuated Salmonella (VNP20009) caused a markedly diminished capacity to induce tumor necrosis factor alpha (TNF-α) in mice10 compared with the parental Salmonella, and these deletions increased the median lethal dose in these animals by 10,000-fold. The purI and msbB deletions of VNP20009 were genetically stable and contained no antibiotic resistance markers.11
Injection of VNP20009 into mice resulted in selective replication in transplanted murine tumors and in a variety of human tumor xenografts. The tumor to normal tissue (liver) ratio was found to be 250 to 25,000 to 1.12 The mechanisms resulting in tumor accumulation are likely a result of a variety of bacterial- and tumor-related factors. Within the tumor, there may be areas of hypoxia that favor the growth of facultative anaerobes or the presence of tumor necrosis that provides additional nutrients, such as purines, required by the organism. In addition, the tumor may provide an immunosuppressive environment that inhibits the clearance of Salmonella. The inhibition of activated neutrophils within the tumor microenvironment by transforming growth factor beta supports the hypothesis that bacterial clearance may be inhibited within solid tumors.13
Although the development of VNP20009 was primarily intended for the delivery of therapeutic proteins to the tumor site, it was found that the unarmed bacteria was capable of inhibiting the growth of tumors in mice.1,2,12 The mechanisms responsible for these antitumor effects may include the production of nonspecific inflammatory mediators, such as TNF-α, which could have a direct effect on tumor vasculature, the consumption of nutrients within the tumor, or the production of toxic proteins that induce tumor apoptosis. Salmonella is able to induce macrophage apoptosis through the translocation of bacterial proteins into the host cell that target Caspase-1 and -2.14 Translocation of bacterial proteins into host cells is mediated by the type III secretion system and may account for some of the antitumor effects observed with VNP20009 in vivo. These results in murine models provided the basis for the phase I study reported here of the intravenous (IV) administration of genetically modified Salmonella typhimurium, VNP20009, to 24 patients with metastatic melanoma and one patient with renal cell carcinoma.
PATIENTS AND METHODS
Clinical Protocol
The clinical protocol was approved by the National Institutes of Health Institutional Review Board and by the Federal Drug Administration. Written informed consent was obtained from all patients. All patients had histologically confirmed metastatic melanoma or renal cell carcinoma and underwent a complete clinical evaluation including standard blood chemistry assays, measurements and X-rays of all assessable tumor sites, cultures of blood, urine, and stool, and baseline serum cytokines. All patients were no longer responsive to conventional treatments and had not received treatment with cytotoxic or biologic agents for 3 weeks before treatment with VNP20009. In addition, at least one patient per dosing cohort had a tumor mass accessible for excisional biopsy or fine-needle aspiration (FNA). Exclusion criteria included patients with permanent artificial implants (eg, prosthetic hips or knees or heart valves), history of valvular disease, severe arteriosclerosis, peripheral vascular disease, arterial aneurysm or arterial or venous malformation, or evidence of cholelithiasis or urolithiasis. Additional exclusion criteria included documented Salmonella infection or vaccination for Salmonella typhi within the last 6 months, any active infection, splenectomy or immunodeficiency, use of corticosteroids or immunosuppressives, life-threatening illness such as chronic obstructive pulmonary disease, unstable angina or end-stage liver or renal disease, or hypersensitivity to quinolones or cephalosporins. The presence of surgical clips was not included as an exclusion criterion.
The trial was a dose-escalation study in which cohorts, consisting of three to six patients, were treated with increasing IV doses of VNP20009. The initial starting dose of 1 ✕ 106 cfu/m² was chosen because it was 1,000 times lower than the dose safely tolerated in mice on a per weight basis (1 ✕ 108 cfu per mouse). The initial dose was escalated in approximately half-log increments until a maximum-tolerated dose (MTD) was determined. The MTD was the highest dose that induced dose-limiting toxicity (DLT) in no more than one patient within a cohort. When DLT was observed in more than one patient of a cohort, dose escalation was terminated and the prior dose was considered the MTD. The National Cancer Institute common toxicity criteria, version 2.0, was used for toxicity grading and adverse reporting (http://ctep.info.nih.gov). DLT was defined as all grade 4 toxicities, most grade 3 toxicities, hypotension (systolic < 80 mm Hg) not correctable within 4 hours, grade 2 fever for more than 6 hours a day that persisted for greater than 3 days, persistent bacteremia for greater than 72 hours, any inflammation induced in the brain or anterior chamber of the eye, or abscess formation not treatable with drainage. Grade 3 toxicities not considered dose-limiting included grade 3 transaminase elevations occurring within the first week and resolving by day 15 or grade 3 constitutional symptoms that persisted for less than 72 hours. Ciprofloxacin or ceftriaxone was given to patients exhibiting any DLT, symptoms of sepsis such as severe dyspnea or confusion in the acute setting, or evidence of progressive disease. All patients received antibiotics when taken off study.
Patients were hospitalized for at least 48 hours after IV administration of VNP20009 and until all toxicity resolved to grade 1 and VNP20009 was no longer detected in blood cultures. Outpatient evaluations were performed at 2 and 4 weeks after discharge. Tumors were biopsied during the first and second week after transfusion by full excision or FNA in appropriate patients. Full tumor restaging was conducted at 4 weeks. Patients meeting the criteria for stable disease or objective response were sometimes offered additional cycles of treatment. An objective clinical response was defined as a 50% or greater decrease in the sum of the products of the greatest perpendicular diameters of all bi-dimensionally measurable lesions lasting at least 1 month and the appearance of no new lesions. Because tumor regression was not observed with VNP20009 therapy, additional cycles were no longer offered after patient 18.
Preparation and Administration of VNP20009
VNP20009 (Vion Pharmaceutical Inc, New Haven, CT) was stored at −80°C in 15% glycerol. Each vial contained 1.2 mL of VNP20009 at greater than 2 ✕ 109 cfu/mL. Vials of VNP20009 were thawed at room temperature for 15 to 20 minutes, and the appropriate amount of VNP20009 (dose [cfu/m²] ✕ body-surface area) was suspended into 100 mL of preservative-free normal saline (0.9%). To obtain an accurate dosing solution of VNP20009, serial dilutions were made in normal saline. The diluted VNP20009 was mixed well to ensure a homogeneous suspension. The dilutions were prepared within 30 minutes of thawing, and the final dosing solution was administered within 1 hour of preparation. From the final prepared bag of VNP20009, a 1-mL sample was removed in a capped syringe and evaluated by the Clinical Microbiology Laboratory for dose verification. Before VNP20009 infusion, patients received 500 mL of D5W with half-normal saline for hydration, and many were premedicated with acetaminophen and indomethacin. VNP20009 was administered as an IV infusion, via an IV catheter, over 30 minutes.
Microbiologic Evaluation
Blood cultures were drawn before treatment and at 15 minutes (during infusion), 1 hour, 4 hours, 12 to 16 hours, 24 hours, 48 hours, 2 weeks, and 1 month after treatment. When Salmonella was isolated from the blood at 24 to 48 hours after infusion, additional blood cultures were drawn until VNP20009 was no longer detected. Urine and stool samples were collected before treatment and 24 hours, 48 hours, 2 weeks, and 1 month after treatment.
Bacteriologic cultures were performed using media and conditions that were optimized for this particular strain of Salmonella (VNP), which grew best at 35°C in air without added CO2. Good growth detectable at 24 hours was obtained on brain-heart infusion (BHI) agars, whereas other agars required 48 hours of incubation.
Blood Cultures
Each blood culture contained 5 mL of blood, in an anaerobic BacTALERT bottle ([BTA], Organon-Teknika, Durham, NC), and 10 mL in an Isolator tube (Wampole Labs, Cranbury, NJ). Isolator concentrate was distributed onto five BHI–sheep blood (BHI-SB) agar plates and incubated at 35°C in air for 4 days. An additional plate inoculated with 100 µL was included for more accurate quantitation of cultures that had very high organism concentrations. The BTA bottles were incubated for 4 days, and, if flagged as positive, they were subcultured to standard culture media as well as to BHI-SB plates, which were incubated at 35°C in air. Gram-negative rods isolated from the Isolator plates and the BTA bottle of each blood culture were identified as described below.
Urine Cultures
One SB agar/MacConkey agar biplate inoculated with a 0.001 calibrated loop of urine was incubated at 35°C in air for 48 hours. All Gram-negative rods, regardless of colony count, were identified.
Stool Cultures
Each stool specimen was plated onto MacConkey agar, xylose-lysine-desoxycholate agar, Hektoen agar, and selenite broth. Agar media were incubated for 48 hours at 35°C in air. Selenite broths were subcultured after overnight incubation onto xylose-lysine-desoxycholate and Hektoen agars, which were incubated for 48 hours. All nonlactose fermenting colonies were screened biochemically for VNP. All Salmonella isolates were typed using Salmonella antisera (groups A through E).
Biopsies or FNAs of Tumors
Biopsies and FNAs with solid particles were disaggregated using 15-mL sterile disposable tissue grinders (Kendall, Mansfield, MA). For FNAs, approximately 20 µL of aspirate was received and resuspended in 1 to 2 mL of normal saline. For excised biopsies, the piece of tumor (less than 1g) was weighed and then disaggregated in 2 mL of saline. A series of 10-fold dilutions (undiluted to 10−6) were prepared from the liquid aspirates or the disaggregated specimens. Colony count plates were set up in duplicate on BHI-SB. Plates were incubated at 35°C in air for 5 days. Colonies were counted and the concentration of organisms per gram of tissue was estimated. The FNA cfu/g calculation was based on 20 µL of aspirate, which weighed approximately 20 mg. Gram-negative rods were identified as VNP, as described below.
Identification and Antibiotic Susceptibility Profile of VNP Strain
Unlike most Salmonella typhimurium, VNP does not produce H2S. Colony morphology can be variable, including smooth, rough, and mucoid colony types. All types agglutinated distinctly with Salmonella group B antisera. Testing of VNP by commercial identification panels yielded identifications of either S paratyphi A/S choleraesuis by NEG Breakpoint Combo Panel (MicroScan; Dade Behring, Inc, West Sacramento, CA) or Salmonella sp. by RapiD 20E or API 20E (Biomerieux Inc, Hazelwood, MO). In vitro antibiotic susceptibility testing using the NEG Breakpoint Combo Panel showed VNP to be susceptible to each group of antibacterial agents deemed as acceptable for testing of Salmonella by the National Committee for Clinical Laboratory Standards. These known biochemical and susceptibility features of VNP, along with agglutination in Salmonella group B antisera, were used to help recognize and identify VNP isolates. All isolates identified by these methods were sent for confirmatory identification as VNP by analysis of the msbB gene deletion by polymerase chain reaction, as previously described by Clairmont et al.11 Other serotypes of Salmonella were identified as Salmonella by the same commercial panels and were serogrouped using Salmonella group-specific antisera (A through E). Non-VNP Salmonella were sent to the Maryland State Health Laboratory for specific serotyping.
Disposal of VNP Strain
Although VNP is an attenuated strain of Salmonella, specimens and culture materials from these patients were sterilized by chemical sterilization, autoclaving, or incineration before disposal.
Cytokine Analysis
Serum samples were analyzed for the presence of interleukin (IL)-1β, TNFα, IL-12, and IL-6 in a standard enzyme-linked immunosorbent assay. Samples that were off-scale were diluted and reanalyzed.
Histopathology
All tumor biopsies (excised and FNA) were evaluated by pathologists in the Clinical Center of the National Institutes of Health for possible changes associated with VNP20009 infection.
Antibody Analysis
An enzyme-linked immunosorbent assay was used to determine the presence of anti-Salmonella antibodies in patient serum. Salmonella-specific antigens were used to capture Salmonella antibodies in serum in microtiter plates. Patient serum was collected and frozen at −20°C and shipped to Specialty Laboratories (Santa Monica, CA) for analysis. Briefly, the Salmonella antigens used consisted of a mixture of LPS and O and H antigens from Salmonella enteritidis (catalog no. L6011; Sigma, St Louis, MO) and Salmonella typhimurium (catalog no. L6511; Sigma). Patient serum was thawed and added to the antigen-containing microtiter plates at various dilutions and allowed to bind. After incubation, alkaline phosphatase–labeled, isotype-specific (immunoglobulin [Ig] G, IgM, and IgA), secondary antibodies were added and allowed to incubate and bind. The plates were washed and incubated with p-nitrophenyl phosphate, and the reaction of p-nitrophenyl phosphate with alkaline phosphatase results in a yellow color that is read at 410 nm. Enzyme immunoassay units were derived by taking the ratio of OD410 of the patient serum to the OD410 of the control serum (IgG: less than five units = not detected, six to 10 units = indeterminate, and more than 10 units = positive; IgM and IgA: less than 10 units = not detected, 10 to 20 units = indeterminate, and more than 20 units = positive).
RESULTS
Patient Clinical Characteristics
Twenty-four patients with metastatic melanoma and one patient with renal cell carcinoma (patient no. 6) received an IV dose of VNP20009. The patients were between the ages of 32 and 72 years (median age, 51 years) and had good performance status (Eastern Cooperative Oncology Group, 0 or 1) (Table 1). All of the patients had prior therapy, including surgery (100%), chemotherapy (60%), radiotherapy (24%), or immunotherapy (100%).
Table 1.
Characteristics of Patients Receiving VNP20009
| No. of Patients (N = 25) | % | |
|---|---|---|
| Sex | ||
| Male | 16 | 64 |
| Female | 9 | 36 |
| Performance status | ||
| 0 | 23 | 92 |
| 1 | 2 | 8 |
| 2 | 0 | |
| 3 | 0 | |
| Prior treatment | ||
| Surgery | 25 | 100 |
| Chemotherapy | 15 | 60 |
| Radiotherapy | 6 | 24 |
| Immunotherapy | 25 | 100 |
Microbiologic Evaluation of Patients
After IV infusion of VNP20009, the quantity of organisms in blood, urine, and stool samples was evaluated. VNP20009 was never isolated from any of the urine or stool samples. Interestingly, in three patients, Salmonella of three different serotypes, which were distinct from S typhimurium, were isolated from stool before treatment. These isolates included serotypes S Enteriditis (patient no. 19), S Montevideo (patient no. 6), and S Orion (patient no. 24). Because the patients demonstrated no signs or symptoms associated with Salmonella infection in the previous 6 months, they were considered chronic carriers and remained on the study.
The blood culture results are listed in Table 2. The starting dose was 1 ✕ 106 cfu/m² and was escalated at approximately half-log increments to 1 ✕ 109 cfu/m² in cohorts of three to six patients. Five of these patients had stable disease after the initial treatment and received a second cycle of VNP20009 at the same dose level. None of the patients treated with VNP20009 showed any evidence of tumor regression. In 24 of 25 patients, VNP20009 was cultured from the blood at 15 minutes after starting the infusion. Overall, as the dose of VNP20009 was escalated, there was a proportional increase in the number of VNP20009 in the blood at 15 minutes. However, significant variation between patients in the same cohort was observed. VNP20009 blood levels decreased rapidly by one hour and the organism was eventually cleared within 12 hours in the majority of the patients in all cohorts except at the highest dose. However, three patients had extended periods of bacteremia. Patient nos. 21 (1 ✕ 109 cfu/m²) and 25 (3 ✕ 108 cfu/m²) had low levels of VNP20009 detectable in the blood for 8 and 7 days, respectively. Both patients eventually required antibiotics for symptomatic relief, and this resulted in the complete clearance of the organism. Patient no. 21 had a focus of infection within a large tumor metastasis located on the chest wall, whereas patient no. 25 had no identifiable source of infection. Patient no. 22 (1 ✕ 109 cfu/m²) also remained bacteremic until day 7 but did not have an apparent source of infection and did not require antibiotics for complete clearance of the organism. In addition, patient nos. 15 (1 ✕ 108 cfu/m²) and 16 (3 ✕ 108 cfu/m²) efficiently cleared VNP20009 from their blood by 12 hours but later demonstrated positive blood cultures at 96 and 48 hours, respectively. No source of infection was identified in either of these patients.
Table 2.
Blood Cultures After the Administration of VNP20009
| Blood Collection Time (hours) (cfu/mL)† |
||||||||
|---|---|---|---|---|---|---|---|---|
| Dose (cfu/m²) | Patient No.* | 25 Minutes | 1 Hour | 4 Hours | 12 Hours | 24 Hours | 48 Hours | > 48 Hours |
| 1 × 106 | 1 | 0 | +b | 0 | 0 | 0 | 0 | ND |
| 2 | 16 | 0 | 0 | 0 | 0 | 0 | ND | |
| 3 | 35 | 0.2 | 0 | 0 | 0 | 0 | ND | |
| 3 × 106 | 4 | 40 | 5 | 0 | 0 | 0.6 | 0 | 0 (72 hours) |
| 5(1) | 7 | 0.1 | 0 | 0 | 0 | 0 | ND | |
| 5(2) | 10 | 0.4 | 0 | 0 | 0 | 0 | ND | |
| 6 | 11 | 0.2 | 0 | 0 | 0 | 0 | ND | |
| 1 × 107 | 7(1) | 39 | 0.2 | 0 | 0 | 0 | 0 | ND |
| 7(2) | 170 | 1 | 0 | 0 | 0 | 0 | ND | |
| 8 | 27 | 0.1 | 0 | 0 | 0 | 0 | ND | |
| 9(1) | 201 | 5 | 0.1 | 0 | 0 | 0 | ND | |
| 9(2) | 2,030 | 2 | +b | 0 | 0 | 0 | ND | |
| 3 × 107 | 10 | 22 | 2 | +b | 0 | 0 | 0 | ND |
| 11 | 162 | 17 | +b | 0 | 0 | 0 | ND | |
| 12 | 254 | 8 | 0.1 | 0 | 0 | 0 | ND | |
| 1 × 108 | 13(1) | 76,000 | 32 | 2 | 0 | 0.2 | 0 | 0 (72 hours) |
| 13(2) | 20,100 | 5 | 0 | 0 | 0 | 0 | ND | |
| 14 | 66,500 | +b | 0 | 0 | 0 | 0 | ND | |
| 15 | 14,700 | 2 | 0 | 0 | 0 | 0 | 0.3 (96 hours) | |
| 3 × 108 | 16 | 117,000 | 32 | +b | 0 | 0 | 0.3 | ND |
| 17 | 3,390 | 31 | 0 | 0 | 0 | 0 | ND | |
| 18(1) | 19,400 | 15 | 0.1 | 0 | 0 | 0 | ND | |
| 18(2) | 38 | 15 | 0.1 | 0 | 0 | 0 | ND | |
| 23 | 26,000 | 15 | +b | 0 | 0 | 0 | ND | |
| 24 | 200,000 | 1 | +b | 0 | 0 | 0 | ND | |
| 25 | 26,300 | 2.4 | 0.3 | 0.2 | 0.2 | +b | 0.2 (72 hours) | |
| 0.1 (168 hours) | ||||||||
| 1 × 109 | 19 | 18,700 | 4 | 0.1 | 0 | 0 | 0 | ND |
| 20 | 56,000 | 0.3 | +b | 33 | 0 | 0 | ND | |
| 21 | 12,100 | 0.2 | 0 | 0.1 | 0.4 | 0 | 12 (72 hours) | |
| 13 (96 hours) | ||||||||
| +b (192 hours) | ||||||||
| 22 | 104,000 | 134.7 | +b | 0.2 | 0.5 | 0.2 | 0.7 (72 hours) | |
| 0.2 (96 hours) | ||||||||
| 0.4 (120 hours) | ||||||||
| +b (168 hours) | ||||||||
Abbreviation: ND, not determined.
Number in parentheses indicates the patient's treatment cycle.
+b indicates isolation of VNP20009 by anaerobic Bact/Alert Blood culture system only.
Assessment of Tumor Colonization
Metastatic tumors were assessed for the presence of VNP20009 by FNA and/or excised biopsies. In addition, every FNA and excised biopsy was evaluated for histologic changes that may be associated with VNP20009 infection. Most biopsies were obtained during the first or second week after infusion. From the first five cohorts (1 ✕ 106 cfu/m² to 1 ✕ 108 cfu/m²), a total of three excised biopsies and 20 FNAs were completed on 11 of 15 patients (Table 3). The patient with renal cell carcinoma (patient no. 6) was not biopsied. None of these samples contained viable VNP20009 or exhibited histologic changes associated with bacterial colonization. However, patient no. 24 (3 ✕ 108 cfu/m²) and patient nos. 19 and 21 (1 ✕ 109 cfu/m²) had detectable levels of VNP20009 within metastatic lesions.
Table 3.
Tumor Biopsy Cultures After the Administration of VNP20009
| Tumor Biopsy |
||||
|---|---|---|---|---|
| Dose (cfu/m²) | Patient No.* | Type* | Day | cfu/g |
| 1 × 106 | 1 | FNA, excised | 5, 14 | 0 |
| 2 | FNA | 13 | 0 | |
| 3 | FNA | 2, 15, 35 | 0 | |
| 3 × 106 | 4 | FNA | 3, 14, 30 | 0 |
| 1 × 107 | 7(1) | FNA | 3 | 0 |
| 8 | FNA | 3, 15 | 0 | |
| 3 × 107 | 10 | FNA | 2, 16 | 0 |
| 12 | FNA | 2, 13, 18 | 0 | |
| 1 × 108 | 13(2) | FNA | 2, 16 | 0 |
| 14 | FNA, excised | 4 | 0 | |
| 15 | Excised, FNA | 6, 15 | 0 | |
| 3 × 108 | 23 | FNA | 3 | 0 |
| 24 | FNA (rt lower leg) | 4 | 0 | |
| Excised (rt lower leg) | 4 | 11,000 | ||
| FNA (rt upper leg) | 4 | 0 | ||
| 25 | FNA (liver) | 5 | 0 | |
| FNA (liver) | 9 | 0 | ||
| 1 × 109 | 19 | FNA | 2 | 100 |
| FNA | 15, 25 | 0 | ||
| 20 | FNA | 3, 14 | 0 | |
| 21 | FNA (ant chest) | 4 | 6.4 × 105 | |
| FNA (ant chest) | 6 | 8.7 × 108 | ||
| FNA (ant chest) | 8 | 7.0 × 109 | ||
| FNA (lat chest) | 4 | 0 | ||
| FNA (lat chest) | 6 | 0 | ||
| FNA (lat chest) | 8 | 36 | ||
| 22 | FNA | 2, 7 | 0 | |
Abbreviations: rt, right; ant, anterior; lat, lateral.
Number in parentheses indicates the patient's treatment cycle.
From patient no. 24, a small (0.9 cm ✕ 0.9 cm ✕ 0.81 cm), subcutaneous, metastatic lesion from the right lower leg was excised 4 days after infusion and contained approximately 11,000 cfu/g of VNP20009. Interestingly, patient no. 24 recorded the highest concentration of VNP20009 in the blood 15 minutes after infusion (2 ✕ 105 cfu/mL), which quickly cleared and remained negative at the time of the biopsy. To evaluate FNA sensitivity, we performed an FNA and an excisional biopsy on the same lesion (right lower leg). The FNA failed to detect any viable VNP20009, although the excisional biopsy was culture positive (11,000 cfu/g). In addition, an FNA aspirate performed on another metastatic lesion (right upper leg [1 cm ✕ 1 cm ✕ 1 cm]) was also negative. Histologic examination of both FNA aspirates revealed the presence of viable melanoma cells; however, the lesion colonized with VNP20009 (right lower leg) contained an abundant amount of necrotic debris and a rare number of malignant cells. Ten days after infusion, patient no. 24 was febrile and exhibited cellulitis in the region of the excised right lower leg lesion. Tissue cultures contained numerous beta-hemolytic Streptococci group G and a scant amount of VNP20009. Blood cultures were negative and antibiotics were started at that time. Patient no. 24 had no evidence of tumor regression.
Patient no. 19 had an FNA performed on a subcutaneous chest lesion (1.1 cm ✕ 1.0 cm) that revealed approximately 100 cfu/g of viable VNP20009 2 days after infusion. Blood cultures were negative at that time. Subsequent FNAs on days 15 and 25 failed to detect any viable VNP20009. Furthermore, while on the VNP20009 protocol, the chest lesion increased in size and continued to grow when the patient was removed from the study.
Patient no. 21 had a 11.5 cm ✕ 6.2 cm chest wall lesion involving the sternum that contained approximately 6.4 ✕ 105 cfu/g of viable VNP20009 on day 4 after infusion. This aspirate was collected from the anterior region of the tumor. Histologically, the aspirate contained very few malignant cells and abundant necrotic debris. By day 6, the concentration of VNP20009 had reached 8.7 ✕ 108 cfu/g, and the patient remained febrile with positive blood cultures. Sixty-five mL of necrotic debris was aspirated on day 8, which contained 7 ✕ 109 cfu/g of VNP20009, and antibiotics were started. Additional aspirates on day 11, 12, and 14 after infusion revealed 1 ✕ 105, 1.6 ✕ 105, and 1.5 ✕ 105 cfu/g of VNP20009, respectively. The tumor site was negative for VNP20009 on day 28 after infusion. In addition, multiple aspirates were collected from the lateral region of the same chest wall tumor but failed to detect viable VNP20009 until day 8 after infusion and at very low concentrations (36 cfu/g). Also, 700 mL of fluid was aspirated from a pleural effusion on day 14, which was negative for VNP20009. While on the protocol, the chest wall lesion and an axillary metastasis continued to grow, and three new brain metastases were identified. The patient died of progressive cancer approximately 3 months after entering the study.
Additional patients receiving 3 ✕ 108 cfu/m² (patient nos. 23 and 25) and 1 ✕ 109 cfu/m² (patient nos. 20 and 22) did not have detectable VNP20009 from metastatic lesions by FNA. Patient nos. 20 and 23 efficiently cleared the organism from the blood by 24 and 12 hours, respectively. However, as mentioned earlier, patient nos. 22 and 25 had prolonged episodes of low-level bacteremia but metastatic lesions that were negative for Salmonella growth by FNA.
Cytokine Analysis
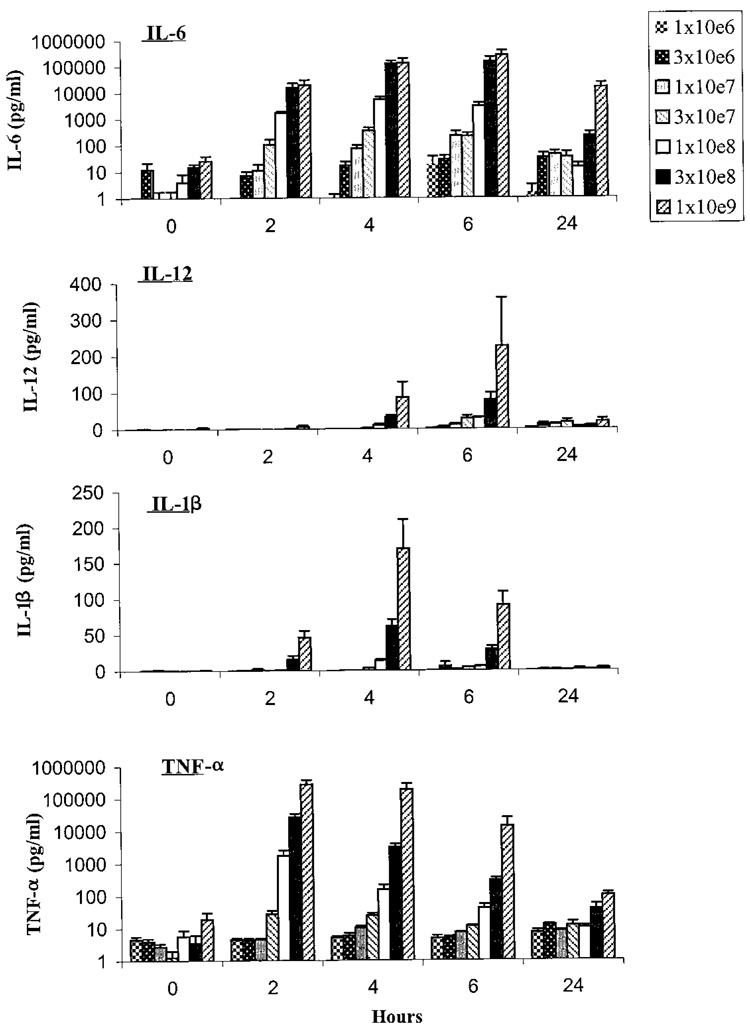
To evaluate the production of proinflammatory cytokines after VNP20009 infusion, serum samples were obtained pretreatment, and at 2, 4, 6, and 24 hours and analyzed for the presence of IL-1β, TNF-α, IL-6, and IL-12 (Fig 1). Significant quantities of IL-1β and TNF-α were initially detected in the serum after infusing 1 ✕ 108 cfu/m² of VNP20009. These levels increased as the dose was escalated. The peak serum concentrations of TNF-α and IL-1β were observed at 2 to 4 hours after infusion, with a mean concentration of 272,008 pg/mL of TNF-α and 169 pg/mL of IL-1β from patients receiving 1 ✕ 109 cfu/m². In addition, IL-6 and IL-12 were initially detected at 1 ✕ 107 cfu/m² and peaked at 6 hours after infusion to 654,501 pg/mL of IL-6 and 225 pg/mL of IL-12 from patients receiving the highest dose of VNP20009.
Fig 1.
Serum cytokine concentrations were measured after the IV injection of VNP20009 at the doses specified. Serum was isolated from peripheral blood at the times indicated and analyzed for the presence of IL-6, IL-12, IL-1β, and TNF-α.
Clinical Effects and Toxicity in Patients Receiving VNP20009
None of the 25 patients who received VNP20009 experienced objective cancer regression, including those tumors colonized with bacteria. Table 4 lists all toxicities observed in patients receiving VNP20009. DLT was observed in cohorts receiving 1 ✕ 109 cfu/m² and, therefore, 3 ✕ 108 cfu/m² was estimated as the MTD. Two patients receiving 3 ✕ 108 cfu/m² (patient nos. 24 and 25) exhibited grade 3 toxicity, which included anemia, fever, and elevated transaminases. The other four patients in the 3 ✕ 108 cfu/m² cohort exhibited only grade 1 to 2 toxicity. All grade 3 toxicities associated with the administration of VNP20009 are listed in Table 5. Grade 3 toxicities included fever, hypotension, thrombocytopenia, anemia, persistent bacteremia, low WBC count (only observed at lower dose levels), hypophosphatemia, hyperbilirubinemia, diarrhea, vomiting, nausea, and elevated alkaline phosphatase and transaminases. No grade 4 toxicities were ever observed. In the 1 ✕ 109 cfu/m² cohort, three of four patients exhibited grade 3 hypotension, which was corrected within 4 hours with fluids and/or pressors and was not considered dose-limiting. Support to maintain the blood pressure at more than 80 mmHg was required in the latter patients for up to 48 hours. Three of four patients from the same cohort (1 ✕ 109 cfu/m²) developed grade 3 hypophosphatemia. Also, two of four patients receiving 1 ✕ 109 cfu/m² experienced grade 3 thrombocytopenia and persistent low-level bacteremia.
Table 4.
Toxicity in Patients Receiving VNP20009
| No. of Patients With Toxicity Receiving VNP20009 Dose* |
|||||||
|---|---|---|---|---|---|---|---|
| Toxicity | 106 cfu/m² (n = 3) | 3 × 106 cfu/m² (n = 3) | 107 cfu/m² (n = 3) | 3 × 107 cfu/m² (n = 3) | 108 cfu/m² (n = 3) | 3 × 108 cfu/m² (n = 6) | 109 cfu/m² (n = 4) |
| Constitutional | |||||||
| Chills | 0 | 3 | 2 | 1 | 0 | 4 | 3 |
| Fever | 1 | 0 | 1 | 0 | 1 | 2(1) | 3(1) |
| Malaise | 0 | 2 | 0 | 0 | 0 | 3 | 1 |
| Cardiovascular | |||||||
| Hypotension | 0 | 0 | 0 | 0 | 0 | 4 | 4(3) |
| Epistaxis | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Hematologic | |||||||
| Thrombocytopenia | 0 | 0 | 0 | 0 | 0 | 4 | 4(2) |
| Elevated WBC | 0 | 0 | 0 | 0 | 1 | 3 | 1 |
| Anemia | 0 | 0 | 0 | 0 | 0 | 1(1) | 2(1) |
| Low WBC | 0 | 1 | 2(1) | 0 | 0 | 0 | 0 |
| Neutrophilia | 0 | 0 | 0 | 0 | 0 | 2 | 2 |
| Serum chemistries | |||||||
| Hypomagnesemia | 0 | 0 | 0 | 0 | 0 | 4 | 2 |
| Hyperbilirubinemia | 0 | 0 | 0 | 0 | 0 | 2 | 3(1) |
| Hypophosphatemia | 0 | 0 | 0 | 0 | 0 | 1 | 3(3) |
| Hypocalcemia | 0 | 0 | 0 | 0 | 0 | 2 | 0 |
| Elevated CPK | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Elevated creatinine | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Elevated BUN | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Liver function | |||||||
| Elevated alk. phos. | 0 | 0 | 0 | 1 | 0 | 3 | 3(1) |
| Prolonged PTT | 0 | 0 | 0 | 0 | 0 | 4 | 3 |
| Elevated AST | 0 | 0 | 1(1) | 1 | 0 | 2(1) | 2(1) |
| Elevated ALT | 0 | 0 | 1(1) | 1 | 0 | 3(1) | 1(1) |
| Prolonged PT | 0 | 0 | 0 | 0 | 0 | 2 | 3 |
| GI | |||||||
| Nausea | 1 | 2 | 1 | 0 | 0 | 4 | 3(1) |
| Vomiting | 0 | 0 | 0 | 1 | 0 | 3 | 2(1) |
| Anorexia | 0 | 0 | 0 | 0 | 0 | 2 | 3 |
| Diarrhea | 0 | 0 | 0 | 0 | 0 | 1 | 2(1) |
| Neurologic | |||||||
| Headache | 1 | 2 | 1 | 0 | 0 | 4 | 2 |
| Dermatologic | |||||||
| Flushing | 0 | 0 | 0 | 0 | 0 | 2 | 0 |
| Rash | 0 | 0 | 1 | 0 | 0 | 1 | 0 |
| Nasal congestion | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Pain | |||||||
| Muscle | 0 | 0 | 0 | 0 | 0 | 3 | 3 |
| Joint | 0 | 1 | 0 | 1 | 0 | 0 | 1 |
| Neuropathic | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Pleuritic | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Chest | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
NOTE. All Grade 1 to 4 toxicities are defined in Patients and Methods.
Numbers in parentheses indicate number of patients with grade 3 toxicity.
Abbreviations: CPK, creatine phosphokinase; BUN, blood urea nitrogen; alk phos, alkaline phosphatase; PTT, partial thromboplastin time; PT, prothrombin time.
Table 5.
Grade 3 Toxicity
| Patient No.* | Dose (cfu/m²) | Toxicity† |
|---|---|---|
| 7(2) | 1 × 107 | Low WBC count, elevated AST and ALT |
| 24 | 3 × 108 | Elevated AST and ALT |
| 25 | 3 × 108 | Fever, anemia, persistent bacteremia |
| 19 | 1 × 109 | Hypotension, thrombocytopenia, hyperbilirubinemia, hypophosphatemia, diarrhea, vomiting, nausea |
| 20 | 1 × 109 | Hypophosphatemia |
| 21 | 1 × 109 | Hypotension, persistent bacteremia, vomiting, hypophosphatemia |
| 22 | 1 × 109 | Hypotension, anemia, fever, cough, persistent bacteremia, thrombocytopenia, elevated alkaline phosphatase, AST, and ALT |
Number in parentheses indicates the patient's treatment cycle.
All grade 3 toxicities are defined in Patients and Methods.
Analysis of VNP20009-Specific Antibodies
The presence of VNP20009-specific antibodies was evaluated at pretreatment, day 15, and day 35. Antibody titers of IgG, IgM, and IgA specific for VNP20009 were evaluated. One patient (patient no. 19) had pre-existing anti-VNP20009 antibodies (IgG), which correlated with the patient’s chronic carrier state (S Enteriditis). In 23 patients treated with VNP20009 and at all dosage levels, 19 patients developed VNP20009-specific IgG by day 35. In addition, patients developed VNP20009-specific IgM (12 patients) and IgA (10 patients) after infusion. At the maximum-tolerated dose (3 ✕ 108 cfu/m²), all patients developed VNP20009-specific IgG. The antibody titers (IgG, IgM, and IgA) did not correlate with the dose of VNP20009 (data not shown).
DISCUSSION
In preclinical studies, systemic administration of an attenuated Salmonella typhimurium (VNP20009) was found to infect and preferentially accumulate in a wide variety of solid tumor types including melanoma.12 VNP20009 was also found to inhibit tumor growth in mice and, therefore, was regarded as a potential antitumor agent in cancer patients.1,2,11 VNP20009 possessed a safety profile that included reduced pathogenicity in animal models based on genetically stable deletions of the msbB and pur I genes and a high degree of antibiotic susceptibility.11 Based on the safety and efficacy demonstrated in animal models, we began a phase I trial of VNP20009 ✕ 30-minute IV injection in patients with advanced cancer. Twenty-four patients with metastatic melanoma and one patient with renal cell carcinoma were evaluated for VNP20009 dose-related toxicities, selective accumulation of the organism within tumors, and antitumor effects.
The MTD of VNP20009 was 3 ✕ 108 cfu/m². At 1 ✕ 109 cfu/m², DLT, which was defined by Food and Drug Administration–approved criteria, was observed in more than one patient. However, with appropriate supportive care, it seems that additional patients could be treated with 1 ✕ 109 cfu/m². The higher doses of VNP20009 were associated with fever, hypotension, thrombocytopenia, anemia, vomiting, diarrhea, nausea, and hypophosphatemia. This toxicity was most likely associated with the systemic release and synergistic action of IL-1β and TNF-α.17 Thus, despite the LPS modification of VNP20009, a significant amount of TNF-α was detected in the peripheral blood at elevated doses, indicating that other bacterial products, such as flagellin, may be capable of inducing the release of proinflammatory cytokines.18 In addition, IL-6 was also elevated in the serum after infusion. IL-6 represents the net effect of biologically active IL-1 and TNF and is also associated with the development of septic shock.17 Despite the release of substantial levels of pro-inflammatory cytokines, most clinical toxicities of VNP20009 at or below the MTD were grade 1 to 2 and rapidly reversible.
VNP20009 was initially developed as a means for delivering therapeutic proteins to the tumor site.1,15,16 Therefore, an important objective of this study was to analyze the efficiency of tumor colonization by VNP20009. Only three patients, all receiving doses of at least 3 ✕ 108/m², had tumors that grew VNP20009 in culture after a tumor biopsy or FNA. Four additional patients from the same cohorts had biopsy-negative tumors after receiving similar doses, even after episodes of prolonged low-level bacteremia (patient nos. 25 and 22). However, other patients may have had metastatic lesions that were colonized with VNP20009 but were not detected by FNA. FNA sensitivity may have been limited by focal tumor colonization or bacterial concentration. For example, patient no. 21 exhibited focal colonization as evidenced by FNA. In addition, patient no. 24 had evidence of colonization by excisional biopsy (11,000 cfu/g) but not by FNA. Although an excised biopsy was clearly more sensitive than an FNA, as illustrated in patient no. 24, we did not have the opportunity to excise the tumor in all patients and often chose to perform an FNA. Therefore, additional clinical studies are required to fully evaluate the benefit of using FNA for assessing VNP20009 tumor colonization.
The inability of VNP20009 to fully colonize tumors in patients in our study, despite the administration of high doses of VNP20009, is different than results obtained in rodent tumor models. The factors that account for efficient tumor colonization and preferential accumulation in the rodent tumor models are not fully defined. Differences between the rodent models and patients may exist with regard to entry of bacteria into tumors, growth of the bacteria within the tumors, or clearance from peripheral circulation and from tumors. In our study, we found that clearance of VNP20009 from peripheral blood occurred very rapidly and much faster than clearance in rodents (unpublished data, Vion Pharmaceuticals, New Haven, CT). The rapid clearance from peripheral blood may prevent the delivery of sufficient organisms to the tumor to establish a full infection of the tumor, and administration of higher doses in the 30-minute infusion schedule was precluded by toxicity. Improvement in tumor colonization may occur if higher doses of VNP20009 could be delivered. Because significant levels of proinflammatory cytokines were induced with VNP20009, the administration of agents, such as TNF-neutralizing antibodies, soluble TNF receptors, or IL-1 receptor antagonists, may reduce the biologic activity of these cytokines and allow patients to tolerate higher doses of VNP20009.17,19 In preclinical studies conducted in monkeys (unpublished data, Vion Pharmaceuticals), high levels of bacteria could be maintained in peripheral blood throughout a 4-hour infusion of VNP20009, without an increase in toxicity, compared with the same dose given by 30-minute infusion. The longer infusions may result in greater delivery of VNP20009 to tumors. Finally, repeated doses of VNP20009 could be considered, particularly because an initial dose of VNP20009 may inhibit cytokine induction from subsequent doses, as observed in some preclinical and clinical studies of cytokine inducers such as LPS.20,21 Other factors, such as tumor viability and preexisting necrosis, may influence the colonization of human tumors. Additional preclinical and clinical studies are required to define the factors affecting tumor colonization.
Once VNP20009 enters and effectively colonizes a tumor, its clearance seems to be blunted. Indeed, in one patient, a substantial inflammatory response developed in a colonized tumor accompanied by considerable systemic toxicity. Patient no. 21 had a large chest wall tumor that eventually contained over 109 cfu/g of VNP20009 by day 8 after infusion. During this period, the patient exhibited signs and symptoms of bacterial sepsis, which included fever, hypotension, and bacteremia, and was subsequently treated with antibiotics on day 8. After antibiotic therapy and the clearance of bacteria, the previously colonized chest wall tumor continued to grow, and the patient eventually died of progressive cancer. In the two other patients that had documented tumor colonization, for example in patient no. 24 where a small tumor was colonized with low levels of VNP20009 (104 cfu/g), systemic effects were not observed. Therefore, the incidence of toxicity after tumor colonization may be related to tumor size and associated VNP20009 tumor concentration, which could potentially be blunted with a short, suppressive course of antibiotics. Additional patients are required to fully delineate the association between tumor size and related toxicity after colonization with VNP20009.
Of the 25 cancer patients treated with VNP20009, no patients experienced objective tumor regression, and all patients have developed progressive disease. In murine tumor models, VNP20009 was capable of inhibiting tumor growth but did not cause tumor regression.1,2 Furthermore, the majority of patients in this phase I study received a relatively low dose of VNP20009 that was rapidly cleared from the blood, and most tumors were not colonized with the organism. If colonization is required for antitumor activity, then the lack of therapeutic effect is not surprising. In one patient receiving 3 ✕ 108 cfu/m² and two other patients receiving 1 ✕ 109 cfu/m², VNP20009 was detected within metastatic lesions but tumor regression was not observed. Interestingly, although tumor regression was not observed, the histologic examination of the two highly colonized tumors (patient nos. 21 and 24) revealed the presence of abundant necrosis and rare tumor cells. It is unclear whether these tumors were necrotic before therapy or became necrotic after colonization. In contrast, in patient no. 21, the lateral region of the same chest wall tumor contained viable melanoma cells, no necrosis, and very low levels of VNP20009.
Currently, we are planning to explore a longer 4-hour infusion to determine whether the efficiency of tumor colonization can be improved, and future studies may include additional modifications in the dosing schedule (eg, multiple doses). However, differences between rodent and human tumors, for example in growth rate, architecture, and blood supply, may influence the antitumor activity of VNP20009. Regardless of whether or not VNP20009 displays an antitumor effect, effective colonization of tumors and achievement of high tumor-to-normal tissue ratios would provide the basis for exploring the antitumor activity of second-generation vectors engineered to produce proteins with increased therapeutic effect. Furthermore, the local inflammatory effect of VNP20009 may enhance the activity of innate or induced antigen-specific T cells, which could be the basis for combinations of VNP20009 with cancer vaccines or IL-2.
REFERENCES
- 1.Pawelek JM, Low KB, Bermudes D. Tumor-targeted Salmonella as a novel anticancer vector. Cancer Res. 1997;57:4537–4544. [PubMed] [Google Scholar]
- 2.Low KB, Ittensohn M, Le T, et al. Lipid A mutant Salmonella with suppressed virulence and TNF-α induction retain tumor-targeting in vivo. Nat Biotechnol. 1999;17:37–41. doi: 10.1038/5205. [DOI] [PubMed] [Google Scholar]
- 3.Giel CP. Abscess formation in pheochromocytoma. N Engl J Med. 1954;251:980–982. doi: 10.1056/NEJM195412092512406. [DOI] [PubMed] [Google Scholar]
- 4.Simmers TA, Mijnhout GS, Van Meyel JJ. Salmonellosis: An unusual complication of hepatocellular carcinoma. Scand J Gastroenterol. 1997;32:1180–1182. [PubMed] [Google Scholar]
- 5.Noguerado A, Cabanyes J, Vivancos J, et al. Abscess caused by Salmonella enteritidis within a glioblastoma multiforme. J Infect. 1987;15:61–63. doi: 10.1016/s0163-4453(87)91476-9. [DOI] [PubMed] [Google Scholar]
- 6.Black PH, Kunz LJ, Swartz MN. Salmonellosis: A review of some unusual aspects. N Engl J Med. 1960;262:921–926. doi: 10.1056/NEJM196005052621806. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez RE, Valero V, Watanakunakorn C. Salmonella focal intracranial infections: Review of the world literature (1884–1984) and report of an unusual case. Rev Infect Dis. 1986;8:31–41. doi: 10.1093/clinids/8.1.31. [DOI] [PubMed] [Google Scholar]
- 8.Grahm FO, Coleman PN. Infection of a secondary carcinoma by Salmonella montevideo. BMJ. 1952;1:1116. doi: 10.1136/bmj.1.4768.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khan SA, Everest P, Servos S. A lethal role for lipid A in Salmonella infection. Mol Microbiol. 1998;29:571–579. doi: 10.1046/j.1365-2958.1998.00952.x. [DOI] [PubMed] [Google Scholar]
- 10.Low KB, Ittensohn M, Le T, et al. VNP20009, a genetically modified Salmonella typhimurium for the treatment of solid tumors. Proc Am Assoc Cancer Res. 1999;40:87. (abstr 581) [Google Scholar]
- 11.Clairmont C, Lee KC, Pike J, et al. Biodistribution and genetic stability of the novel antitumor agent VNP20009, a genetically modified strain of Salmonella typhimurium. J Infect Dis. 2000;181:1996–2002. doi: 10.1086/315497. [DOI] [PubMed] [Google Scholar]
- 12.Luo X, Ittensohn M, Liu Y, et al. Genetically modified Salmonella typhimurium inhibited growth of primary tumors and metastases. Proc Am Assoc Cancer Res. 1999;40:87. (abstr 3146) [Google Scholar]
- 13.Chen JJ, Sun Y, Nabel GJ. Regulation of the proinflammatory effects of Fas ligand (CD95L) Science. 1998;282:1714–1717. doi: 10.1126/science.282.5394.1714. [DOI] [PubMed] [Google Scholar]
- 14.Jesenberger V, Procyk KJ, Yuan J, et al. Salmonella-induced caspase-2 activation in macrophages: A novel mechanism in pathogenmediated apoptosis. J Exp Med. 2000;192:1035–1045. doi: 10.1084/jem.192.7.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carrier MJ, Chatfield SN, Dougan G. Expression of human IL-1 beta in Salmonella typhimurium: A model system for the delivery of recombinant therapeutic proteins in vivo. J Immunol. 1992;148:1176–1181. [PubMed] [Google Scholar]
- 16.Saltzman DA, Katsanis E, Heise CP. Antitumor mechanism of attenuated Salmonella typhimurium containing the gene for human interleukin-2: A novel antitumor agent? J Ped Surg. 1997;32:301–306. doi: 10.1016/s0022-3468(97)90198-6. [DOI] [PubMed] [Google Scholar]
- 17.Dinarello CA. Proinflammatory and anti-inflammatory cytokines as mediators in the pathogenesis of septic shock. Chest. 1997;112:321S–329S. doi: 10.1378/chest.112.6_supplement.321s. [DOI] [PubMed] [Google Scholar]
- 18.Eaves-Pyles T, Murthy K, Liaudet L. Flagellin, a novel mediator of Salmonella-induced epithelial activation and systemic inflammation: IkappaB alpha degradation, induction of nitric oxide synthase, induction of proinflammatory mediators, and cardiovascular dysfunction. J Immunol. 2001;166:1248–1260. doi: 10.4049/jimmunol.166.2.1248. [DOI] [PubMed] [Google Scholar]
- 19.Dinarello CA. Proinflammatory cytokines. Chest. 2000;118:503–508. doi: 10.1378/chest.118.2.503. [DOI] [PubMed] [Google Scholar]
- 20.Freudenberg MA, Galanos C. Induction of tolerance to LPS-D-galactosamine lethality by pretreatment with LPS is mediated by macrophages. Infect Immun. 1988;56:1352–1357. doi: 10.1128/iai.56.5.1352-1357.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Otto F, Schmid P, Mackensen A. Phase II trial of intravenous endotoxin in patients with colorectal and non-small cell lung cancer. Eur J Cancer. 1996;32A:1712–1718. doi: 10.1016/0959-8049(96)00186-4. [DOI] [PubMed] [Google Scholar]