Abstract
Caspase-8, a member of the caspase family, plays an important role in apoptotic signal transduction in mammals. Here we report the identification and characterization of the caspase-8 (casp8) gene in the zebrafish Danio rerio. The zebrafish casp8 gene has a genomic organization similar to mammalian casp8 genes, consisting of 10 exons. By chromosome mapping, we found that casp8 maps on linkage group 6 (LG6), a zebrafish chromosome segment orthologous to the long arm of human Chr. 2, which carries CASP8. In contrast, the zebrafish casp10-like gene and the cflar gene separately localize on LG9 and LG11, respectively, and these genes form a cluster with CASP8 on the human chromosome. This chromosomal segregation is unique to fish but not other vertebrates. Furthermore, we examined the function of zebrafish Casp8 protein in mammalian cells, and showed that it has pro-apoptotic activity when overexpressed. In addition, this molecule was capable of transmitting apoptotic signals mediated through not only Fas but also the TNF receptor in mouse Casp8-deficient cells. Expression analysis showed that casp8 is maternally expressed, and transcripts continue to be present throughout embryogenesis and into larval stages. These results show that zebrafish casp8 has a structure and function similar to mammalian CASP8 orthologs, and our study suggests that the role of caspase-8 in the apoptotic signal pathway has been conserved over at least 450 million years of vertebrate evolution.
Keywords: caspase-8, caspase-10, c-FLIP, evolution, zebrafish
1. Introduction
Apoptosis, or programmed cell death, plays an important role in the development of multicellular organisms and is critical for the maintenance of normal homeostasis (Raff, 1992; Jacobson et al., 1997; Vaux and Korsmeyer, 1999; Meier et al., 2000). During apoptosis, caspases, members of a conserved family of cysteine proteases, play a central role as executioners (Cryns and Yuan, 1998). Currently, 15 caspases in mammals, seven in flies, and four in worms have been identified, and there appears to be an increased number of caspases with increasing evolutionary complexity (Lamkanfi et al., 2002). In vertebrates, most mammalian caspase homologs have also been identified in Xenopus (Yaoita and Nakajima, 1997; Nakajima et al., 2000) as well as in birds and fish (Inohara and Nunez, 2000; Lamkanfi et al., 2002). Among these, caspase-8 (also known as FLICE/MACH1/Mch5) has an extended amino-terminal prodomain, the “death effector domain” (DED), and a carboxyl-terminal catalytic domain (CASc) (Boldin et al., 1996; Muzio et al., 1996; Sakamaki et al., 1998; Nakajima et al., 2000). Caspase-8 is a key effector molecule in apoptotic induction mediated through cell surface “death receptors” such as Fas (APO-1/CD95) in mammals. Oligomerization of Fas by an agonistic antibody or its ligand FasL recruits the adaptor molecule FADD (Fas-associated death domain protein, also termed MORT1) to the cytosolic domain of Fas. Procaspase-8 then associates with FADD through homophilic interactions mediated by the DEDs. The Fas-FADD-procaspase-8 complex is referred to as the “death-inducing signaling complex” (DISC) (Kischkel et al., 1995) and induces the auto-cleavage and activation of procaspase-8. Activated caspase-8 subsequently triggers a downstream caspase cascade leading to cell death (Lavrik et al., 2003). Cells deficient in caspase-8 fail to undergo Fas-mediated apoptosis (Juo et al., 1998; Kawahara et al., 1998; Varfolomeev et al., 1998). Apoptotic signals induced by ligation of tumor necrosis factor type I receptor (TNFR1) and receptors for TNF-related apoptosis-inducing ligand (TRAIL) also require caspase-8 (Thorburn, 2004). Thus, caspase-8 is indispensable for the induction of apoptosis downstream of multiple different death receptors in mammals. Furthermore, an essential role for caspase-8 during development was identified using mice deficient in caspase-8 expression. Deletion of caspase-8 is embryonic lethal, and these mice exhibit gross developmental defects in multiple tissues (Varfolomeev et al., 1998; Sakamaki et al., 2002). In humans, deletion or inactivation of caspase-8 causes aggressive neuroblastoma when accompanied by amplification of the myc gene in these cells (Teitz et al., 2000). Human caspase-8 is also thought to function as a tumor suppressor in these circumstances, but its precise function remains unclear. Thus, caspase-8 plays multiple, essential roles in mammals.
The zebrafish Danio rerio is a useful model organism for the study of development because of its short gestation period, only two to three days, and mutations causing developmental defects are easily detected. Apoptosis is also easily detected during zebrafish embryogenesis. In eggs and several tissues including the brain, apoptosis occurs during the course of normal development (Furutani-Seiki et al., 1996; Chan and Yager, 1998; Ikegami et al., 1999; Goltzene et al., 2000; Williams et al., 2000; Cole and Ross, 2001; Yamashita, 2003). In addition, several apoptosis-regulating genes have been identified in zebrafish based on their high homology with mammalian genes (Inohara and Nunez, 2000; Eimon et al., 2006). Recently, two death receptors were identified in zebrafish; one is specifically expressed in embryonic hematopoietic cells and the other is detected in the ovary (Long et al., 2000; Bobe and Goetz, 2001). These death receptors may function similarly to their mammalian orthologs for the extrinsic apoptotic pathway. Thus, zebrafish is a suitable organism for improving our understanding of the molecular mechanisms regulating apoptosis in vivo.
To understand the physiological roles of caspase-8 in vertebrates, we isolated the zebrafish ortholog of human caspase-8 and investigated its functions. In the present study, we report the genomic structure of the zebrafish caspase-8 gene, its chromosomal location, its expression profile and its role in inducing cell death and in embryogenesis. Our studies clearly demonstrate that zebrafish caspase-8 has strong structural and functional similarities to its mammalian orthologs, and these data suggest that the physiological role of caspase-8 has been conserved among vertebrates for at least 450 million years since the divergence of human and zebrafish lineages.
2. Materials and methods
2.1. Animals, cell lines and reagents
The zebrafish Danio rerio used in this study were derived from an AB strain. Animals were kept in a light and temperature controlled facility and maintained at optimal breeding conditions (Westerfield, 1994). Embryos produced by natural mating were staged according to the method of Kimmel et al (Kimmel et al., 1995). Mouse embryonic fibroblasts (MEF) isolated from wild-type and caspase-8-deficient mouse embryos (Sakamaki et al., 2002), human HeLa cells and HEK293T were cultured in Dulbecco’s Modified Eagle’s medium with 10% fetal calf serum. Monoclonal anti-human Fas (CH11) and anti-mouse Fas (Jo2) antibodies were obtained from MBL Co. (Nagoya, Japan) and BD-PharMingen (San Diego, CA). TNFα (Sigma Chemicals Co., St. Louis, MO), the peptide caspase inhibitor carbobenzoyl-Val-Ala-Asp-fluoromethylketone (z-VAD-fmk) (Kamiya Biomedical Co., Seattle, WA), and 5-bromo-4-chloro-3-indolyl-β-D-galactoside (X-Gal, Nacali Tesque, Kyoto, Japan) were purchased.
2.2. Genetic mapping
For meiotic mapping of the zebrafish caspase-8 (casp8) gene, we identified genetic polymorphisms segregating in the heat shock (HS) meiotic mapping panel. This mapping panel consists of 42 homozygous diploid individuals produced by HS treatment of haploid embryos at the one-cell stage (HS diploids) (Kelly et al., 2000; Woods et al., 2000 and 2005). HS diploid individuals are homozygous at all loci, and allow the identification of slight polymorphic differences between individuals. In addition, HS diploids can survive to become adults and provide more DNA for mapping assays (Postlethwait et al., 1998; Gates et al., 1999). The casp8 gene was examined among over 4500 PCR-based markers, including genes, expressed sequence tags (ESTs), and simple sequence length polymorphisms (Shimoda et al., 1999) already scored on the HS mapping panel (Postlethwait et al., 2000; Woods et al., 2000). Genetic polymorphisms were detected using single-strand conformation polymorphism (SSCP) analysis (Beier, 1993). For SSCP analysis, genomic DNA from the mapping cross was amplified using primers specific for the 3’ untranslated region of casp8 (EST clone, fd18f10); forward primer 5’-CAATCTTCCGTTTTTCTGTTACTGG-3’ and reverse primer 5’-ATCTTTGTGTTCCTAGTTCAGCCATTT-3’ were used. In each mapping PCR, one primer was end-labeled using [α-32P] ATP for detection by autoradiography on acrylamide gels. Strain distribution patterns in the mapping panel were analyzed using MapManager (Manly, 1993). Sequences for simple sequence length polymorphisms were obtained from Shimoda et al. (Shimoda et al., 1999).
2.3. Computational analysis
Comparative mapping was performed by first determining the human (Homo sapiens) and mouse (Mus musculus) orthologs by the reciprocal best hit protocol (Hirsh and Fraser, 2001), and then consulting public databases to identify the genomic location of the orthologs. Zebrafish genes and EST sequences downloaded from GenBank were assigned putative human orthologs by BLASTX searches with the concatenated 5’ and 3’ sequence of the EST against the NCBI human nonredundant protein sequence database. If the results of these searches gave expected scores (e-scores) of 1e-15 or less, the putative orthologs were further tested with reciprocal TBLASTN searches against the zebrafish subset of nonredundant sequences and dbEST databases. A human ortholog was confirmed if the original zebrafish gene or EST (or a gene or EST in the same UniGene cluster) was in the top five matches of the reciprocal search by TBLASTN. Map positions for orthologs were found using the OMIM and LocusLink databases. Mouse orthologs were identified using the HomoloGene database, and their genomic locations were found using Locuslink and the Mouse Genome Database (http://www.informatics.jax.org).
For comparative mapping of the casp8, caspase-10 (casp10), casp8/10 (caspase-8/10 homolog) and cflar loci, the genome sequences of chicken (Gallus gallus) and human from the GenBank database, the genome sequences of zebrafish, West African clawed frog (Xenopus tropicalis) and pufferfish (Takifugu rubripes) from the Ensembl (http://www.ensembl.org/) and JGI (http://genome.jgi-psf.org/) databases and the genome sequence of freshwater pufferfish (Tetraodon nigroviridis) from the Genoscope database (http://www.genoscope.cns.fr/externe/tetranew/) were used (Supplementary Table 1).
2.4. Molecular phylogenetic analysis
We used the published or predicted amino acid sequences for Casp8, Casp10, Casp8/10, cFlar and CARD-Casp8 from available mammal (human and mouse), bird (chicken), amphibian (frog), fish [Medaka (Oryzias latipes), fugu, halibut (Paralichthys olivaceus) and zebrafish] and for Casp8/10/cFlar from ascidian (Ciona intestinalis, Ciona savignyi and Molgula tectiformis) databases (Supplementary Table 2). For construction of the molecular phylogenetic tree, we aligned amino acid sequence of the CASc using the T-COFFEE program (Notredame et al., 2000). Based on this alignment, a molecular phylogenetic tree was constructed using the Maximum likelihood method implemented in the phyml program (Guindon and Gascuel, 2003) with the WAG amino acid substitution matrix (Whelan and Goldman, 2001), the proportion of invariant sites calculated from the alignment, and four rate categories with a gamma distribution parameter estimated from the data. The trees were tested with 100 bootstrap replications. For confirmation of phylogenetic relevancy, the Bayesian tree was also constructed by the MRBAYES program (ver3.1.2) (Ronquist and Huelsenbeck, 2003). One hundred thousand generations of two independent runs were conducted (each with four chains).
2.5. Construction and transfection of expression vectors
To express zebrafish casp8 cDNA in mammalian cell lines, cloned fish cDNA was fused with DNA sequence encoding a Flag tag and inserted into the mammalian expression vector pME18S (Sakamaki et al., 1992). To generate a protease-deficient zebrafish Casp8 mutant, Casp8C355S, the cysteine residue (amino acid 355) in the QACQG protease active site of the CASc domain was mutated to serine by a performing polymerase chain reaction (PCR) with an oligonucleotide encoding the appropriate codon change. This mutant casp8C355S cDNA was also inserted into the pME18S-Flag vector. The plasmid pME18S-hCASP8 carrying human CASP8 cDNA has been previously generated (Hirata et al., 1998). The viral gene encoding a cytokine response modifier A (CrmA), the kind gift of Dr. D. J. Pickup (Duke University), was cloned into the pCAGGS expression vector (Niwa et al., 1991) to generate pCX-CrmA. The reporter plasmids pJ7-LacZ carrying the lacZ gene (Sakamaki et al., 1998) and pCX-EGFP carrying enhanced green fluorescent protein (EGFP) (Okabe et al., 1997) were used for cytotoxicity assays. The plasmid phLBR1TM-EGFP, which is a kind gift of Dr. J. Ellenberg (EMBL), was used for flow cytometric analysis.
Transfection of these plasmid constructs into cells was performed using LipofectAMINE PLUS Reagent (Invitrogen, Carlsbad, CA), according to manufacturer’s instructions.
2.6. Cytotoxicity assays
Twenty-four hours after transfection with zebrafish casp8 or human CASP8 cDNA plus pJ7-LacZ, HeLa cells were fixed in 2% formaldehyde and 0.2% glutaraldehyde, stained with X-Gal as described previously (Sakamaki et al., 1998), and were examined for cell viability microscopically (DMIRE2, Leica Microsystems, Wetzlar, Germany). For examination of susceptibility of transfectants to Fas- or TNFα-induced apoptotic stimulation, HeLa or MEF cells expressing zebrafish Casp8 or mutant Casp8C355S were treated with either anti-human Fas antibody CH11 (100 ng/ml), anti-mouse Fas antibody Jo2 (100 ng/ml) or TNFα (100 ng/ml) in the presence of 10 μg/ml cycloheximide (CHX). Following 6 h incubation MEF cells transfected with pJ7-LacZ were examined under a phase-contrast microscope for enumeration of β-galactosidase (βGal)-positive blue cells, and cells were photographed after staining with X-Gal. The percentage of surviving MEF cells was determined by the number of cells treated with anti-Fas antibody or TNFα divided by the number of untreated cells. The viability of HeLa cells cotransfected with pCX-EGFP was examined by monitoring EGFP-expressing cells under the microscope, and positive transfectants were photographed in phase contrast and fluorescence over the same area.
2.7. Flow cytometric analysis
Twenty-four hours after transfection with zebrafish casp8 or the casp8 mutant with or without the CrmA gene, HeLa cells were fixed in 70% ethanol at -20 °C for 1 h as described previously (Sakamaki et al., 2004). For selection of transfected and untransfected cells, we cotransfected with phLBR1TM-EGFP, which encodes a nuclear fusion protein consisting of LBR and EGFP. Following fixation, cells were washed with PBS, treated with RNase A (50 μg/ml) in PBS at 37°C for 30 min, and stained with 50 μg/ml of propidium iodide (PI) in PBS for 30 min. The DNA content of EGFP-positive cells was then analyzed by flow cytometry (XL™, BECKMAN-COULTER, Miami, FL).
2.8. PCR and reverse-transcriptase-PCR (RT-PCR) analyses
For sequencing of exon 9 of the casp8 gene, genomic DNA was isolated from the liver of adult fish and amplified by PCR with primers (forward: 5′-AGCAGAATGCATTTCCAGATTGAG-3′, reverse: 5′-GCTCCTGGATAAAGATAGAGCCCT-3′) with an initial denaturation at 96°C for 3 min followed by 30 cycles at 94°C for 45 sec, 58°C for 60 sec, 72°C for 90 sec and extension at 72°C for 5 min. Amplified PCR products were subcloned into pCRII (Invitrogen) and sequenced.
Total RNA was isolated from 20 embryos collected at various stages using Isogen (Nippongene, Toyama, Japan) according to the manufacturer′s recommendations. For RT-PCR analysis, 2 μg of each RNA sample was used for first-strand cDNA synthesis with an oligo-dT primer on Ready-To-Go RT-PCR beads (Amersham Biosciences, Arlington Heights, IL), according to the manufacturer′s recommendations. First-strand cDNAs were then amplified with two sets of primers: primers (forward: 5′-CCAGACAATCTGGATGAACTTTAC-3′, reverse: 5′-TGCAAACTGCTTTATCTCATCT-3′) for the detection of caspase-8 transcripts, and primers (forward: 5′-TC AAGCCTGGTATGGTTGTGACCT-3′, reverse: 5′-GTGCAGACTTTGTGACCTTGCCAG-3′) for EF1α transcripts as a positive control. PCR was carried out as above and resulting products were analyzed by 1% agarose-gel electrophoresis.
3. Results
3.1. Characterization of the zebrafish caspase-8 gene
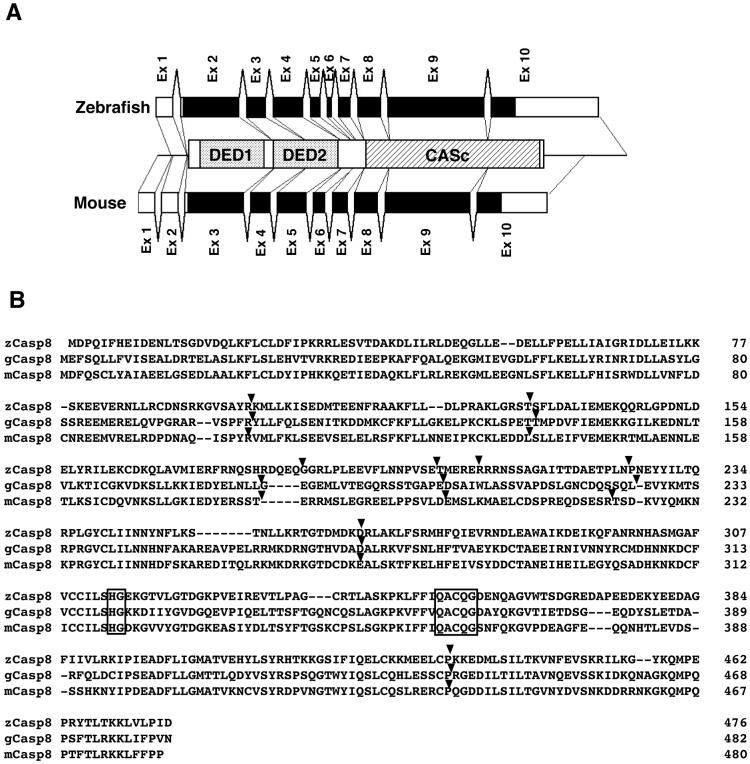
We searched for zebrafish homologs of the mammalian caspase-8 (casp8) gene in the GenBank DNA database and identified two EST clones, fd18f10 and fc87g09. Sequencing of these clones demonstrated that they encode a protein similar to mammalian caspase-8 (Supplementary Fig. 1). The identical gene was previously identified and suggested to be casp8 (Inohara and Nunez, 2000), although the precise characterization of this gene has not yet been performed. First we examined the genomic organization of this zebrafish casp8 gene and compared it to that of the mammalian casp8 gene. By searching the GenBank genome database, we found several Whole Genome Shotgun (WGS) sequences containing the putative zebrafish casp8 gene (Supplementary Table 3), but the complete sequence of the casp8 gene had not yet been published. We amplified genomic DNA by PCR and determined the entire sequence of exon 9 of the casp8 gene. By comparing the genomic sequences to the obtained casp8 cDNA sequence, we confirmed that the casp8 gene consists of 10 exons and 9 introns (Fig. 1A). Additionally, the observed differences between the two EST clones (fd18f10 and fc87g09) arise from allelic polymorphisms because the WGS and direct sequences were intermediate between these clones (data not shown). The genomic structure of the zebrafish casp8 gene completely corresponded to those of the mouse and chicken Casp8 genes with coincident splice junction sites (Fig. 1B). The nucleotide sequences of the exon-intron boundaries of the zebrafish gene, shown in Supplementary Table 3, agree with the GT-AG rule (Breathnach and Chambon, 1981). These results show that the genomic structure of the casp8 is conserved between fish and mammals
Fig. 1.
Analysis of the genomic structure of the zebrafish caspase-8 (casp8) gene.
(A) Schematic diagram of the casp8 genes. Both zebrafish and mouse casp8 genes are organized into 10 exons. Black and white boxes indicate the coding and non-coding regions, respectively. (B) Comparison of the splice junction sites in zebrafish, chicken and mouse caspase-8. Arrowheads on the amino acid alignment indicate the splice junction sites in the zebrafish, chicken and mouse genes, respectively. Amino acids essential for catalytic activity are indicated by a box.
3.2. Chromosome mapping of the zebrafish caspase-8 gene
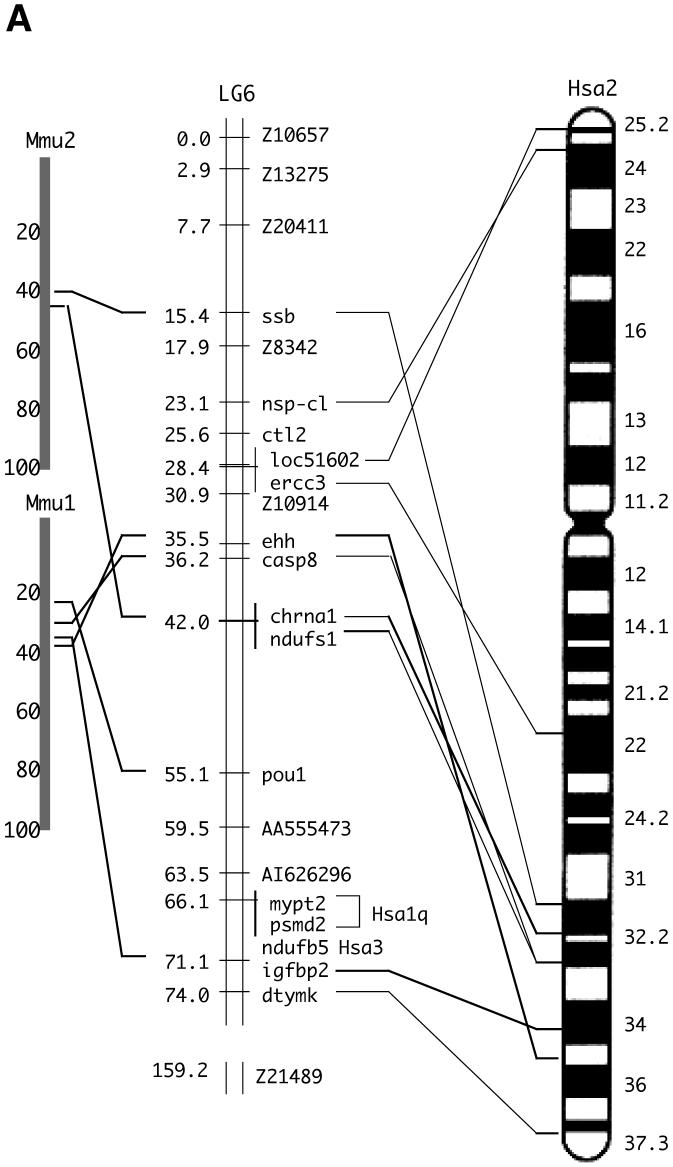
We next examined the genomic location of the EST clones fd18f10 and fc87g09 on the zebrafish chromosomes by meiotic mapping and SSCP analysis. Both clones mapped to the upper arm of linkage group 6 (LG6) with high statistical significance (Table 1). By ortholog analysis, we identified that this linkage group has conserved syntenies with the long arm of human Chr. 2 (Hsa2q) (Fig. 2A). The human CASP8 gene resides on this chromosomal segment. Additional genes distributed along much of the length of Hsa2q have putative orthologs on the upper arm of LG6 (Fig. 2A). These comparative mapping data support the assignment of the zebrafish casp8 gene as an ortholog of human CASP8.
Table 1.
Mapping statistics for the zebrafish casp8 gene.
| Marker | Acc no | Pos | Mat | Pat | X | N | Map | ±SE | 95% | interval | LOD |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Z10914 | G39842 | 30.9 | 19 | 22 | |||||||
| 1 | 40 | 2.50 | 2.47 | 0.1 | 13.2 | 10.0 | |||||
| Z22712 | G39512 | 33.4 | 19 | 22 | |||||||
| 1 | 35 | 2.86 | 2.82 | 0.1 | 14.9 | 8.6 | |||||
| casp8 | AY518734/AY518735 | 36.2 | 17 | 18 | |||||||
| 2 | 35 | 5.71 | 3.92 | 0.7 | 19.2 | 7.2 | |||||
| ndufs1 | AI437455 | 42.0 | 18 | 24 | |||||||
| 5 | 38 | 13.16 | 5.48 | 4.4 | 28.1 | 5.0 | |||||
| pou1 | D13045 | 55.1 | 17 | 21 | |||||||
| 1 | 23 | 4.35 | 4.25 | 0.1 | 21.9 | 5.1 | |||||
| AA555473 AA555473 | 59.5 | 12 | 13 | ||||||||
Locus, Simple sequence length polymorphism, cloned gene, or EST placed on the HS panel (only a few of the 81 loci mapped on this chromosome are shown); Acc no, the accession number at GenBank; Pos, position in centiMorgan (cM) from upper end of the chromosome on the HS panel; Mat, number of segregants with the maternal genotype; Pat, number of segregants with the paternal genotype; X, number of recombinants between the two adjacent markers; N, number of segregants scored for the two adjacent markers; Map, the genetic distance between the two adjacent markers in cM; ±SE, ±standard error in cM; 95%, lower and upper range of the 95% confidence interval in cM; LOD, logarithm of the odds.
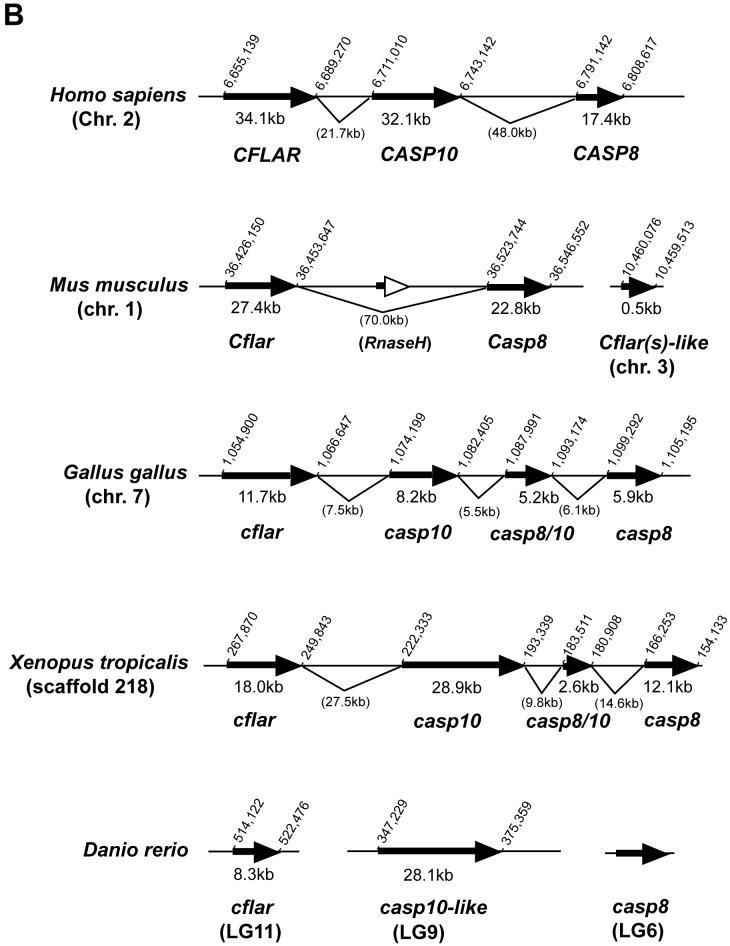
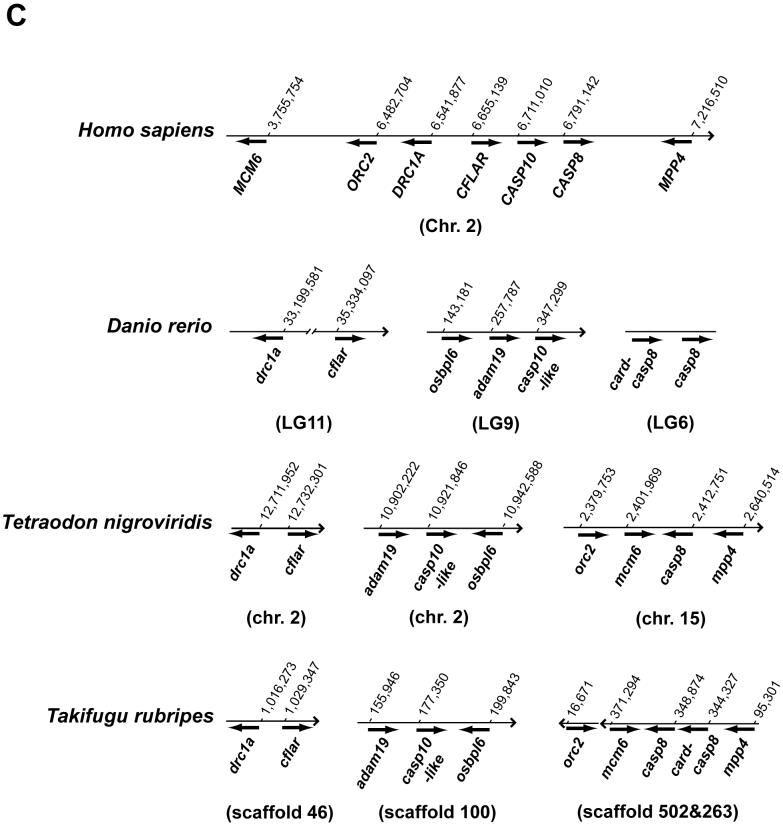
Fig. 2.
Chromosomal analyses of the caspase-8 gene and its orthologs.
(A) Chromosomal mapping of the zebrafish casp8 gene. Center, zebrafish linkage group 6 (LG6), numbers indicate distance from the top of the chromosome in centiMorgans, with casp8 at 36.2 centiMorgans. Left and right, locations of mouse (left) and human (right) orthologs of human Chr. 2 genes mapped on LG6. (B) Physical map of the region including the casp8 gene and its related genes in vertebrates. The bold arrows indicate the coding region and orientation of the gene. In human, the CASP8, CASP10 and CFLAR genes form a cluster on Chr. 2. In zebrafish, the casp8, casp10 and cflar genes localize on different chromosomes. Rodents have lost the Casp10 gene following the insertion of RnaseH. The chicken and frog have an additional caspase-8/10-homologus gene, casp8/10 between the casp8 and casp10 genes. (C) Comparative mapping of the CASP8, CASP10 and CFLAR genes in human and fish. The bold arrows indicate the coding region and orientation of the gene. The casp8-like gene resides near the casp8 gene on both zebrafish and fugu chromosomes. Abbreviated genes: adam19; a disintegrin and metalloproteinase domain 19; DRC1A, down-regulated by CTNNB1 protein A, MCM6, minichromosome maintenance protein 6; MPP4; MAGUK p55 subfamily member 4; osbpl6, oxysterol-binding protein-like protein 6; ORC2, origin recognition complex, subunit 2.
Caspase-10 (Casp10) possesses two DEDs and an active CASc protease domain, and its overall structure is similar to Casp8 (Lamkanfi et al., 2002). Although Casp8 has an established role as an initiator of death receptor-mediated apoptosis, the function of Casp10 is almost completely unknown even in humans (Fischer et al., 2006). Cflar (also termed as c-FLIP) also consists of two DEDs and an inactive CASc, and it is an important regulator of Casp8 (Thorburn, 2004). Therefore, we hypothesized that the casp8, casp10 and cflar genes may be phylogenetically related. In the human genome, these three genes are aligned within a 153-kb region on Hsa2q33 according to LocusLink (Fig. 2B). Similarly, we determined that these genes in the mouse, chicken and West African clawed frog genomes localize within 120-kb, 50-kb and 113-kb regions on the same chromosome, respectively, with the exception of the absent mouse Casp10 gene (Fig. 2B). Thus, these genes form a cluster in mammals, birds and amphibians. Additionally, we found a third casp8/10-homologus gene (casp8/10) localizing between the casp8 and casp10 genes in both chicken and frog. Based on the sequence of the chicken casp8/10 cDNA, we confirmed that the casp8/10 gene has a similar genomic structure as casp8, casp10 and cflar (Supplementary Fig. 2).
By searching the Ensembl database of the zebrafish genome sequence, we identified ENSDARG00000058335 on scaffold2888 as highly homologous to casp8 (Fig. 2B). Within the Zv6 assembly, this scaffold corresponds to LG20, and this genomic location is inconsistent with our direct identification. Using two primer sets in independent experiments to map casp8, however, we confirmed casp8 localization on LG6 with very strong statistical support. Therefore, we cannot exclude the possibility that the zebrafish genome is incorrectly assembled in this region. Further analysis of the genomic sequence of the casp10 and cflar homologs showed that the casp10-like gene (ENSDART00000014766 listed in Supplementary Table 1) corresponds to the casp10 or a second casp8 gene located on LG9, and the cflar gene (GenBank accession no. NM_194399 listed in Supplementary Table 1) localizes on LG11 in the zebrafish genome on our HS mapping panel (Woods et al., 2005). These computational analyses clearly indicate chromosomal rearrangement of these three genes in zebrafish relative to tetrapods. To confirm whether this event is unique in fish, we analyzed the genomes of two pufferfish species, Tetraodon nigroviridis and Takifugu rubripes, available in public databases (Fig. 2C and Supplementary Table 1). The chromosome segment including the casp8, casp10 and cflar genes is also rearranged in these fish. Additionally, putative orthologs of DRC1A, MCM6, MMP4 and ORC2, which are located near the CASP8, CASP10 and CFLAR genes on human Chr. 6, also localize with either the casp8 or cflar gene in these three fish genomes. Furthermore, we identified an additional casp8-like gene (GenBank accession nos. CT693310 and AL728411, tentatively termed as card-casp8) closely localizing to the casp8 gene in the zebrafish, fugu and Medaka genomes (Fig. 2C and Sakamaki et al, manuscript submitted). Based on the amino acid sequence of the card-casp8 genes analyzed by SMART, the predicted proteins possess an active CASc and caspase recruitment domain (CARD), but lack N-terminal DED motifs (Sakamaki et al, manuscript submitted). Thus, chromosome mapping of the casp8 and related genes in vertebrates indicates that these genes form a cluster of genes that are highly similar structurally in a number of different species, and they are independently segregated in fish after the divergence of vertebrates.
3.3. Molecular phylogenetic analysis of caspase-8 and related genes in animals
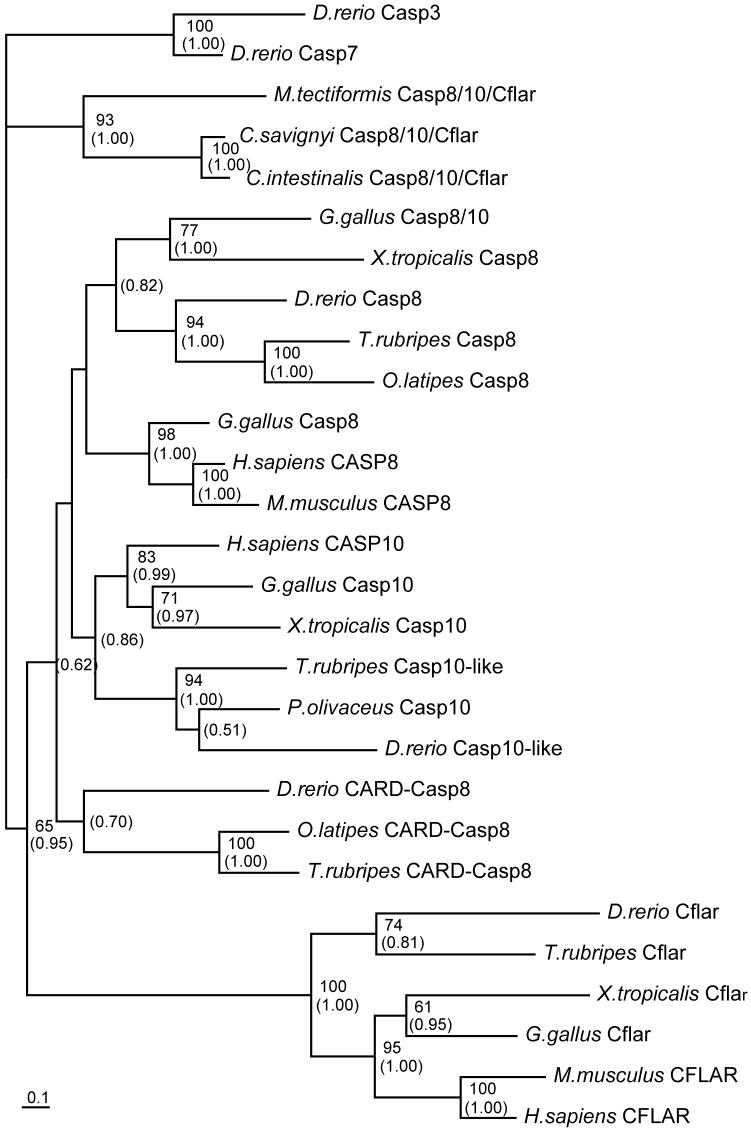
To examine phylogenetic relationships among these Casp8-related molecules, we constructed a molecular phylogenetic tree including molecules identified in this and previous studies (Supplementary Table 2). The phylogenetic tree was constructed based on an alignment of a CASc protease domain (Fig. 3). Zebrafish Casp8 resides in a clade containing fugu and Medaka Casp8. Phylogenetic analysis also suggests that fish Casp8 forms a branch with Xenopus Casp8 and chicken Casp8/10. Zebrafish Casp8-related molecules, Casp10-like and CARD-Casp8 constitute each clade different from Casp8. We conclude that an ancient fish, the last common ancestor of zebrafish, fugu and Medaka also had these casp8, casp10-like and card-casp8 genes (Fig. 3 and Sakamaki et al., manuscript submitted). Although the generated tree was not sufficient to show orthologies between Casp8 of amniotes and fish/amphibian Casp8, it clearly showed that Cflar and Casp10/Casp10-like molecules form a discrete subfamily. In the unchordate ascidian genome, one gene similar to casp8 was identified (Terajima et al., 2003). The tree also indicated that the ascidian gene product represents an ancestral form of vertebrate Casp8, Casp8/10, Casp10 and Cflar. Namely, the last common ancestor of ascidians and vertebrates, had only one ancestral gene casp8/10/cflar, and after the divergence of ascidians and vertebrates, this casp8/10/cflar gene diverged into casp8, casp8/10, casp10 and cflar in vertebrates. In this study, we confirmed this hypothesis by determining a cDNA sequence of casp8/10/cflar of another ascidian, Molgula tectiformis (accession number DQ900614). Our hypothesis is also supported by a report that there is only one gene orthologous for casp8 in the Drosophila genome (Chen et al., 1998).
Fig. 3.
A molecular phylogenetic tree of caspase-8 and related proteins generated by the Maximum likelihood method.
The amino acid sequences of caspase-8 (Casp8), caspase-8/10-homologue (Casp8/10), caspase-10 (Casp10), Cflar and CARD-Casp8 and ancestral Casp8/10/Cflar were analyzed from the databases. A rooted tree was generated based on the alignment of the amino acid sequences deduced from eleven species of animals. CASc protease domains of zebrafish Casp3 and Casp7 were used as the outgroup proteins for rooting the tree. The upper number noted at a given branch indicates the percentage of times that a node was supported in 100 bootstrap replications and the lower number indicates the posterior probability of the Bayesian phylogenetic analysis. These numbers are shown only on branches with greater than 60% for the bootstrap value or greater than 0.50 for posterior probability. The scale bar indicates an evolutionary distance of 0.1 amino acid substitutions per position. Ascidians (C. intestinalis, C. savignyi and M. tectiformis), chicken (G. gallus), fugu (T. rubripes), halibut (P. olivaceus), human (H. sapiens), Medaka (O. latipes), mouse (M. musculus), West African clawed frog (X. tropicalis) and zebrafish (D. rerio) listed with the sequence accession numbers in Supplementary Table 2 were analyzed.
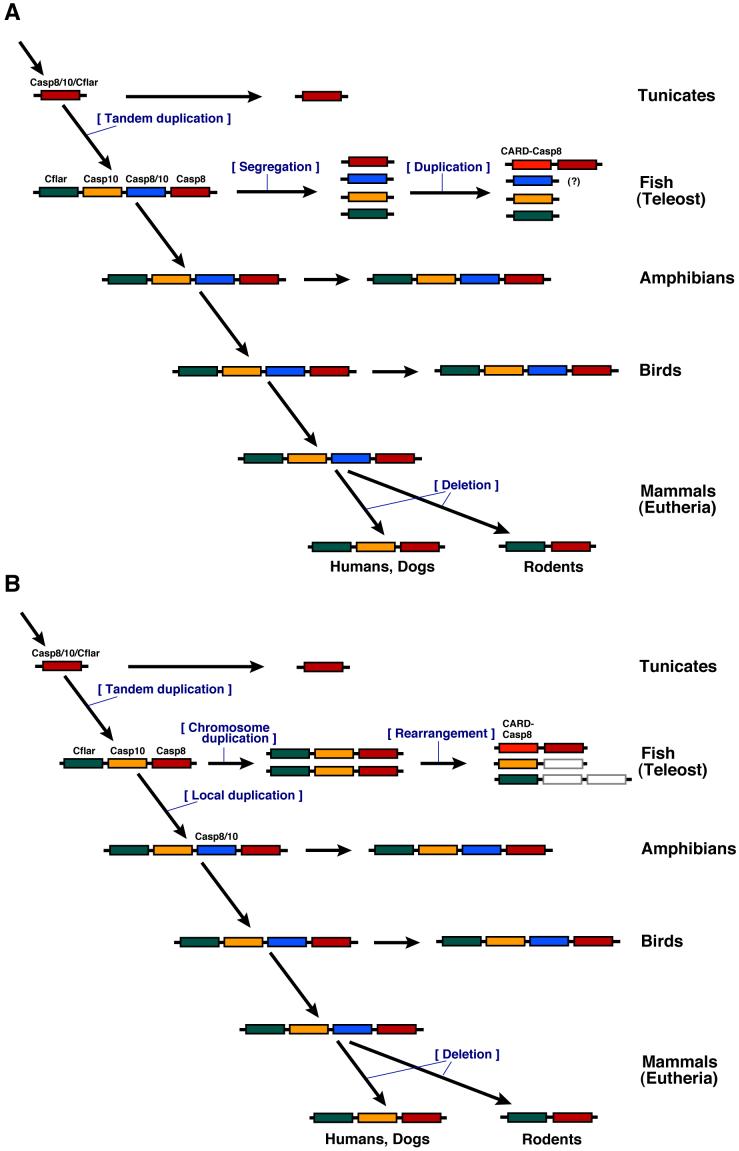
Taking into account of all of the above data, we present a hypothesis for evolution of this gene family (Fig. 4). Before the emergence of vertebrates, there was only one casp8/10/cflar gene in the ancestral genome, which is highly similar to the extant ascidian gene. Early during vertebrate evolution, the casp8, casp10, casp8/10 and cflar genes arose from tandem duplication events of the ancestral casp8/10/cflar gene. After the divergence of vertebrates, casp10 or a third casp8/10 gene was deleted from the genome of some animals. In the fish lineage, the duplicated genes were further translocated to different chromosomes and the ancient casp8 gene is locally duplicated to become both casp8 and card-casp8, which might occur once in the fish genome. As an alternative explanation, the fish casp8, casp10 and cflar genes became doubled on chromosomes duplicated by the teleost genome duplication event. In zebrafish, the doublet localizing on two chromosomes, one of which became LG6 and one LG9, which have been shown to be duplicated chromosomes (Amores et al., 1998; Postlethwait et al., 1998 and 2000; Woods et al., 2005), caused rearrangement during the course of evolution. Namely, casp8 but not casp10 was lost from LG9, and casp10 but not casp8 was lost from LG6. A translocation may then have separated cflar from the other genes.
Fig.4.
Summary of the evolutionary insights regarding casp8 and related genes provided by phylogenetic and chromosome mapping analyses.
(Explanation A) Early during vertebrate evolution, the four casp8, casp10, casp8/10 and cflar genes arose from tandem duplication events of the ancestral casp8/10/cflar gene. In the fish lineage, the duplicated genes were further translocated to different chromosomes and the ancient casp8 gene is locally duplicated to become both casp8 and card-casp8. In mammals, the casp10 or a third casp8/10 gene was deleted from the genome. The casp8/10 gene has not yet been identified in the fish. (Explanation B) Early during vertebrate evolution, the three casp8, casp10 and cflar genes arose from tandem duplication of the ancestral casp8/10/cflar gene. In fish, these genes became doubled on chromosomes duplicated by the teleost genome duplication event. During the course of evolution, casp8 but not casp10 was lost from one chromosome, and casp10 but not casp8 was lost from the duplicated chromosome. A chromosome rearrangement also led to separation of the cflar gene from the other genes and a local duplication event generated the casp8 and card-casp8 genes. After evolution to tetrapods, an additional casp8/10 gene arose from duplication and the casp10 or a third casp8/10 gene was deleted from the genome in mammals.
3.4. Functional analyses of zebrafish caspase-8 in mammalian cells
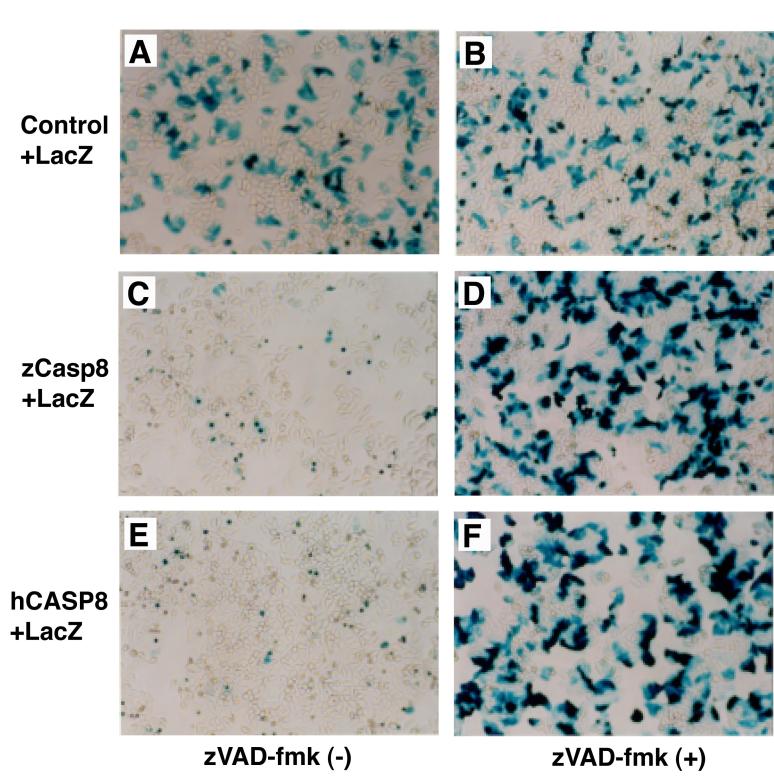
Given the high degree of homology between zebrafish and mammalian Casp8 proteins, we hypothesized that Casp8 also conserves a role in regulating apoptosis. Overexpression of mammalian and amphibian Casp8 molecules induces cell death without exogenous apoptotic stimulation (Boldin et al., 1996; Muzio et al., 1996; Sakamaki et al., 1998; Sakamaki et al., 2004). Therefore, we examined whether zebrafish Casp8 proteins expressed in mammalian cell lines could induce cell death. The Fas-mediated apoptotic signaling pathway is intact in human HeLa cells, and we used these cells to examine the pro-apoptotic activity of zebrafish caspase-8. HeLa cells were transfected with either zebrafish or human casp8 cDNA and a reporter plasmid carrying the lacZ gene. After 24 h culture and staining with X-Gal, cell viability was examined by detecting βGal-positive cells under the microscope. A large number of cells cotransfected with control vector and reporter plasmids were βGal-positive demonstrating the efficiency of transfection (Fig. 5A). In contrast, few βGal-positive cells were observed after cotransfection with zebrafish casp8 and lacZ, indicating that ectopic expression of zebrafish Casp8 induced cell death (Fig. 5C). In the presence of the pan-caspase inhibitor z-VAD-fmk, however, the number of βGal-positive cells increased and was comparable to cells transfected with the control plasmid (Fig. 5B and D). These data suggest that zebrafish Casp8-induced cell death is apoptotic death associated with caspase activation. Confirming the activity of the inhibitor, we found that the killing activity and sensitivity to z-VAD-fmk of zebrafish Casp8 were consistent with human CASP8 (Fig. 5E and F). Taken together, these data support a role for zebrafish Casp8 in regulating apoptosis in mammalian cells.
Fig. 5.
Overexpressed zebrafish caspase-8 induces apoptosis in human HeLa cells.
(A-F) Control plasmid vector pME18S (A and B) or pME18S-Flag/zCasp8 carrying zebrafish casp8 cDNA (C and D) or pME18S-hCASP8 carrying human CASP8 cDNA (E and F) were transfected along with a reporter plasmid pJ7-LacZ into HeLa cells in the absence (A, C and E) or presence (B, D and F) of 100 μM z-VAD-fmk, a peptide caspase inhibitor. After 24 h, cells were fixed, stained with X-Gal and photographed under the microscope. Viable transfectants were observed as βGal-positive blue cells.
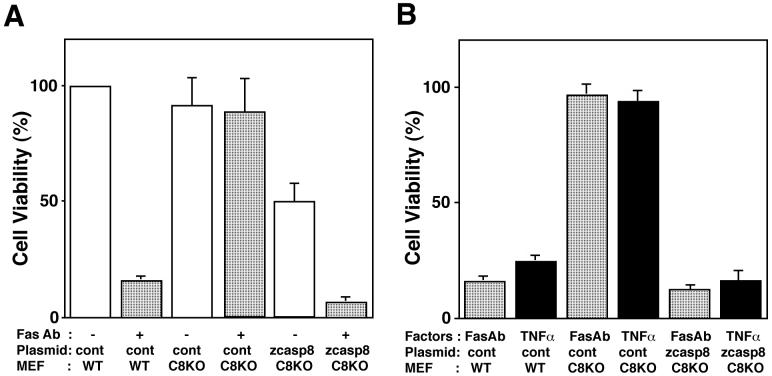
Using Casp8-deficient mouse embryonic fibroblast (MEF) cells (Sakamaki et al., 2002), we next examined the regulation of zebrafish Casp8 activity following ligation of Fas with an agonistic anti-Fas antibody, a potent apoptotic stimulant. We hypothesized that zebrafish Casp8 could substitute for the function of its mammalian orthologs. To examine this hypothesis, we introduced zebrafish casp8 cDNA with the lacZ gene into MEF cells deficient in caspase-8 expression. Although wild-type MEF cells were sensitive to apoptosis induced by the anti-mouse Fas antibody Jo2, Casp8-deficient MEF cells, which are unable to transmit Fas-mediated apoptotic signals, survived even in the presence of Jo2 (Fig. 6A). Exogenous expression of zebrafish Casp8 in Casp8-deficient MEF cells triggered cell death without ligation of Fas. Additionally, Casp8-expressing cells were highly sensitive to anti-Fas treatment and 80% of the transfectants died following Fas ligation (Fig. 6A). These results clearly indicate that zebrafish Casp8 directly transmits apoptotic signals via Fas to downstream molecules, showing that zebrafish Casp8 is able to replace the function of its mouse homologue in the apoptotic signaling pathway.
Fig. 6.
Zebrafish caspase-8 substitutes for the function of its mammalian orthologs.
(A, B) Wild-type (WT) and Casp8-deficient (C8KO) MEF cells transiently transfected with plasmid pME18S-Flag/zCasp8 (zcasp8) or control vector (cont) and reporter plasmid pJ7-LacZ were stimulated for 6 h with 100 ng/ml of anti-Fas antibody Jo-2 (A and B) and 100 ng/ml of TNFα (B), respectively, in the presence of cycloheximide (CHX). Cell viability was calculated by counting βGal-positive cells after treatment as described in Materials and methods.
Caspase-8 is also required for TNFR1- and TRAIL receptor-mediated apoptosis (Thorburn, 2004). To address whether zebrafish Casp8 functions as an initiator for death receptor-mediated apoptosis, we examined the susceptibility of mouse Casp8-deficient MEF transfectants expressing zebrafish Casp8 to TNFα (Fig. 6B). Mouse cells expressing zebrafish Casp8 were sensitive to TNFα as well as anti-Fas antibody whereas control Casp8-deficient MEF cells showed no response to these stimuli. This confirms a role for zebrafish Casp8 in transmitting apoptotic signals downstream of mouse death receptors.
3.5. Characterization of zebrafish caspase-8-induced cell death
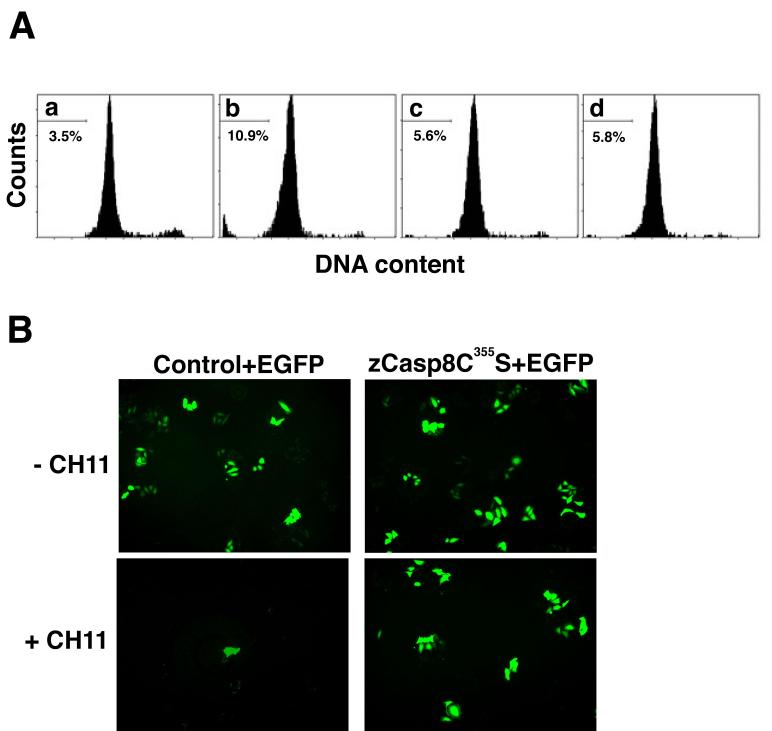
We next characterized transfectants expressing zebrafish Casp8 with a quantitative flow cytometric method designed to determine the apoptotic potential of this molecule. As shown in Fig. 7A, overexpression of zebrafish Casp8 in HeLa cells increased the proportion of the cellular population found in a sub-G1 fraction, indicating one of apoptotic characteristics (Lamm et al., 1997). In contrast, coexpression of zebrafish Casp8 and CrmA, an apoptosis inhibitor that specifically blocks the protease activity of caspase-8 as well as caspase-1 (Zhou et al., 1997), reduced the number of sub-G1 cells. Expression of a protease-deficient zebrafish caspase-8 mutant (Casp8C355S) in HeLa cells did not induce cell death. We also examined the ability of zebrafish Casp8 to activate effector caspases in HeLa cells by immunoblot analysis. Zebrafish Casp8-expressing HeLa cells had increased active caspase-3 (data not shown). Thus, zebrafish Casp8 activates the caspase cascade associated with activation of downstream effector caspases and leads to apoptotic cell death in mammalian cells.
Fig. 7.
Characterization of zebrafish caspase-8-induced apoptotic signal transduction.
(A) The DNA content of transfectants expressing zebrafish caspase-8 or its mutant was assessed by flow cytometry. Transfectants carrying pME18S (panel a), pME18S-Flag/zCasp8 (panel b), both pME18S-Flag/zCasp8 and pCX-CrmA (panel c) or pME18S-Flag/zCasp8C355S (panel d) were analyzed for the detection of the DNA content stained with PI. Percentage indicates the cellular population detected in the sub-G1 fraction of DNA content. (B) HeLa cells were transfected with pME18S-Flag/zCasp8C355S (right panels) or control vector (left panels) together with pCX-EGFP, cultured for 48 h and treated with (lower panels) or without (upper panels) 100 ng/ml of anti-Fas antibody CH11 and 10 μg/ml of CHX for 6 h. Viable cells were detected as EGFP-positive cells under the fluorescent microscope.
3.6. Interaction of zebrafish caspase-8 with human apoptotic signaling molecules
In mammals, the adaptor molecule FADD associates with Casp8 through homophilic DED interactions (Boldin et al., 1996; Muzio et al., 1996). We investigated whether human FADD interacts with zebrafish Casp8. HA-tagged human FADD was coexpressed with Flag-tagged zebrafish Casp8 in HEK293T cells, and we examined their interaction by coimmunoprecipitation. Zebrafish Casp8 and human FADD were detected in immunoprecipitates (Supplementary Fig. 3). These data suggest that zebrafish Casp8 is able to interact with a mammalian molecule through a homophilic DED interaction. Furthermore, we examined whether mutant Casp8C355S expressed in HeLa cells disturbs Fas-mediated apoptosis. HeLa cells, which endogenously express Fas, are sensitive to apoptotic stimuli following treatment with the anti-human Fas antibody CH11. When cells were cotransfected with Casp8C355S and EGFP, however, we observed greatly reduced cell death following treatment with CH11 compared with control transfected cells (Fig. 7B). These data suggest that Casp8C355S acts as a dominant-negative molecule by interacting with FADD, inhibiting the activation of endogenous human CASP8. These results indicate that zebrafish Casp8 functions in human cells in a manner indistinguishable from mammalian CASP8.
3.7. Expression of caspase-8 during embryonic development
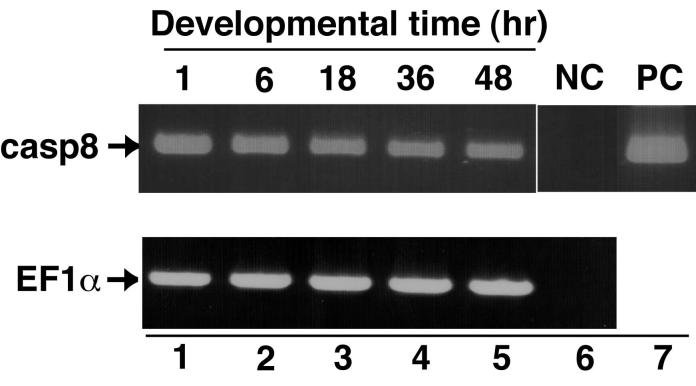
Finally, we investigated caspase-8 expression during zebrafish embryonic development. We isolated total RNA at different zebrafish developmental stages and examined caspase-8 mRNA expression by RT-PCR. Caspase-8 transcripts were detected in all samples prepared from cleavage (pre-midblastula transition), gastrula, segmentation, pharyngula and hatching periods (Fig. 8). Although we also examined the spatial profile of caspase-8 expression in embryos by whole-mount in situ hybridization, a specific pattern of caspase-8 expression was not observed (data not shown). Taken together, these results show that caspase-8 mRNA exists in both maternal and zygotic transcripts during embryonic development.
Fig. 8.
Expression of zebrafish caspase-8 in embryos.
The expression profile of caspase-8 during embryonic development. RT-PCR analysis of caspase-8 transcripts was performed with total RNA isolated from embryos collected at 1, 6, 18, 36 and 48 h after fertilization (lanes 1-5). Aliquots of the synthesized first strand cDNAs were amplified with primers for casp8 (Upper) or EF1α as a control (Bottom) and analyzed by agarose-gel electrophoresis. No template DNA and zebrafish casp8 cDNA samples as negative and positive controls were examined in the lanes 6 and 7, respectively.
4. Discussion
Using a combination of functional assays and computational genomic structural analyses we clearly identified the zebrafish ortholog of mammalian caspase-8. The caspase-8 gene appears to be evolutionarily conserved between fish and mammals at both the sequence and functional level. Furthermore, we confirmed that the caspase-8 and related caspase-10 and cflar genes form a cluster on the same chromosome in mammals, birds and amphibians but they are chromosomally segregated in fish. We also showed that overexpression of zebrafish Casp8 protein promotes apoptosis in mammalian cells downstream of death receptors, and in MEFs lacking endogenous Casp8, zebrafish Casp8 can restore normal apoptotic activity. Based on these results, we conclude that, like its mammalian homolog, zebrafish caspase-8 is an executioner caspase within the apoptotic signaling pathway. Thus, our results strongly suggest that the extrinsic signals regulating apoptosis are highly evolutionarily conserved from fish to mammals.
Comparative genomic mapping revealed that the chromosomal segment containing the caspase-8 gene has remained largely intact in zebrafish and humans (Fig. 2A) since the two lineages diverged 450 million years ago (Kumar and Hedges, 1998). In contrast, this region was split between two chromosomes in the mouse genome, Mmu1 and Mmu2. This genomic region appears to be more conserved between zebrafish and human than between mice and humans, suggesting that a chromosomal rearrangement occurred during rodent evolution. Indeed the Casp10 gene has been deleted in rodents (Fig. 2B). A genome duplication event occurred in the zebrafish lineage at the base of the teleost radiation (Amores et al., 1998; Postlethwait et al., 1998; Naruse et al., 2000). As a consequence of this duplication, zebrafish has two orthologs of many human genes -- it appears that about 30% of loci in the zebrafish genome are duplicates retained from this duplication event in the ray fin fish lineage (Postlethwait et al., 2000; Taylor et al., 2001 and 2003). Because LG6 is duplicated on a portion of LG9 (Postlethwait and Talbot, 1997; Postlethwait et al., 1998), any casp8 duplicates should reside on LG9. Consistent with this, we identified a candidate gene on LG9. Although further analysis is required for a definitive conclusion, the zebrafish casp10-like gene likely represents a fish counterpart to the Casp10 gene identified in other vertebrates. In the Tetraodon and fugu genome databases, the gene (GIDT00023376001 and NEWSINFRUG00000161667 listed in Supplementary Table 1) corresponding to the zebrafish casp10-like gene showed a high degree of similarity to human CASP10 at the amino acid level with e-scores of 1e-66 and 1e-53. In addition, these casp10-like genes showed high similarity to the halibut casp10 (Fig. 3 and data not shown). Thus, based on the mapping of the caspase-8 and related genes, we demonstrated that chromosomal segregation of these genes has independently occurred in fish despite the preservation of this genomic segment through the course of 450 million years of evolution since the divergence of zebrafish and humans.
In the Zv6 assembly close to the casp8 gene, we identified a casp8-like gene, which we tentatively designated card-casp8 (Fig. 2C). In the fugu and Medaka genomes, the same card-casp8 gene was also identified next to the casp8 gene (Fig. 2C and Sakamaki et al., manuscript submitted). These gene products showed high similarity to human and other vertebrate CASP8 and CASP10 sequences when their protease domains were compared. However, the card-casp8 gene lacks the region corresponding to the DED, which is essential for CASP8 and CASP10 function, and, in its place, it encodes an N-terminal CARD (Sakamaki et al., manuscript submitted). Strictly speaking, the card-casp8 gene is not a third casp8/10 gene, as identified in the frog and chicken genomes. The third casp8/10 gene may be missing from the fish genome or has not yet been identified. Over the course of fish evolution, an ancestral casp8 gene has diverged into both casp8 and casp8-like genes by local duplication and the casp8-like gene may have replaced its DED with a CARD motif.
During embryogenesis, we detected caspase-8 transcripts in 4-cell eggs during the cleavage period, long before the mid-blastula transition when zygotic genes begin to be expressed (Kane et al., 1992). This evidence suggests that caspase-8 mRNA exists as maternal transcripts. Furthermore, we detected caspase-8 transcripts in embryos of the gastrula, segmentation, pharyngula and hatching periods during development (Fig. 8). Consistent with this, several caspase-8 EST clones registered in the GenBank database originated in zebrafish embryos. By whole-mount in situ hybridization, we examined the spatial profile of caspase-8 expression and observed ubiquitous expression of caspase-8 transcripts in whole embryos (data not shown). In mouse, caspase-8 transcripts were detected in embryos at mid-gestation (Sakamaki et al., 1998) and in ES cells (data not shown). In previous studies, we and another group showed the abnormal development of several tissues in Casp8-deficient mouse embryos, suggesting that caspase-8 is an essential molecule for embryogenesis (Varfolomeev et al., 1998; Sakamaki et al., 2002). Furthermore, caspase-8 is expressed in early stages of Xenopus embryonic development (Kominami et al., 2006), and the exogenous expression of a protease-defect caspase-8 mutant disturbed normal development (data not shown). Therefore, we speculate that caspase-8 plays an important role during embryonic development of fish embryos as well as mammal/amphibian embryos.
Zebrafish are frequently used for genetic research on vertebrate development, immune system function, and human disease (Zon, 1999; Roest Crollius and Weissenbach, 2005). Few reports have investigated apoptotic signal transduction pathways in zebrafish, but a role for apoptosis in fish development is well documented (Furutani-Seiki et al., 1996; Chan and Yager, 1998; Ikegami et al., 1999; Goltzene et al., 2000; Williams et al., 2000; Yamashita, 2003). In addition to casp8, several other genes involved in apoptosis have been identified in the zebrafish with high homology to mammalian genes (Inohara and Nunez, 2000; Long et al., 2000; Bobe and Goetz, 2001; Yabu et al., 2001). Recently, Fas ligand, Fas, and FADD, essential components forming the DISC complex with caspase-8, have been identified in zebrafish (Eimon et al., 2006). Taken together with the present report, these data argue for a high degree of functional and structural conservation of apoptotic signaling in fish and mammals. Additionally, there is an increased role for zebrafish as a model organism for understanding the common conserved components of the apoptotic machinery.
We provide the first direct evidence of the evolutionary conservation of caspase-8 between fish and mammals based on the genomic organization, protein structure, and function. The remarkable conservation between zebrafish and mammalian caspase-8 implies similar physiological roles for caspase-8 among these animals. Conversely, the identification of the casp8/10 and card-casp8 genes in the genomes of birds, amphibians and fish suggests that further consideration of the interrelationship of mammalian caspase-8 and caspase-10 may be needed. Further genomic analysis of the caspase-8 gene and its relatives in zebrafish should resolve the conservation and diversification of apoptotic pathways throughout evolution. In addition, zebrafish are an excellent model for increasing our understanding of apoptosis in embryonic development and homeostasis.
Supplementary Material
Acknowledgments
The authors are grateful to Drs. D. Pickup (Duke University) and J. Ellenberg (EMBL) for providing the CrmA gene and the LBR-EGFP construct and Drs. T. Hirano and S. Yamashita (Osaka University) for their experimental support. The complete sequences of zebrafish caspase-8, chicken caspase-8/10-like and Molgula tectiformis caspase-8 cDNAs have been deposited in the GenBank database with accession numbers AY518734, AY518735, DQ010055 and DQ900614, respectively. This work was supported in part by a Grant-in-Aid from the Ministry of Education, Science and Culture of Japan (K.S.) and by NIH grants 9 P01HD22486 and R01RR10715 in USA (J.H.P.).
Abbreviations
- ADAM19
a disintegrin and metalloproteinase domain 19
- βGal
β-galactosidase
- CARD
caspase recruitment domain
- CASc
caspase catalytic domain
- CHX
cycloheximide
- CrmA
cytokine response modifier A
- DD
death domain
- DED
death effector domain
- DISC
death-inducing signaling complex
- DRC1A
down-regulated by CTNNB1 protein A
- EGFP
enhanced green fluorescent protein
- EST
expressed sequence tag
- e-score
expected score
- FADD
Fas-associated death domain protein
- HS
heat shock
- LG
linkage group
- MCM6
minichromosome maintenance protein 6
- MEF
mouse embryonic fibroblasts
- MPP4
MAGUK p55 subfamily member 4
- MO
morpholino oligonucleotide
- OSBPL6
oxysterol-binding protein-like protein 6
- ORC2
origin recognition complex, subunit 2
- PI
propidium iodide
- RT-PCR
reverse-transcriptase-polymerase chain reaction
- SSCP
single-strand conformation polymorphism
- SSLP
simple sequence length polymorphisms
- TNFα
tumor necrosis factor-α
- TNFR1
tumor necrosis factor receptor type 1
- TRAIL
TNF-related apoptosis-inducing ligand
- WGS
Whole Genome Shotgun
- X-Gal
5-bromo-4-chloro-3-indolyl-β-D-galactoside
- zVAD-fmk
carbobenzoyl-Val-Ala-Asp-fluoromethylketone
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amores A, Force A, Yan YL, Joly L, Amemiya C, Fritz A, Ho RK, Langeland J, Prince V, Wang YL, Westerfield M, Ekker M, Postlethwait JH. Zebrafish hox clusters and vertebrate genome evolution. Science. 1998;282:1711–1714. doi: 10.1126/science.282.5394.1711. [DOI] [PubMed] [Google Scholar]
- Beier DR. Single-strand conformation polymorphism (SSCP) analysis as a tool for genetic mapping. Mamm. Genome. 1993;4:627–631. doi: 10.1007/BF00360898. [DOI] [PubMed] [Google Scholar]
- Bobe J, Goetz FW. Molecular cloning and expression of a TNF receptor and two TNF ligands in the fish ovary. Comp. Biochem. Physiol. B, Biochem. Mol. Biol. 2001;129:475–481. doi: 10.1016/s1096-4959(01)00353-0. [DOI] [PubMed] [Google Scholar]
- Boldin MP, Goncharov TM, Goltsev YV, Wallach D. Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1- and TNF receptor-induced cell death. Cell. 1996;85:803–815. doi: 10.1016/s0092-8674(00)81265-9. [DOI] [PubMed] [Google Scholar]
- Breathnach R, Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu. Rev. Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Chan DW, Yager TD. Preparation and imaging of nuclear spreads from cells of the zebrafish embryo. Evidence for large degradation intermediates in apoptosis. Chromosoma. 1998;107:39–60. doi: 10.1007/s004120050280. [DOI] [PubMed] [Google Scholar]
- Chen P, Rodriguez A, Erskine R, Thach T, Abrams JM. Dredd, a novel effector of the apoptosis activators reaper, grim, and hid in Drosophila. Dev. Biol. 1998;201:202–216. doi: 10.1006/dbio.1998.9000. [DOI] [PubMed] [Google Scholar]
- Cole LK, Ross LS. Apoptosis in the developing zebrafish embryo. Dev. Biol. 2001;240:123–142. doi: 10.1006/dbio.2001.0432. [DOI] [PubMed] [Google Scholar]
- Cryns V, Yuan J. Proteases to die for. Genes Dev. 1998;12:1551–1570. doi: 10.1101/gad.12.11.1551. [DOI] [PubMed] [Google Scholar]
- Eimon PM, Kratz E, Varfolomeev E, Hymowitz SG, Stern H, Zha J, Ashkenazi A. Delineation of the cell-extrinsic apoptosis pathway in the zebrafish. Cell Death Differ. 2006;13:1619–1630. doi: 10.1038/sj.cdd.4402015. [DOI] [PubMed] [Google Scholar]
- Fischer U, Stroh C, Schulze-Osthoff K. Unique and overlapping substrate specificities of caspase-8 and caspase-10. Oncogene. 2006;25:152–159. doi: 10.1038/sj.onc.1209015. [DOI] [PubMed] [Google Scholar]
- Furutani-Seiki M, et al. Neural degeneration mutants in the zebrafish, Danio rerio. Development. 1996;123:229–239. doi: 10.1242/dev.123.1.229. [DOI] [PubMed] [Google Scholar]
- Gates MA, Kim L, Egan ES, Cardozo T, Sirotkin HI, Dougan ST, Lashkari D, Abagyan R, Schier AF, Talbot WS. A genetic linkage map for zebrafish: comparative analysis and localization of genes and expressed sequences. Genome Res. 1999;9:334–347. [PubMed] [Google Scholar]
- Goltzene F, Skalski M, Wolff CM, Meyer D, Mager-Heckel AM, Darribere T, Remy P. Heterotopic expression of the Xl-Fli transcription factor during xenopus embryogenesis: modification of cell adhesion and engagement in the apoptotic pathway. Exp. Cell Res. 2000;260:233–247. doi: 10.1006/excr.2000.5005. [DOI] [PubMed] [Google Scholar]
- Guindon S, Gascuel O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 2003;52:696–704. doi: 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
- Hirata H, Takahashi A, Kobayashi S, Yonehara S, Sawai H, Okazaki T, Yamamoto K, Sasada M. Caspases are activated in a branched protease cascade and control distinct downstream processes in Fas-induced apoptosis. J. Exp. Med. 1998;187:587–600. doi: 10.1084/jem.187.4.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsh AE, Fraser HB. Protein dispensability and rate of evolution. Nature. 2001;411:1046–1049. doi: 10.1038/35082561. [DOI] [PubMed] [Google Scholar]
- Ikegami R, Hunter P, Yager TD. Developmental activation of the capability to undergo checkpoint-induced apoptosis in the early zebrafish embryo. Dev. Biol. 1999;209:409–433. doi: 10.1006/dbio.1999.9243. [DOI] [PubMed] [Google Scholar]
- Inohara N, Nunez G. Genes with homology to mammalian apoptosis regulators identified in zebrafish. Cell Death Differ. 2000;7:509–510. doi: 10.1038/sj.cdd.4400679. [DOI] [PubMed] [Google Scholar]
- Jacobson MD, Weil M, Raff MC. Programmed cell death in animal development. Cell. 1997;88:347–354. doi: 10.1016/s0092-8674(00)81873-5. [DOI] [PubMed] [Google Scholar]
- Juo P, Kuo CJ, Yuan J, Blenis J. Essential requirement for caspase-8/FLICE in the initiation of the Fas-induced apoptotic cascade. Curr. Biol. 1998;8:1001–1008. doi: 10.1016/s0960-9822(07)00420-4. [DOI] [PubMed] [Google Scholar]
- Kane DA, Warga RM, Kimmel CB. Mitotic domains in the early embryo of the zebrafish. Nature. 1992;360:735–737. doi: 10.1038/360735a0. [DOI] [PubMed] [Google Scholar]
- Kawahara A, Ohsawa Y, Matsumura H, Uchiyama Y, Nagata S. Caspase-independent cell killing by Fas-associated protein with death domain. J. Cell Biol. 1998;143:1353–1360. doi: 10.1083/jcb.143.5.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly PD, et al. Genetic linkage mapping of zebrafish genes and ESTs. Genome Res. 2000;10:558–567. doi: 10.1101/gr.10.4.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Kischkel FC, Hellbardt S, Behrmann I, Germer M, Pawlita M, Krammer PH, Peter ME. Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO J. 1995;14:5579–5588. doi: 10.1002/j.1460-2075.1995.tb00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kominami K, Takagi C, Nozaki M, Kurata T, Kitayama A, Nozaki M, Sawasaki T, Kuida K, Endo Y, Manabe N, Ueno N, Sakamaki K. The initiator caspase, caspase-10β, and the BH-3-only molecule, Bid, demonstrate evolutionary conservation in Xenopus of their pro-apoptotic activities in the extrinsic and intrinsic pathways. Genes Cells. 2006;11:701–711. doi: 10.1111/j.1365-2443.2006.00983.x. [DOI] [PubMed] [Google Scholar]
- Kumar S, Hedges SB. A molecular timescale for vertebrate evolution. Nature. 1998;392:917–920. doi: 10.1038/31927. [DOI] [PubMed] [Google Scholar]
- Lamkanfi M, Declercq W, Kalai M, Saelens X, Vandenabeele P. Alice in caspase land. A phylogenetic analysis of caspases from worm to man. Cell Death Differ. 2002;9:358–361. doi: 10.1038/sj.cdd.4400989. [DOI] [PubMed] [Google Scholar]
- Lamm GM, Steinlein P, Cotten M, Christofori G. A rapid, quantitative and inexpensive method for detecting apoptosis by flow cytometry in transiently transfected cells. Nucleic Acids Res. 1997;25:4855–4857. doi: 10.1093/nar/25.23.4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavrik I, Krueger A, Schmitz I, Baumann S, Weyd H, Krammer PH, Kirchhoff S. The active caspase-8 heterotetramer is formed at the CD95 DISC. Cell Death Differ. 2003;10:144–145. doi: 10.1038/sj.cdd.4401156. [DOI] [PubMed] [Google Scholar]
- Long Q, Huang H, Shafizadeh E, Liu N, Lin S. Stimulation of erythropoiesis by inhibiting a new hematopoietic death receptor in transgenic zebrafish. Nat. Cell Biol. 2000;2:549–552. doi: 10.1038/35019592. [DOI] [PubMed] [Google Scholar]
- Manly KF. A Macintosh program for storage and analysis of experimental genetic mapping data. Mamm. Genome. 1993;4:303–313. doi: 10.1007/BF00357089. [DOI] [PubMed] [Google Scholar]
- Meier P, Finch A, Evan G. Apoptosis in development. Nature. 2000;407:796–801. doi: 10.1038/35037734. [DOI] [PubMed] [Google Scholar]
- Muzio M, Chinnaiyan AM, Kischkel FC, O′Rourke K, Shevchenko A, Ni J, Scaffidi C, Bretz JD, Zhang M, Gentz R, Mann M, Krammer PH, Peter ME, Dixit VM. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death--inducing signaling complex. Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- Nakajima K, Takahashi A, Yaoita Y. Structure, expression, and function of the Xenopus laevis caspase family. J. Biol. Chem. 2000;275:10484–10491. doi: 10.1074/jbc.275.14.10484. [DOI] [PubMed] [Google Scholar]
- Notredame C, Higgins DG, Heringa J. T-Coffee: A novel method for fast and accurate multiple sequence alignment. J. Mol. Biol. 2000;302:205–217. doi: 10.1006/jmbi.2000.4042. [DOI] [PubMed] [Google Scholar]
- Naruse K, et al. A detailed linkage map of medaka, Oryzias latipes: comparative genomics and genome evolution. Genetics. 2000;154:1773–1784. doi: 10.1093/genetics/154.4.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193–199. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- Okabe M, Ikawa M, Kominami K, Nakanishi T, Nishimune Y. ′Green mice′ as a source of ubiquitous green cells. FEBS Lett. 1997;407:313–319. doi: 10.1016/s0014-5793(97)00313-x. [DOI] [PubMed] [Google Scholar]
- Postlethwait JH, Talbot WS. Zebrafish genomics: from mutants to genes. Trends Genet. 1997;13:183–190. doi: 10.1016/s0168-9525(97)01129-3. [DOI] [PubMed] [Google Scholar]
- Postlethwait JH, et al. Vertebrate genome evolution and the zebrafish gene map. Nat. Genet. 1998;18:345–349. doi: 10.1038/ng0498-345. [DOI] [PubMed] [Google Scholar]
- Postlethwait JH, Woods IG, Ngo-Hazelett P, Yan YL, Kelly PD, Chu F, Huang H, Hill-Force A, Talbot WS. The zebrafish gene map and origins of mammalian chromosomes. Genome Res. 2000;10:1890–1902. doi: 10.1101/gr.164800. [DOI] [PubMed] [Google Scholar]
- Raff MC. Social controls on cell survival and cell death. Nature. 1992;356:397–400. doi: 10.1038/356397a0. [DOI] [PubMed] [Google Scholar]
- Roest Crollius H, Weissenbach J. Fish genomics and biology. Genome Res. 2005;15:1675–1682. doi: 10.1101/gr.3735805. [DOI] [PubMed] [Google Scholar]
- Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- Sakamaki K, Miyajima I, Kitamura T, Miyajima A. Critical cytoplasmic domains of the common beta subunit of the human GM-CSF, IL-3 and IL-5 receptors for growth signal transduction and tyrosine phosphorylation. EMBO J. 1992;11:3541–3549. doi: 10.1002/j.1460-2075.1992.tb05437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamaki K, Tsukumo S, Yonehara S. Molecular cloning and characterization of mouse caspase-8. Eur. J. Biochem. 1998;253:399–405. doi: 10.1046/j.1432-1327.1998.2530399.x. [DOI] [PubMed] [Google Scholar]
- Sakamaki K, Inoue T, Asano M, Sudo K, Kazama H, Sakagami J, Sakata S, Ozaki M, Nakamura S, Toyokuni S, Osumi N, Iwakura Y, Yonehara S. Ex vivo whole-embryo culture of caspase-8-deficient embryos normalize their aberrant phenotypes in the developing neural tube and heart. Cell Death Differ. 2002;9:1196–1206. doi: 10.1038/sj.cdd.4401090. [DOI] [PubMed] [Google Scholar]
- Sakamaki K, Takagi C, Kominami K, Sakata S, Yaoita Y, Kubota HY, Nozaki M, Yonehara S, Ueno N. The adaptor molecule FADD from Xenopus laevis demonstrates evolutionary conservation of its pro-apoptotic activity. Genes Cells. 2004;9:1249–1264. doi: 10.1111/j.1365-2443.2004.00802.x. [DOI] [PubMed] [Google Scholar]
- Shimoda N, Knapik EW, Ziniti J, Sim C, Yamada E, Kaplan S, Jackson D, de Sauvage F, Jacob H, Fishman MC. Zebrafish genetic map with 2000 microsatellite markers. Genomics. 1999;58:219–232. doi: 10.1006/geno.1999.5824. [DOI] [PubMed] [Google Scholar]
- Taylor JS, Van de Peer Y, Braasch I, Meyer A. Comparative genomics provides evidence for an ancient genome duplication event in fish. Philos. Trans. R. Soc. Lond., B, Biol. Sci. 2001;356:1661–1679. doi: 10.1098/rstb.2001.0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JS, Braasch I, Frickey T, Meyer A, Van de Peer Y. Genome duplication, a trait shared by 22000 species of ray-finned fish. Genome Res. 2003;13:382–390. doi: 10.1101/gr.640303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitz T, Wei T, Valentine MB, Vanin EF, Grenet J, Valentine VA, Behm FG, Look AT, Lahti JM, Kidd VJ. Caspase 8 is deleted or silenced preferentially in childhood neuroblastomas with amplification of MYCN. Nat. Med. 2000;6:529–535. doi: 10.1038/75007. [DOI] [PubMed] [Google Scholar]
- Terajima D, Shida K, Takada N, Kasuya A, Rokhsar D, Satoh N, Satake M, Wang HG. Identification of candidate genes encoding the core components of the cell death machinery in the Ciona intestinalis genome. Cell Death Differ. 2003;10:749–753. doi: 10.1038/sj.cdd.4401223. [DOI] [PubMed] [Google Scholar]
- Thorburn A. Death receptor-induced cell killing. Cell. Signal. 2004;16:139–144. doi: 10.1016/j.cellsig.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Varfolomeev EE, et al. Targeted disruption of the mouse Caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity. 1998;9:267–276. doi: 10.1016/s1074-7613(00)80609-3. [DOI] [PubMed] [Google Scholar]
- Vaux DL, Korsmeyer SJ. Cell death in development. Cell. 1999;96:245–254. doi: 10.1016/s0092-8674(00)80564-4. [DOI] [PubMed] [Google Scholar]
- Westerfield M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio rerio) University of Oregon Press; Eugene: 1994. [Google Scholar]
- Whelan S, Goldman N. A general empirical model of protein evolution derived from multiple protein families using a maximum-likelihood approach. Mol. Biol. Evol. 2001;18:691–699. doi: 10.1093/oxfordjournals.molbev.a003851. [DOI] [PubMed] [Google Scholar]
- Williams JA, Barrios A, Gatchalian C, Rubin L, Wilson SW, Holder N. Programmed cell death in zebrafish rohon beard neurons is influenced by TrkC1/NT-3 signaling. Dev. Biol. 2000;226:220–230. doi: 10.1006/dbio.2000.9860. [DOI] [PubMed] [Google Scholar]
- Woods IG, Kelly PD, Chu F, Ngo-Hazelett P, Yan YL, Huang H, Postlethwait JH, Talbot WS. A comparative map of the zebrafish genome. Genome Res. 2000;10:1903–1914. doi: 10.1101/gr.10.12.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods IG, Wilson C, Friedlander B, Chang P, Reyes DK, Nix R, Kelly PD, Chu F, Postlethwait JH, Talbot WS. The zebrafish gene map defines ancestral vertebrate chromosomes. Genome Res. 2005;15:1307–1314. doi: 10.1101/gr.4134305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabu T, Kishi S, Okazaki T, Yamashita M. Characterization of zebrafish caspase-3 and induction of apoptosis through ceramide generation in fish fathead minnow tailbud cells and zebrafish embryo. Biochem. J. 2001;360:39–47. doi: 10.1042/0264-6021:3600039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita M. Apoptosis in zebrafish development. Comp. Biochem. Physiol. B, Biochem. Mol. Biol. 2003;136:731–742. doi: 10.1016/j.cbpc.2003.08.013. [DOI] [PubMed] [Google Scholar]
- Yaoita Y, Nakajima K. Induction of apoptosis and CPP32 expression by thyroid hormone in a myoblastic cell line derived from tadpole tail. J. Biol. Chem. 1997;272:5122–5127. doi: 10.1074/jbc.272.8.5122. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Snipas S, Orth K, Muzio M, Dixit VM, Salvesen GS. Target protease specificity of the viral serpin CrmA. Analysis of five caspases. J. Biol. Chem. 1997;272:7797–7800. doi: 10.1074/jbc.272.12.7797. [DOI] [PubMed] [Google Scholar]
- Zon LI. Zebrafish: a new model for human disease. Genome Res. 1999;9:99–100. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.