Abstract
This is a double blind placebo controlled study of sustained release bupropion as a smoking cessation aid in alcoholics undergoing treatment for their alcoholism. Participants (N=58) were enrolled within one week of entry into alcohol treatment from community and Veterans Affairs Substance Use Disorder programs. All participants received nicotine patch and were invited to attend a smoking cessation lecture and group. Cigarette smoking and alcohol outcomes were measured at six months. Bupropion when added to nicotine patch did not improve smoking outcomes. One-third of participants on bupropion reported discontinuing the drug during weeks 1-4. Participants reported cigarette outcomes with nicotine patch which are similar to those seen in the general population. All study participants significantly reduced cigarette use. Co-morbid affective disorder or anti-personality disorder did not affect outcomes. Alcohol outcomes were improved in those who discontinued cigarettes.
Keywords: Smoking cessation, Alcoholism, Bupropion, Nicotine replacement, Nicotine dependence
Introduction
The World Health Organization (WHO) reports that 1.3 billion children, women and men are cigarette smokers worldwide. According to the WHO, almost 5 million tobacco-related deaths occur each year and almost half of cigarette smokers will die as a result of their nicotine addiction (Graul and Prous, 2005). While cigarette smoking has decreased to 24.9% of the United States population age twelve or older (2005), approximately 80-95% of alcoholics smoke cigarettes (Friend and Pagano, 2005; Hughes, 1995). Smoking alcoholics smoke more than non-alcoholics (Hurt et al., 1995; Marks et al., 1997), are more nicotine dependent (Hughes and Kalman, 2006), may experience more severe nicotine withdrawal symptoms (Marks et al., 1997) and are particularly vulnerable to significant medical sequelae of concomitant use of both drugs (Blot et al., 1988; Vaillant et al., 1991). Tobacco, rather than alcohol, has been shown to be the leading cause of death among alcohol dependent patients previously treated for their alcoholism in an inpatient setting (Hurt et al., 1996).
Previous studies of smoking cessation in alcoholics
Smoking quit attempts result in quit rates at 6 months of 10-40% in the general population and 0-29% in alcoholics. In a study of lifetime quit attempts, remarkably only 7% of alcoholics reported being successful in their quit attempts while 49% of the general population were able to discontinue cigarette use (DiFranza and Guerrera, 1990). In a meta-analysis of persons with substance use disorders, smoking abstinence rates for comparison and intervention groups were 6 and 7% respectively for those in addictions treatment and 15 and 20% for those in recovery at 6-12 months (Prochaska et al., 2004).
The authors found the greatest intervention effects in those recent studies that provided nicotine replacement therapy. When alcoholics are compared across various stages of their disease, individual quit attempts result in quit rates of 3-19% at 4-6 months while in alcoholism treatment (Bobo et al., 1996; Bobo et al., 1998; Grant KM, 2003; Joseph et al., 2004; Kalman et al., 2001) and 9-29% at 4-6 months in alcoholics who reported they were no longer drinking (Hays et al., 1999; Kalman et al., 2006; Martin et al., 1997) (Table 1).
Table 1.
Cigarette Smoking Quit Rates
| 6 Months | 12 Months | ||
|---|---|---|---|
|
General Population |
Placebo ± PSS | 10.3-18.8%(Daughton et al., 1998; Hurt et al., 1997; Jorenby et al., 1999) |
8.7-15.6%(Daughton et al., 1998; Hurt et al., 1997; Jorenby et al., 1999) |
| Nicotine Replacement + PSS |
18.5-28%(Daughton et al., 1998; Fiore et al., 1994; Hays et al., 1999; Jorenby et al., 1999) |
14.7-16.4%(Daughton et al., 1998; Jorenby et al., 1999) |
|
| Bupropion + PSS |
24.2-34.8%(Hurt et al., 1997) (Aubin et al., 2004; Jorenby et al., 1999) |
19.6-30.3%(Hurt et al., 1997) (Jorenby et al., 1999) |
|
| Bupropion + NRT + PSS |
38.8%(Jorenby et al., 1999) |
35.5%(Jorenby et al., 1999) |
|
| Alcohol Population | |||
|
Community Sample |
NRT+PSS | 25%(Hays et al., 1999) | |
|
Alcohol Treatment |
Psychosocial support |
3-11%(Bobo et al., 1996; Bobo et al., 1998; Grant KM, 2003a) | 0-9%(Bobo et al., 1998; Grant KM, 2003a) |
| NRT+ PSS | 10.8-19% (Joseph et al., 2004; Kalman et al., 2001 b) | 12.8-17.6%(Joseph et al., 2004) | |
|
Recovery from Alcoholism |
PSS | 21-29%(Martin et al., 1997) | 26-27%(Martin et al., 1997) |
| Bupropion + PSS |
24.3%(Hayford et al., 1999) | ||
Results from previous cigarette smoking cessation studies employing psychosocial support (PSS), nicotine replacement (NRT) and/or bupropion. PSS=variety of interventions including brief individual counseling, primary care intervention, behavioral counseling, group therapy, individual counseling, education sessions. NRT=nicotine patch or gum.
participants offered but not required to utilize nicotine patch.
16 weeks f/u.
Two studies found no adverse effects of concurrently treating alcoholism and nicotine dependence (Hurt et al., 1994) (Kalman et al., 2001) while Joseph showed that delayed treatment may be superior to concurrent treatment (Joseph et al., 2004). Another study suggests that smoking cessation reduces the risk of alcohol relapse (Sobell, 1995) and an analysis of Project MATCH (a large multi-site study comparing various alcohol treatments) participants showed that persons who discontinued cigarettes had a significant and durable reduction in alcohol intake at the time of smoking cessation which persisted for six months post cigarette cessation (Friend and Pagano, 2005). A number of studies have found a positive association between abstinence from cigarettes and alcohol in alcoholics (De Soto et al., 1989; Miller et al., 1983; Stuyt, 1997).
Bupropion as a potential treatment for smoking cessation in alcoholics
Sustained release bupropion, an antidepressant, has been approved for the treatment of cigarette smoking. In a review of 19 placebo-controlled trials, bupropion doubled the odds of smoking cessation (Hughes JR, 2006). It has multiple pharmacologic effects including blocking the reuptake of dopamine and noradrenaline (Ferris, 1993; Horst and Preskorn, 1998) and the nicotinic acetylcholine receptors (Slemmer et al., 2000). Sustained release (SR) bupropion has been shown to be efficacious in the treatment of smoking cessation in the general population, in African-Americans and in those who have relapsed to smoking (Ahluwalia et al., 2002; Gonzales et al., 2001; Hurt et al., 1997; Jorenby et al., 1999). Preliminary studies suggest that bupropion may be useful in smoking cessation attempts in persons with schizophrenia and chronic post traumatic stress disorder (Evins et al., 2001; Hertzberg et al., 2001; Weiner et al., 2001). Bupropion has also been shown to have efficacy in cigarette smokers with a former history of alcoholism with and without a history of major depression (Hayford et al., 1999). However, in a study of bupropion in outpatient smoking clinics in France, participants with mild to moderate alcohol problems quit smoking at about half the rate of persons with no alcohol problems at 6 months (Aubin et al., 2004). Bupropion has not been studied as a smoking cessation aid in individuals being treated for their alcoholism.
Materials and Methods
Overview
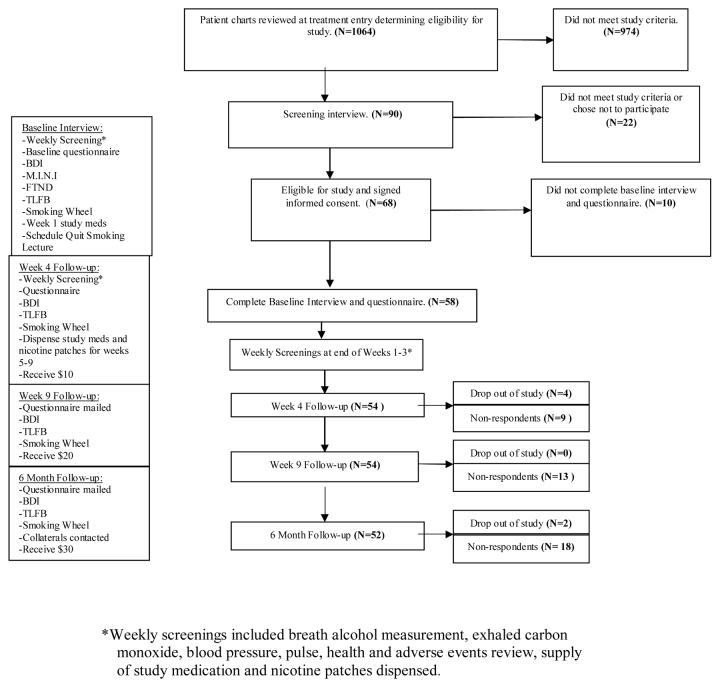
This is a double blind placebo controlled study of bupropion SR as a smoking cessation aid in alcoholics undergoing treatment for their alcoholism. All participants received nicotine patch and participated in treatment as usual for their alcoholism. Cigarette smoking and alcohol outcomes were measured at 4 and 9 weeks and 6 months. See study flow chart in Figure 1.
Fig. 1.
Study Flow Chart. Beck Depression Inventory (BDI), Timeline Follow-back Drinking Calendar (TLFB), Fagerstrom Test for Nicotine Dependence (FTND), Mini-International Neuropsychiatric Interview (MINI).
Treatment Settings
Participants were recruited between September 2002 and October 2004 from two substance use disorder (SUD) treatment programs in Eastern Nebraska: the Veterans Affairs Nebraska Western Iowa Health Care System (VA NWIHCS) Omaha site and Catholic Charities Campus for Hope (CFH). Participants were recruited at admission to residential and nonresidential outpatient programs. The study protocol and consent form were approved by the VA NWIHCS Institutional Review Board and Catholic Charities.
Enrollment
A total of 1,064 persons entering SUD treatment at the study sites were screened for study eligibility within seven days of treatment entry. If a review of the patient's chart indicated that the individual met criteria for study entry, a screening interview with the prospective participant was conducted. Patients were excluded if any of the following criteria were met: did not meet DSM-IV criteria for alcohol dependence/abuse, smoking less than twenty cigarettes per day, current use of bupropion or past use of bupropion in a research study, history of seizures including alcohol withdrawal seizures, history of cerebral infract, intra-cerebral hemorrhage or brain tumor, unstable angina, respiratory or liver failure, history of schizophrenia or bipolar affective disorder, unwillingness to quit smoking (stage 1 pre-contemplation of Prochaska's Stages of Change) (Prochaska and DiClemente, 1992), refusal to provide collateral information, current unstable depression (any depressive symptoms in the previous six months), women who reported being pregnant or anticipated becoming pregnant in the subsequent 12 weeks or current use of any of the following medications: tricylic antidepressants, monoamine oxidase inhibitors, fluoxetine, antipsychotics, benzodiazepines, protriptyline, theophylline or any investigational drug in the previous four weeks. Other antidepressants (e.g. paroxetine, escitalopram, mirtazepine, venlafaxine, sertraline) did not preclude study entry. The most common reasons for study ineligibility were lack of alcohol diagnosis (278), current use of contraindicated medication (328), current unstable depression (98), history of bipolar affective disorder (126) and enrollment in SUD treatment for greater than 7 days (146). Patients who were screened may have met more than one criteria for ineligibility. Patients with active SUD in addition to their alcoholism were eligible for the study.
Ninety patients were eligible for study entry and attended a screening interview. Seventy-five percent (75%) or 68 individuals were enrolled in the study. All participants enrolled in the study completed written informed consent. The procedures followed were in accordance with the ethical standards of the committee on human experimentation and with the Helsinki Declaration of 1975 and revised in 1983. Ten participants did not complete the baseline questionnaire. Data were analyzed for the 58 participants who completed the baseline questionnaire. Six participants later dropped out of the study and data from these participants are included in the analysis until they chose to withdraw from study participation. A “quit smoking” date was set and collateral information collected. A serum beta-HCG was obtained in appropriate female participants to confirm pregnancy status.
Intervention
Participants were randomized in a double blind manner into the treatment or control group. The treatment group received bupropion SR 150 mg per day for three days to be followed by 150 mg twice daily for 60 days. The control group received identical placebo capsules and was instructed to follow the same medication regimen. All participants were instructed to begin their capsules eight days before their quit smoking date. Both groups received nicotine patches and were instructed in their appropriate use. Participants were asked to initiate the patches on their targeted quit date and to follow a tapering regimen of 21 mg (four weeks), 14 mg (two weeks), and 7 mg (two weeks).
Study Protocol
At the time of study enrollment, participants completed a baseline questionnaire. Each of the following three weeks the participants met with study staff to assess tolerability and compliance with study medication and participation in alcoholism treatment. Participants were asked to attend a single one hour smoking cessation group in which an educational video was shown and followed by a staff-lead discussion of smoking cessation techniques. At week four, medication tolerability and compliance, cigarette smoking and alcohol outcomes were measured. At week nine and at six months, cigarette smoking and alcohol outcomes were measured.
Participants received $10, $20, and $30 dollars at week 4, week 9 and 6 months respectively upon completing follow-up questionnaires. Participants were asked to return any unused nicotine patches and all bupropion/placebo medication bottles. The majority of participants were seen by the study coordinator at their treatment site during the first four weeks of study participation. Those participants who discontinued alcohol treatment during study weeks 1-4 were contacted by study staff and completed the questionnaire by telephone. However, blood pressure, pulse, exhaled carbon monoxide, and breath alcohol measurement were not measured in these participants. Ninety four percent (94%) of participants completed all weekly screenings in person. At week 9 and 6 months, participants responded to the follow-up questionnaire via mail or telephone. At 6 months, collateral informants were contacted to confirm cigarette and alcohol use history provided by participant. Adverse event information was collected and reviewed every four weeks throughout the study by study staff until the last enrolled participant completed the 6 month follow-up.
Assessment instruments
At study enrollment participants completed the Mini-International Neuropsychiatric Interview (MINI) (Sheehan et al., 1998) to determine substance use diagnoses and assess psychiatric symptoms and diagnoses, the Fagerstrom Test for Nicotine Dependence (FTND) (Heatherton et al., 1991) to determine severity of nicotine dependence, and the Beck Depression Inventory (BDI) (Beck and Beamesderfer, 1974) to evaluate severity of depressive symptoms.
Outcomes
Cigarette smoking outcomes were measured by seven day point prevalence abstinence rates (i.e. percentage who report abstinence from cigarettes over the previous seven days) at 4 and 9 weeks and 6 months (Velicer and Prochaska, 2004). At the same follow up points the Timeline Follow-Back (TLFB) drinking calendar was used to determine the number of drinks per day, drinks per drinking day, percent days abstinent and continuous abstinence in the previous 30 days (Sobell, 1992).
Statistical Methods
Data were analyzed with SPSS and SAS statistical packages. T-tests and chi-square tests were used to compare the baseline characteristics between the treatment groups. Fisher's exact test was used for comparisons for small sample situations and the Wilcoxon rank sum test was used for continuous data when normality assumptions were not met. An a priori sample size of 130 (65 in each group) was powered to detect a difference of medium size (.18). However, use of contraindicated medication and other factors noted above limited the number of prospective participants to 90. Seventy-five percent of them were enrolled in the study. In order to help control the type I error rate due to testing multiple endpoints, p-values less than 0.01 will be considered statistically significant. When comparing the follow-up rates between the groups, the rates calculated account for the number of drop outs at each time point. In general, only data from participants who responded to the surveys were analyzed. These are called “respondent” analyses. For the smoking cessation rates, we also did an “intention to treat” analysis, where non-responders are assumed to be smoking cigarettes.
Results
Sample Characteristics
Eighty-four percent of study participants were male. All females were enrolled from the community site. The bupropion and placebo groups were similar in gender, racial and ethnic composition, and age at study entry. The mean number of cigarettes per day at study entry was 27 in the placebo group and 23 in the bupropion group. The mean Fagerstrom Test for Nicotine Dependence (FTND) was 6 in both groups. Fifty-three percent of participants had at least one other substance dependence in addition to alcohol and nicotine. Drinking measures and mental health history were similar in the two groups. Two (7%) of the placebo group and four (13%) of the bupropion group were on antidepressants at study entry. Many of the study participants reported a history of mental illness, most commonly an affective disorder or anti-social personality disorder. Twenty-two percent of participants reported desire to quit smoking within the next 6 months and 78% reported wanting to quit within the next month (Table 2).
Table 2.
Baseline Characteristics of Study Participants
| Total (n=58) |
Placebo (n=28) |
Bupropion (n=30) |
Test statistic, df |
P-value | |
|---|---|---|---|---|---|
| Demographics | |||||
| Mean Age (SD) | 39.6 (11.5) | 40.8 (11.3) | 38.5 (11.7) | T=0.76, 56 | 0.45 |
| Male (N, %) | 49 (84%) | 24 (86%) | 25 (83%) | Χ2=0.06, 1 | 0.80 |
| Non-Hispanic, Caucasian (N, %) | 33 (58%) | 16 (57%) | 17 (59%) | Χ2=0.01, 1 | 0.91 |
| Smoking History | |||||
| Mean cigarettes/day smoked (SD) | 25.0 (8.2) | 27.0 (9.8) | 23.2 (5.9) | T=1.78, 44 | 0.083 |
| Mean FTND (SD) range: 3-9 | 6.1 (1.7) | 6.0 (1.7) | 6.1 (1.8) | T=−0.07, 56 | 0.95 |
| Mean Expired CO ppm (SD) | 20.4 (10.3) | 19.1 (9.3) | 21.5 (11.1) | T=−0.88, 56 | 0.38 |
| Mean Age onset regular smoker (SD) | 16.0 (5.8) | 15.1 (4.7) | 16.9 (6.7) | T=−1.18, 54 | 0.24 |
| Number serious quit attempts (N, %) | |||||
| 0-1 | 26 (46%) | 12 (43%) | 14 (50%) | Χ2=0.44, 2 | 0.80 |
| 2 | 16 (29%) | 8 (29%) | 8 (29%) | ||
| >=3 | 14 (25%) | 8 (20%) | 6 (21%) | ||
| Quit smoking for 7 days in the past year (N, %) | 22 (38%) | 13 (46%) | 9 (30%) | Χ2=1.7, 1 | 0.20 |
| Prochaska's stage of change (N, %) | |||||
| 2 – quit smoking next 6 months | 13 (22%) | 4 (14%) | 9 (30%) | Χ2=2.1, 1 | 0.15 |
| 3 – quit smoking next month | 45 (78%) | 24 (86%) | 21 (70%) | ||
| Alcohol History | |||||
| Mean age onset regular drinking (SD) | 16.4 (4.9) | 15.5 (3.8) | 17.2 (5.7) | T=−1.26, 53 | 0.21 |
| Mean age onset problem drinking (SD) | 26.9 (9.5) | 25.4 (7.7) | 28.0 (10.7) | T=−0.89, 41 | 0.38 |
| Number serious quit attempts (N, %) | |||||
| 0-1 | 10 (18%) | 4 (14%) | 6 (21%) | Χ2=0.42, 2 | 0.81 |
| 2 | 18 (32%) | 9 (32%) | 9 (31%) | ||
| >=3 | 29 (52%) | 15 (54%) | 14 (48%) | ||
| Number prior SUD treatment (N, %) | |||||
| 0-1 | 20 (35%) | 9 (32%) | 11 (38%) | Χ2=0.21, 1 | 0.65 |
| >=2 | 37 (65%) | 19 (68%) | 18 (62%) | ||
| Mean number drinking days past 30 days (SD) | 7.4 (7.8) | 9.3 (7.3) | 5.8 (8.0) | T=1.66, 51 | 0.10 |
| Mean number of drinks/day (SD) | 4.0 (5.4) | 4.5 (5.5) | 3.6 (5.5) | T=0.60, 51 | 0.55 |
| Mean number of drinks/drinking day (SD) (n=36) | 11.3 (5.7) | 12.0 (5.9) | 10.4 (5.5) | T=0.87, 34 | 0.39 |
| Other Substance Use | |||||
| Marijuana (N, %) | 51 (88%) | 24 (86%) | 27 (90%) | Exact p | 0.70 |
| Cocaine (N, %) | 44 (76%) | 23 (82%) | 21 (70%) | Χ2=1.2, 1 | 0.28 |
| Methamphetamine (N, %) | 35 (60%) | 17 (61%) | 18 (60%) | Χ2=0.003,1 | 0.96 |
| Substance dependence, non-alcohol (N, %) | 30 (53%) | 15 (54%) | 15 (52%) | Χ2=0.02,1 | 0.89 |
| Substance abuse, non-alcohol (N, %) | 14 (25%) | 7 (25%) | 7 (24%) | Χ2=0.006, 1 | 0.94 |
| Mental Health History | |||||
| Mean Beck Depression Inventory (SD) (range 0 - 63) | 11.6 (6.0) | 12.0 (6.5) | 11.1 (5.6) | T=0.58, 55 | 0.56 |
| Affective Disorder (MINI) (N, %) | 27 (47%) | 14 (50%) | 13 (43%) | Χ2=0.26, 1 | 0.61 |
| Current MDD (MINI) (N, %) | 7 (12%) | 4 (14%) | 3 (10%) | Exact p | 0.71 |
| Suicidiality (MINI) (N, %) | 22 (39%) | 12 (43%) | 10 (34%) | Χ2=0.42, 1 | 0.52 |
| Anxiety (MINI) (N, %) | 14 (25%) | 8 (29%) | 6 (21%) | Χ2=0.48, 1 | 0.49 |
| Anti-Social Personality Disorder, MINI (N, %) | 24 (42%) | 13 (46%) | 11 (38%) | Χ2=0.42, 1 | 0.52 |
Standard Deviation=SD, df= degrees of freedom, FTND= Fagerstrom Test for Nicotine Dependence, SUD=Substance use disorder, CO=Carbon monoxide measured in parts per million (ppm), MDD=Major depressive disorder, MINI=Mini-International Neuropsychiatric Interview.
Affective Disorder = having at least one of the following: Current, recurrent or remitting MDD, MDD with melancholic features, Dysthymia, Current or past manic or hypomanic episode, Mood disorder with psychotic features.
Anxiety = having at least one of the following: Current or lifetime Panic Disorder, Panic Disorder, Agoraphobia, Social Phobia, Current Post Traumatic Stress Disorder, Generalized anxiety disorder, Obsessive Compulsive Disorder.
Beck Depression Inventory (BDI): 0-9 Assymptomatic, 10-18 Mild-Moderate Depression, 19-29 Moderate-Severe Depression, ≥ 30 Severe Depression.
Response Rates and Analysis
The follow-up rates at 4 and 9 weeks and 6 months were 93%, 83% and 75% respectively. There was no significant difference in follow-up rates between the bupropion and placebo group at any of the follow-up points. Fifty-six percent of collateral contacts were reached at the 6 month follow-up.
Cigarette Smoking
At each follow-up point there was no significant difference in cigarette smoking outcomes between the placebo and bupropion groups. At week four there appeared to be a trend toward benefit with bupropion, however, by week 9 this trend was reversed and maintained through the remainder of the follow-up period in both the “intention to treat” and “respondents only” analyses (Table 3). Interestingly, at weeks 4, 9 and 6 months there was a significant change (all P<0.001) from baseline in the number of cigarettes smoked in both the placebo and bupropion groups. The mean carbon monoxide measurement decreased from baseline, 20.4 parts per million (ppm) (SD 10.3), to week four, 12.1 ppm (SD 12.0), for all participants (P=0.0095). No significant changes were seen between weeks 4, 9 and month 6. (Figure 2) However, of the 13 respondents who reported abstinence from cigarettes in the previous seven days at the six month follow-up, at least one collateral contact of seven respondents reported that they were currently smoking. Although there was no difference between the treatment and control groups, abstinence from cigarettes at 6 months was higher than previously reported in “in treatment” alcoholics (33% and 22% in respondents and intention to treat analysis respectively.)
Table 3.
Seven Day Point Prevalence of Cigarette Abstinence
| Respondents | ||||
|---|---|---|---|---|
| Total (n=58) |
Placebo (n=28) |
Bupropion (n=30) |
P-value a | |
| 7-day point prevalence of smoking abstinence | ||||
| Week 4 | 14/49 (29%) | 5/24 (21%) | 9/25 (36%) | 0.24 |
| Week 9 | 12/45 (27%) | 7/23 (30%) | 5/22 (23%) | 0.74 |
| Month 6 | 13/40 (33%) | 8/22 (36%) | 5/18 (28%) | 0.74 |
| Intention to treat b | ||||
| 7-day point prevalence of smoking abstinence | ||||
| Week 4 | 14/58 (24%) | 5/28 (18%) | 9/30 (30%) | 0.36 |
| Week 9 | 12/58 (21%) | 7/28 (25%) | 5/30 (17%) | 0.52 |
| Month 6 | 13/58 (22%) | 8/28 (29%) | 5/30 (17%) | 0.35 |
All are p-values from Fisher's exact test.
Includes non-respondents to the survey and assumes that they are currently smoking cigarettes.
Fig. 2.
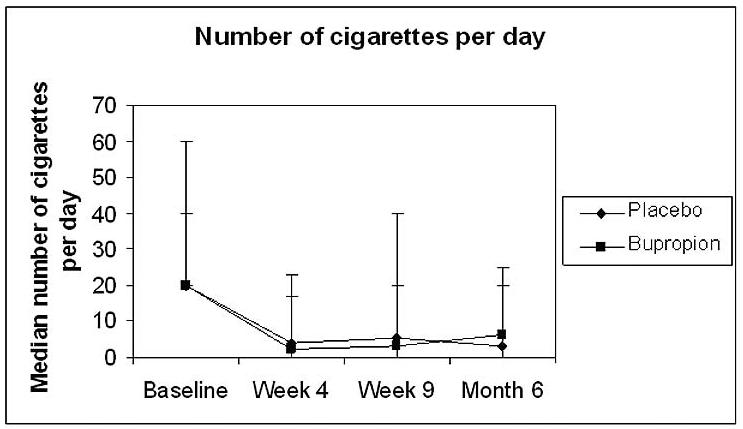
Median number of cigarettes smoked per day at baseline and each follow-up point in bupropion and placebo groups. Each point represents median and error bars are the range of the data.
Alcohol
Continuous abstinence, drinks per day, drinks per drinking day and percent days abstinent in the previous 30 days were measured at each follow-up point. At week 4, the placebo group were more likely to be continuously abstinent from alcohol, had fewer drinks per day, and greater percent days abstinent in the previous 30 days but these were not statistically significant (Table 4). When asked about continuous abstinence from alcohol since study entry, collateral contacts for 7 of 30 respondents who reported 30 days of continuous abstinence at 6 months reported at least a single episode of drinking several drinks since study entry. Also of note, participants who successfully discontinued smoking at 6 months reported greater continuous abstinence from alcohol, fewer drinks per day, and more abstinent days in the previous 30 days but, again, these differences were not statistically significant. (Table 5).
Table 4.
Alcohol Use by Treatment Group (respondents)
| Total | Placebo | Bupropion | Test statistic* |
P-value | |
|---|---|---|---|---|---|
|
Continuous abstinence from alcohol (in past 30 days) questionnaire self report: N (%) |
|||||
| Week 4 | 44 (90%) | 24 (100%) | 20 (80%) | Exact test | 0.050 |
| Week 9 | 37 (82%) | 21 (91%) | 16 (73%) | Exact test | 0.13 |
| Month 6 | 30 (75%) | 18 (82%) | 12 (67%) | Exact test | 0.30 |
|
Mean drinks per day, in past 30 days – timeline (SD) |
|||||
| Baseline | 4.0 (5.4) | 4.5 (5.5) | 3.6 (5.5) | 754 | 0.15 |
| Week 4 | 0.67 (2.8) | 0.04 (0.17) | 1.3 (3.7) | 461 | 0.051 |
| Week 9 | 0.87 (2.4) | 0.89 (2.6) | 0.84 (2.4) | 339 | 0.12 |
| Month 6 | 1.1 (2.7) | 0.91 (2.4) | 1.4 (3.0) | 222.5 | 0.076 |
|
Mean drinks per drinking day, in past 30 days (SD) |
|||||
| Baseline (n=36, 20 placebo, 16 Bupropion) | 11.3 (5.7) | 12.0 (5.9) | 10.4 (5.5) | 272 | 0.45 |
| Week 4 (n=7, 1 placebo, 6 Bupropion) | 7.6 (6.3) | 8 (NA) | 7.6 (6.9) | 5 | 0.80 |
| Week 9 (n=9, 2 placebo, 7 Bupropion) | 5.8 (2.9) | 8.5 (0.71) | 5.1 (2.9) | 15 | 0.19 |
| Month 6 (n=12, 3 placebo, 9 Bupropion) | 6.5 (4.5) | 5.4 (3.0) | 6.9 (5.0) | 19 | 1.0 |
|
Mean percent days abstinent, in past 30 days (SD) |
|||||
| Baseline | 67% (34%) | 65% (28%) | 69% (39%) | 627.5 | 0.40 |
| Week 4 | 91% (26%) | 99% (2%) | 83% (34%) | 573 | 0.051 |
| Week 9 | 88% (31%) | 89% (32%) | 86% (31%) | 420.5 | 0.13 |
| Month 6 | 83% (34%) | 86% (34%) | 80% (34%) | 304.5 | 0.083 |
Fisher's exact test and Wilcoxon rank sum test.
Table 5.
Alcohol Use by 6 Month Smoking Status
| Non- Quitters (n=27) |
Quitters (n=13) |
Test statistic * |
P-value | |
|---|---|---|---|---|
|
Continuous abstinence from alcohol, in past 30 days questionnaire report (N, %) |
||||
| Week 4 | 22 (85%) | 13 (100%) | Exact test | 0.28 |
| Week 9 | 21 (78%) | 12 (92%) | Exact test | 0.39 |
| Month 6 | 17 (63%) | 13 (100%) | Exact test | 0.016 |
| Mean drinks per day, in past 30 days – timeline (SD) | ||||
| Baseline | 3.9 (6.1) | 2.2 (2.1) | 186 | 0.90 |
| Week 4 | 0.49 (1.6) | 0.08 (0.24) | 177 | 0.51 |
| Week 9 | 1.4 (3.0) | 0.02 (0.05) | 144 | 0.14 |
| Month 6 | 1.6 (3.1) | 0.04 (0.13) | 107 | 0.048 |
| Mean drinks per drinking day, in past 30 days (SD) | ||||
| Baseline (n=24, 17 non-quitters, 7 quitters) | 11.2 (6.3) | 9.7 (4.3) | 82.5 | 0.77 |
| Week 4 (n=6, 5 non-quitters, 1 quitter) | 5.7 (5.7) | 8 (NA) | 5 | 0.56 |
| Week 9 (n=9, 8 non-quitters, 1 quitter) | 5.9 (3.0) | 5.0 (NA) | 3.5 | 0.70 |
| Month 6 (n=12, 11 non-quitters, 1 quitter) | 6.0 (4.4) | 12.0 (NA) | 11 | 0.27 |
| Mean percent abstinent days, in past 30 days (SD) | ||||
| Baseline | 69% (34%) | 77% (22%) | 199.5 | 0.75 |
| Week 4 | 89% (28%) | 99% (3%) | 203 | 0.51 |
| Week 9 | 80% (38%) | 99% (1%) | 206.5 | 0.13 |
| Month 6 | 76% (38%) | 99% (1%) | 191.5 | 0.040 |
Fisher's exact test and Wilcoxon rank sum test.
NA=Not applicable
Affective and Personality Disorders
There was no difference in mean Beck Depression Inventory between cigarette quitters and non-quitters (P=0.42) and no significant difference in abstinence from cigarettes in participants with and without a history of an affective disorder (Table 6). There was no significant difference in cigarette outcomes in participants with and without anti-social personality disorder (Table 6).
Table 6.
Outcomes in Respondents with Affective Disorder or Anti-Social Personality Disorder
|
Affective Disorder (n=27) |
No Affective Disorder (n=31) |
Test Statistic |
P-value | |
|---|---|---|---|---|
|
Mean Beck Depression Inventory (SD) |
14.3 (6.4) | 9.1 (4.4) | T=3.6, 45.3 | 0.0009 |
|
Mean Baseline Drinks/day in past 30 days (SD) |
5.0 (6.7) | 3.2 (4.0) | W=724 | 0.38 |
|
Mean Baseline Number of days drinking in past 30 days (SD) |
7.0 (6.7) | 7.8 (8.7) | W=683 | 0.89 |
|
7-day point prevalence of smoking abstinence: n/N (%) |
||||
| Week 4 | 8/23 (35%) | 6/26 (23%) | Exact test | 0.53 |
| Week 9 | 6/20 (30%) | 6/25 (24%) | Exact test | 0.74 |
| Month 6 | 6/18 (33%) | 7/22 (32%) | Exact test | 1.0 |
|
Median Number of cigarettes per day (range) |
||||
| Week 4 | 3.4 (0 – 23.1) | 3.1 (0 – 16.9) | W=558 | 0.74 |
| Week 9 | 5.0 (0 – 27.1) | 3.0 (0 – 40.0) | W=483.5 | 0.59 |
| Month 6 | 4.9 (0 – 25.0) | 4.0 (0 – 15.0) | W=374 | 0.51 |
|
ASPD (n=24) |
No ASPD (n=33) |
Statistic | P-value | |
|
Mean Beck Depression Inventory (SD) |
12.4 (6.7) | 11.0 (5.6) | T=0.85, 54 | 0.40 |
|
Mean Baseline Drinks/day in past 30 (SD) |
6.0 (6.8) | 2.8 (4.0) | W=647 | 0.089 |
|
Mean Baseline Number of days drinking in past 30 days (SD) |
8.8 (8.4) | 6.7 (7.4) | W=602.5 | 0.39 |
|
7-day point prevalence of smoking abstinence: n/N (%) |
||||
| Week 4 | 5/21 (24%) | 8/27 (30%) | Exact test | 0.75 |
| Week 9 | 5/18 (28%) | 7/26 (27%) | Exact test | 1.0 |
| Month 6 | 5/17 (29%) | 7/22 (32%) | Exact test | 1.0 |
|
Median Number of cigarettes per day (range) |
||||
| Week 4 | 3.3 (0 – 17.5) | 2.9 (0 – 23.1) | W=490 | 1.0 |
| Week 9 | 4.2 (0 – 20) | 3.9 (0 – 40) | W=407.5 | 0.96 |
| Month 6 | 6.6 (0 – 20) | 4.8 (0 – 25) | W=329.5 | 0.44 |
Affective Disorder = having at least one of the following: Current, Recurrent or Remitting Major Depressive Disorder (MDD), MDD with melancholic features, dysthymia, current or past manic episode, current or past hypo-manic episode, mood disorder with psychotic features.
Anti-Social Personality Disorder (ASPD),W=Wilcoxon rank sum test, T=t-test, Exact test=Fisher's exact test.
Compliance and Tolerability
There was no significant difference in medication compliance between participants on bupropion and placebo weeks 1-4 however, at each week participants in the bupropion group reported taking fewer of the capsules than those on placebo. We attempted to assess medication compliance during weeks 5-9 by asking participants to return medication bottles (with any unused bupropion/placebo) and any unused nicotine patches with their week 9 follow-up questionnaires. However, the majority of participants did not comply with our request and we are unable to assess medication compliance for weeks 5-9.
There were no seizures in the study population. The most common side effects reported with bupropion were insomnia and headache (Table 7). Ten (33%) of the participants on bupropion reported discontinuing drug during weeks one through four while only three (11%) of participants on placebo reported discontinuing medication during the same time period (P=0.059). Insomnia was reported by eleven of the participants in the bupropion SR group during weeks 1-4 but by only two participants in the placebo group.
Table 7.
Adverse Events Reported Weeks 1-4
|
Placebo |
Bupropion |
|||
|---|---|---|---|---|
| # times reported | # participants reporting |
# times reported | # participants reporting |
|
| Vomiting | 0 | 0 | 1 | 1 |
| Nausea | 1 | 1 | 3 | 3 |
| Abdominal pain | 0 | 0 | 4 | 3 |
| Diarrhea | 1 | 1 | 3 | 3 |
| Exhaustion | 0 | 0 | 1 | 1 |
| Headache | 5 | 5 | 7 | 4 |
| Dry Mouth | 3 | 2 | 4 | 3 |
| Shortness of breath | 0 | 0 | 1 | 1 |
| Mental confusion | 0 | 0 | 1 | 1 |
| Sweating | 0 | 0 | 1 | 1 |
| Insomnia | 2 | 2 | 16 | 11 |
No Seizures Reported.
Discussion
Effect of bupropion on cigarette and alcohol use
To our knowledge this is the first study evaluating the efficacy of bupropion for smoking cessation in alcoholics early in their alcoholism treatment. The primary outcome of this study indicates that bupropion, when added to nicotine patch therapy, did not improve smoking outcomes in this population of “in treatment” alcoholics. In this preliminary study, there was a trend toward poorer alcohol outcomes in three of the four alcohol outcomes measured at week four in the bupropion group.
Given that bupropion has been been shown to be efficacious in multiple other populations, its lack of efficacy in early in treatment alcoholics may be associated with a feature unique to this population. It is possible that the side effects associated with bupropion may be less tolerable or more frequent in newly sober alcoholics prompting discontinuation of bupropion. The current study is remarkable for the number of participants in the bupropion group who reported insomnia. By the end of week four, 33% of participants on bupropion had reported discontinuing the drug. In previous studies 6% (Hurt et al., 1997), 11-12% (Jorenby et al., 1999), and 10% (Aubin et al., 2004) of participants discontinued bupropion due to side effects. Compliance with bupropion and placebo was not assessed weeks 5 – 9. It is possible that the side effects associated with bupropion prompted discontinuation of drug in weeks1-4 in one-third of participants and less compliance in the remaining subjects during the remaining 5 weeks of the study which went undetected. At week 4, participants on bupropion had better (but not significant) cigarette outcomes and worse alcohol outcomes. Early use of bupropion may have resulted in less cigarette use at week 4 but simultaneously contributed to increased alcohol use to combat insomnia. Alcoholics typically report higher rates of insomnia (36% - 67%) than the general population (17%-30%) (Mellinger et al., 1985) with 60% - 70% reporting using alcohol to assist with sleep (Skoloda TE, 1979). Insomnia in alcoholics has been associated with relapse to alcohol (Brower et al., 2001). In clinical practice, a patient's report of insomnia while on bupropion dosed twice daily would likely result in a dosage or schedule change. The study protocol did not allow for such an adjustment and it is conceivable that a modifed dosage or schedule may have been more tolerable in this population.
Effect of Nicotine replacement on cigarette use in alcoholics
Participants in this study, all of whom utilized nicotine patch with or without bupropion, showed a significant reduction in cigarette smoking at each follow-up point. Study participants reported a decrease from a median of 20 cigarettes per day at baseline to 4.8 cigarettes per day at 6 months. There was a significant change from baseline in the median number of cigarettes smoked in both the placebo and bupropion groups at weeks 4, 9 and month 6 (all p< 0.001). No significant changes were seen between weeks 4, 9 and month 6. Similar findings were seen in a study of persons hospitalized for alcohol, cocaine and opiate use disorders utilizing nicotine patch with a significant reduction from 24 to 10 cigarettes per day at 6 months (Gariti et al., 2002). This observation underscores the findings from a meta-analysis reported by Prochaska which found that the greatest intervention effects on smoking abstinence in those with SUD were seen in those studies which employed nicotine replacement therapy (Prochaska et al., 2004).
Cigarette abstinence of 22% (intention to treat) and 32% (respondents) at six months in alcoholics while in alcohol treatment is encouraging and similar to outcomes seen with nicotine replacement in the general population. Alcoholics are heavily nicotine dependent (Hughes and Kalman, 2006) and nicotine replacement therapy may be particularly important in this population. Few previous studies have examined nicotine replacement in alcoholics in the early stages of their alcohol treatment. However, two studies that included nicotine replacement in alcoholics in early alcohol treatment resulted in 14.1 % cigarette abstinence at six months (Joseph et al., 2004) and 19% at 4 months (Kalman et al., 2001). Study participation, increased awareness of cigarette smoking and/or the nicotine patch may have all played a role in the 6 month outcomes. Enthusiasm for these findings must be tempered, however, given that at least one of the collateral contacts for seven of the respondents did not substantiate their report of cigarette abstinence in the previous seven days at the 6 month follow-up, which, if accurate, results in a total quit rate of 10% (intention to treat) and 15% (respondents).
Relationship between smoking cessation and alcohol sobriety
In our study, 75% of respondents reported continuous alcohol abstinence in the previous 30 days at 6 months. These findings are similar to those seen by Joseph at 6 months in alcoholics who were treated for cigarette smoking concurrent to their alcohol treatment (67%) or in those treated six months later (74%) (Joseph et al., 2004). While our findings, given the modest number of subjects, must be considered preliminary, we found no evidence that those who were successful at smoking cessation in this study jeopardized their sobriety from alcohol. Those who quit cigarettes had better alcohol outcomes at six months in three of four alcohol outcome measures supporting earlier studies which reported improved drug or alcohol outcomes in persons who discontinued cigarettes (Gariti et al., 2002; Kalman et al., 2001; Miller et al., 1983). Participants who discontinued cigarettes reported no non-alcohol drug use at six months.
Affective and Anti-Social Personality Disorder
To our knowledge this is the first study to examine smoking quit rates in persons undergoing treatment for alcoholism with a history of affective disorder or with antisocial personality disorder (ASPD). Smoking cessation outcomes were similar in the affected and non-affected groups. While eligibility criteria excluded persons with currently symptomatic depression and any diagnosis of bipolar affective disorder, smoking outcomes were similar in those participants who reported a history of or symptoms suggestive of depression or mood instability as those who did not. Likewise, the Mean Beck Depression Inventory score was similar in those who were and were not abstinent from cigarettes at six months. Similarly, persons with and without antisocial personality disorder had comparable outcomes. Clearly alcoholics with ASPD or stable depressive disorders may be candidates for a cigarette quit attempt. Persons with cooccurring disorders (mood disorder + alcoholism or ASPD + alcoholism) are reported to have poorer SUD outcomes but, in this setting, had quit rates similar to those without the dual disorder.
Strengths and weaknesses
This study of in treatment alcoholics has a number of strengths. Alcoholics who are recently symptomatic are typically excluded from cigarette cessation studies. This is the first study of bupropion in alcoholics undergoing alcoholism treatment. Participants were recruited in a naturalistic fashion within one week of entry into multiple levels of care (residential, intensive outpatient or low intensity outpatient alcohol treatment) in a community and VA setting. They were heavy smokers and the majority had made multiple alcohol and cigarette quit attempts. Other drug use or treatment with many frequently prescribed anti-depressants did not preclude study enrollment. Persons with stable depression, anxiety, post traumatic stress disorder and anti-social personality disorder participated in the study. All participants were given an 8 week course of nicotine patch and encouraged to attend a one hour education and group session and continued with alcohol treatment as usual. The population studied is typical for many community and VA based treatment settings. Alcohol and cigarette outcomes were measured at six months.
There are several factors which contribute to the limitations of this study. Given the modest number of participants in this study, the findings discussed above must be considered preliminary. Secondly, we did not confirm participant reports of alcohol or cigarette use/abstinence bio-chemically and relied on self-report and the information provided by collaterals (in some cases the two collateral contacts disagreed with each other).While this practice is consistent with many previous smoking cessation studies, it does contribute to a lack of confidence about our findings. We were not reliably able to ascertain compliance with either the nicotine replacement product or bupropion/placebo capsules weeks 5-9 of the study which may have diluted any differences between the intervention and control groups. Given the apparent robust response to nicotine patch treatment in all participants and the size of the study population, a small difference in the two study groups would be difficult to detect. Participants were recruited from two publicly funded sites which largely serve low income alcoholics. Additionally, we did not enroll persons with a history of alcohol withdrawal seizures. These factors may make our findings less generalizable to a broader alcoholic population. Participants were paid a nominal amount for study participation which may have effected an individual's decision to participate in the study. Lastly, this study did not include a control group who received nicotine replacement only without the potential placebo effect afforded by the neutral capsules. Likewise, there was no group who received alcohol treatment as usual with delayed cigarette treatment.
This study of smoking cessation treatment in alcoholics resulted in a significant decrease in cigarette use in study participants which was sustained at six months. Subjects participated in the intervention at the onset of their alcohol treatment and those who quit smoking, in this preliminary study, showed a trend towards improved alcohol outcomes at six months.
Acknowledgements
This study was funded by the National Institute on Alcohol Abuse and Alcoholism (R21 AA13689-01). We would like to thank the staff at Catholic Charities Campus for Hope and at the Veterans Administration Nebraska Western Iowa Health Care System (Omaha site) Substance Use Disorders Program for their support, without which this project would not have been possible. We would also like to thank Dr Stephen I. Rennard for his thoughtful review of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- SAMHSA, editor. Results from the 2004 National Survey On Drug Use and Health: National Findings. United States Department of Health and Human Services; 2005. [Google Scholar]
- Ahluwalia JS, Harris KJ, Catley D, Okuyemi KS, Mayo MS. Sustained-release Bupropion for Smoking Cessation in African Americans: A Randomized Controlled Trial. JAMA. 2002;288:468–474. doi: 10.1001/jama.288.4.468. [DOI] [PubMed] [Google Scholar]
- Aubin HJ, Lebargy F, Berlin I, Bidaut-Mazel C, Chemali-Hudry J, Lagrue G. Efficacy of bupropion and predictors of successful outcome in a sample of French smokers: a randomized placebo-controlled trial. Addiction. 2004;99:1206–1218. doi: 10.1111/j.1360-0443.2004.00814.x. [DOI] [PubMed] [Google Scholar]
- Beck AT, Beamesderfer A. Assessment of depression: the depression inventory. Mod. Probl. Pharmacopsychiatry. 1974;7:151–169. doi: 10.1159/000395074. [DOI] [PubMed] [Google Scholar]
- Blot WJ, McLaughlin JK, Winn DM, Austin DF, Greenberg RS, Preston-Martin S, Bernstein L, Schoenberg JB, Stemhagen A, Fraumeni JF., Jr. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res. 1988;48:3282–3287. [PubMed] [Google Scholar]
- Bobo JK, Lando HA, Walker RD, McIlvain HE. Predictors of Tobacco Quit Attempts among Recovering Alcoholics. Journal of Substance Abuse. 1996;8:431–443. doi: 10.1016/s0899-3289(96)90004-8. [DOI] [PubMed] [Google Scholar]
- Bobo JK, McIlvain HE, Lando HA, Walker RD, Leed-Kelly A. Effect of smoking cessation counseling on recovery from alcoholism: findings from a randomized community intervention trial. Addiction. 1998;93:877–887. doi: 10.1046/j.1360-0443.1998.9368779.x. [DOI] [PubMed] [Google Scholar]
- Brower KJ, Aldrich MS, Robinson EA, Zucker RA, Greden JF. Insomnia, self-medication, and relapse to alcoholism. Am. J. Psychiatry. 2001;158:399–404. doi: 10.1176/appi.ajp.158.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Soto CB, O'Donnell WE, De Soto JL. Long-term recovery in alcoholics. Alcohol. Clin. Exp. Res. 1989;13:693–697. doi: 10.1111/j.1530-0277.1989.tb00406.x. [DOI] [PubMed] [Google Scholar]
- DiFranza JR, Guerrera MP. Alcoholism and smoking. J. Stud. Alcohol. 1990;51:130–135. doi: 10.15288/jsa.1990.51.130. [DOI] [PubMed] [Google Scholar]
- Evins AE, Mays VK, Rigotti NA, Tisdale T, Cather C, Goff DC. A pilot trial of bupropion added to cognitive behavioral therapy for smoking cessation in schizophrenia. Nicotine Tob. Res. 2001;3:397–403. doi: 10.1080/14622200110073920. [DOI] [PubMed] [Google Scholar]
- Ferris R. a. C. Mechanism of antidepressant activity of bupropion. Journal of Clinical Psychiatry Monograph. 1993;11:2–14. BR. [Google Scholar]
- Friend KB, Pagano ME. Smoking cessation and alcohol consumption in individuals in treatment for alcohol use disorders. J. Addict. Dis. 2005;24:61–75. doi: 10.1300/J069v24n02_06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gariti P, Alterman A, Mulvaney F, Mechanic K, Dhopesh V, Yu E, Chychula N, Sacks D. Nicotine intervention during detoxification and treatment for other substance use. Am. J. Drug Alcohol Abuse. 2002;28:671–679. doi: 10.1081/ada-120015875. [DOI] [PubMed] [Google Scholar]
- Gonzales DH, Nides MA, Ferry LH, Kustra RP, Jamerson BD, Segall N, Herrero LA, Krishen A, Sweeney A, Buaron K, Metz A. Bupropion SR as an aid to smoking cessation in smokers treated previously with bupropion: a randomized placebo-controlled study. Clin. Pharmacol. Ther. 2001;69:438–444. doi: 10.1067/mcp.2001.115750. [DOI] [PubMed] [Google Scholar]
- Grant KM, Agrawal S, Olsen DM, McIvor C, Romberger DJ. Smoking cessation in outpatient alcohol treatment. Addictive Disorders and Their Treatment. 2003;2:41–46. N. J. [Google Scholar]
- Graul AI, Prous JR. Executive summary: nicotine addiction. Drugs Today (Barc) 2005;41:419–425. doi: 10.1358/dot.2005.41.6.914907. [DOI] [PubMed] [Google Scholar]
- Hayford KE, Patten CA, Rummans TA, Schroeder DR, Offord KP, Croghan IT, Glover ED, Sachs DPL, Hurt RD. Efficacy of bupropion for smoking cessation in smokers with a former history of major depression of alcoholism. British Journal of Psychiatry. 1999;174:174–178. doi: 10.1192/bjp.174.2.173. [DOI] [PubMed] [Google Scholar]
- Hays JT, Schroeder DR, Offord KP, Croghan IT, Patten CA, Hurt RD, Jorenby DE, Fiore MC. Response to nicotine dependence treatment in smokers with current and past alcohol problems. Ann. Behav. Med. 1999;21:244–250. doi: 10.1007/BF02884841. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br. J. Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hertzberg MA, Moore SD, Feldman ME, Beckham JC. A preliminary study of bupropion sustained-release for smoking cessation in patients with chronic posttraumatic stress disorder. J. Clin. Psychopharmacol. 2001;21:94–98. doi: 10.1097/00004714-200102000-00017. [DOI] [PubMed] [Google Scholar]
- Horst WD, Preskorn SH. Mechanisms of action and clinical characteristics of three atypical antidepressants: venlafaxine, nefazodone, bupropion. J. Affect. Disord. 1998;51:237–254. doi: 10.1016/s0165-0327(98)00222-5. [DOI] [PubMed] [Google Scholar]
- Hughes JR. In: Clinical implications of the association between smoking and alcoholism. M. NR, editor. Vol. 30. NIH Publications; 1995. pp. 171–185. [Google Scholar]
- Hughes JR, Kalman D. Do smokers with alcohol problems have more difficulty quitting? Drug Alcohol. Depend. 2006;82:91–102. doi: 10.1016/j.drugalcdep.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Lancaster T. Antidepressants for smoking cessation (Review) The Cochrane Collaboration; 2006. S. L. [Google Scholar]
- Hurt RD, Dale LC, Offord KP, Croghan IT, Hays JT, Gomez-Dahl L. Nicotine patch therapy for smoking cessation in recovering alcoholics. Addiction. 1995;90:1541–1546. doi: 10.1046/j.1360-0443.1995.9011154112.x. [DOI] [PubMed] [Google Scholar]
- Hurt RD, Eberman KM, Croghan IT, Offord KP, Davis LJ, Jr., Morse RM, Palmen MA, Bruce BK. Nicotine dependence treatment during inpatient treatment for other addictions: a prospective intervention trial. Alcohol. Clin. Exp. Res. 1994;18:867–872. doi: 10.1111/j.1530-0277.1994.tb00052.x. [DOI] [PubMed] [Google Scholar]
- Hurt RD, Offord KP, Croghan IT, Gomez-Dahl L, Kottke TE, Morse RM, Melton LJ., 3rd Mortality following inpatient addictions treatment. Role of tobacco use in a community-based cohort. JAMA. 1996;275:1097–1103. doi: 10.1001/jama.275.14.1097. [DOI] [PubMed] [Google Scholar]
- Hurt RD, Sachs DP, Glover ED, Offord KP, Johnston JA, Dale LC, Khayrallah MA, Schroeder DR, Glover PN, Sullivan CR, et al. A comparison of sustained-release bupropion and placebo for smoking cessation. N. Engl. J. Med. 1997;337:1195–1202. doi: 10.1056/NEJM199710233371703. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Leischow SJ, Nides MA, Rennard SI, Johnston JA, Hughes AR, Smith SS, Muramoto ML, Daughton DM, Doan K, et al. A controlled trial of sustained-release bupropion, a nicotine patch, or both for smoking cessation. N. Engl. J. Med. 1999;340:685–691. doi: 10.1056/NEJM199903043400903. [DOI] [PubMed] [Google Scholar]
- Joseph AM, Willenbring ML, Nugent SM, Nelson DB. A randomized trial of concurrent versus delayed smoking intervention for patients in alcohol dependence treatment. J. Stud. Alcohol. 2004;65:681–691. doi: 10.15288/jsa.2004.65.681. [DOI] [PubMed] [Google Scholar]
- Kalman D, Hayes K, Colby SM, Eaton CA, Rohsenow DJ, Monti PM. Concurrent versus delayed smoking cessation treatment for person in early alcohol recovery: A pilot study. J. Subst. Abuse Treat. 2001;20:233–238. doi: 10.1016/s0740-5472(00)00174-4. [DOI] [PubMed] [Google Scholar]
- Kalman D, Kahler CW, Garvey AJ, Monti PM. High-dose nicotine patch therapy for smokers with a history of alcohol dependence: 36-week outcomes. J. Subst. Abuse Treat. 2006;30:213–217. doi: 10.1016/j.jsat.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Marks JL, Hill EM, Pomerleau CS, Mudd SA, Blow FC. Nicotine dependence and withdrawal in alcoholic and nonalcoholic ever-smokers. J. Subst. Abuse. Treat. 1997;14:521–527. doi: 10.1016/s0740-5472(97)00049-4. [DOI] [PubMed] [Google Scholar]
- Martin JE, Calfas KJ, Patten CA, Polarek M, Hofstetter CR, Noto J, Beach D. Prospective evaluation of three smoking interventions in 205 recovering alcoholics: one-year results of Project SCRAP-Tobacco. J. Consult. Clin. Psychol. 1997;65:190–194. doi: 10.1037//0022-006x.65.1.190. [DOI] [PubMed] [Google Scholar]
- Mellinger GD, Balter MB, Uhlenhuth EH. Insomnia and its treatment. Prevalence and correlates. Arch. Gen. Psychiatry. 1985;42:225–232. doi: 10.1001/archpsyc.1985.01790260019002. [DOI] [PubMed] [Google Scholar]
- Miller WR, Hedrick KE, Taylor CA. Addictive behaviors and life problems before and after behavioral treatment of problem drinkers. Addict. Behav. 1983;8:403–412. doi: 10.1016/0306-4603(83)90041-2. [DOI] [PubMed] [Google Scholar]
- Prochaska JJ, Delucchi K, Hall SM. A meta-analysis of smoking cessation interventions with individuals in substance abuse treatment or recovery. J. Consult. Clin. Psychol. 2004;72:1144–1156. doi: 10.1037/0022-006X.72.6.1144. [DOI] [PubMed] [Google Scholar]
- Prochaska JO, DiClemente CC. Stages of change in the modification of problem behaviors. Prog. Behav. Modif. 1992;28:183–218. [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry. 1998;59(Suppl 20):22–33. quiz 34-57. [PubMed] [Google Scholar]
- Skoloda TE, Gottheil E. Sleep quality reported by drinking and non-drinking alcoholics. Pergamon Press; Elmsford, NY: 1979. A. A. [Google Scholar]
- Slemmer JE, Martin BR, Damaj MI. Bupropion is a nicotinic antagonist. J. Pharmacol. Exp. Ther. 2000;295:321–327. [PubMed] [Google Scholar]
- Sobell L. a. S. Timeline Follow-Back: A technique for assessing self-reported alcohol consumption. Humana Press; Totowa, NJ: 1992. MB. [Google Scholar]
- Sobell M, Sobell LC, Kozlowski LT. In: Alcohol and Tobacco: From Basic Science to Clinical Practice In Addiction. N. NM, editor. Vol. 30. U.S. Department of Health and Human Services, Public Health Service; 1995. pp. 207–224. [Google Scholar]
- Stuyt EB. Recovery rates after treatment for alcohol/drug dependence. Tobacco users vs. non-tobacco users. Am. J. Addict. 1997;6:159–167. [PubMed] [Google Scholar]
- Vaillant GE, Schnurr PP, Baron JA, Gerber PD. A prospective study of the effects of cigarette smoking and alcohol abuse on mortality. J. Gen. Intern. Med. 1991;6:299–304. doi: 10.1007/BF02597425. [DOI] [PubMed] [Google Scholar]
- Velicer WF, Prochaska JO. A comparison of four self-report smoking cessation outcome measures. Addict. Behav. 2004;29:51–60. doi: 10.1016/s0306-4603(03)00084-4. [DOI] [PubMed] [Google Scholar]
- Weiner E, Ball MP, Summerfelt A, Gold J, Buchanan RW. Effects of sustained-release bupropion and supportive group therapy on cigarette consumption in patients with schizophrenia. Am. J. Psychiatry. 2001;158:635–637. doi: 10.1176/appi.ajp.158.4.635. [DOI] [PubMed] [Google Scholar]