Abstract
Studying the neural mechanisms underlying complex goal-directed behaviors, such as social behavior, reward seeking or punishment avoidance, has become increasingly tractable in humans, nonhuman primates and rodents. In most experiments, however, goal-directed behaviors are measured in a laboratory setting, which is vastly different from the context in which these behaviors naturally occur. This study adapted a reward assessment paradigm, previously conducted with nonhuman primates in the controlled environment of a WGTA (Machado and Bachevalier, 2007), to a more naturalistic context. We used this new paradigm to examine the effects of bilateral amygdaloid, hippocampal or orbital frontal cortex lesions on established food and nonfood preferences. Behavioral modification following reinforcer devaluation was also measured. Consistent with our previous study, none of the lesions produced changes in preference for palatable foods relative to pre-surgery, but animals with amygdala lesions displayed heightened preference for unpalatable foods that control or other operated animals typically avoided. In contrast to several previous WGTA-based experiments, nonfood preference was not affected by any of the lesions. Finally, animals with orbital frontal cortex lesions continued to select preferred foods after satiation, but those with amygdala, hippocampal or sham lesions altered their foraging behavior appropriately and selected less of the sated food. These findings parallel food devaluation results obtained with these same animals when tested in the WGTA. Overall, this study stresses the importance of testing context when measuring decision-making abilities in nonhuman primates with selective brain lesions.
Keywords: monkey, food preference, reinforcer devaluation, foraging, neurotoxin, decision-making
Defining the neural network responsible for sophisticated goal-directed behaviors, such as social behavior, procurement of food and avoidance of danger, has become a more tractable research aim in recent years. This renewed potential for progress is due largely to the development of equally sophisticated methods that selectively manipulate or measure brain function while research subjects produce quantifiable behavior in controlled testing paradigms. Early studies with nonhuman primates using nonspecific lesions encompassing the amygdala and adjacent temporal neocortex (Weiskrantz, 1956; Aggleton and Passingham, 1981; 1982; Baylis and Gaffan, 1991) or large portions of the ventral and medial frontal lobe (Butter et al., 1969; Ursin et al., 1969; Baylis and Gaffan, 1991) clearly demonstrated that these areas were essential for normal food preferences and avoidance of inappropriate or potentially dangerous foods, such as raw meat. More recently, similar nonhuman primate studies using axon-sparing, neurotoxic amygdala lesions have demonstrated that this structure is mainly involved in avoidance of unsavory foods and inedible objects that normal animals find of little interest (Murray et al., 1996; Stefanacci et al., 2003; Machado and Bachevalier, 2007). Similarly, aspiration lesions restricted only to orbital frontal areas 11, 13 and 14 play little if any role in normal food and nonfood selection frequencies (Izquierdo et al., 2004; Machado and Bachevalier, 2007).
As another example, initial neurophysiological experiments demonstrated that neurons in the nonhuman primate amygdala and orbital frontal cortex respond strongly to the presentation of familiar reinforcers and punishers (Thorpe et al., 1983; Ono and Nishijo, 1992). However, more recent studies have demonstrated that an intricate interaction occurs between these two structures when animals learn about the positive or negative qualities of stimuli (Schoenbaum et al., 1999; 2000; 2003). Additionally, amygdala and orbital frontal neurons appear to modulate their activity when contingencies change and new responses must be learned, or after motivational state or expected reward quantity change (Tremblay and Schultz, 1999; Hikosaka and Watanabe, 2000; Ono and Nishijo, 2000; Rolls, 2000; Tremblay and Schultz, 2000; Wallis and Miller, 2003). Consistent with these results, the ability to change goal-directed behavior depending on motivational state or reward value is also disrupted by bilateral amygdala lesions (rodents: Hatfield et al., 1996; Balleine et al., 2003; nonhuman primates: Málková et al., 1997; Izquierdo and Murray, 2007; Machado and Bachevalier, 2007), temporary inactivation of the amygdala (Wellman et al., 2005), bilateral orbital frontal lesions (rodents: Gallagher et al., 1999; Pickens et al., 2003; nonhuman primates: Izquierdo et al., 2004; Machado and Bachevalier, 2007), and disconnection (Baxter et al., 2000) or combined unilateral lesions of these structures (Izquierdo and Murray, 2004). Similarly, functional neuroimaging studies in humans have shown that the orbital frontal cortex is selectively activated during gambling tasks (Rogers et al., 1999) and when subjects are asked to make food choices after selective satiation (Arana et al., 2003).
These recent studies with animals and humans have greatly advanced our understanding of how the brain navigates an individual towards reinforcement and away from punishment in an efficient and flexible manner. However, these results must be interpreted within the context in which they were generated. All previous studies on this topic have been conducted in non-naturalistic and highly restrictive environments, which are far removed from the contexts in which these behaviors ordinarily occur. Nonhuman primate studies of food preferences and reinforcer devaluation have consistently been conducted using forced-choice tasks in a laboratory setting. The impact of brain lesions on other forms of goal directed behavior (i.e., social behavior) appears to be highly dependent on context (Kling and Brothers, 1992; Emery and Amaral, 2000; Bachevalier and Málková, 2006). It is possible that the results described above from artificial testing environments may again not fully reflect how the primate brain functions in more complex or naturalistic settings. This issue was addressed in the current study. We adapted an established behavioral paradigm, used previously to measure forced-choice food preferences and reinforcer devaluation within the controlled environment of a WGTA (Machado and Bachevalier, 2007), to measure these same goal-directed behaviors in a semi-naturalistic setting. This paradigm had three important advantages over the WGTA version. First, the effects of selective brain lesions on food preferences and reinforcer devaluation could be assessed when stimulus presentation order was not constrained by the experimenter. Second, animals were not required to make any particular type or number of choices. Finally, food and nonfood preferences could be measured not only by the total number selected, but also in terms of priority (selection latency) upon entering the enclosure.
This semi-naturalistic paradigm was used to concurrently assess the effects of selective amygdaloid, hippocampal, and orbital frontal lesions in adult nonhuman primates on food or nonfood preferences, as well as behavioral modification following reinforcer devaluation. The results were compared to those obtained with the same animals in two forced-choice reward assessment paradigms (Machado and Bachevalier, 2007) to further define the role of each structure in guiding goal-directed behavior. Preliminary reports of this work have appeared in abstract form (Machado and Bachevalier, 2002a; 2002b).
Experimental procedures
Subjects
Twenty-four adult male rhesus monkeys (Macaca mulatta) participated in this study, weighing 3 - 6 kilograms and ranging between 2.4 and 3.2 years old at the beginning of the pre-surgical phase. Prior to this study, all animals received pre- and post-surgical assessments of social behavior (Machado and Bachevalier, 2006), a WGTA-based assessment of food/nonfood preference and reinforcer devaluation (Machado and Bachevalier, 2007), as well as one measure of emotional reactivity (Human Intruder task) which will be reported elsewhere. These animals were also tested in the Visual Paired Comparison task one month following surgery to assess their ability to recognize pictures of objects (data also reported elsewhere).
Animals were randomly assigned to one of the following five experimental groups, which were balanced with respect to pre-surgical social dominance rank (determined from pre-surgical social behavior testing, see Machado and Bachevalier, 2006): Sham-operated control (C; n = 6), neurotoxic hippocampal lesion (H-ibo; n = 6), neurotoxic amygdala lesion (A-ibo; n = 6), neurotoxic orbital frontal lesion (O-ibo; n = 3) and aspiration orbital frontal lesion (O-asp; n = 3). Animals were housed individually at the University of Texas Medical School Animal Care Facility (an AAALAC accredited institution), given water ad libitum and fed fresh fruit, vegetables and high-protein monkey chow (Lab Diet #5045, PMI Nutrition International Inc., Brentwood, MO) daily. Animal housing rooms were maintained on a twelve hour light/dark cycle. All procedures were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals and approved by the Animal Care and Use Committee of the University of Texas Health Science Center, Houston.
Neuroimaging
Magnetic resonance imaging (MRI) procedures have been detailed in four previous studies from our laboratory (Nemanic et al., 2002; 2004; Machado and Bachevalier, 2006; Machado and Bachevalier, 2007), and therefore only a brief account is offered here. Animals were initially sedated with Ketamine Hydrochloride and Xylazine (10 mg/kg of 7:3 Ketamine Hydrochloride, 100mg/ml, and Xylazine, 20mg/ml, i.m.), intubated with an endotracheal canula and maintained under gas anesthesia (Isoflurane; 1.0 - 3.0%, v/v, to effect) throughout the scanning procedure. After being transported to the MRI scanner, each animal’s head was secured in a non-ferromagnetic stereotaxic apparatus (Crist Instruments Co., Inc., Damascus, MD) and centered with respect to the magnet. The MRI protocol included two sessions performed with a GE Signa 1.5 Tesla Echo Speed scanner (GE Medical Systems, Milwaukee, WI). The first session occurred 1 - 3 weeks prior to surgery, and included two series of coronal images through the entire brain: one T1-weighted structural scan (1 mm thick) and three Fluid Attenuated Inversion Recovery (FLAIR, 3 mm thick, each offset by 1 mm) scans. The second scanning session was performed 7 - 10 days after surgery and included the same two MR series.
Pre-surgical T1-weighted images were used to precisely select and calculate stereotaxic coordinates for neurotoxin injections in each target area (Saunders et al., 1990) or to visualize the individual sulcal pattern that served as landmarks for orbital frontal cortex lesions. Post-surgical T1-weighted images were compared to matched pre-surgical T1-weighted images to identify the location and quantify the extent of orbital frontal cortex aspiration lesions (Group O-asp). Post-surgical FLAIR images were compared to matched pre-surgical FLAIR and T1-weighted images to accurately identify localized areas of edema indicative of neurotoxin-induced cell death, and were therefore used to quantify the extent of lesion for all animals in Groups H-ibo, A-ibo and O-ibo (Málková et al., 2001; Nemanic et al., 2002).
Surgery
A full description of all surgical techniques has been previously published (Machado and Bachevalier, 2006). Briefly, all surgical procedures were performed under deep anesthesia (Ketamine Hydrochloride, 10 mg/kg, i.m., followed by Isoflurane gas, 1.0 - 2.0%, v/v, to effect) using aseptic techniques. Animals in Groups C, A-ibo and H-ibo were repositioned in the same stereotaxic apparatus used for pre-surgical neuroimaging, whereas those in Groups O-ibo and O-asp were placed in a head-holder that permitted easy rotation of the animal’s head during surgery. After a midline longitudinal incision from a mid-point on the supra-orbital ridge to the occipital notch, the skin, connective tissue and temporalis muscles were gently retracted.
After the procedures specific to each lesion (described below), the wound was closed in anatomical layers. The animal was removed from anesthesia and observed until it could breathe on its own. Beginning twelve hours prior to surgery and continuing until one week after surgery, all animals were treated with dexamethazone sodium phosphate (0.4 mg/kg, i.m.) and Cephazolin (25 mg/kg, i.m.) to prevent excessive immunoreactivity and protect against infection, respectively. For three days following surgery, animals also received an analgesic (acetaminophen, 10 mg/kg, p.o.) to minimize pain.
During recovery from surgical procedures, none of the animals displayed any changes in food and water consumption, or arousal state. However, reduced locomotor behaviors and weakness of the limbs were observed in the two cases that sustained additional damage to the ventral putamen (i.e. cases C-1-inj and H-ibo-1). These locomotor deficits progressively disappeared in 2 - 3 weeks. All fur on the animals’ scalp had re-grown by the start of the post-surgery testing phase, such that scars from surgery were not visible.
Neurotoxic hippocampal formation lesions
Neurotoxic hippocampal formation lesions were intended to damage all ammonic fields, the dentate gyrus, the prosubiculum and subiculum. The number of injection sites and their positions in the anterior/posterior, medial/lateral and dorsal/ventral planes were determined from each animal’s pre-surgical T1-weighted MR images. For the posterior two-thirds of the hippocampal formation, one injection site was selected every 1.5 mm and was centered within the body of the hippocampal formation. For the most anterior portion, where the uncus was clearly visible, two injection sites were selected every 1.5 mm. One was situated laterally, again within the body of the hippocampal formation, and the other located more medially within the uncus.
Small bilateral craniotomies were created above the injection sites and small slits were cut in the dura bilaterally to allow the needle of the 10 μl Hamilton syringe, held by a Kopf electrode manipulator (David Kopf Instruments, Tujunga, CA), to be lowered to the appropriate injection coordinates. Two Hamilton syringes were filled with ibotenic acid (Biosearch Technologies, Novato, CA, 10 mg/ml in PBS, pH 7.4) and used to simultaneously inject 1.5 or 2.4 μl ibotenic acid (0.4 μl/minute) at each of the eleven sites selected for each hemisphere.
Neurotoxic amygdala lesions
Neurotoxic amygdala lesions were intended to damage all amygdaloid nuclei, and were also guided by pre-surgical T1-weighted MR images. The coronal image through the mid-portion of the amygdala, usually including a complete view of the anterior commissure, was identified. On this image, 11 injection sites were selected and spaced 2 mm apart in the medial/lateral and dorsal/ventral directions. Two additional coronal images located 2 mm anterior and 2 mm posterior to this central image were also selected. For these two additional images, two injection sites were placed 1 mm lateral and medial to the center of the amygdala. During surgery, small bilateral craniotomies were created above the injection sites and small slits were cut in the dura bilaterally to allow for a total of fifteen injections per amygdala. Similar to hippocampal lesions (above), two 10 μl Hamilton syringes were used to simultaneously deliver 0.2 - 0.6 μl ibotenic acid (0.4 μl/min) to each site bilaterally.
Orbital frontal cortex lesions
Orbital frontal cortex lesions (both ibotenic and aspiration) were intended to damage those areas of the ventral frontal cortex that are heavily interconnected with the amygdala (Amaral et al., 1992; Cavada et al., 2000; Ghashghaei et al., 2006), namely areas 11 and 13 (as defined by Carmichael and Price, 1994). Because the shape and length of the orbital sulci, which were used as landmarks, vary between animals, pre-surgical T1-weighted MR images were used to reconstruct the ventral surface of the frontal lobe for each animal and approximate locations for 17 - 36 injection sites (all spaced ∼2 mm apart) through areas 11 and 13 for each hemisphere.
To access the orbital surface, a large craniotomy was created just above each orbit, and the bone of the supra-orbital ridge was gently eroded with an electric drill to gain a full view of the orbital frontal surface. The dura was cut and retracted, and with the aid of a surgical microscope, the lateral and medial orbital sulci and the olfactory stria were visualized. The boundaries of areas 11 and 13 on the ventral surface of the frontal lobe were defined as follows: The anterior border was set as a line joining the anterior tips of the medial and lateral orbital sulci. The posterior border was a line joining the medial lip of the lateral orbital sulcus to the olfactory stria just anterior to its division into the medial and lateral olfactory tracts. The medial border followed the olfactory stria, and the lateral border followed the medial lip of the lateral orbital sulcus from its anterior tip to the posterior border of the lesion. These borders approximate the extent of areas 11 and 13 in the macaque monkey and mostly avoid area 14 more medially.
For animals in Group O-ibo, a 2 mm × 2 mm grid of injections was placed in the cortex within these borders. A 30 gauge needle attached to a 10 μl Hamilton syringe by polyethylene tubing was used to manually inject 0.4 - 0.8 μl ibotenic acid (0.4 μl/min) at each site. For animals in Group O-asp, 21 and 23 gauge suckers were used first to coagulate the pia matter along the defined borders of the lesion and, then, to gently aspirate the cortical tissue contained within these limits. Care was taken to not severe the white matter beneath the cortical mantle in all cases.
Sham lesions
After opening the skin, bilateral craniotomies (similar to those used for hippocampal formation or amygdala lesions) were made as described above. For five of the six cases, the dura was cut bilaterally, but no needle penetrations occurred. The remaining sham-operated animal (case C-1-inj) was prepared to serve as a control animal for one of the hippocampal formation lesion animals (case H-ibo-1) that sustained inadvertent damage to the putamen. Case C-1-inj received ibotenic acid injections into the section of the putamen which lies dorsal to the posterior one-third of the amygdala and the anterior one-third of the hippocampal formation. A total of seventeen injections were made per hemisphere and 0.4 - 0.6 μl ibotenic acid were injected at each site at a rate of 0.4 μl/min.
MRI-based lesion evaluation
All lesions were evaluated using MRI techniques (Málková et al., 2001; Nemanic et al., 2002) since the animals of the present study died in the flooding of Tropical Storm Allison in June 2001. These techniques provide an accurate estimate of actual cell loss following neurotoxic hippocampal lesions in nonhuman primates (Málková et al., 2001; Nemanic et al., 2002). Because these neuroimaging techniques have not been validated for neurotoxic lesions amygdala and orbital frontal cortex, hypersignals measured following these lesions should only be used as an approximation of actual damage.
For case C-1-inj and all animals of Groups H-ibo, A-ibo and O-ibo, pre-surgical T1-weighted images (1 mm slice thickness) were used as an aid to match the lower resolution pre- and post-surgical FLAIR images (3 mm slice thickness) to digital drawings of coronal sections from a normalized rhesus monkey template brain at 1 mm intervals. Hypersignals on post-surgical FLAIR MR images were then identified and, using the pre-surgical T1-weighted images to maximize accuracy, plotted onto corresponding coronal drawings from the template brain using Adobe Photoshop software (v. 6). These drawings were imported into a Java-based image analysis program (ImageJ®; http://rsb.info.nih.gov/ij/) to measure the surface area (in pixels2) of damage for intended targets, as well as all areas sustaining unintended damage. For any given region of interest (ROI), the measured surface area of damage on each section through each hemisphere was summed and then multiplied by image thickness to calculate a total volume of damage (Gundersen and Jensen, 1987). The volume of damage was then divided by the normal volume of the ROI (obtained from the template brain in a similar manner) to estimate a percent of the total volume damaged.
For each animal of Group O-asp, pre- and post-surgical T1-weighted images were used to measure the total volume of orbital frontal cortex (areas 11 and 13) and adjacent cortical regions damaged. Pre- and post-surgical T1-weighted images were again matched to corresponding coronal drawings from the normal rhesus monkey template brain. Within each hemisphere, the total volume of aspirated tissue from the orbital frontal cortex and adjacent regions was measured as above and expressed as a percentage of the normal volume for that region.
Behavioral testing
Apparatus
A large indoor enclosure (3.1 m long × 1.6 m wide × 1.9 - 2.3 m tall) made of galvanized steel mesh (0.6 cm diameter, spaced 4.7 cm apart vertically and 14 cm horizontally) located in a separate room adjacent to the animal housing quarters was used for this experiment. One side of this enclosure was composed of clear lexan Plexiglass (2 cm thick) to permit optimal video recording of the animals’ behavior. This enclosure also contained two galvanized steel perches (75 cm long × 25 cm wide × 4 cm thick), two rope swings and a single horizontal PVC pole perch (4 cm diameter, 1.6 m long) that spanned the width of the cage near its center. Twelve stainless steel boxes (9.2 cm wide × 7 cm tall × 7 cm deep) with open tops were hung on the three steel-mesh walls (four per wall) in a rectangular pattern. Animals were well habituated to this enclosure prior to pre-surgery testing in this paradigm since the assessments of their group social behavior were also conducted in this enclosure (Machado and Bachevalier, 2006).
Food/nonfood Preference
Procedures for assessing food and nonfood preferences in a semi-naturalistic environment were adapted from a WGTA-based task used previously in our laboratory (Machado and Bachevalier, 2007). Animals were tested both pre- and post-surgery, with approximately nine months intervening between these phases. Food and nonfood preferences were tested over three days in each phase. Six foods, each cut to be approximately the same size, were selected to be different from those used in our previous study and included: grapes, apples, sweetened chocolate cereal (Cocoa Puffs, General Mills, Inc., Minneapolis, MN), monkey chow (Lab Diet #5045, PMI Nutrition International Inc., Brentwood, MO), garlic cloves and lemon. The three former items were chosen to be highly preferred foods for macaques, whereas the three latter foods were chosen to be edible, but significantly less preferred. Six nonfoods, cut to similar sizes as the six foods, were also selected to be different from those used in our previous report and included felt fabric, cardboard, cotton balls, latex, wood shavings and metal keys (chained to the cage wall to preclude ingestion).
Before each testing session, five pieces of each food or nonfood were placed in a different box hanging on the wall of the enclosure (i.e., one box contained five grapes, another contained five cotton balls, etc.). The animal was then released into the enclosure, using the same door on each day, and allowed to freely forage in all boxes for 15 minutes. A Sony Handycam video camera (model CCD-FX710) was used to record animals’ latency to remove an item from each box (Selection Latency) and the number of items removed from each box during the entire trial (Selection Frequency). To control for any temporal biases in the testing procedures or animals’ motivation, all testing for this experiment was conducted between 0800 and 1000 hours (i.e., 16 hours after the last feed and prior to the daily feeding). The daily testing order was generated randomly, but kept constant across the pre- and post-surgery phases.
Twenty-four hours after the three days of post-surgery food/nonfood preference testing, the ability of each animal to inhibit previously learned responses was assessed by pseudorandomly changing the spatial location of each box within the enclosure (“shuffled” phase) and the animals were again given three additional days of testing using these new food/nonfood locations. Testing procedures and data collection in the shuffled phase were identical to those used in the pre-and post-surgical phases.
Reinforcer devaluation
We also evaluated each animal’s ability to change their foraging preference following satiation with their favorite food by adapting procedures from Thornton and colleagues (1998). Twenty-four hours after the last day of post-surgery food/nonfood preference testing in the shuffled phase, all animals were given 200 grams of their favorite food (determined from the post-surgery food/nonfood preference testing) in their living quarters and allowed to eat freely for 30 minutes. An additional 100 grams of the same food were given every 15 minutes until five consecutive minutes passed without any further food ingestion. Each animal’s body weight, along with the amount of food consumed (in grams) and the total time required for the animal to become satiated were also recorded.
Animals were then transported to the testing enclosure and allowed to forage freely for 15 minutes. Baiting of food boxes was done prior to releasing the animal into the test cage exactly as described above for the food/nonfood preference testing, and the animal’s behavior was again videotaped to measure Selection Latency and Selection Frequency.
Data analysis
Food and nonfood preference between the pre-surgery, post-surgery and shuffled phases were analyzed using General Linear Model ANOVAs (SPSS v. 12) for each measure with Group (4) and Phase (3) as main factors and repeated measures on Phase. Similar between groups comparisons were made within the post-surgery and shuffled phases, but with test day as the repeated measure (4 Group × 3 Day ANOVAs). In both cases, a Huynh-Feldt correction was used to adjust the degrees of freedom if sphericity could not be assumed. Since all animals were presumably normal during the pre-surgery testing phase, changes in food and nonfood preference across test days were assessed with paired-sample t-tests, Bonferroni corrected for multiple comparisons. Main effects of Group were investigated further using two-sided Dunnett’s tests for differences between Group C and the three lesion groups and Tukey tests for comparisons between the three lesion groups. Main effects of Phase were examined using Bonferroni post-hoc tests. Significant interactions between Group and Phase were investigated with paired-sample t-tests, which were also Bonferroni corrected for multiple comparisons. Statistical significance was set at p < .05. However, given the low number of animals and heterogeneity of lesion extent in each experimental group, we occasionally report results for which p values fall above this threshold. Results are identified as “marginally significant” if their p value is greater than .05 but less than .08.
To investigate if orbital frontal lesion method itself impacted differentially on behavioral measures, data from Groups O-asp and O-ibo were compared to each other. No significant main effects of Group or Group × Phase interactions were found for these comparisons. Furthermore, animals with orbital frontal lesions were compared to the other three experimental groups both when segregated into Groups O-ibo and O-asp and also when combined into a single Group O. Significant main effects of Group or interactions between Group and Phase were only found when all animals with orbital frontal lesions were considered together as Group O. Therefore, in the following Results section, Groups O-ibo and O-asp are pooled into a single Group O.
Finally, for all experimental groups, Pearson product moment correlation matrices were generated to determine if the extent of damage to any brain region (intended or unintended) was significantly related to the behavioral parameters measured. Only those regions displaying greater than 5% mean damage across hemispheres were included in this final analysis.
Results
Lesion extent
Lesion extent for the 24 animals tested in this experiment have been described previously (see Results section in Machado and Bachevalier, 2006). Therefore, the reader is directed to that publication for full description of the intended and unintended damage for each group. Table 1 is provided here to summarize the major areas of intended and unintended damage for each group. Briefly, neurotoxic amygdala lesions (range: 50.1 - 90.0%) and neurotoxic hippocampal lesions (range: 66.2 - 99.1%) were largely as intended, and only resulted in mild to moderate unintended damage in adjacent regions (i.e., the hippocampus for Group A-ibo and areas TH/TF for Group H-ibo). Aspiration orbital frontal cortex lesions resulted in damage largely confined to areas 11 and 13 (range: 87.5 - 91.8%), as well as the anterior one-third of the agranular insular area (5.7 - 22.6%). By contrast, the three cases with ibotenic acid orbital frontal lesions received damage not only to areas 11 and 13 (range: 28.5 - 45.2%), but also to significant portions of neighboring area 14 (range: 7.1 - 36.4%), area 12 (range: 1.6 - 34.9%) and a more substantial portion of the agranular insular area (range: 31.2 - 41.4%). In addition, the damage to these orbital frontal regions was confined mostly to the superficial cortical layers and spared the deepest layers, resulting in comparably lower total volume, but larger total surface area of damage relative to Group O-asp.
Table 1.
Intended and unintended damage for all experimental groups
| Cases | Hippocampal Formation |
Amygdala |
TH |
TF |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L | R | Avg | W | L | R | Avg | W | L | R | Avg | W | L | R | Avg | W | |
| H-ibo-1 | 76.3 | 97.9 | 87.1 | 74.7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| H-ibo-2 | 75.7 | 81.3 | 78.5 | 61.6 | 0 | 5.9 | 2.9 | 0 | 53.1 | 20.1 | 36.6 | 10.7 | 60.3 | 27.6 | 43.9 | 16.6 |
| H-ibo-3 | 67.5 | 74.1 | 70.8 | 50.0 | 0 | 0 | 0 | 0 | 26.7 | 15.3 | 21.0 | 4.1 | 29.9 | 44.0 | 37.0 | 13.2 |
| H-ibo-4 | 56.2 | 76.2 | 66.2 | 42.9 | 0 | 0 | 0 | 0 | 13.6 | 27.8 | 20.7 | 3.8 | 18.5 | 19.4 | 18.9 | 3.6 |
| H-ibo-5 | 98.8 | 99.3 | 99.1 | 98.1 | 0 | 0 | 0 | 0 | 15.2 | 15.9 | 15.6 | 2.4 | 38.8 | 8.5 | 23.7 | 3.3 |
| H-ibo-6 | 88.8 | 94.8 | 91.8 | 84.3 | 0 | 0 | 0 | 0 | 29.6 | 45.6 | 37.6 | 13.5 | 21.2 | 17.2 | 19.2 | 3.6 |
| X | 77.2 | 87.3 | 82.3 | 68.6 | 0.0 | 1.0 | 0.5 | 0.0 | 23.0 | 20.8 | 21.9 | 5.8 | 28.1 | 19.5 | 23.8 | 6.7 |
| Cases |
Amygdala |
Hippocampal Formation |
ERh |
PRh |
||||||||||||
| L | R | Avg | W | L | R | Avg | W | L | R | Avg | W | L | R | Avg | W | |
| A-ibo-1 | 20.6 | 82.2 | 51.4 | 17.0 | 10.6 | 1.6 | 6.1 | 0.2 | 0 | 1.8 | 0.9 | 0 | 0 | 0 | 0 | 0 |
| A-ibo-2 | 48.9 | 88.1 | 68.5 | 43.1 | 1.2 | 0 | 0.6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| A-ibo-3 | 27.1 | 73.1 | 50.1 | 19.8 | 15.7 | 13.6 | 14.6 | 2.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| A-ibo-4 | 79.1 | 92.5 | 85.8 | 73.2 | 3.4 | 3.0 | 3.2 | 0.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| A-ibo-5 | 88.7 | 91.3 | 90.0 | 81.0 | 1.5 | 0.1 | 0.8 | 0 | 0 | 5.5 | 2.8 | 0 | 0 | 0 | 0 | 0 |
| A-ibo-6 | 70.3 | 90 | 80.2 | 63.3 | 21.1 | 10.3 | 15.7 | 2.2 | 0.8 | 0 | 0.4 | 0 | 0.1 | 0 | 0.1 | 0 |
| X | 55.8 | 86.2 | 71.0 | 49.6 | 8.9 | 4.8 | 6.8 | 0.8 | 0.1 | 1.2 | 0.7 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Cases |
Orbital Frontal Cortex (Areas 11 & 13) |
Area 12 |
Area 14 |
Ia |
||||||||||||
| L | R | Avg | W | L | R | Avg | W | L | R | Avg | W | L | R | Avg | W | |
| O-ibo-1 | 37.1 | 19.9 | 28.5 | 7.4 | 2.3 | 0.9 | 1.6 | 0 | 49.0 | 23.8 | 36.4 | 11.7 | 37.0 | 25.3 | 31.2 | 9.4 |
| O-ibo-2 | 33.9 | 37.5 | 35.7 | 12.7 | 28.1 | 41.7 | 34.9 | 11.7 | 8.3 | 5.9 | 7.1 | 0.5 | 28.2 | 34.3 | 31.3 | 9.6 |
| O-ibo-3 | 43.0 | 47.3 | 45.2 | 20.3 | 9.9 | 20.9 | 15.4 | 2.1 | 25.4 | 11.7 | 18.5 | 3.0 | 44.9 | 38.0 | 41.4 | 17.0 |
| X | 38.0 | 34.9 | 36.5 | 13.5 | 13.4 | 21.2 | 17.3 | 4.6 | 27.6 | 13.8 | 20.7 | 5.1 | 36.7 | 32.5 | 34.6 | 12.0 |
| O-asp-1 | 88.4 | 95.3 | 91.8 | 84.2 | 3.9 | 28.9 | 16.4 | 1.1 | 15.2 | 21.7 | 18.5 | 3.3 | 20.6 | 21.1 | 20.9 | 4.3 |
| O-asp-2 | 83.0 | 92.0 | 87.5 | 76.3 | 7.0 | 9.3 | 8.2 | 0.7 | 10.7 | 5.9 | 8.3 | 0.6 | 21.4 | 23.7 | 22.6 | 5.1 |
| O-asp-3 | 90.3 | 87.6 | 88.9 | 79.1 | 4.5 | 10.2 | 7.4 | 0.5 | 0.7 | 0 | 0.3 | 0 | 5.5 | 5.9 | 5.7 | 0.3 |
| X | 87.2 | 91.6 | 89.4 | 79.9 | 5.1 | 16.1 | 10.7 | 0.8 | 8.9 | 9.2 | 9.0 | 1.3 | 15.8 | 16.9 | 16.4 | 3.2 |
Data are the estimated percentage of normal volume as assessed from MR images. Areas 11, 12, 13, and 14 - cytoarchitectonic subregions of the macaque frontal lobe as defined by Carmichael and Price (1994); Ia - agranular insular area as defined by Carmichael and Price (1994); ERh - entorhinal cortex and PRh - perirhinal cortex as defined by Amaral and colleagues (Amaral et al., 1987; Insausti et al., 1987); L - percentage of damage to the left hemisphere; R - percentage of damage to the right hemisphere; Avg - average of L and R; W = (L × R)/100 [weighted index as defined by Hodos and Bobko (1984)]; X - group mean.
Categorization of items
Three foods were purposely chosen by the experimenter to be more desirable (grapes, apples and chocolate cereal) than the remaining three (garlic, lemon and monkey chow). However, it was necessary to determine if these subjective food categories were reflected in the behavior of our animals prior to surgery. It was also conceivable that the six nonfoods used were not all equally appealing to normal rhesus monkeys. Therefore, the Selection Latency data from all 24 animals on Day 3 of the pre-surgery testing phase (all locations highly familiar) were used to determine if this variable differed significantly from the maximum session duration (e.g., fifteen minutes or 900 seconds) for any particular food or nonfood. Selection Latencies that were significantly less than the maximum session duration would indicate that these items (either food or nonfood) were consistently selected by normal macaques.
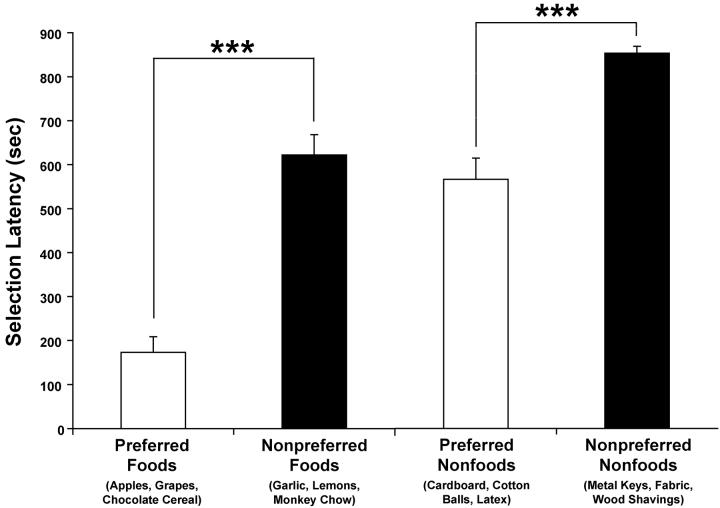
All six foods were taken significantly faster than the 900-sec trial duration (one-sample t-tests, Bonferroni corrected for six tests, all ps ≤ .01), indicating that none of the foods were consistently refused. However, inspection of the mean Selection Latencies for the six foods revealed a bimodal distribution. As predicted by the experimenter, three foods (apples, grapes and chocolate cereal) appeared to be highly preferred (mean Selection Latency = 190 sec) and the remaining three (garlic, lemons and monkey chow) were less preferred (mean Selection Latency = 621 sec). When the mean Selection Latencies for these two food categories (Preferred and Nonpreferred, respectively) were compared, Preferred Foods were selected significantly faster than Nonpreferred Foods (t = -7.876; p < .001; Figure 1).
Figure 1.
The mean Selection Latency (maximum = 900 sec) for all 24 animals on Day 3 of the pre-surgery testing phase for Preferred Foods, Nonpreferred Foods, Preferred Nonfoods and Nonpreferred Nonfoods. Vertical bars indicate the Standard Errors of the Mean. *** p < .001
The six nonfoods also showed a bimodal distribution, with three (cardboard, cotton balls and latex) being more preferred than the remaining three (metal keys, fabric and wood shavings; t = - 6.609; p < .001; Figure 1). Therefore, in addition to examining lesion effects on individual food and nonfood preferences, we also examined group differences for Preferred Foods, Nonpreferred Foods, Preferred Nonfoods and Nonpreferred Nonfoods.
Foraging behavior
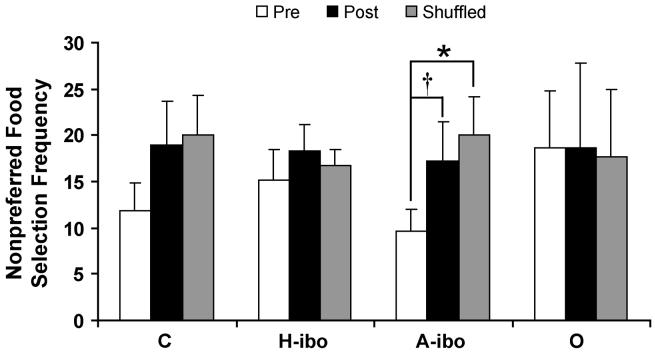
Two parameters were used to measure food and nonfood preferences (Selection Frequency and Selection Latency) across the three testing phases (pre-surgery, post-surgery and shuffled). For each individual item and the four general categories, we calculated the total Selection Frequency and average Selection Latency across the three test days of each phase. These values were used for between groups comparisons across the three phases. There were no significant main effects of Group, indicating that none of the animals displayed profound differences in dietary preferences pre- and post-surgery. Only one marginally significant Group × Phase interaction was detected, specifically for Selection Frequency of Nonpreferred Foods [FHunyh-Feldt(3,20) = 2.132, p = .08; Figure 2]. This interaction resulted from Group A-ibo showing increased selection of Nonpreferred Foods (such as garlic, lemons and monkey chow) in the post-surgery (t = -2.953; p = .07) and shuffled (t = -3.599; p < .05) phases relative to pre-surgery.
Figure 2.
Total Selection Frequency for the category of Nonpreferred Foods (garlic, lemons and monkey chow) for each experimental group across the pre-surgery, post-surgery and shuffled testing phases. A maximum of 45 Nonpreferred Foods were available in each phase. Vertical bars indicate the Standard Errors of the Mean. * p < .05; † p = .07; relative to pre-surgery; C - sham-operated controls; H-ibo - animals with bilateral, ibotenic lesions of the hippocampal formation; A-ibo - animals with bilateral, ibotenic lesions of the amygdala; O - animals with bilateral, ibotenic or aspiration lesions of orbital frontal areas 11 and 13.
This initial analysis also gave numerous indications of significant food and nonfood preference adaptation (likely due to learning) across the three testing phases (Table 2). Regardless of lesion, all animals selected progressively fewer pieces of garlic and cotton balls across phases and Selection Latencies increased for cotton balls and Preferred Nonfoods, both of which indicate decreased preference for these items. By contrast, all animals selected more pieces of lemon, fabric, Nonpreferred Foods and Nonpreferred Nonfoods with increasing experience in the task. Similarly, average Selection Latencies decreased for apples, chocolate cereal, metal keys, wood shavings, Preferred Foods and Nonpreferred Nonfoods across phases.
Table 2.
Food and nonfood preference adaptation across testing phase
| Selection Frequency | Pre | Post | Shuffled | Phase effect | Post-hoc |
|---|---|---|---|---|---|
| Preferred Foods | 38.9 | 41.5 | 42.2 | NS | |
| Apples | 10.5 | 12.3 | 13.1 | NS | |
| Grapes | 15.0 | 15.0 | 14.8 | NS | |
| Chocolate Cereal | 13.4 | 14.3 | 14.3 | NS | |
| Nonpreferred Foods | 12.4 | 17.3 | 17.2 | F(2,40) = 9.239, p < .01 | Pre < (Post = Shuffled), ps < .05 |
| Garlic | 5.2 | 6.7 | 4.4 | F(2,40) = 7.451, p < .01 | Post > Shuffled, p < .01 |
| Lemon | 2.5 | 4.2 | 5.4 | F(2,40) = 8.056, p < .01 | Pre < Shuffled, p < .05 |
| Monkey Chow | 4.8 | 6.4 | 7.4 | NS | |
| Preferred Nonfoods | 20.0 | 20.6 | 17.8 | NS | |
| Cardboard | 2.8 | 2.9 | 3.8 | NS | |
| Cotton Balls | 9.8 | 9.2 | 5.9 | F(2,40) = 8.013, p < .01 | (Pre = Post) > Shuffled, ps < .05 |
| Latex | 7.4 | 8.5 | 8.1 | NS | |
| Nonpreferred Nonfoods | 3.8 | 8.8 | 16.0 | F(2,40) = 31.057, p < .001 | Pre < Post < Shuffled, ps < .01 |
| Metal Keys | 1.1 | 3.8 | 6.7 | NS | |
| Fabric | 1.1 | 2.8 | 3.5 | F(2,40) = 6.431, p < .01 | Pre < (Post = Shuffled), ps < .05 |
| Wood Shavings | 1.6 | 2.2 | 5.8 | NS | |
| Selection Latency | Pre | Post | Shuffled | Phase effect | Post-hoc |
| Preferred Foods | 196.1 | 132.7 | 123.7 | F(2,40) = 4.409, p < .05 | Pre > Post, p < .05 |
| Apples | 362.6 | 274.4 | 192.3 | F(2,40) = 4.616, p < .05 | Pre > Shuffled, p < .05 |
| Grapes | 45.7 | 22.3 | 39.3 | NS | |
| Chocolate Cereal | 180.0 | 101.3 | 139.6 | F(2,40) = 4.089, p < .05 | Pre > Post, p < .05 |
| Nonpreferred Foods | 529.2 | 490.6 | 488.0 | NS | |
| Garlic | 454.0 | 442.5 | 498.4 | NS | |
| Lemon | 611.0 | 536.6 | 537.8 | NS | |
| Monkey Chow | 522.6 | 492.8 | 427.8 | NS | |
| Preferred Nonfoods | 474.1 | 469.8 | 584.8 | F(2,40) = 11.678, p < .001 | (Pre = Post) < Shuffled, ps < .05 |
| Cardboard | 620.7 | 605.7 | 643.6 | NS | |
| Cotton Balls | 306.9 | 371.5 | 556.4 | F(2,40) = 6.582, p < .01 | (Pre = Post) < Shuffled, ps < .05 |
| Latex | 494.7 | 432.3 | 554.3 | NS | |
| Nonpreferred Nonfoods | 778.9 | 687.1 | 550.0 | F(2,40) = 21.514, p < .001 | (Pre = Post) > Shuffled, ps < .05 |
| Metal Keys | 815.5 | 718.3 | 553.2 | F(2,40) = 18.065, p < .001 | (Pre = Post) > Shuffled, ps < .05 |
| Fabric | 785.7 | 706.3 | 696.5 | NS | |
| Wood Shavings | 735.5 | 636.6 | 400.3 | F(2,40) = 18.065, p < .001 | (Pre = Post) > Shuffled, ps < .001 |
Data are the total Selection Frequency (top) and average Selection Latency (bottom) for all animals, regardless of lesion, in each of the three testing phases. Values are provided for each individual item, as well as the four general food and nonfood categories. Significant main effects of Phase are identified, along with their post-hoc evaluation. Pre - Pre-surgery phase, Post - Post-surgery phase, Shuffled - Shuffled phase.
Theses findings prompted a more thorough investigation of changes in foraging pattern or sequence across test days. Selection Latency data, as opposed to Selection Frequency, were used for these analyses since this measure more closely reflects sequential foraging priority. Since animals also showed substantial individual variability in specific food and nonfood preferences, this analysis was conducted only with the four food and nonfood categories. On the first day of pre-surgical testing, animals entered the enclosure quickly and began visiting each box in a systematic circular pattern, typically starting with the boxes closest to the entry door or those adjacent to the preferred (highest) perch. Even on the first day of pre-surgery testing, animals selected from boxes containing Preferred Foods faster than the other three categories (paired-sample t-tests; ps < .001). On subsequent pre-surgical test days, the animals’ foraging pattern was further refined. Decreases in Selection Latency were observed for Preferred Foods (t = 3.094; p < .01) and Selection Latencies for Nonpreferred Foods, Preferred Nonfoods and Nonpreferred Nonfoods all increased (t = -4.512, t = -3.658 and t = -2.480 respectively, ps < .05).
The animals then underwent their assigned lesion or sham surgeries. Approximately nine months passed before animals were tested in this paradigm again, but the spatial location of each item did not change in the interim. Between groups comparisons were then made with Selection Latencies collected on Day 3 of the pre-surgery phase and Day 1 of the post-surgery phase (4 Groups × 2 Days ANOVA). No significant Group × Day interactions were found for any of the categories, indicating that none of the lesions drastically altered animals’ established foraging priorities. Analysis across the three post-surgery test days detected only main effects of Day for each of the general food and nonfood categories, indicating further refinement to foraging priority by all animals [Preferred Foods: F(2,40) = 11.434, p < .001, Nonpreferred Foods: F(2,40) = 3.237, p = .05, Preferred Nonfoods: F(2,40) = 4.421, p < .05, Nonpreferred Nonfoods: F(2,40) = 8.281, p = .001]. Similar to the pre-surgery phase, all animals decreased Selection Latencies for Preferred Foods (p < .05) and increased Selection Latencies for the other three categories (ps < .05) between Day 1 and Day 2 of the post-surgery phase. Selection Latencies for each category did not change appreciably between Days 2 and 3.
Item locations were shuffled following Day 3 of the post-surgery phase, and foraging behavior was tested 24 hours later. Selection Latency was again compared between groups from Day 3 of the post-surgery phase with Day 1 of the shuffled phase. No significant Group × Day interactions were found, again indicating that all groups were able to quickly adapt their foraging pattern following spatial rearrangement. Analysis of Selection Latencies across test days again demonstrated several main effects of Day, but only for Preferred Nonfoods [F(2,40) = 9.322, p < .001] and Nonpreferred Nonfoods [F(2,40) = 20.067, p < .001]. All animals showed significant increases in Selection Latency for these categories between Days 1 and 2 (ps < .01), but no further changes between Days 2 and 3.
Reinforcer devaluation
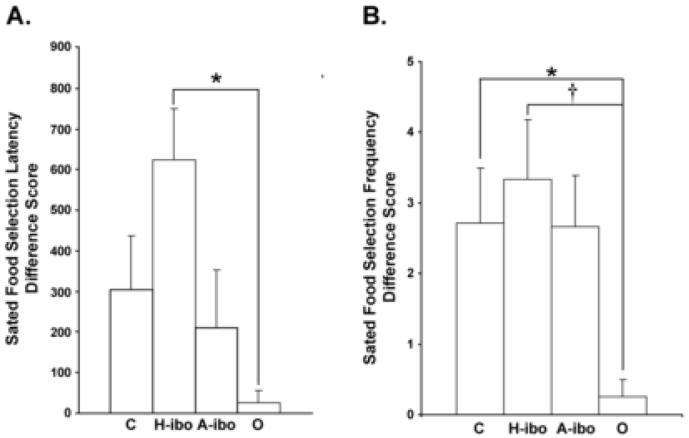
Two animals, both from Group O (O-asp-2 and O-ibo-3), did not select any foods or nonfoods during the testing session following reinforcer devaluation, indicating a more global defect of motivation. Therefore, these two animals were removed from the following statistical analyses. Difference Scores were calculated for the Selection Frequency and Selection Latency of the sated food (each animal’s favorite food), the animal’s second and third most preferred foods combined and all nonfoods combined (Difference Score = Shuffled Phase - Reinforcer Devaluation Phase) to determine if satiation with one food impacted only on the selection of that particular food, or also influenced preference for nonsated foods and nonfoods. A positive difference score for Selection Frequency indicates that animals selected fewer pieces of the sated food, other preferred foods, or nonfoods following reinforcer devaluation. Conversely, a negative difference score for the Selection Latency indicates that animals took longer to make a selection from a box containing the sated food, the other highly preferred foods or all nonfoods after reinforcer devaluation. For illustration purposes (Figure 3), the absolute value of the Selection Latency Difference Score is shown.
Figure 3.
A) Absolute value of the Sated Food Selection Latency Difference Score and B) the Sated Food Selection Frequency Difference Scores are shown for each experimental group. Vertical bars indicate the Standard Errors of the Mean, and maximum scores are indicated by the ordinates. * p < .05, † p = .06, difference relative to another experimental group as indicated. The data for each individual animal used to calculate these two Difference Scores is provided in Table 2. Group abbreviations are the same as in Figure 2.
Group differences were found for the Sated Food Selection Latency (F(3,18) = 3.570, p < .05) and Sated Food Selection Frequency (F(3,18) = 2.697, p = .08) Difference Scores, but not for animals’ second and third most preferred foods combined or for all nonfoods combined. The raw data for each animal which were used to calculate the Sated Food Selection Latency and Sated Food Selection Frequency Difference Scores are provided in Table 3. As shown in Figure 3A, Groups C, A-ibo and H-ibo took longer to approach the box containing the sated food than did Group O, although this difference reached significance only for Group H-ibo (Tukey: H-ibo > O, p < .05). Additionally, the Sated Food Selection Latency Difference Score was significantly greater than zero (indicating a change in preference) for Group H-ibo (p < .01) and was marginally significant for Group C (p = .07), but was not significant for Groups A-ibo and O (ps > .10). Similarly, for the Sated Food Selection Frequency Difference Score (Figure 3B), Groups C, A-ibo, and H-ibo took less sated food than Group O, although this difference reached significance for Groups C and H-ibo only [Dunnett’s O < C, p = .05; Tukey O < H-ibo, p = .06]. Additionally, the Sated Food Selection Frequency Difference Score was significant relative to zero for Groups C, H-ibo and A-ibo (ps < .02), but not Group O.
Table 3.
Individual difference scores
| Cases | Sated Food Selection Latency |
Sated Food Selection Frequency |
||||
|---|---|---|---|---|---|---|
| Shuffled Phase | Reinforcer Devaluation | Difference Score | Shuffled Phase | Reinforcer Devaluation | Difference Score | |
| C-1 | 10.3 | 116.0 | -105.7 | 5 | 4 | 1 |
| C-2 | 1.3 | 900.0 | -898.67 | 5 | 0 | 5 |
| C-3 | 31.3 | 9 | 22.3 | 5 | 5 | 0 |
| C-4 | 28.0 | 251.0 | -223.0 | 5 | 1 | 4 |
| C-5 | 27.7 | 201.0 | -173.3 | 5 | 2 | 3 |
| C-6 | 455.0 | 900.0 | -445.0 | 3.3 | 0 | 3.3 |
| X | 92.3 ± 72.7 | 396.2 ± 162.8 | -303.9 ± 134.5 | 4.7 ± 0.3 | 2.0 ± 0.9 | 2.7 ± 0.8 |
| H-ibo-1 | 157.0 | 900.0 | -743.0 | 5 | 0 | 5 |
| H-ibo-2 | 31.3 | 900.0 | -868.7 | 5 | 0 | 5 |
| H-ibo-3 | 43.0 | 570.0 | -527.0 | 5 | 5 | 0 |
| H-ibo-4 | 18.0 | 900.0 | -882.0 | 5 | 0 | 5 |
| H-ibo-5 | 10.3 | 64.0 | -53.67 | 5 | 2 | 3 |
| H-ibo-6 | 20.7 | 688 | -667.3 | 5 | 3 | 2 |
| X | 46.7 ± 22.5 | 670.3 ± 133.7 | -623.6 ± 126.1 | 5.0 ± 0.0 | 1.7 ± 0.8 | 3.3 ± 0.8 |
| A-ibo-1 | 15.7 | 9.0 | 6.7 | 5 | 5 | 0 |
| A-ibo-2 | 96.3 | 21.0 | 75.3 | 5 | 2 | 3 |
| A-ibo-3 | 13.0 | 229.0 | -216.0 | 5 | 1 | 4 |
| A-ibo-4 | 57.7 | 219.0 | -161.3 | 5 | 3 | 2 |
| A-ibo-5 | 292.7 | 377.0 | -84.3 | 5 | 3 | 2 |
| A-ibo-6 | 15.7 | 900.0 | -884.3 | 5 | 0 | 5 |
| X | 81.8 ± 44.2 | 292.5 ± 134.1 | -210.7 ± 141.6 | 5.0 ± 0.0 | 2.3 ± 0.7 | 2.7 ± 0.7 |
| O-ibo-1 | 19.7 | 131.0 | -111.3 | 5 | 5 | 0 |
| O-ibo-2 | 30.0 | 19.0 | 11.0 | 5 | 5 | 0 |
| O-ibo-3 | 20.7 | 900.0 | -879.3 | 5 | 0 | 5 |
| O-asp-1 | 2.7 | 2.0 | 0.7 | 5 | 5 | 0 |
| O-asp-2 | 5.7 | 900.0 | -894.3 | 5 | 0 | 5 |
| O-asp-3 | 14.7 | 21.0 | -6.3 | 5 | 4 | 1 |
| X | 16.8 ± 5.7 | 43.3 ± 29.6 | -26.5 ± 28.5 | 5.0 ± 0.0 | 4.8 ± 0.3 | 0.3 ± 0.3 |
Data for individual animals, showing the latency to select the sated food (left side, in seconds, maximum = 900) during the Shuffled Phase (mean across three testing days) and following reinforcer devaluation, as well as the calculated difference score for each animal and the group means (±SEM). Also provided are the individual data for the selection frequency of the sated food (right side, maximum = 5) during the two phases and the calculated difference sores. Note that two members of Group O (cases O-ibo-3 and O-asp-2) were excluded from the statistical analyses. Their data are highlighted in gray and do not factor into the Group O mean scores. X - mean for all animals included in the statistical analyses.
Finally, the lack of devaluation effect observed for animals in Group O did not reflect a difference in the satiation level for this group relative to the other groups, since there were no significant group differences in total time (in seconds) and total weight (in grams) of food eaten to become satiated, or in body weight (in kilograms).
Correlations
No significant correlations were found between behavioral measures and intended or unintended damage for any experimental group.
Discussion
This is the first study to examine the effects bilateral amygdaloid, hippocampal or orbital frontal cortex lesions on learned food/nonfood preferences and flexible reward assessment in a semi-naturalistic setting. Three important findings emerged. First, animals with amygdala lesions, but not control animals or those with hippocampal or orbital frontal lesions, displayed increased selection of foods that normal animals typically refuse (lemons, garlic and monkey chow). Second, the foraging sequence for foods and nonfoods was not altered by any lesion following surgery or after spatial rearrangement of items. Finally, animals with orbital frontal cortex lesions were unable to alter their established selection frequency and latency when their favorite food was devalued via satiation. Control animals and those with amygdala or hippocampal lesions did not show such deficits. These results will be discussed in turn and compared to those obtained when these same animals were similarly tested in a more conventional WGTA setting.
Food and nonfood selections
None of the groups showed changes in the total number of palatable foods selected between the pre- and post-surgery testing phases of the current experiment. A similar result was found when these same animals were tested in the more restrictive context of a WGTA (Machado and Bachevalier, 2007). However, two previous reports have indicated that aspiration (Baylis and Gaffan, 1991) or neurotoxic (Izquierdo and Murray, 2007) amygdala lesions result in decreased preference for highly palatable foods. There is no clear reason for this discrepancy, since the palatable foods used between these studies were quite similar (apples, M&M candies, raisins, etc.). It is possible that these incongruent results stem from the animals in each study having differential experience with these highly-palatable food rewards. Nevertheless, these results indicate that the hippocampal formation and areas 11 and 13 of the orbital frontal cortex are not necessary for judging the reinforcement value of familiar, palatable foods regardless of context, but further research regarding the amygdala and preference for highly palatable foods is warranted.
None of the groups showed any changes in nonfood preferences from pre to post-surgery. This result was expected for control and orbital frontal lesion animals since they did not show any abnormal nonfood preferences when tested in the WGTA environment. It was surprising, however, that animals with amygdala or hippocampal lesions did not show heightened nonfood preferences in the semi-naturalistic setting, since these animals displayed such behavior when tested in the WGTA environment (Machado and Bachevalier, 2007). This difference suggests that the context in which the effects of amygdaloid or hippocampal lesions are assessed may have a significant impact on the behavioral changes observed. One potential explanation for these contrasting results may lie in the amount of effort required to retrieve foods and nonfoods in each paradigm. In a WGTA environment, foods and nonfoods are presented on a test tray and are easily within arms’ reach. In the current foraging experiment, however, small caches of foods or nonfoods were scattered around a much larger enclosure (3.1 m long × 1.6 m wide × 1.9 - 2.3 m tall). Therefore, the amount of time and energy the animal must expend to ingest a food or explore an inedible nonfood is considerably greater in the larger foraging arena relative to the WGTA. It is possible that animals with amygdala or hippocampal lesions were willing to habitually select and explore nonfoods when they were easily accessible at arms’ length, but did not typically make that same choice when the nonfoods required more work to acquire. Another complementary explanation for these discrepant results pertains to greater experience with inedible nonfoods due to prior testing. Because the current experiment was performed after the WGTA-based food preference test, it is possible that animals with amygdala or hippocampal lesions had acquired enough knowledge regarding the low reinforcement value of inedible nonfoods that exploring them in the post-surgery and shuffled phases of the current experiment was no longer a high priority.
Animals with amygdala lesions were not completely devoid of abnormal behaviors. In both post-surgery testing phases, these animals showed abnormally high preferences for foods which normal macaques typically refused (lemons, garlic and monkey chow). This result is consistent with the only two other nonhuman primate food preference studies that included both palatable and unpalatable foods (Baylis and Gaffan, 1991; Stefanacci et al., 2003), both of which were conducted in a WGTA. Thus the primate amygdala appears to play a role in avoidance of unpalatable foods across multiple contexts. More generally, the amygdala may be critical for associating the perceptual properties of a visual stimulus with its intrinsic reward value, as already suggested by several earlier reports (Málková et al., 1997; Balleine et al., 2003).
Foraging sequence following surgery and shuffled locations
Given that hippocampal lesions in nonhuman primates disrupt spatial memory (Hampton et al., 2004; Banta-Lavenex et al., 2006), we also investigated whether damage to the hippocampal formation could have impacted spatial memory abilities by contrasting pre- and post-surgery Selection Latencies while item location was held constant. Animals with hippocampal lesions went to the locations of their preferred foods as rapidly as did animals in the other three groups, thus demonstrating that they remembered the locations of preferred foods. Similarly, given that orbital frontal cortex damage in nonhuman primates (Butter, 1969; Iversen and Mishkin, 1970; Jones and Mishkin, 1972; Dias et al., 1996; Meunier et al., 1997) produces severe deficits in altering previously learned actions once reward contingencies change, we also investigated whether animals with orbital frontal lesions had difficulty changing their foraging strategy during the Shuffled condition. Animals with orbital frontal lesions were as able as animals in the other three groups to alter their foraging patterns after the foods and nonfoods were spatially shuffled. The most likely reason for both of these discrepancies stems from our use of boxes with open tops. Most of our animals quickly adopted a strategy of moving along the top of each wall. From that vantage point, they could look down on several boxes to see what item each contained. Thus, animals could quickly identify the location of several items without actually approaching any boxes. Olfactory cues were also likely present since the boxes were open. Even if our operated groups could not remember where items were from day to day, or phase to phase, they could use this alternate strategy and/or olfactory cues to survey the cage and prioritize their foraging path like normal animals. Therefore, our methods were likely insensitive to any spatial memory or response inhibition impairments produced by amygdala, hippocampal or orbital frontal lesions.
Primary reinforcer devaluation
Animals with amygdala or hippocampal lesions were able to alter their established foraging pattern when the incentive value of their favorite food was diminished by selective satiation. These results were expected since all previous nonhuman primate lesion studies of reinforcer devaluation in a controlled setting have demonstrated that amygdala or hippocampal dysfunction, either via bilateral neurotoxic lesions (Málková et al., 1997; Izquierdo and Murray, 2007; Machado and Bachevalier, 2007) or transient inactivation (Wellman et al., 2005), do not diminish animals’ ability to avoid devalued foods.
Animals with orbital frontal cortex lesions, by contrast, were not able to modify their behavior according to a change in motivational state. This impairment was demonstrated both in the number of sated foods taken and the latency to select a sated food upon entering the testing enclosure. These results closely resemble those generated when these animals were similarly tested in a WGTA (Machado and Bachevalier, 2007). These results also converge upon nonhuman primate electrophysiological recording studies describing neurons in the orbital frontal cortex that specifically decrease their firing rate when the animal views a food eaten to satiation (Tremblay and Schultz, 1999; Hikosaka and Watanabe, 2000; Rolls, 2000; Tremblay and Schultz, 2000; Wallis and Miller, 2003). However, a similar nonhuman primate lesion study conducted by Izquierdo and colleagues (2004) did not find similar impairments of reinforcer devaluation following lesions targeting areas 11, 13 and 14 of the orbital frontal cortex. One possible explanation for this discrepancy could stem from differential damage to orbital frontal areas across studies. Our orbital frontal lesions primarily damaged areas 11 and 13, but mostly spared area 14 (see Table 1). Further, animals with orbital frontal lesions in our current study, especially those created with ibotenic acid, also sustained an average of 25.5% damage to the agranular insular area. This caudal sector of the nonhuman primate orbital frontal cortex is known to contain the secondary olfactory (medially) and gustatory (laterally) cortices (Rolls, 2000). Neurons in the agranular insular area have also been shown to decrease their established firing rate to an odor or taste following satiation (Rolls, 2000). Damage to the agranular insular area, either alone or combined with damage to areas 11 and 13, may have resulted in more severe disruption of our animals’ ability to use information about their internal bodily state to modulate food selections adaptively. Despite these inconsistencies, the overlying theme is that the orbital frontal cortex (especially areas 11, 13 and the anterior third of the agranular insular area) is critically important for judging the value of a primary reinforcer with respect to one’s current bodily or motivational state.
Controlled versus naturalistic testing paradigms
The current experiment further suggests that testing context can have a large impact on behavioral observations of nonhuman primates with brain lesions. This idea has been demonstrated in several recent assessments of social behavior and social dominance rank with adolescent or adult monkeys (Kling and Brothers, 1992; Emery et al., 2001; Bauman et al., 2006; Machado and Bachevalier, 2006; and see commentary by Bachevalier and Málková, 2006), but now also appears relevant to reward assessment studies. The choice between either a controlled, laboratory-based testing paradigm or one that is either semi- or fully-naturalistic should not be entered into lightly. Both options bring with them positive and negative consequences. First, laboratory-based testing paradigms give the experimenter a high level of control over how, when and where stimuli are presented, and similar control over the type of responses that the animals can make or be recorded, all of which aid in testing specific hypotheses. Conversely, laboratory-based tasks arguably have less external validity. Since they are vastly different from the context in which behaviors naturally occur, the results are less applicable to explaining behavior in the general population. On the other hand, semi- or fully-naturalistic experiments are certainly advantageous because they measure subjects’ behavior as it typically occurs in nature, without the significant influence of experimenter-generated stimulus presentation schedules, fixed trial and inter-trial lengths, or a restrictive range of responses. However, the external validity gained in naturalistic paradigms is countered by the difficulty of reliable data collection, the impact of environmental factors (i.e., weather), the increased potential for animals to develop alternative behavioral strategies and the significant monetary expense of testing animals in large, open enclosures or in the wild.
The current report does not aim to discount any finding from controlled testing paradigms. On the contrary, this study highlights the benefits of testing animals in both controlled and semi-naturalistic settings when examining how neural structures contribute to behavior. In our case, this experiment served as a bridge between two controlled studies of how the amygdala, hippocampal formation and orbital frontal cortex contribute to reward assessment (Machado and Bachevalier, 2007) and a separate study of how these structures influence unrestrained social interactions with familiar peers (Machado and Bachevalier, 2006). Conducting this series of experiments has produced converging evidence that each of these neural regions makes a distinct contribution to reward assessment and social cognition.
Acknowledgements
This work was supported by grants from the National Institutes of Mental Health (MH- 58846), the National Institute of Child Health and Human Development (HD-35471), and the National Institutes of Health (RR00165) to Dr. Bachevalier, and by a National Research Service Award Predoctoral Fellowship from the National Institutes of Mental Health (MH-63577) to Dr. Machado. The data described here served as partial fulfillment of the requirements for the Ph.D. degree from The University of Texas Graduate School of Biomedical Sciences at Houston to Dr. Machado. We thank the University of Texas Health Science Center at Houston veterinary and animal husbandry staff for expert animal care, Roger E. Price and Belinda Rivera for the care and handling of the animals during the MR imaging procedures, Edward F. Jackson for expert assistance in neuroimaging techniques, and David Lane for statistical advice and guidance in data analysis. We also extend thanks to the two anonymous reviewers who offered valuable comments on a previous version of this paper. Christopher Machado is now at The M.I.N.D. Institute, University of California, Davis, 2805 50th Street, Room 1411, Sacramento, CA 95817 and Jocelyne Bachevalier is now at the Yerkes National Primate Research Center, Emory University, 954 Gatewood Rd., Atlanta, GA 30329.
Abbreviations
- AAALAC
Association for Assessment and Accreditation of Laboratory Animal Care
- A-ibo
Animals with bilateral, ibotenic acid amygdala lesions
- ANOVA
Analysis of Variance
- C
Sham-operated control animals
- FLAIR
Fluid Attenuated Inversion Recovery
- H-ibo
Animals with bilateral, ibotenic acid hippocampal formation lesions
- MRI
Magnetic Resonance Imaging
- O
Animals with either ibotenic acid or aspiration lesions of the orbital frontal cortex (areas 11 and 13)
- O-ibo
Animals with bilateral, ibotenic acid lesion of the orbital frontal cortex (areas 11 and 13)
- O-asp
Animals with bilateral, aspiration lesions of the orbital frontal cortex (areas 11 and 13)
- PBS
phosphate buffered saline
- PVC
Polyvinyl chloride
- ROI
Region of Interest
- WGTA
Wisconsin General Testing Apparatus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Section Editor: Dr. Joan I. Morrell (Behavioral Neuroscience)
References
- Aggleton JP, Passingham RE. Syndrome produced by lesions of the amygdala in monkeys (Macaca mulatta) J Comp Physiol Psychol. 1981;95:961–977. doi: 10.1037/h0077848. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Passingham RE. An assessment of the reinforcing properties of foods after amygdaloid lesions in rhesus monkeys. J Comp Physiol Psychol. 1982;96:71–77. doi: 10.1037/h0077861. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Insausti R, Cowan WM. The entorhinal cortex of the monkey: I. Cytoarchitectonic organization. J Comp Neurol. 1987;264:326–355. doi: 10.1002/cne.902640305. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Price JL, Pitkanen A, Carmichael ST. Anatomical organization of the primate amygdaloid complex. In: Aggleton JP, editor. The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction. John Wiley & Sons, Inc; New York: 1992. pp. 1–66. [Google Scholar]
- Arana FS, Parkinson JA, Hinton E, Holland AJ, Owen AM, Roberts AC. Dissociable contributions of the human amygdala and orbitofrontal cortex to incentive motivation and goal selection. J Neurosci. 2003;23:9632–9638. doi: 10.1523/JNEUROSCI.23-29-09632.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachevalier J, Málková L. The amygdala and development of social cognition: theoretical comment on Bauman, Toscano, Mason, Lavenex, and Amaral (2006) Behav Neurosci. 2006;120:989–991. doi: 10.1037/0735-7044.120.4.989. [DOI] [PubMed] [Google Scholar]
- Balleine BW, Killcross AS, Dickinson A. The effect of lesions of the basolateral amygdala on instrumental conditioning. J Neurosci. 2003;23:666–675. doi: 10.1523/JNEUROSCI.23-02-00666.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banta-Lavenex P, Amaral DG, Lavenex P. Hippocampal lesion prevents spatial relational learning in adult macaque monkeys. J Neurosci. 2006;26:4546–4558. doi: 10.1523/JNEUROSCI.5412-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman MD, Toscano JE, Mason WA, Lavenex P, Amaral DG. The expression of social dominance following neonatal lesions of the amygdala or hippocampus in rhesus monkeys (Macaca mulatta) Behav Neurosci. 2006;120:749–760. doi: 10.1037/0735-7044.120.4.749. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Parker A, Lindner CC, Izquierdo AD, Murray EA. Control of response selection by reinforcer value requires interaction of amygdala and orbital prefrontal cortex. J Neurosci. 2000;20:4311–4319. doi: 10.1523/JNEUROSCI.20-11-04311.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylis LL, Gaffan D. Amygdalectomy and ventromedial prefrontal ablation produce similar deficits in food choice and in simple object discrimination learning for an unseen reward. Exp Brain Res. 1991;86:617–622. doi: 10.1007/BF00230535. [DOI] [PubMed] [Google Scholar]
- Butter CM. Perseveration in extinction and in discrimination reversal tasks following selective frontal ablations in Macaca mulatta. Physiology & Behavior. 1969;4:163–171. [Google Scholar]
- Butter CM, McDonald JA, Snyder DR. Orality, preference behavior, and reinforcement value of nonfood object in monkeys with orbital frontal lesions. Science. 1969;164:1306–1307. doi: 10.1126/science.164.3885.1306. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Architectonic subdivision of the orbital and medial prefrontal cortex in the macaque monkey. J Comp Neurol. 1994;346:366–402. doi: 10.1002/cne.903460305. [DOI] [PubMed] [Google Scholar]
- Cavada C, Company T, Tejedor J, Cruz-Rizzolo RJ, Reinoso-Suarez F. The anatomical connections of the macaque monkey orbitofrontal cortex. A review. Cereb Cortex. 2000;10:220–242. doi: 10.1093/cercor/10.3.220. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Dissociation in prefrontal cortex of affective and attentional shifts. Nature. 1996;380:69–72. doi: 10.1038/380069a0. [DOI] [PubMed] [Google Scholar]
- Emery NJ, Amaral DG. The role of the amygdala in primate social cognition. In: Lane RD, Nadel L, editors. Cognitive Neuroscience of Emotion. Oxford University Press; Oxford: 2000. [Google Scholar]
- Emery NJ, Capitanio JP, Mason WA, Machado CJ, Mendoza SP, Amaral DG. The effects of bilateral lesions of the amygdala on dyadic social interactions in rhesus monkeys (Macaca mulatta) Behav Neurosci. 2001;115:515–544. [PubMed] [Google Scholar]
- Gallagher M, McMahan RW, Schoenbaum G. Orbitofrontal cortex and representation of incentive value in associative learning. J Neurosci. 1999;19:6610–6614. doi: 10.1523/JNEUROSCI.19-15-06610.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghashghaei HT, Hilgetag CC, Barbas H.Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala Neuroimage Epub ahead of print 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen HJ, Jensen EB. The efficiency of systematic sampling in stereology and its prediction. J Microsc. 1987;147(Pt 3):229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- Hampton RR, Hampstead BM, Murray EA. Selective hippocampal damage in rhesus monkeys impairs spatial memory in an open-field test. Hippocampus. 2004;14:808–818. doi: 10.1002/hipo.10217. [DOI] [PubMed] [Google Scholar]
- Hatfield T, Han JS, Conley M, Gallagher M, Holland P. Neurotoxic lesions of basolateral, but not central, amygdala interfere with Pavlovian second-order conditioning and reinforcer devaluation effects. J Neurosci. 1996;16:5256–5265. doi: 10.1523/JNEUROSCI.16-16-05256.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka K, Watanabe M. Delay activity of orbital and lateral prefrontal neurons of the monkey varying with different rewards. Cereb Cortex. 2000;10:263–271. doi: 10.1093/cercor/10.3.263. [DOI] [PubMed] [Google Scholar]
- Insausti R, Amaral DG, Cowan WM. The entorhinal cortex of the monkey: II. Cortical afferents. J Comp Neurol. 1987;264:356–395. doi: 10.1002/cne.902640306. [DOI] [PubMed] [Google Scholar]
- Iversen SD, Mishkin M. Perseverative interference in monkeys following selective lesions of the inferior prefrontal convexity. Exp Brain Res. 1970;11:376–386. doi: 10.1007/BF00237911. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Murray EA. Combined unilateral lesions of the amygdala and orbital prefrontal cortex impair affective processing in rhesus monkeys. J Neurophysiol. 2004;91:2023–2039. doi: 10.1152/jn.00968.2003. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Murray EA. Selective bilateral amygdala lesions in rhesus monkeys fail to disrupt object reversal learning. J Neurosci. 2007;27:1054–1062. doi: 10.1523/JNEUROSCI.3616-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Suda RK, Murray EA. Bilateral orbital prefrontal cortex lesions in rhesus monkeys disrupt choices guided by both reward value and reward contingency. J Neurosci. 2004;24:7540–7548. doi: 10.1523/JNEUROSCI.1921-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B, Mishkin M. Limbic lesions and the problem of stimulus--reinforcement associations. Exp Neurol. 1972;36:362–377. doi: 10.1016/0014-4886(72)90030-1. [DOI] [PubMed] [Google Scholar]
- Kling AS, Brothers L. The amygdala and social behavior. In: Aggleton JP, editor. The Amygdala: Neurobiological Aspects of Emotion. Wiley-Liss; New York: 1992. pp. 353–377. [Google Scholar]
- Machado CJ, Bachevalier J. Effects of slective amygdala or orbital frontal cortex lesions on decision-making in monkeys; Annual Meeting of the Cognitive Neuroscience Society 48; 2002a. [Google Scholar]
- Machado CJ, Bachevalier J. Using two distinct testing environments to assess decision-making skills in rhesus monkeys (Macaca mulatta) after selective amygdala, hippocampal or orbital frontal lesions; Measuring Behavior 2002 - 4th Annual Conference on Methods and Techniques in Behavioral Research; 2002b. [Google Scholar]
- Machado CJ, Bachevalier J. The impact of selective amygdala, orbital frontal cortex or hippocampal formation lesions on established social relationships in monkeys. Behav Neurosci. 2006;120:761–786. doi: 10.1037/0735-7044.120.4.761. [DOI] [PubMed] [Google Scholar]
- Machado CJ, Bachevalier J. The effects of selective amygdala, orbital frontal cortex or hippocampal formation lesions on reward assessment in nonhuman primates. Eur J Neurosci. 2007;25:2885–2904. doi: 10.1111/j.1460-9568.2007.05525.x. [DOI] [PubMed] [Google Scholar]
- Málková L, Gaffan D, Murray EA. Excitotoxic lesions of the amygdala fail to produce impairment in visual learning for auditory secondary reinforcement but interfere with reinforcer devaluation effects in rhesus monkeys. J Neurosci. 1997;17:6011–6020. doi: 10.1523/JNEUROSCI.17-15-06011.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Málková L, Lex CK, Mishkin M, Saunders RC. MRI-Based evaluation of locus and extent of neurotoxic lesions in monkeys. Hippocampus. 2001;11:361–370. doi: 10.1002/hipo.1050. [DOI] [PubMed] [Google Scholar]
- Meunier M, Bachevalier J, Mishkin M. Effects of orbital frontal and anterior cingulate lesions on object and spatial memory in rhesus monkeys. Neuropsychologia. 1997;35:999–1015. doi: 10.1016/s0028-3932(97)00027-4. [DOI] [PubMed] [Google Scholar]
- Murray EA, Gaffan EA, Flint RWJ. Anterior rhinal cortex and amygdala: dissociation of their contributions to memory and food preference in rhesus monkeys. Behav Neurosci. 1996;110:30–42. [PubMed] [Google Scholar]
- Nemanic S, Alvarado MC, Bachevalier J. The hippocampal/parahippocampal regions and recognition memory: insights from visual paired comparison versus object-delayed nonmatching in monkeys. J Neurosci. 2004;24:2013–2026. doi: 10.1523/JNEUROSCI.3763-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemanic S, Alvarado MC, Price RE, Jackson EF, Bachevalier J. Assessment of locus and extent of neurotoxic lesions in monkeys using neuroimaging techniques: a replication. J Neurosci Methods. 2002;121:199–209. doi: 10.1016/s0165-0270(02)00264-9. [DOI] [PubMed] [Google Scholar]
- Ono T, Nishijo H. Neurophysiology basis of the Kluver-Bucy Syndrome: Responses of monkey amygdaloid neurons to biologcially significant objects. In: Aggleton JP, editor. The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction. John Wiley & Sons, Inc; New York: 1992. pp. 167–190. [Google Scholar]
- Ono T, Nishijo H. Neurophysiological basis of emotion in primates: Neuronal responses in the monkey amygdala and anterior cingulate cortex. In: Gazzaniga M, editor. The new cognitive neurosciences. The MIT Press; London: 2000. pp. 1099–1114. [Google Scholar]
- Pickens CL, Saddoris MP, Setlow B, Gallagher M, Holland PC, Schoenbaum G. Different roles for orbitofrontal cortex and basolateral amygdala in a reinforcer devaluation task. J Neurosci. 2003;23:11078–11084. doi: 10.1523/JNEUROSCI.23-35-11078.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RD, Owen AM, Middleton HC, Williams EJ, Pickard JD, Sahakian BJ, Robbins TW. Choosing between small, likely rewards and large, unlikely rewards activates inferior and orbital prefrontal cortex. J Neurosci. 1999;19:9029–9038. doi: 10.1523/JNEUROSCI.19-20-09029.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET. The orbitofrontal cortex and reward. Cereb Cortex. 2000;10:284–294. doi: 10.1093/cercor/10.3.284. [DOI] [PubMed] [Google Scholar]
- Saunders RC, Aigner TG, Frank JA. Magnetic resonance imaging of the rhesus monkey brain: use for stereotactic neurosurgery. Exp Brain Res. 1990;81:443–446. doi: 10.1007/BF00228139. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Chiba AA, Gallagher M. Neural encoding in orbitofrontal cortex and basolateral amygdala during olfactory discrimination learning. J Neurosci. 1999;19:1876–1884. doi: 10.1523/JNEUROSCI.19-05-01876.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Chiba AA, Gallagher M. Changes in functional connectivity in orbitofrontal cortex and basolateral amygdala during learning and reversal training. J Neurosci. 2000;20:5179–5189. doi: 10.1523/JNEUROSCI.20-13-05179.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B, Saddoris MP, Gallagher M. Encoding predicted outcome and acquired value in orbitofrontal cortex during cue sampling depends upon input from basolateral amygdala. Neuron. 2003;39:855–867. doi: 10.1016/s0896-6273(03)00474-4. [DOI] [PubMed] [Google Scholar]
- Stefanacci L, Clark RE, Zola SM. Selective neurotoxic amygdala lesions in monkeys disrupt reactivity to food and object stimuli and have limited effects on memory. Behav Neurosci. 2003;117:1029–1043. doi: 10.1037/0735-7044.117.5.1029. [DOI] [PubMed] [Google Scholar]
- Thornton JA, Málková L, Murray EA. Rhinal cortex ablations fail to disrupt reinforcer devaluation effects in rhesus monkeys (Macaca mulatta) Behav Neurosci. 1998;112:1020–1025. doi: 10.1037//0735-7044.112.4.1020. [DOI] [PubMed] [Google Scholar]
- Thorpe SJ, Rolls ET, Maddison S. The orbitofrontal cortex: neuronal activity in the behaving monkey. Exp Brain Res. 1983;49:93–115. doi: 10.1007/BF00235545. [DOI] [PubMed] [Google Scholar]
- Tremblay L, Schultz W. Relative reward preference in primate orbitofrontal cortex. Nature. 1999;398:704–708. doi: 10.1038/19525. [DOI] [PubMed] [Google Scholar]
- Tremblay L, Schultz W. Modifications of reward expectation-related neuronal activity during learning in primate orbitofrontal cortex. J Neurophysiol. 2000;83:1877–1885. doi: 10.1152/jn.2000.83.4.1877. [DOI] [PubMed] [Google Scholar]
- Ursin H, Rosvold HE, Vest B. Food preference in brain lesioned monkeys. Physiology & Behavior. 1969;4:609–612. [Google Scholar]
- Wallis JD, Miller EK. Neuronal activity in primate dorsolateral and orbital prefrontal cortex during performance of a reward preference task. Eur J Neurosci. 2003;18:2069–2081. doi: 10.1046/j.1460-9568.2003.02922.x. [DOI] [PubMed] [Google Scholar]
- Weiskrantz L. Behavioral changes associated with ablation of the amygdaloid complex in monkeys. Journal of Comparitive and Physiological Psychology. 1956;49:381–391. doi: 10.1037/h0088009. [DOI] [PubMed] [Google Scholar]
- Wellman LL, Gale K, Málková L. GABAA-mediated inhibition of basolateral amygdala blocks reward devaluation in macaques. J Neurosci. 2005;25:4577–4586. doi: 10.1523/JNEUROSCI.2257-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]