Abstract
The ability to recognize a previously experienced stimulus is supported by two processes: recollection of the stimulus in the context of other information associated with the experience, and a sense of familiarity with the features of the stimulus. Although familiarity and recollection are functionally distinct, there is considerable debate about how these kinds of memory are supported by regions in the medial temporal lobes (MTL). Here, we review evidence for the distinction between recollection and familiarity and then consider the evidence regarding the neural mechanisms of these processes. Evidence from neuropsychological, neuroimaging, and neurophysiological studies of humans, monkeys, and rats indicates that different subregions of the MTL make distinct contributions to recollection and familiarity. The data suggest that the hippocampus is critical for recollection but not familiarity. The parahippocampal cortex also contributes to recollection, possibly via the representation and retrieval of contextual (especially spatial) information, whereas perirhinal cortex contributes to and is necessary for familiarity-based recognition. The findings are consistent with an anatomically guided hypothesis about the functional organization of the MTL and suggest mechanisms by which the anatomical components of the MTL interact to support of the phenomenology of recollection and familiarity.
Keywords: recollection, familiarity, hippocampus, perirhinal cortex, parahippocampal cortex, entorhinal cortex, amnesia
INTRODUCTION
Imagine an occasion when you are walking across campus and see someone who seems vaguely familiar. When she greets you, you are quite sure you know this person, and yet you can not recall when you met her or why you know her. A casual conversation ensues and you search for clues with innocuous questions. Further embarrassment is avoided when she says something about a meeting last week. Suddenly you recall her name, where the meeting was, and some of the topics discussed there.
This common scenario illustrates the two kinds of subjective experiences that occur during recognition. One is a sense of familiarity, which is experienced rapidly and varies from a weak intuition to a compellingly strong belief. The other experience is recollection, which involves the recovery of qualitative associations prompted by a critical cue. Beyond the distinction in subjective experiences, considerable evidence now demonstrates that familiarity and recollection are two different forms of memory, which exhibit very different functional characteristics (see Yonelinas 2002). It remains unclear, however, whether recollection and familiarity are supported by distinct brain processes or instead emerge from a single neural mechanism. In particular, scholars debate how these kinds of memory are supported by regions in the medial temporal lobes (MTL). Here, we begin with an overview of the behavioral and event-related potential studies indicating that recollection and familiarity are distinct and then focus on studies that shed light on the neural mechanisms of recognition. Guided by the neuroanatomy of the MTL, we review studies of recognition memory in humans with amnesia following MTL lesions and results from functional imaging studies in healthy subjects. We also consider animal models that have been used to identify brain areas that are critical to aspects of recognition memory and to characterize neuronal activity related to recognition performance.
Medial temporal lobe (MTL).
the combination of the parahippocampal region and hippocampus
Methodological limitations are associated with any particular approach. For example, it is often difficult to ascertain the precise locus and extent of damage in amnesic patients, and conflicting results in these studies are often attributed to the possibility of damage outside the medial temporal region. Functional imaging studies of humans can be used to localize neural correlates of recognition processes, but these methods do not test whether a given region is truly necessary for these processes. Animal models provide precise anatomical localization of lesions and recording sites, but it can be difficult to map concepts from psychological studies of humans onto the kinds of tasks used in animal models. Given that any research method has unique strengths and limitations, our approach is to seek convergence across different methods. Evidence from these different approaches is indeed converging on an anatomically based model in which different medial temporal areas implement distinct information processing functions in the service of recollection and familiarity.
RECOLLECTION AND FAMILIARITY
Studies of human memory have indicated that recollection and familiarity reflect two functionally distinct memory processes. Although evidence for this distinction comes from a variety of different experimental paradigms (see Table 1), here we focus on three methods (i.e., response time, receiver operating characteristics, and event-related potential studies) that have been particularly important in motivating this distinction and in providing insight into the nature of these processes (for reviews see Diana et al. 2006, Rugg & Yonelinas 2003, Yonelinas 2002).
Table 1.
An overview of the functional characteristics of recollection and familiarity. The effects of encoding and retrieval manipulations on recollection and familiarity (for details see text and Yonelinas 2002). **, large effect; *, moderate effect; –, no effect
| Manipulations | Recollection | Familiarity |
|---|---|---|
| Encoding | ||
| Study time | ** | ** |
| Elaboration | ** | * |
| Attention | ** | * |
| Rote repetition | - | * |
| Retrieval | ||
| Speeding | ** | - |
| Attention | ** | - |
| Fluency | - | ** |
| Bias | - | ** |
| Perceptual match (verb) | - | * |
| Brief delay | - | * |
| Long delay | ** | ** |
As illustrated in the opening vignette, familiarity typically becomes available more quickly than does recollection. For example, forcing subjects to make speeded recognition responses has only a small effect on recognition tests in which subjects are required only to discriminate between studied and nonstudied items. However, it leads to robust reductions in performance on relational recognition tests (sometimes called associative, source, or context recognition tests) that require the retrieval of specific information about each item, such as where or when it was studied (e.g., Gronlund et al. 1997, Hintzman & Caulton 1997, Yonelinas & Jacoby 1994). In some recognition experiments, familiarity and recollection processes are placed in opposition by requiring participants to reject items that are highly familiar (e.g., either items that were studied on a particular list or foil items that are highly similar to studied items). As the amount of time allowed to make a recognition response is increased, the probability of incorrectly accepting these highly familiar items first increases then decreases (e.g., Dosher 1984, Gronlund & Ratcliff 1989, Hintzman & Curran 1994, McElree et al. 1999). The increasing, then decreasing nature of these functions indicates that at least two temporally distinct processes or memory components are operating in recognition memory: a rapidly available familiarity process that leads to incorrect responses when fast responses are required, and a slower recollective process that allows subjects to reject those items when participants are given more time.
A second line of evidence indicating that recognition involves two distinct memory processes comes from the analysis of recognition memory receiver operating characteristics (ROCs). ROCs are examined typically by collecting recognition confidence responses then plotting hits against false alarms as a function of confidence. As illustrated in Figure 1, ROCs observed in item-recognition tests are curvilinear when plotted in probability space and approximately linear when plotted in z-space. The z-space transformation allows for a relatively simple way of quantifying the nature of the functions and testing various recognition theories. Figure 1a shows that increasing study time leads the ROCs to increase in sensitivity (the height or “bowing” of the probability curve and an increase in the intercept in z-space) but to retain the same degree of ROC asymmetry in the probability curve (constant slope in z-space; for reviews, see Ratcliff et al. 1994, A. Yonelinas & C. Parks, submitted). In contrast, conditions where the meaning of items (“deep” encoding), compared with conditions where the perceptual features of items (“shallow” encoding), are emphasized during study (Figure 1b), increase overall performance but lead the ROC to become more asymmetrical (i.e., an increase in z-intercept and a decrease in z-slope; for reviews see Glanzer et al. 1999, A. Yonelinas & C. Parks, submitted). The dissociation between sensitivity and asymmetry indicates the existence of two or more functionally independent memory processes or components.
Figure 1.
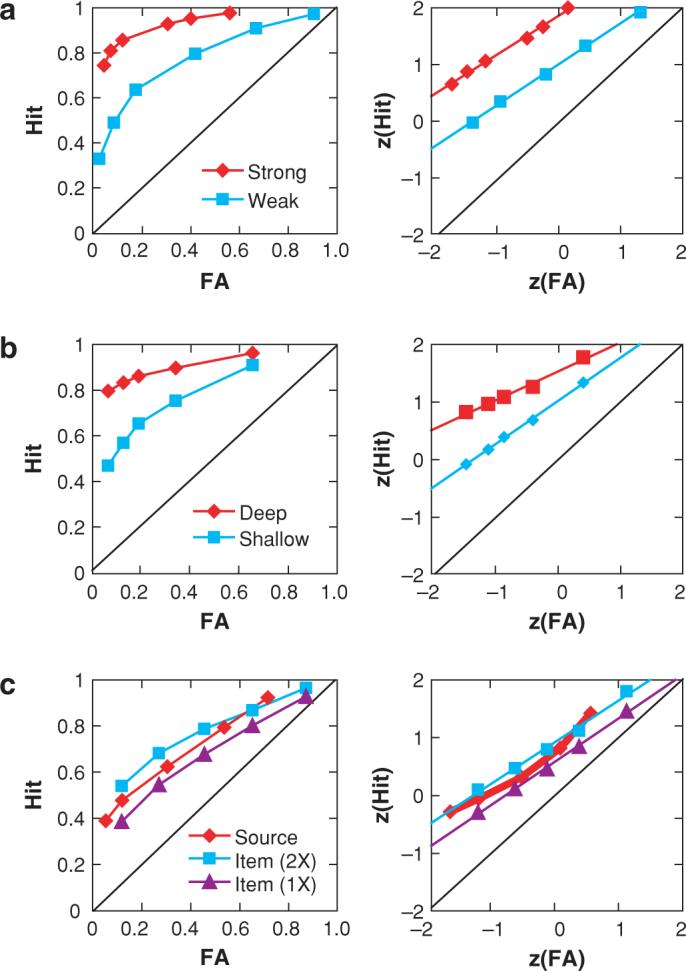
Receiver operating characteristic (ROC) analyses of recognition memory. Left panels plot probability of hits versus false alarms (FA). Right panels plot the same data normalized (z-Hit and zFA). Chance performance is represented by the diagonal in each panel. (a) ROCs for strongly encoded items (studied twice) and weakly encoded items (studied once) from Egan (1958, experiment 1). (b) ROCs for items studied under deep encoding conditions (i.e., rate the pleasantness of the word) and shallow encoding conditions (i.e., count the number of syllables in the word) (from Yonelinas et al. 1996). (c) ROCs in which subjects studied verbal items spoken once or twice by a male or female speaker. At test they were visually presented with the words and were to indicate how confident they were that the item had been presented earlier and whether it was presented by the male or female source (from Yonelinas 1997, experiment 3).
Another important finding was that ROCs in relational-recognition tests tend to be much flatter than item recognition ROCs, and z-transformed ROCs (z-ROCs) exhibit a pronounced U-shape (see Figure 1c). U-shaped z-ROCs are seen almost always in relational-recognition tests including both source memory (e.g., Glanzer et al. 2004, Hilford et al. 2002, Slotnick et al. 2000, Yonelinas 1999) and associative memory (Healy et al. 2005, Kelley & Wixted 2001, Rotello et al. 2000, Yonelinas et al. 1999). The “U” shape is predicted if recollection contributes to high-confidence recognition responses (Yonelinas 1994). This finding rules out other pure familiarity-based signal-detection models that predict that z-ROCs should always be linear (e.g., the unequal variance signal-detection model).
The processes that determine the shape of the recognition ROC are likely the same as those underlying subjective reports of recollection and familiarity, as well as the ability to retrieve relational information. That is, the ROC observed in item-recognition tasks can be quantified by fitting a nonlinear function to the observed data to derive estimates of recollection and familiarity (Yonelinas 1994, 2001a). In brief, the y-intercept provides a measure of recollection (i.e., the proportion of old items that are recollected), and the degree of curvilinearity in the ROC provides a measure of familiarity (i.e., the difference in familiarity strength between old and new items). The reasoning behind these interpretations is that recollection supports high-confidence recognition responses, whereas familiarity increases gradually across a wide range of recognition confidence. There are two additional methods commonly used to estimate recollection and familiarity. One method involves requiring that subjects distinguish items they “remember” using qualitative information about the study event versus items they merely “know” were studied on the basis of familiarity in the absence of recollection (Yonelinas & Jacoby 1995). The other method involves a process-dissociation procedure in which one measures recollection as the ability to retrieve where or when an item was studied, and familiarity as the ability to recognize an item given that it was not recollected ( Jacoby 1991). The convergence of results across these various measurement methods attests to the construct validity of the recollection/familiarity distinction and suggests that recollection and familiarity can be indexed using either the ROC, remember/know, or relational-recognition methods.
Receiver operating characteristic (ROC).
Comparison of the proportion of hits to false alarms under varying confidence levels or response biases
Hit.
correct recognition of an experienced item
False alarm.
incorrect identification of a new item as old
ERP.
event-related potential
Recordings of event-related brain potentials (ERPs) during memory tasks have provided further evidence to support the distinction between recollection and familiarity (for reviews see Friedman & Johnson 2000, Rugg & Yonelinas 2003). Numerous studies have reported temporally, topographically, and functionally distinct ERP correlates of recollection and familiarity during retrieval (for evidence of encoding differences see also Yovel & Paller 2005). For example, Figure 2 illustrates two distinct ERP modulations commonly observed during recognition: a mid-frontal negativity onsetting ∼400 ms after stimulus onset that is associated with familiarity, and a parietally distributed positivity beginning ∼500 ms after stimulus onset that is associated with recollection. The parietal effect is evident for correct, but not incorrect, relational-recognition judgments (e.g., Curran 2004), for remember-but-not-know responses (Curran 2004, Duarte et al. 2006, Duzel et al. 1997, Smith 1993), and with the most confidently recognized items (Woodruff et al. 2006). The mid-frontal effect is also related to successful recognition, but it tends to increase gradually with increases in recognition confidence (Woodruff et al. 2006). It also does not differentiate between recognized, recollected items and recognized, nonrecollected items. The earlier onset of the familiarity effects is in good agreement with the behavioral results indicating that familiarity is available earlier than recollection. The finding that recollection and familiarity are related to ERP modulations that exhibit distinct scalp topographies suggests that these processes are associated with activity in distinct neural generators.
Figure 2.
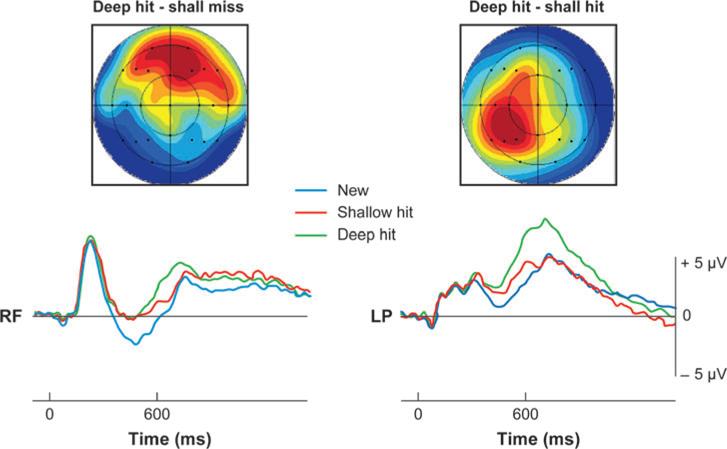
ERPs from Rugg et al. (2002) illustrating a mid-frontal ERP modulation (left panel ) associated with familiarity and a parietally distributed (right panel ) related to recollection. In this study, the familiarity effect was evident for deeply and shallowly encoding items, whereas the recollection effect was most pronounced for the deeply encoding items.
Implications of the Behavioral and ERP Data
The behavioral data reviewed above indicate that recollection and familiarity have qualitatively different characteristics during both the encoding of new information into memory and the retrieval of previously encoded information (see Table 1; for reviews see Diana et al. 2006, Yonelinas 2002). These studies indicate that there is a broad set of measurement tools that can be used to measure recollection and familiarity and that can thus be used to help identify the neural underpinnings of these processes. Moreover, the finding that the ERP correlates of recollection and familiarity are qualitatively different provides preliminary evidence that these processes depend on partially distinct brain regions.
ANATOMICAL ORGANIZATION OF THE MEDIAL TEMPORAL LOBE MEMORY SYSTEM
At least 50 years of evidence has established the importance of structures within the MTL as critical to memory. Anatomical studies have identified the major subregions of the MTL in humans, monkeys, and rodents (Figure 3, top) and have suggested a hypothetical functional organization for memory processing (Figure 3; for reviews see Burwell 2000, Witter et al. 1989).
Figure 3.
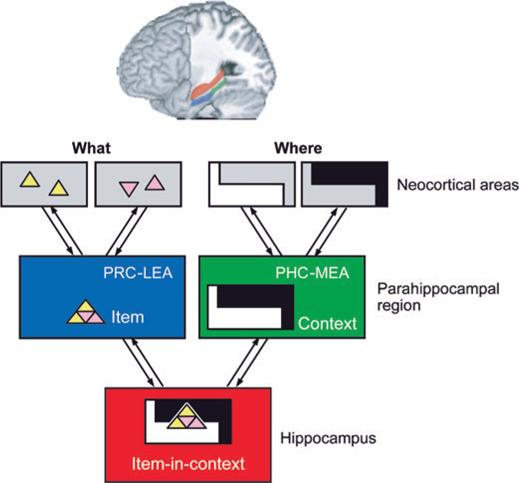
Functional organization of the MTL system. Neocortical input regarding the object features (“what”) converges in the perirhinal cortex (PRC) and lateral entorhinal area (LEA), whereas details about the location (“where”) of objects converge in the parahippocampal cortex (PHC) and medial entorhinal area (MEA). These streams converge in the hippocampus, which represents items in the context in which they were experienced. Reverse projections follow the same pathways back to the parahippocampal and neocortical regions. Back projections to the PHC-MEA may support recall or context, whereas back projections to the PHC-LEA may support recall of item associations.
At the broadest level, the MTL can be subdivided into the perirhinal cortex, the parahippocampal cortex, and entorhinal cortex (collectively referred to as the parahippocampal region), and the hippocampus (including the dentate gyrus, Ammon's horn, and subiculum). Most of the neocortical input to the perirhinal cortex comes from association areas that process unimodal sensory information about qualities of objects (i.e., “what” information), whereas most of the neocortical input to the parahippocampal cortex (called postrhinal cortex in rodents) comes from areas that process polymodal spatial (“where”) information. Subsequently, the “what” and “where” streams of processing remain largely segregated as the perirhinal cortex projects primarily to the lateral entorhinal area, whereas the parahippocampal cortex projects mainly to the medial entorhinal area. Some connections exist between the perirhinal and parahippocampal cortices and between the entorhinal areas, but the “what” and “where” information converge mainly within the hippocampus. The cortical outputs of hippocampal processing involve feedback connections from the hippocampus successively back to the entorhinal cortex, then perirhinal and parahippocampal cortices, and finally, neocortical areas from which the inputs to the MTL originated.
Implications of the Anatomical Data
This anatomical evidence suggests the following hypothesis about how information is encoded and retrieved during memory processing. During encoding, representations of distinct items (e.g., people, objects, events) are formed in the perirhinal cortex and lateral entorhinal area. These representations along with back projections to the “what” pathways of the neocortex can then support subsequent judgments of familiarity. In addition, during encoding, item information is combined with contextual (“where”) representations that are formed in the parahippocampal cortex and medial entorhinal area, and the hippocampus associates items and their context (as first proposed by Mishkin et al. 1983). When an item is subsequently presented as a memory cue, the hippocampus completes the full pattern and mediates a recovery of the contextual representation in the parahippocampal cortex and medial entorhinal area. Hippocampal processing may also recover specific item associates of the cue and reactivate those representations in the perirhinal cortex and lateral entorhinal area. The recovery of context and item associations constitutes the experience of recollection. In the succeeding sections, we consider the evidence on the functional roles of these brain areas in support of this hypothesis.
Parahippocampal region.
a set of cortical areas surrounding the hippocampus, including the perirhinal cortex, parahippocampal cortex (called postrhinal cortex in rodents), and entorhinal cortex
RECOGNITION MEMORY AND AMNESIA
Studies of recognition memory in amnesia have been driven by two major classes of theories: those that posit a single process that supports recognition and those that posit two distinct underlying processes.
One-Process Theories
One of the earliest views stated that the MTL is critical for recollection, whereas association cortex supports familiarity (e.g., Huppert & Piercy 1978, Mayes et al. 1985, Wickelgren 1979). According to this view, patients with MTL damage should exhibit recollection deficits, whereas familiarity-based recognition should remain unaffected. An alternative one-process theory argues that the MTL is equally important for both recollection and familiarity (Squire 1994, Squire & Zola 1998). By this view, MTL damage should lead to equivalent deficits in recollection and familiarity.
Neither of these theories provides an adequate account of MTL amnesia. For example, several studies have shown that MTL patients can discriminate between old and new items relatively well, but they are profoundly impaired at discriminating between recently and frequently presented items (e.g., Huppert & Piercy 1978, Mayes et al. 1989, Meudell et al. 1985). These results indicate that recollection is more impaired than familiarity in MTL patients, which is inconsistent with the claim that the MTL is equally important for both processes. Even when item recognition is matched, MTL patients typically perform poorly in relational-recognition tests that require them to remember when or where an item was presented (see Yonelinas 2002 for review). Studies using ROC and other estimation methods have verified that recollection was more impaired than familiarity, but in addition they have indicated that familiarity was also disrupted in MTL patients relative to controls (see Yonelinas et al. 1998). This pattern of broad deficits in recollection and familiarity following MTL damage has been observed in studies using the remember/know procedure (e.g., Blaxton & Theodore 1997, Kishiyama et al. 2004, Knowlton & Squire 1995, Moscovitch & McAndrews 2002, Schacter et al. 1996), the process dissociation procedure (Verfaellie & Treadwell 1993), and the ROC procedure (Yonelinas et al. 1998). These results are inconsistent with theories that assume that the MTL supports recollection but plays no role in supporting familiarity.
MRI.
magenetic resonance imaging
Hypoxia/ischemia.
temporary loss of oxygen typically resulting in neuronal damage
Two-Process Theories
In 1994, Eichenbaum et al. proposed that two separate processes within the MTL contribute to recognition memory. They argued that the hippocampus is critical for recollecting associations of a memory cue, whereas the parahippocampal region can support recognition of familiar cues in isolation. According to this proposal, only one process is affected by selective hippocampal damage, leading to partial or complete sparing of performance based on the other process; the extent of sparing and appearance of the deficit on any given task would depend on task parameters that favor one or the other process. Brown & Aggleton (2001) extended this distinction, suggesting that the hippocampus is critical for episodic recollection, whereas the perirhinal cortex supports judgments about the recency and familiarity of specific stimuli.
Both of these models predict that hippocampal damage should disrupt recollection but not familiarity, whereas damage to the surrounding parahippocampal region should lead to deficits in familiarity. These models can account for the typical pattern of severely impaired recollection and mildly deficient familiarity in MTL patients. That is, disproportionate recollection deficits are expected if the damage disproportionately affected the hippocampus as compared with the parahippocampal region. However, the strongest prediction of these models is that patients with selective hippocampal damage should exhibit a selective impairment in recollection.
More recent research has tested these theories by attempting to dissociate recollection and familiarity in patients with selective hippocampal damage. Typically, these patients developed memory loss following transient cerebral hypoxia. The hippocampus is particularly vulnerable to hypoxic-ischemic damage, and postmortem studies as well as structural imaging studies have demonstrated that mild hypoxia results in neuronal loss confined largely to the hippocampus (e.g., Gadian et al. 2000, Hopkins et al. 1995, Zola-Morgan et al. 1986). An important caveat about these studies is that it is impossible to rule out damage to regions outside the hippocampus, particularly in cases in which the hypoxic event is severe. Notably, structural magnetic resonance imaging (MRI) scans can fail to reveal neuronal loss that is apparent in histological examinations (Rempel-Clower et al. 1996).
Several studies have reported that hypoxic patients exhibit disproportional deficits in relational compared with item recognition (Giovanello et al. 2003b, Holdstock et al. 2005, Mayes et al. 2002, Turriziani et al. 2004). In some cases, the relational-recognition deficit appears to be reduced when the paired items are from the same category (e.g., face pairs compared with word-face pairs; Vargha-Khadem et al. 1997), but not in others (Turriziani et al. 2004). Studies using estimation methods have largely confirmed that recollection can be selectively impaired in hypoxic patients. For example, one study (Yonelinas et al., 2002) examining ROCs showed that mildly hypoxic patients exhibited severe deficits in recollection but demonstrated normal familiarity (Figure 4a,b). The results were verified by using remember/know measures in the same patients (Figure 4c). Moreover, the covariation between recall, recognition, and hypoxic severity (as indexed by coma duration) was examined using structural equation modeling methods in a large sample of hypoxic patients and indicated that hypoxic severity predicted the degree to which recollection, but not familiarity, was impaired (Figure 4d). A similar pattern of deficient recollection and preserved familiarity was reported in a patient with relatively selective hippocampal atrophy related to meningitis (Aggleton et al. 2005).
Figure 4.
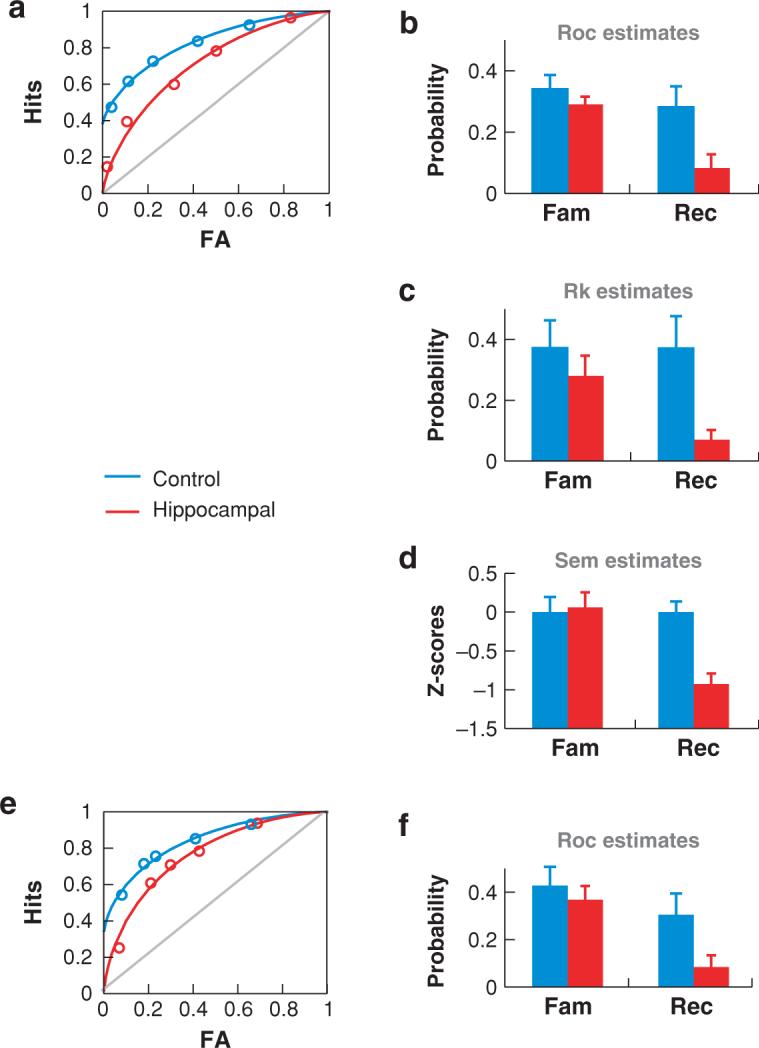
Measures of recollection and familiarity in amnesic patients with expected hippocampal damage (Yonelinas et al. 2002) and in rats with selective hippocampal lesions (Fortin et al. 2004). (a) ROCs for the amnesic patients are lower and more symmetrical than those of the controls. (b) ROC measures of recollection (R) and familiarity (F) indicate that recollection, but not familiarity, is reduced in the amnesics (H) compared with controls (C). (c) R and F estimates derived from a separate remember/know experiment also indicate that recollection was disrupted in the amnesic patients whereas familiarity was unaffected. (d ) R and F estimates derived using structural equation modeling (SEM) in a larger group of amnesic patients showed that increases in hypoxic severity led to a decrease in recollection but left familiarity unaffected. (e) ROCs for rats with hippocampal lesions are lower and more symmetrical than are those of control animals. ( f ) ROC measures indicated that familiarity is unaffected by hippocampal lesions.
However, some studies of severely impaired hypoxic patients have failed to find selective recollection deficits following limited hippocampal damage. For example, Cipolotti et al. (2006) found that a severely hypoxic patient exhibited deficits in recollection and familiarity indexed using the ROC procedure. However, in addition to hippocampal atrophy, the patient also exhibited parahippocampal region atrophy, which might have led to the familiarity deficits. Global impairments in item and relational recognition, and in familiarity and recollection, have also been observed in a group of amnesic patients with diverse etiologies (Wais et al. 2006, Gold et al. 2006, Manns et al. 2003, Stark & Squire 2003). In the absence of histological evidence, however, it is possible that the familiarity deficits observed in this group were also due to damage outside the hippocampus (Yonelinas et al. 2004).
Although human studies of amnesia have been useful in differentiating the role of the hippocampus from the surrounding parahippocampal gyrus, they have provided only limited evidence regarding the differential roles of the perirhinal and parahippocampal cortex in recognition memory because both regions are typically damaged together in MTL patients. Nonetheless, some evidence indicates that the parahippocampal cortex may be important particularly for the recall of spatial information (e.g., Bohbot et al. 2000).
Implications of the Findings on Amnesia
Extensive MTL damage leads to deficits in recollection and smaller but consistent deficits in familiarity. These results indicate the MTL is not a unified memory system that is critical only for recollection (Mayes et al. 1985) or that it is equally important for recollection and familiarity (Squire 1994). The results are more consistent with models stating that recollection is dependent on the hippocampus, whereas familiarity can be supported by the surrounding parahippocampal region (Brown & Aggleton 2001, Eichenbaum et al. 1994). Studies on amnesic patients have not provided definitive information on putative functional distinctions between the perirhinal and parahippocampal cortical areas.
FUNCTIONAL IMAGING OF RECOLLECTION AND FAMILIARITY
Functional imaging studies have linked activity throughout the MTL to successful recognition performance (see Henson 2005), but these studies have led to some confusion regarding the precise roles of different MTL subregions in recognition memory. For example, many studies have contrasted MTL activity during retrieval tests between studied (“old”) items and items that were not previously studied (“new”). Activation differences between old and new items could be related to increased recollection and/or familiarity elicited by old items (Rugg & Yonelinas 2003), increased encoding of new items (Stark & Okado 2003), or possibly nondeclarative memory processes such as repetition priming (Grill-Spector et al. 2006). Other studies have contrasted activation during encoding of items that were subsequently remembered against items that were subsequently forgotten (e.g., Brewer et al. 1998). In these contrasts, activations could reflect the formation of representations that support recollection, familiarity, or both processes. Accordingly, differentiating MTL activity associated with recollection and familiarity requires contrasts that more specifically isolate these processes.
Neuroimaging studies typically identify neural correlates of recollection by contrasting activity between recollected items and recognized items that were not recollected. For example, some studes use a remember/know test (Tulving 1985) and then contrast activity between items that elicited “remember” responses (presumably using recollection) and items that elicited “know” responses (presumably using high familiarity in the absence of recollection). Another approach is to use relational-recognition tests and then contrast activation between recognized items that were associated with successful retrieval of contextual information against recognized items for which the participant could not retrieve the specific contextual association.
Neural correlates of familiarity are typically examined by contrasting activation elicited during processing of nonrecollected items that generated varying levels of familiarity strength. As described above, most imaging studies have identified neural correlates of familiarity by contrasting activity between items that were recognized and not recollected (i.e., items eliciting a “know” response or items that were recognized but associated with incorrect source/relational judgments) against items that were not recognized (i.e., “misses”). Another potentially more powerful method capitalizes on the fact that familiarity varies in a continuous manner as a function of response confidence (see Recollection and Familiarity). A number of studies (Daselaar et al. 2006, Montaldi et al. 2006, Ranganath et al. 2003, Yonelinas et al. 2005) have used graded confidence ratings to identify regions where encoding- or retrieval-related activity increases or decreases monotonically with recognition confidence.
For several reasons, one might expect some variability in findings among studies. First, it is unlikely that any given imaging contrast will be 100% process-pure. Thus, a “recollection” contrast might also index some activity related to familiarity. Also, structures within the MTL are in close proximity, near the resolution of current functional MRI methodology, and these structures are highly interconnected. Thus, neural processing that originates in one region can be expected to activate closely connected neighboring regions, resulting in more extensive MRI signal changes (see Logothetis & Wandell 2004). For these reasons, we considered the overall pattern of findings in the imaging literature and asked whether activity in different MTL subregions tends to be correlated with recollection and/or familiarity (Table 2).
Table 2.
MTL activation in studies that examined neural correlates of recollection and/or familiarity1
| Study | Method | Materials | Stage | Contrast | Hipp | PPHG | APHG |
|---|---|---|---|---|---|---|---|
| A. Recollection of items | |||||||
| Davachi et al. 2003 | SC/SI/Miss | words | En | SC > SI | b | l | none |
| Gold et al. 2006 | SC/SI/Miss | words | En | SC > SI | none | none | l |
| Kensinger & Schacter 2006 | SC/SI/Miss | emotional pictures | En | SC > SI | l | r | none |
| Kensinger & Schacter 2006 | SC/SI/Miss | emotional words | En | SC > SI | l | none | none |
| Ranganath et al. 2003 | SC/SI/1−6 | words | En | SC > SI | r | r | none |
| Uncapher & Rugg 2005 | RKN | words | En | R > K | l | none | none |
| Uncapher et al. 2006 | SC/SI/Miss | words+2 sources | En | Both SC > 1 or 2 SI | r | none | none |
| Cansino et al. 2002 | SC/SI/Miss | words | Re | SC > SI | r | l | none |
| Daselaar et al. 2006 | 1−6 conf | words | Re | 6 > 1−5 | l | none | none |
| Dolcos et al. 2005 | RKN | neutral pictures | Re | R > K | b | r | none |
| Dolcos et al. 2005 | RKN | emotional pictures | Re | R > K | b | b | b |
| Eldridge et al. 2000 | RKN | words | Re | R > K | b | r | none |
| Kahn et al. 2004 | SC/SI/Miss | words | Re | SC > SI | none | b | none |
| Montaldi et al. 2006 | 1−4R | scenes | Re | R > all else | b | none | none |
| Sharot et al. 2004 | RKN | scenes | Re | R > K | none | r | none |
| Weis et al. 2004b | SC/SI/Miss | scenes | Re | SC > SI | b | none | none |
| Wheeler & Buckner 2004 | RKN | words | Re | R > K | b | none | none |
| Woodruff et al. 2005 | RKN | words | Re | R > K | r | r | none |
| Yonelinas et al. 2005 | 1−4R | words | Re | R > 4 | b | l | none |
| Reported activations | 16 | 11 | 2 | ||||
| Total contrasts | 19 | 19 | 19 | ||||
| % | 84% | 58% | 11% | ||||
| B. Recollection of associations | |||||||
| Jackson & Schacter 2004 | Assoc. rec. | word pairs | En | Intact hit > intact called recombined | l | none | l |
| Kirwan & Stark 2004 | Assoc. rec. | face-name | En | Intact hit > intact called recombined | r | r | none |
| Eldridge et al. 20052 | RKN | picture-word | Re | R > K | l* | none | b |
| Fenker et al. 2005 | RKN | word-fearful face | Re | R > K | r | none | r |
| Fenker et al. 2005 | RKN | word-neutral face | Re | R > K | b | l | none |
| Kirwan & Stark 2004 | Assoc. rec. | face-name | Re | Intact hit > intact called recombined | r | r | b |
| Reported activations | 6 | 3 | 4 | ||||
| Total contrasts | 6 | 6 | 6 | ||||
| % | 100% | 50% | 67% | ||||
| Study | Method | Materials | Stage | Contrast | Hipp | PPHG | APHG |
| C. Familiarity | |||||||
| Davachi et al. 2003 | SC/SI/Miss | words | En | SC = SI > Miss | none | none | l |
| Gold et al. 2006 | SC/SI/Miss | words | En | SC = SI > Miss | l | b | r |
| Henson et al. 1999 | RKN | words | En | K > R | none | none | r |
| Kensinger & Schacter 2006 | SC/SI/Miss | emotional pictures | En | SI > Miss | none | none | l |
| Kensinger & Schacter 2006 | SC/SI/Miss | emotional words | En | SI > Miss | none | none | l |
| Kirwan & Stark 2004 | Assoc. rec. | face-name | En | intact recognized > intact called new | none | r | r |
| Ranganath et al. 2003 | SC/SI/1−6 | words | En | 1−4 linear increase | none | none | l |
| Uncapher & Rugg 2005 | RKN | words | En | K > Miss | none | none | r |
| Uncapher et al. 2006 | SC/SI/Miss | words+2 sources | En | All recognized > forgotten | none | none | l |
| Daselaar et al. 2006 | 1−6 conf | words | Re | 1−6 linear decrease | l | none | l |
| Eldridge et al. 20051 | RKN | picture-word | Re | RK < Miss = CR | r* | r | none |
| Gonsalves et al. 2005 | RKN | faces | Re | R < K < Miss < CR | none | l | b |
| Montaldi et al. 2006 | 1−4R | scenes | Re | 1−4 linear decrease | none | none | b |
| Weis et al. 2004b | SC/SI/Miss | scenes | Re | SI < Miss | none | none | r |
| Yonelinas et al. 2005 | 1−4R | words | Re | 1−4 linear decrease | b | none | none |
| Reported activations | 4 | 4 | 13 | ||||
| Total contrasts | 15 | 15 | 15 | ||||
| % | 27% | 27% | 87% |
Notes: Only experiments and contrasts that reported activation in at least one MTL subregion were included in the table. Also, contrasts not specific to familiarity or recollection (see text) were excluded. Numbers 1−6 refer to recognition confidence ratings, in which higher ratings indicate higher confidence that an item was studied. Abbreviations: SC, source (and item) correct; SI, source incorrect but item correct; Miss, old item judged new; Assoc. rec., associative recognition; R, remember; K, know; N, new; En, encoding; Re, retrieval; l, left; r, right; b, bilateral.
This study used a flat-mapping procedure to differentiate activations in different hippocampal subregions. Although data were collected during encoding and retrieval, the encoding data were contaminated by an unexplained artifact that precludes a clear interpretation of the data. Accordingly, only the retrieval data are summarized here. Within the hippocampus, recollection-related activation was reported for the left subiculum, and familiarity-related activation was reported for the CA2/CA3/dentate gyrus region.
Finally, although anatomical studies in nonhuman primates and rats suggest an important functional distinction between lateral and medial areas of the entorhinal cortex, these areas have not yet been differentiated in humans. Given our current understanding of these regions (3), we consider signal in the anterior parahippocampal gyrus to reflect mainly activation of the perirhinal and lateral entorhinal areas and signal in the posterior parahippocampal region to reflect mainly activation of the parahippocampal cortex with or without medial entorhinal area activation.
Activation in the Hippocampus and Posterior Parahippocampal Gyrus
Table 2 summarizes results from studies that have identified neural correlates of items, recollection of item-item associations, and/or familiarity in the MTL. Of the 19 contrasts that identified activation related to recollection, 16 (84%) reported hippocampal activation and 11 reported posterior parahippocampal activation (58%). A similar pattern was seen in the 6 contrasts related to recollection of associations: all 6 reported hippocampal activation (100%) and 3 (50%) reported posterior parahippocampal activation. Of the 15 contrasts that identified neural correlates of familiarity-based recognition, only 4 (27%) revealed hippocampal activation and 4 (27%) revealed posterior parahippocampal activation. In two of the studies reporting hippocampal activation correlated with familiarity (Daselaar et al. 2006, Yonelinas et al. 2005), the analyses included responses to old and new items. Given that old items typically receive high confidence ratings and new items receive low ratings, the hippocampal activations may have reflected encoding of new item-context associations (e.g., Stark & Okado 2003) rather than familiarity.
Overall, the results suggest that hippocampal activation during both encoding and retrieval is consistently higher for recollected than nonrecollected items and is generally insensitive to changes in familiarity strength. This pattern of results was consistent across different measurement techniques and different stimulus materials. A similar pattern of results, although not as consistent, is apparent in the posterior parahippocampal gyrus.
Activation in the Anterior Parahippocampal Gyrus
Table 2 reveals a very different pattern of results in the anterior parahippocampal gyrus. Only 2 of 19 (11%) contrasts related to item recollection reported activation in the anterior parahippocampal gyrus, whereas 13 of the 15 (87%) familiarity contrasts reported anterior parahippocampal activation. Anterior parahippocampal activation was reported in 4 of the 6 contrasts (67%) related to recollection of item-item associations. Thus, anterior parahippocampal activation is generally correlated with familiarity and rarely correlated with item recollection.
The relationship between anterior parahippocampal activation and familiarity differs between encoding and retrieval: Activation during encoding is higher for items that were subsequently rated as highly familiar than for items that were subsequently forgotten (Davachi et al. 2003, Kensinger & Schacter 2006, Kirwan & Stark 2004, Ranganath et al. 2003, Uncapher & Rugg 2005), whereas during retrieval, activation is lower for items that were highly familiar than for items that were forgotten (Daselaar et al. 2006, Gonsalves et al. 2005, Montaldi et al. 2006, Weis et al. 2004a). Results from studies using confidence ratings have progressed further by showing that activation during encoding increases monotonically with subsequent confidence ratings (Ranganath et al. 2003), whereas activity during recognition decreases monotonically with recognition confidence (Daselaar et al. 2006, Montaldi et al. 2006). Although this pattern might seem counterintuitive, it is strikingly consistent with results from imaging and physiological studies of rodents and monkeys that are reviewed below (see also Brozinsky et al. 2005, Henson et al. 2003).
As shown in Table 2c, anterior parahippocampal activation was sometimes (4 of 6 contrasts; 67%) correlated with recollection in studies that required participants to learn associations between two items, such as faces and names (Kirwan & Stark 2004), faces and words (Fenker et al. 2005), pictures and words (Eldridge et al. 2005), or pairs of unrelated words ( Jackson & Schacter 2004). These findings suggest that, at least under some conditions, the perirhinal cortex may support memory for associations between items, consistent with findings from studies on rats (Bunsey & Eichenbaum 1993) and monkeys (Murray et al. 1993).
Implications of the Imaging Data
Findings from the imaging literature are inconsistent with the idea that all MTL subregions contribute to an equivalent degree to recollection and familiarity-based recognition. Most compelling in support of this conclusion are within-study dissociations (e.g., Figure 5) in which anterior parahippocampal activation was selectively associated with familiarity and activity in the hippocampus and posterior parahippocampal gyrus was selectively associated with recollection (Daselaar et al. 2006, Davachi et al. 2003, Kensinger & Corkin 2004, Ranganath et al. 2003, Uncapher & Rugg 2005, Weis et al. 2004b). The findings are also inconsistent with a simple distinction between the hippocampus and the parahippocampal region. Instead, the results summarized above suggest a functional dissociation between the perirhinal cortex, where activation changes are consistently associated with familiarity, and the hippocampus and parahippocampal cortex, where activation changes are consistently associated with recollection. The results are similar across studies that examined subsequent memory effects at encoding and studies that examined activity during retrieval and across studies that used very different techniques to measure familiarity and recollection.
Figure 5.
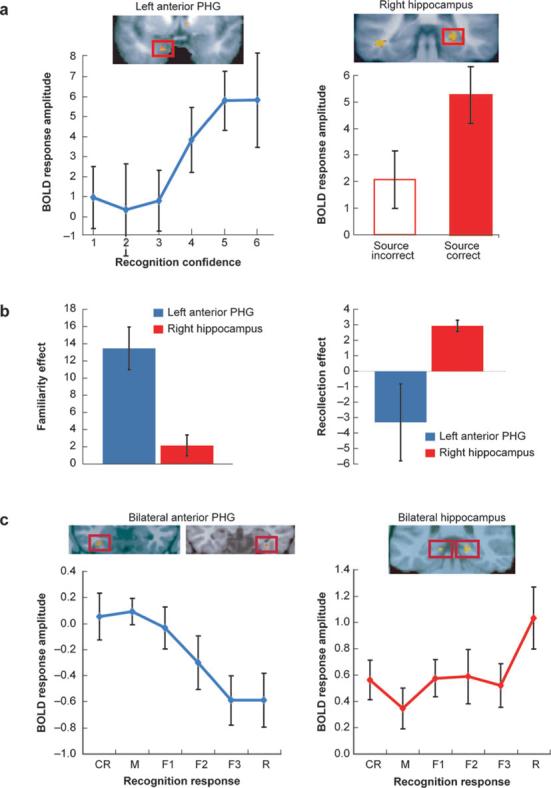
Double dissociations between hippocampal and anterior parahippocampal activity. (a) Data from Ranganath et al. (2003). Participants were scanned during encoding of words shown in red or green and subsequently were asked to rate their confidence regarding whether each word was shown in the scanner and to decide whether the word was shown in red or green during the study phase. Activity in the left anterior parahippocampal gyrus (PHG; left) during encoding was correlated with subsequent confidence ratings, suggesting a role in familiarity-based recognition. Encoding activity in the right hippocampus was correlated with accuracy on the relational-recognition test, suggesting a role in recollection. (b) Comparison of the magnitudes of subsequent familiarity (left) and recollection (right) effects in the left anterior PHG and right hippocampal regions. The comparison revealed a reliable double dissociation between the two regions. (c) Data from Montaldi et al. (2006). Participants studied scenes and were scanned during recognition testing. For each item that was judged old, participants were to indicate if it was recollected (R) or to rate its familiarity on a 1–3 scale (F1–F3). Activation is separately plotted for correctly rejected new items (CR) and for old items that elicited new (M), F1–F3, and R responses. Activation in bilateral anterior parahippocampal regions (left) monotonically decreased with increasing confidence and did not differ between highly familiar items (F3) and items that were recollected. In contrast, activation in bilateral hippocampal regions (right) was increased specifically for recollected items, as compared with nonrecollected items, and was insensitive to gradations in familiarity strength.
The findings from the imaging literature, along with the anatomical characteristics of the MTL (Figure 3), suggest that the hippocampus, the perirhinal cortex, and the parahippocampal cortex may each form unique representations that support recognition memory. In perirhinal cortex, encoding an item may elicit increases in the selectivity or efficiency (i.e., “strength”) of item representations. Consequently, during retrieval, increased familiarity is manifest as reduced activation of these representations (Brown & Aggleton 2001, Eichenbaum et al. 1994, Yonelinas 2002). In contrast, the parahippocampal cortex may encode representations of the global context in which an item was encountered (Bar & Aminoff 2003, Davachi et al. 2003, Ranganath et al. 2003), and the hippocampus may represent item-context associations (Davachi et al. 2003, Ranganath et al. 2003). Activation of these representations during retrieval may support recollection.
ANIMAL MODELS OF RECOGNITION MEMORY
Animal models offer a substantial improvement in the resolution with which we can examine the effects of selective damage to particular MTL areas and allow us to identify the behavioral correlates of neural activation at the level of the units of information processing. These methods have been applied in recognition-memory models in which monkeys or rats are trained to respond differentially to new and previously experienced stimuli or in which we observe their natural tendency to explore novel stimuli.
Delayed Nonmatch to Sample (DNMS)
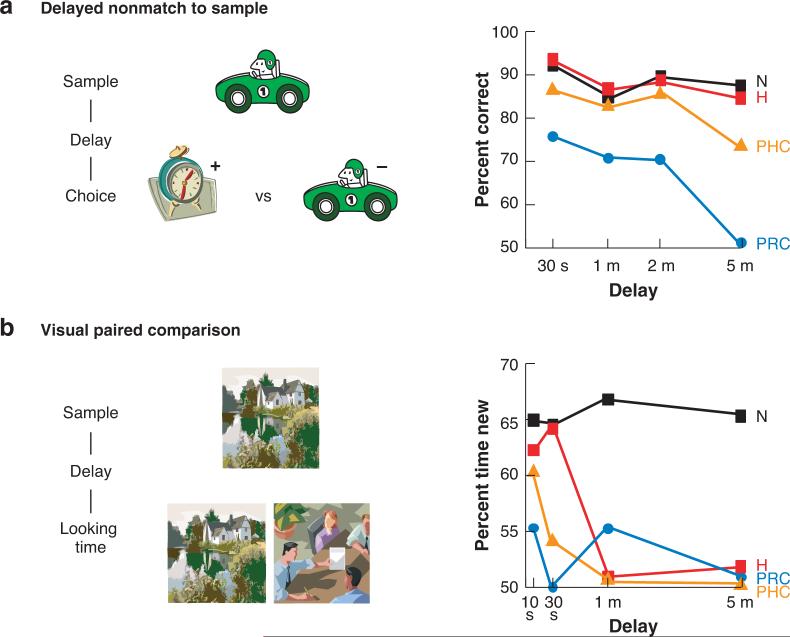
In the delayed nonmatch to sample (DNMS) paradigm an initially novel “sample” object is presented, and after a variable delay, the subject is rewarded for selecting another novel (i.e., nonmatching) object over the sample (Figure 6). Monkeys with extensive MTL lesions perform well if the delay is a few seconds, but recognition deteriorates rapidly at longer delays (Mishkin 1978). This effect is analogous to the rapid forgetting rate observed in amnesic patients (Squire et al. 1988). A similar severe and rapid decline in recognition is also observed after damage limited to the perirhinal cortex alone (Nemanic et al. 2004) (Figure 6) or in combination with parahippocampal or entorhinal cortex in monkeys (Meunier et al. 1993, Zola-Morgan et al. 1989) and in rats (Otto & Eichenbaum 1992a, reviewed in Brown & Aggleton 2001, Steckler et al. 1998).
Figure 6.
Performance of normal monkeys (N) and monkeys with lesions of the hippocampus (H), perirhinal cortex (PRC), or parahippocampal cortex (PHC) on two recognition memory tasks. (a) In the delayed nonmatch to sample task, animals must remember a sample object over a delay period, then select against it (nonmatch) in the choice phase. Normal monkeys show robust recognition over long memory delays, as do animals with hippocampal or PHC damage. PRC lesions result in rapid decay of memory. (b)In the visual paired comparison task, animals remember a once-viewed scene and later prefer to view a novel scene. Normal monkeys also show robust recognition over long delays in this task, but memory in animals with H, PHC, or PRC lesions rapidly decays (data from Nemanic et al. 2004).
Delayed nonmatch to sample (DNMS).
recognition test wherein the subject is rewarded for choosing a novel stimulus over a familiar one
Spontaneous novelty exploration.
recognition test that exploits the tendency to preferentially investigate novel over previously experienced stimuli
In contrast, monkeys with selective damage to the hippocampus (Nemanic et al. 2004) (Figure 6) or entorhinal cortex (Buckmaster et al. 2004) can perform normally on DNMS even at substantial memory delays. In some studies, a small but statistically significant deficit was observed at a very long delay (Zola et al. 2000), but in other studies, no deficit was observed even at long memory delays or when animals were required to remember a long list of stimuli (Murray & Mishkin 1998). In rats, DNMS performance is also intact following selective hippocampal damage (see Mumby 2001 and Steckler et al. 1998 for detailed reviews), although some studies report partial impairment at long delay intervals or with very large lists of sample objects (Clark et al. 2001, Dudchencko et al. 2000, Steele & Rawlins 1993; reviewed in Mumby 2001).
Spontaneous Novelty Exploration
In studies that examine recognition by monitoring spontaneous exploration of familiar and novel stimuli, an object or picture is initially presented and then represented after a delay along with a novel stimulus (Figure 6). Animals typically spend approximately twice as much time investigating the novel stimulus over the previously experienced stimulus. Superficially, these paradigms seem to tap into the same recognition memory processes as does the DNMS task. However, there are differences in the nature of the stimuli and behavioral responses, as well as in the motivational basis for performance, that could influence the use of different memory strategies in the expression of recognition memory (Nemanic et al. 2004). Indeed, monkeys with selective hippocampal or parahippocampal cortex damage that show little or no deficit in DNMS have severe and rapidly apparent deficits on the spontaneous novelty exploration task (Figure 6) (Nemanic et al. 2004, Zola et al. 2000). In rats, some studies report no deficit (e.g., Mumby et al. 2002, Winters et al. 2004; reviewed in Mumby 2001) whereas other studies have observed impairment at long delays (Clark et al. 2000, Hammond et al. 2004), and observation of the deficit may depend on the amount of damage to the hippocampus (e.g., Broadbent et al. 2004). In contrast to the modest and variable deficit observed following damage to the hippocampus, ablation of the perirhinal cortex consistently results in a severe and rapidly developing deficit in monkeys (Nemanic et al. 2004) (Figure 6) and in rats (e.g., Ennaceur et al. 1996, Mumby et al. 2002, Norman & Eacott 2005, Winters et al. 2004, Winters & Bussey 2005).
In variants of recognition tasks during which rats must remember places, the hippocampus consistently plays an important role. Hippocampal damage causes severe and immediate deficits on DNMS tasks in which animals must remember arms of a recently visited maze (Olton et al. 1979) or complex visual cues that are composed as visually elaborated arms of a maze (e.g., Cassaday & Rawlins 1995; Prusky et al. 2004). In a variant of the spontaneous novelty exploration task where an initially presented object is moved to a new location or to a new environment during the test phase, selective hippocampal lesions consistently result in deficits even following relatively small lesions of the hippocampus that have no effect on exploration of novel objects (e.g., Eacott & Norman 2004, Mumby et al. 2002). Similarly, damage to the parahippocampal cortex does not affect exploration of novel objects but results in severe impairment in recognizing objects after a change in position or context (Eacott and Norman 2004, Norman & Eacott 2005). These results suggest that the hippocampus and parahippocampal cortex may be involved particularly in memory for spatial scenes or context.
Resolving the Discrepancies: Two Components of Recognition Memory in Animals
One explanation for the partial and variable effects of hippocampal damage on object recognition memory is that the hippocampus supports only one of two processes that contribute to recognition performance (i.e., recollection), and the demand for this component varies across paradigms. This hypothesis was recently tested by training rats on a variant of DNMS in which they initially sampled a series of odors and then judged old and new test stimuli across a range of response criteria. These data were used to derive ROC functions, similar to the approach used in humans (See Recollection and Familiarity) (Fortin et al. 2004). Similar to the findings in healthy humans, the ROC curve of normal rats was asymmetrical and curvilinear, indicating both recollection and familiarity components of recognition (Figure 3e, f ). By contrast, the ROC curve of rats with selective hippocampal damage was entirely symmetrical and identical to the familiarity component of the ROC of control animals, which indicated that recognition was supported primarily by familiarity. A strong recollection component was retained in normal rats when the memory delay was elongated to equate their overall accuracy with that of rats with hippocampal damage at the shorter delay. This observation indicates that deterioration of the recollection component of the ROC curve following hippocampal damage is not a consequence of a generally weakened memory but rather a selective loss of the contribution of recollection. The combined findings strongly support two-process over single-process models of recognition and identify the hippocampus as selectively critical to recollection. Furthermore, these findings explain the inconsistent effects of hippocampal damage on recognition memory in previous studies. In animals, as in humans, the presence and magnitude of deficits depend on the relative contributions of recollection and familiarity processes to each particular task.
Activity Patterns of Single Neurons in the Medial Temporal Region
During the performance of delayed matching and DNMS tasks, cells in the perirhinal and lateral entorhinal areas of monkeys and rats exhibit properties that suggest a role in recognition memory (reviewed in Brown & Xiang 1998, Desimone et al. 1995, Fuster 1995, Suzuki & Eichenbaum 2000). For example, many cells exhibit stimulus-specific responses that are enhanced or suppressed when the preferred stimulus is represented. Some of these neurons show diminished responses to stimulus repetition within a recognition memory trial but then recover their maximal responses; these cells could signal the recency of stimulus presentations. Other cells exhibit long-lasting decrements in responsiveness to stimuli, which could support recognition over extended periods. In most studies, more cells show suppressed rather than enhanced responses to stimulus repetition, and this mixture of responses has been attributed to a sharpening of the ensemble representation of items as a result of experience. Although stimulus-selective repetition effects are robust in perirhinal and lateral entorhinal area, they are not often observed in parahippocampal and medial entorhinal area. Instead, parahippocampal and medial entorhinal area neurons demonstrate strong spatial coding (Burwell & Hafeman 2003, Fyhn et al. 2004, Hargreaves et al. 2005), consistent with lesion studies demonstrating the importance of the parahippocampal cortex in recognition that relies on spatial context.
Hippocampal neurons in monkeys (Brown & Xiang 1998), rats (e.g., Otto & Eichenbaum 1992a), and humans (Rutishauser et al. 2006) do not show increased or decreased responses associated with repetition of specific stimuli during delayed matching and nonmatching tasks that test item recognition. Instead, hippocampal neurons show only general responses to novelty or familiarity across a broad range of stimuli, suggesting a role in encoding the outcome of recognition experiences. In contrast with the absence of stimulus-specific responses in item-recognition tasks, results from studies of rats (e.g., Hampson et al. 1993; Moita et al. 2003; Wood et al. 1999, 2000), monkeys (e.g., Cahusac et al. 1993, Wirth et al. 2003), and humans (e.g., Ekstrom et al. 2003, Kreiman et al. 2000) suggest that hippocampal neurons encode associations between specific stimuli and a unique location or behavioral context. These results indicate that hippocampal firing patterns reflect unique conjunctions of stimuli with their significance, the animal's specific behaviors, and the places and contexts in which the stimuli occur (Eichenbaum 2004).
The observations from single neuron recordings have been confirmed by differential activation of the immediate early gene fos in neurons in the MTL (e.g., Wan et al. 1999). In these studies, rats are trained to view visual stimuli that are novel and familiar or familiar but spatially rearranged. Fos is activated by novel stimuli in the perirhinal and lateral entorhinal area but not in the hippocampus or postrhinal (parahippocampal) cortex. Conversely, fos is expressed in response to novel spatial arrangements of familiar stimuli, as well as in spatial learning (Vann et al. 2000), selectively in the hippocampus and postrhinal cortex but not in perirhinal cortex.
Implications of the Data from Animal Models
The convergence of findings from lesion and recording approaches in animals strongly supports the idea that different components of the MTL make distinct contributions to recognition memory. The combined findings on monkeys and rats suggest that perirhinal cortex lesions have a devastating effect on object-recognition memory. Conversely, the perirhinal cortex can support relatively intact recognition memory even when the hippocampus or neocortical input to the hippocampus is eliminated. Consistent with these findings, neurons in the perirhinal cortex encode and maintain representations of individual stimuli and signal their familiarity. In contrast, damage to the parahippocampal or medial entorhinal areas results in deficits in spatial recognition, and correspondingly, neurons in these areas encode spatial features of the environment and not individual stimuli.
Damage to or disconnection of the hippocampus has a relatively modest effect on item recognition but results in severe deficits in memory for spatial context and in the recollection component of item recognition. Correspondingly, hippocampal neurons encode configurations of items in the behavioral and spatial context in which they were experienced, a central feature of recollective memory.
CONCLUSIONS
The diverse lines of evidence reviewed above suggest that recollection and familiarity are functionally dissociable processes and that different MTL subregions make distinct contributions to recognition memory. Evidence from neuropsychological, neuroimaging, and neurophysiological studies of humans, monkeys, and rats indicates a specific role for the hippocampus in recollection and not familiarity. The parahippocampal cortex also contributes to recollection, possibly via the representation and retrieval of contextual (especially spatial) information, whereas perirhinal cortex contributes to and is necessary for familiarity-based recognition.
The findings are consistent with a novel, anatomically guided (Figure 3) hypothesis regarding the mechanisms by which different regions of the MTL may interact to support the phenomenology of recollection and familiarity: During encoding, information about stimuli to be remembered, processed by the perirhinal and lateral entorhinal areas, and information about their context, processed by parahippocampal and medial entorhinal areas, converge in the hippocampus. When a previously encountered stimulus is processed, perirhinal and lateral entorhinal areas can signal its match to a preexisting item representation, observed as overall suppressed activation. This match signal can be propagated back to neocortical areas, which may be sufficient to generate the sense of familiarity without participation of the hippocampus. Additionally, processing of the stimulus may drive the recovery of object-context associations in the hippocampus that, via back projections, reactivate a representation of the contextual associations in the parahippocampal and medial entorhinal areas. These areas, in turn, project back to the neocortical areas that processed the context in which the item was previously encountered, thereby eliciting the subjective experience of recollection.
Our working hypothesis leads to several predictions, some of which have been tested in experiments described above or in other published studies, and some of which remain challenges for future experiments. One prediction is that the perirhinal and lateral entorhinal cortices encode individual items and thereby support familiarity-based recognition. Consistent with this prediction, damage to perirhinal cortex results in deficits in familiarity in human amnesic patients and severe impairment in item recognition in monkeys and rats across different recognition tests. Correspondingly, functional imaging studies in humans and single-neuron recording studies in monkeys and rats have revealed differential activation of the perirhinal cortex, as well as lateral entorhinal cortex in animals, when subjects view novel versus familiar stimuli. Furthermore, the pattern of decreased neural responses to familiar stimuli is similar across species and physiological measures and distinct from activation patterns associated with recollection (reviewed in Brown & Aggleton 2001, Brozinsky et al. 2005, Eichenbaum et al. 1994, Henson et al. 2003). One outstanding question is whether the human entorhinal cortex can be subdivided into functionally distinct lateral and medial areas similar to those described in animals.
Another expectation derived from the anatomical framework is that the parahippocampal and medial entorhinal cortices process spatial context and could play a more general contextual processing role. A paucity of evidence exists on this issue from studies of human patients. However, functional imaging studies have shown that the parahippocampal cortex is selectively activated when human subjects examine spatial scenes or objects that strongly evoke spatial as well as nonspatial contexts (Bar & Aminoff 2003) and during recollection of spatial or nonspatial contexts (Table 2a,b), whereas the perirhinal cortex is disproportionately activated during the object processing (Pihlajamaki et al. 2004). Converging results come from lesion studies in rats (Gaffan et al. 2004, Norman & Eacott 2005) and monkeys (Alvarado & Bachevalier 2005, Malkova & Mishkin 2003) have demonstrated that object-location recognition is impaired following damage to the parahippocampal cortex, whereas object recognition is impaired following perirhinal damage. Furthermore, results from single-unit recording studies and fos imaging indicate that neurons in rodent homologs of parahippocampal and medial entorhinal cortices show strong spatial coding, whereas perirhinal and lateral entorhinal neurons have poor spatial-coding capacities. In future studies it will be important to determine whether the parahippocampal region is generally activated during recollection or more specifically activated during retrieval of particular contextual information as a part of the recalled experience. Recollection of specific contexual information might be observed in imaging studies on human subjects as region-specific activations that match the kind of material retrieved and in single-neuron recording studies as reinstantiation of specific firing patterns that match the retrieved contextual information. In addition, our hypothesis predicts that the perirhinal and lateral entorhinal cortices may be involved in recovery of specific item-item associations during recollection. Some evidence from the imaging (Table 2b) and single-neuron recording studies (Naya et al. 2001) is consistent with this prediction.
Our hypothesis also suggests that the hippocampus plays a specialized role in associating items and their contexts in the service of recollection. This claim is strongly supported by several lines of evidence. Recent studies on human amnesic patients indicate that the hippocampus supports our ability to associate items in memory and to recollect contextual associations, as compared with recognition of single items based on familiarity. Parallel evidence from functional imaging studies strongly indicates that the hippocampus is selectively activated during item associations and contextual associations in support of recollection (Table 2; see also Addis et al. 2004, Davachi & Wagner 2002, Giovanello et al. 2003a, Prince et al. 2005). In animals, the hippocampus is selectively involved in the recollection component of recognition memory and recognition of spatial context. Correspondingly, hippocampal neurons are activated by stimuli in the spatial or temporal contexts in which they were experienced. These findings converge on the notion that the hippocampus supports recollective memory by associating items and their contexts. Future research can build on these findings by strengthening the connections between findings from animal models and the phenomenology of recollection in humans. A critical step toward this goal will be to determine whether the parameters that differentially influence recollection and familiarity in humans (see Table 1) have similar effects in animals in order to develop valid animal models that can support detailed analyses of information coding within subfields of the hippocampus.
Available evidence also suggests roles for the prefrontal cortex, parietal cortex, and diencephalon in familiarity and recollection (e.g., Aggleton & Brown 1999, Duarte et al. 2005, Kishiyama et al. 2005, Uncapher et al. 2006). A comprehensive understanding of the neurobiology of recognition will require a consideration of the contributions of these and other areas as they coordinate with the MTL. Although there remain many unanswered questions within the framework offered here, it provides a simple and testable working hypothesis about the functional organization of recognition memory by the MTL system.
ACKNOWLEDGEMENTS
NIMH MH71702, MH51570, MH52090 to H.E., MH059352, NS40813 to A.Y, and MH67821 to C.R.
LITERATURE CITED
- Addis DR, Moscovitch M, Crawley AP, McAndrews MP. Recollective qualities modulate hippocampal activation during autobiographical memory retrieval. Hippocampus. 2004;14:752–62. doi: 10.1002/hipo.10215. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Brown MW. Episodic memory, amnesia, and the hippocampal-anterior thalamic axis. Behav. Brain Sci. 1999;22:425–44. [PubMed] [Google Scholar]
- Aggleton JP, Vann SD, Denby C, Dix S, Mayes AR, et al. Sparing of the familiarity component of recognition memory in a patient with hippocampal pathology. Neuropsychologia. 2005;43(12):1810–23. doi: 10.1016/j.neuropsychologia.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Alvarado MC, Bachevalier J. Comparison of the effects of damage to the perirhinal and parahippocampal cortex on transverse patterning and location memory in rhesus macaques. J. Neurosci. 2005;25:1599–609. doi: 10.1523/JNEUROSCI.4457-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar M, Aminoff E. Cortical analysis of visual context. Neuron. 2003;38:347–58. doi: 10.1016/s0896-6273(03)00167-3. [DOI] [PubMed] [Google Scholar]
- Blaxton TA, Theodore WH. The role of the temporal lobes in recognizing visuospatial materials: remembering versus knowing. Brain Cogn. 1997;35(1):5–25. doi: 10.1006/brcg.1997.0902. [DOI] [PubMed] [Google Scholar]
- Bohbot VD, Allen JJ, Nadel L. Memory deficits characterized by patterns of lesions to the hippocampus and parahippocampal cortex. Ann. N.Y. Acad. Sci. 2000;911:355–68. doi: 10.1111/j.1749-6632.2000.tb06737.x. [DOI] [PubMed] [Google Scholar]
- Brewer JB, Zhao Z, Desmond JE, Glover GH, Gabrieli JD. Making memories: brain activity that predicts how well visual experience will be remembered. Science. 1998;281:1185–87. doi: 10.1126/science.281.5380.1185. [DOI] [PubMed] [Google Scholar]
- Broadbent NJ, Squire LR, Clark RE. Spatial memory, recognition memory, and the hippocampus. Proc. Natl. Acad. Sci. USA. 2004;101(40):14515–20. doi: 10.1073/pnas.0406344101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MW, Aggleton JP. Recognition memory: What are the roles of the perirhinal cortex and hippocampus? Nat. Rev. Neurosci. 2001;2:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- Brown MW, Xiang JZ. Recognition memory: neuronal substrates of the judgment of prior occurrence. Prog. Neurobiol. 1998;55:149–89. doi: 10.1016/s0301-0082(98)00002-1. [DOI] [PubMed] [Google Scholar]
- Brozinsky CJ, Yonelinas AP, Kroll NE, Ranganath C. Lag-sensitive repetition suppression effects in the anterior parahippocampal gyrus. Hippocampus. 2005;15(5):557–61. doi: 10.1002/hipo.20087. [DOI] [PubMed] [Google Scholar]
- Buckmaster CA, Eichenbaum H, Amaral DG, Suzuki WA, Rapp P. Entorhinal cortex lesions disrupt the relational organization of memory in monkeys. J. Neurosci. 2004;24:9811–25. doi: 10.1523/JNEUROSCI.1532-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunsey M, Eichenbaum H. Critical role of the parahippocampal region for paired-associate learning in rats. Behav. Neurosci. 1993;107:740–47. doi: 10.1037//0735-7044.107.5.740. [DOI] [PubMed] [Google Scholar]
- Burwell RD. The parahippocampal region: corticocortical connectivity. Ann. N.Y. Acad. Sci. 2000;911:25–42. doi: 10.1111/j.1749-6632.2000.tb06717.x. [DOI] [PubMed] [Google Scholar]
- Burwell RD, Hafeman DM. Positional firing properties of postrhinal cortex neurons. Neuroscience. 2003;119:577–88. doi: 10.1016/s0306-4522(03)00160-x. [DOI] [PubMed] [Google Scholar]
- Cahusac PMB, Rolls ET, Miyashita Y, Niki H. Modification of the responses of hippocampal neurons in the monkey during the learning of a conditional spatial response task. Hippocampus. 1993;3:29–42. doi: 10.1002/hipo.450030104. [DOI] [PubMed] [Google Scholar]
- Cansino S, Maquet P, Dolan RJ, Rugg MD. Brain activity underlying encoding and retrieval of source memory. Cereb. Cortex. 2002;12:1048–56. doi: 10.1093/cercor/12.10.1048. [DOI] [PubMed] [Google Scholar]
- Cassaday HJ, Rawlins JNP. Fornix-fimbria section and working memory deficits in rats: Stimulus complexity and stimulus size. Behav Neurosci. 1995;109:594–606. doi: 10.1037//0735-7044.109.4.594. [DOI] [PubMed] [Google Scholar]
- Cipolotti L, Bird C, Good T, Macmanus D, Rudge P, Shallice T. Recollection and familiarity in dense hippocampal amnesia: a case study. Neuropsychologia. 2006;44(3):489–506. doi: 10.1016/j.neuropsychologia.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Clark RE, West AN, Zola SM, Squire LR. Rats with lesions of the hippocampus are impaired on the delayed nonmatching-to-sample task. Hippocampus. 2001;11:176–86. doi: 10.1002/hipo.1035. [DOI] [PubMed] [Google Scholar]
- Clark RE, Zola SM, Squire LR. Impaired recognition memory in rats after damage to the hippocampus. J. Neurosci. 2000;20(23):8853–60. doi: 10.1523/JNEUROSCI.20-23-08853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran T. Effects of attention and confidence on the hypothesized ERP correlates of recollection and familiarity. Neuropsychologia. 2004;42:1088–106. doi: 10.1016/j.neuropsychologia.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Fleck MS, Cabeza R. Triple dissociation in the medial temporal lobes: recollection, familiarity, and novelty. J. Neurophysiol. 2006;96:1902–11. doi: 10.1152/jn.01029.2005. [DOI] [PubMed] [Google Scholar]
- Davachi L, Mitchell JP, Wagner AD. Multiple routes to memory: distinct medial temporal lobe processes build item and source memories. Proc. Natl. Acad. Sci. USA. 2003;100:2157–62. doi: 10.1073/pnas.0337195100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davachi L, Wagner AG. Hippocampal contributions to episodic encoding, insights from relational and item-based learning. J. Neurophysiol. 2002;88:982–90. doi: 10.1152/jn.2002.88.2.982. [DOI] [PubMed] [Google Scholar]
- Desimone R, Miller EK, Chelazzi L, Lueschow A. Multiple memory systems in the visual cortex. In: Gazzaniga MS, editor. The Cognitive Neurosciences. MIT Press; Cambridge, MA: 1995. pp. 475–86. [Google Scholar]
- Diana RA, Reder LM, Arndt J, Park H. Models of recognition: a review of arguments in favor of a dual-process account. Psychon. Bull. Rev. 2006;131:1–21. doi: 10.3758/bf03193807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R. Remembering one year later: role of the amygdala and the medial temporal lobe memory system in retrieving emotional memories. Proc. Natl. Acad. Sci. USA. 2005;102:2626–31. doi: 10.1073/pnas.0409848102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosher BA. Discriminating preexperimental semantic from learned episodic associations: a speed/accuracy study. Cogn. Psychol. 1984;164:519–55. [Google Scholar]
- Duarte A, Ranganath C, Knight RT. Effects of unilateral prefrontal lesions on familiarity, recollection, and source memory. J. Neurosci. 2005;25(36):8333–37. doi: 10.1523/JNEUROSCI.1392-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte A, Ranganath C, Trujillo C, Knight RT. Intact recollection memory in high-performing older adults: ERP and behavioral evidence. J. Cogn. Neurosci. 2006;18(1):33–47. doi: 10.1162/089892906775249988. [DOI] [PubMed] [Google Scholar]
- Dudchencko P, Wood E, Eichenbaum H. Neurotoxic hippocampal lesions have no effect on odor span and little effect on odor recognition memory, but produce significant impairments on spatial span, recognition, and alternation. J. Neurosci. 2000;20:2964–77. doi: 10.1523/JNEUROSCI.20-08-02964.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duzel E, Yonelinas AP, Mangun GR, Heinze HJ, Tulving E. Event-related brain potential correlates of two states of conscious awareness in memory. Proc. Natl. Acad. Sci. USA. 1997;94(11):5973–78. doi: 10.1073/pnas.94.11.5973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eacott MJ, Norman G. Integrated memory for object, place, and context in rats: a possible model of episodic-like memory? J. Neurosci. 2004;24(8):1948–53. doi: 10.1523/JNEUROSCI.2975-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan JP. USAF Tech. Note. No. 58–51, 32. Oper. Appl. Lab.; Indianapolis IN: 1958. Recognition memory and the operating characteristic. [Google Scholar]
- Eichenbaum H. Hippocampus: cognitive processes and neural representations that underlie declarative memory. Neuron. 2004;44:109–20. doi: 10.1016/j.neuron.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Otto T, Cohen NJ. Two functional components of the hippocampal memory system. Behav. Brain Sci. 1994;17:449–517. [Google Scholar]
- Ekstrom AD, Kahana MJ, Caplan JB, Fields TA, Isham EA, et al. Cellular networks underlying human spatial navigation. Nature. 2003;425:184–87. doi: 10.1038/nature01964. [DOI] [PubMed] [Google Scholar]
- Eldridge LL, Engel SA, Zeineh MM, Bookheimer SY, Knowlton BJ. A dissociation of encoding and retrieval processes in the human hippocampus. J. Neurosci. 2005;25:3280–86. doi: 10.1523/JNEUROSCI.3420-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge LL, Knowlton BJ, Furmanski CS, Bookheimer SY, Engel SA. Remembering episodes: a selective role for the hippocampus during retrieval. Nat. Neurosci. 2000;3:1149–52. doi: 10.1038/80671. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Neave N, Aggleton JP. Neurotoxic lesions of the perirhinal cortex do not mimic the behavioural effects of fornix transection in the rat. Behav. Brain Res. 1996;80:9–25. doi: 10.1016/0166-4328(96)00006-x. [DOI] [PubMed] [Google Scholar]
- Fenker DB, Schott BH, Richardson-Klavehn A, Heinze HJ, Duzel E. Recapitulating emotional context: activity of amygdala, hippocampus and fusiform cortex during recollection and familiarity. Eur. J. Neurosci. 2005;21:1993–99. doi: 10.1111/j.1460-9568.2005.04033.x. [DOI] [PubMed] [Google Scholar]
- Fortin NJ, Wright SP, Eichenbaum H. Recollection-like memory retrieval in rats is dependent on the hippocampus. Nature. 2004;431:188–91. doi: 10.1038/nature02853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman D, Johnson RJ. Event-related potential (ERP) studies of memory encoding and retrieval: a selective review. Microscopy Res. Tech. 2000;51:6–28. doi: 10.1002/1097-0029(20001001)51:1<6::AID-JEMT2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Fuster JM. Memory in the Cerebral Cortex: An Empirical Approach to Neural Networks in the Human and Nonhuman Primate. MIT Press; Cambridge, MA: 1995. [Google Scholar]
- Fyhn M, Molden S, Witter MP, Moser EI, Moser MB. Spatial representation in the entorhinal cortex. Science. 2004;305:1258–64. doi: 10.1126/science.1099901. [DOI] [PubMed] [Google Scholar]
- Gadian DG, Aicardi J, Watkins KE, Porter DA, Mishkin M, Vargha-Khadem F. Developmental amnesia associated with early hypoxic-ischaemic injury. Brain. 2000;123:499–507. doi: 10.1093/brain/123.3.499. [DOI] [PubMed] [Google Scholar]
- Gaffan EA, Healey AN, Eacott MJ. Objects and positions in visual scenes: effects of perirhinal and postrhinal cortex lesions in the rat. Behav. Neurosci. 2004;118:992–1010. doi: 10.1037/0735-7044.118.5.992. [DOI] [PubMed] [Google Scholar]
- Giovanello KS, Schnyer DM, Verfaellie M. A critical role for the anterior hippocampus in relational memory: evidence from an fMRI study comparing associative and item recognition. Hippocampus. 2003a;14:5–8. doi: 10.1002/hipo.10182. [DOI] [PubMed] [Google Scholar]
- Giovanello KS, Verfaellie M, Keane MM. Disproportionate deficit in associative recognition relative to item recognition in global amnesia. Cog. Aff. Behav. Neurosci. 2003b;3:186–94. doi: 10.3758/cabn.3.3.186. [DOI] [PubMed] [Google Scholar]
- Glanzer M, Hilford A, Kim K. Six regularities of source recognition. J. Exp. Psychol. Learn Mem. Cogn. 2004;30:1176–95. doi: 10.1037/0278-7393.30.6.1176. [DOI] [PubMed] [Google Scholar]
- Glanzer M, Kim K, Hilford A, Adams JK. Slope of the receiver-operating characteristic in recognition memory. J. Exp. Psychol: Learn Mem. Cogn. 1999;252:500–13. [Google Scholar]
- Gold JJ, Smith CN, Bayley PJ, Shrager Y, Brewer JB, et al. Item memory, source memory, and the medial temporal lobe: concordant findings from fMRI and memory-impaired patients. Proc. Natl. Acad. Sci. USA. 2006;103:9351–56. doi: 10.1073/pnas.0602716103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonsalves BD, Kahn I, Curran T, Norman KA, Wagner AD. Memory strength and repetition suppression: multimodal imaging of medial temporal cortical contributions to recognition. Neuron. 2005;47:751–61. doi: 10.1016/j.neuron.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Henson R, Martin A. Repetition and the brain: neural models of stimulus-specific effects. Trends Cogn. Sci. 2006;10:14–23. doi: 10.1016/j.tics.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Gronlund SD, Edwards MB, Ohrt DD. Comparison of the retrieval of item versus spatial position information. J. Exp. Psychol. Learn Mem. Cogn. 1997;235:1261–74. doi: 10.1037//0278-7393.23.5.1261. [DOI] [PubMed] [Google Scholar]
- Gronlund SD, Ratcliff R. Time course of item and associative information: implications for global memory models. J. Exp. Psychol. Learn Mem. Cogn. 1989;155:846–58. doi: 10.1037//0278-7393.15.5.846. [DOI] [PubMed] [Google Scholar]
- Hammond RS, Tull LE, Stackman RW. On the delay-dependent involvement of the hippocampus in object recognition memory. Neurobiol. Learn Mem. 2004;82(1):26–34. doi: 10.1016/j.nlm.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Hampson RE, Heyser CJ, Deadwyler SA. Hippocampal cell firing correlates of delayed-match-to-sample performance in the rat. Behav. Neurosci. 1993;107:715–39. doi: 10.1037//0735-7044.107.5.715. [DOI] [PubMed] [Google Scholar]
- Hargreaves EL, Rao G, Lee I, Knierim JJ. Major dissociation between medial and lateral entorhinal input to dorsal hippocampus. Science. 2005;308(5729):1792–94. doi: 10.1126/science.1110449. [DOI] [PubMed] [Google Scholar]
- Healy MR, Light LL, Chung C. Dual-process models of associative recognition in young and older adults: evidence from receiver operating characteristics. Exp. Psychol. Learn Mem. Cogn. 2005;31:768–88. doi: 10.1037/0278-7393.31.4.768. [DOI] [PubMed] [Google Scholar]
- Henson RN. A mini-review of fMRI studies of human medial temporal lobe activity associated with recognition memory. Q. J. Exp. Psychol. B. 2005;58:340–60. doi: 10.1080/02724990444000113. [DOI] [PubMed] [Google Scholar]
- Henson RN, Cansino S, Herron JE, Robb WG, Rugg MD. A familiarity signal in human anterior medial temporal cortex? Hippocampus. 2003;13:259–62. doi: 10.1002/hipo.10117. [DOI] [PubMed] [Google Scholar]
- Henson RNA, Rugg MD, Shallice T, Josephs O, Dolan RJ. Recollection and familiarity in recognition memory: an event-related functional magnetic resonance imaging study. J. Neurosci. 1999;19:3962–72. doi: 10.1523/JNEUROSCI.19-10-03962.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilford A, Glanzer M, Kim K, DeCarlo LT. Regularities of source recognition: ROC analysis. J. Exp. Psychol. Gen. 2002;131:494–510. doi: 10.1037//0096-3445.131.4.494. [DOI] [PubMed] [Google Scholar]
- Hintzman DL, Caulton DA. Recognition memory and modality judgments: a comparison of retrieval dynamics. J. Mem. Lang. 1997;371:1–23. [Google Scholar]
- Hintzman DL, Curran T. Retrieval dynamics of recognition and frequency judgments: evidence for separate processes of familiarity and recall. J. Mem. Lang. 1994;331:1–18. [Google Scholar]
- Holdstock JS, Mayes AR, Gong QY, Roberts N, Kapur N. Item recognition is less impaired than recall and associative recognition in a patient with selective hippocampal damage. Hippocampus. 2005;15(2):203–15. doi: 10.1002/hipo.20046. [DOI] [PubMed] [Google Scholar]
- Hopkins RO, Kesner RP, Goldstein M. Item and order recognition memory in subjects with hypoxic brain injury. Brain Cogn. 1995;27:180–201. doi: 10.1006/brcg.1995.1016. [DOI] [PubMed] [Google Scholar]
- Huppert FA, Piercy M. The role of trace strength in recency and frequency judgments by amnesic and control subjects. Q. J. Exp. Psychol. 1978;30:347–54. doi: 10.1080/14640747808400681. [DOI] [PubMed] [Google Scholar]
- Jackson O, Schacter DL. Encoding activity in anterior medial temporal lobe supports subsequent associative recognition. Neuroimage. 2004;21:456–62. doi: 10.1016/j.neuroimage.2003.09.050. [DOI] [PubMed] [Google Scholar]
- Jacoby LL. A process dissociation framework: separating automatic from intentional uses of memory. J. Mem. Lang. 1991;30:513–41. [Google Scholar]
- Kahn I, Davachi L, Wagner AD. Functional-neuroanatomic correlates of recollection: implications for models of recognition memory. J. Neurosci. 2004;24:4172–80. doi: 10.1523/JNEUROSCI.0624-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley R, Wixted JT. On the nature of associative information in recognition memory. J. Exp. Psychol. Learn Mem. Cogn. 2001;27:701–22. [PubMed] [Google Scholar]
- Kensinger EA, Corkin S. Two routes to emotional memory: distinct neural processes for valence and arousal. Proc. Natl. Acad. Sci. USA. 2004;101:3310–15. doi: 10.1073/pnas.0306408101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensinger EA, Schacter DL. Amygdala activity is associated with the successful encoding of item, but not source, information for positive and negative stimuli. J. Neurosci. 2006;26:2564–70. doi: 10.1523/JNEUROSCI.5241-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirwan CB, Stark CE. Medial temporal lobe activation during encoding and retrieval of novel face-name pairs. Hippocampus. 2004;14:919–30. doi: 10.1002/hipo.20014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishiyama MM, Yonelinas AP, Kroll NE, Lazzara MM, Nolan EC, et al. Bilateral thalamic lesions affect recollection and familiarity-based recognition judgments. Cortex. 2005;41:778–88. doi: 10.1016/s0010-9452(08)70296-x. [DOI] [PubMed] [Google Scholar]
- Kishiyama MM, Yonelinas AP, Lazzara MM. The Von Restorff effect in amnesia: the contribution of the hippocampal system to novelty-related memory enhancements. J. Cogn. Neurosci. 2004;16:15–23. doi: 10.1162/089892904322755511. [DOI] [PubMed] [Google Scholar]
- Knowlton BJ, Squire LR. Remembering and knowing: two different expressions of declarative memory. J. Exp. Psychol. Learn Mem. Cogn. 1995;21:699–710. doi: 10.1037//0278-7393.21.3.699. [DOI] [PubMed] [Google Scholar]
- Kreiman K, Kock C, Fried I. Category specific visual responses of single neurons in the human medial temporal lobe. Nat. Neurosci. 2000;3:946–53. doi: 10.1038/78868. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Wandell BA. Interpreting the BOLD signal. Annu. Rev. Physiol. 2004;66:735–69. doi: 10.1146/annurev.physiol.66.082602.092845. [DOI] [PubMed] [Google Scholar]
- Malkova L, Mishkin M. One-trial memory for object-place associations after separate lesions of hippocampus and posterior parahippocampal region in the monkey. J. Neurosci. 2003;23:1956–65. doi: 10.1523/JNEUROSCI.23-05-01956.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns JR, Hopkins RO, Reed JM, Kitchener EG, Squire LR. Recognition memory and the human hippocampus. Neuron. 2003;37:171–80. doi: 10.1016/s0896-6273(02)01147-9. [DOI] [PubMed] [Google Scholar]
- Mayes AR, Baddeley AD, Cockburn J, Meudell PR. Why are amnesic judgments of recency and frequency made in a qualitatively different way from those of normal people? Cortex. 1989;25(3):479–88. doi: 10.1016/s0010-9452(89)80061-9. [DOI] [PubMed] [Google Scholar]
- Mayes AR, Holdstock JS, Isaac CL, Hunkin NM, Roberts N. Relative sparing of item recognition memory in a patient with adult-onset damage limited to the hippocampus. Hippocampus. 2002;12:325–40. doi: 10.1002/hipo.1111. [DOI] [PubMed] [Google Scholar]
- Mayes AR, Isaac CL, Holdstock JS, Cariga P, Gummer A, Roberts N. Long-term amnesia: a review and detailed illustrative case study. Cortex. 2003;39:567–603. doi: 10.1016/s0010-9452(08)70855-4. [DOI] [PubMed] [Google Scholar]
- Mayes AR, Meudell PR, Pickering A. Is organic amnesia caused by a selective deficit in remembering contextual information? Cortex. 1985;21:167–202. doi: 10.1016/s0010-9452(85)80026-5. [DOI] [PubMed] [Google Scholar]
- McElree B, Dolan PO, Jacoby LL. Isolating the contributions of familiarity and source information to item recognition: a time course analysis. J. Exp. Psychol. Learn Mem. Cogn. 1999;253:563–82. doi: 10.1037//0278-7393.25.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meudell PR, Mayes AR, Ostergaard A, Pickering A. Recency and frequency judgments in alcoholic amnesics and normal people with poor memory. Cortex. 1985;21(4):487–511. doi: 10.1016/s0010-9452(58)80001-5. [DOI] [PubMed] [Google Scholar]
- Meunier M, Bachevalier J, Mishkin M, Murray EA. Effects on visual recognition of combined and separate ablations of the entorhinal and perirhinal cortex in rhesus monkeys. J. Neurosci. 1993;13(12):5418–32. doi: 10.1523/JNEUROSCI.13-12-05418.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishkin M. Memory in monkeys severely impaired by combined but not by separate removal of amygdala and hippocampus. Nature. 1978;273(5660):297–98. doi: 10.1038/273297a0. [DOI] [PubMed] [Google Scholar]
- Mishkin M, Ungerleider LG, Macko KA. Object vision and spatial vision: two cortical pathways. Trends Neurosci. 1983;6:414–17. [Google Scholar]
- Moita MAP, Moisis S, Zhou Y, LeDoux JE, Blair HT. Hippocampal place cells acquire location specific location specific responses to the conditioned stimulus during auditory fear conditioning. Neuron. 2003;37:485–97. doi: 10.1016/s0896-6273(03)00033-3. [DOI] [PubMed] [Google Scholar]
- Montaldi D, Spencer TJ, Roberts N, Mayes AR. The neural system that mediates familiarity memory. Hippocampus. 2006;16:504–20. doi: 10.1002/hipo.20178. [DOI] [PubMed] [Google Scholar]
- Moscovitch DA, McAndrews MP. Material-specific deficits in “remembering” in patients with unilateral temporal lobe epilepsy and excisions. Neuropsychologia. 2002;40:1335–42. doi: 10.1016/s0028-3932(01)00213-5. [DOI] [PubMed] [Google Scholar]
- Mumby DG. Perspectives on object-recognition memory following hippocampal damage: lessons from studies in rats. Behav. Brain Res. 2001;127(1–2):159–81. doi: 10.1016/s0166-4328(01)00367-9. [DOI] [PubMed] [Google Scholar]
- Mumby DG, Gaskin S, Glenn MJ, Schramek TE, Lehmann H. Hippocampal damage and exploratory preferences in rats: memory for objects, places, and contexts. Learn Mem. 2002;9:49–57. doi: 10.1101/lm.41302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EA, Gaffan D, Mishkin M. Neural substrates of visual stimulus-stimulus association in rhesus monkeys. J. Neurosci. 1993;13:4549–61. doi: 10.1523/JNEUROSCI.13-10-04549.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EA, Mishkin M. Object recognition and location memory in monkeys with excitotoxic lesions of the amygdala and hippocampus. J. Neurosci. 1998;18:6568–82. doi: 10.1523/JNEUROSCI.18-16-06568.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naya Y, Yoshida M, Miyashita Y. Backward spreading of memory-retrieval signal in the primate temporal cortex. Science. 2001;291:661–64. doi: 10.1126/science.291.5504.661. [DOI] [PubMed] [Google Scholar]
- Nemanic S, Alvarado MC, Bachevalier J. The hippocampal/parahippocampal regions and recognition memory: insights from visual paired comparison versus object-delayed nonmatching in monkeys. J. Neurosci. 2004;24:2013–26. doi: 10.1523/JNEUROSCI.3763-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman G, Eacott MJ. Dissociable effects of lesions to the perirhinal cortex and the postrhinal cortex on memory for context and objects in rats. Behav. Neurosci. 2005;119:557–66. doi: 10.1037/0735-7044.119.2.557. [DOI] [PubMed] [Google Scholar]
- Olton DS, Becker JT, Handlemann GE. Hippocampus, space, and memory. Brain Behav Sci. 1979;2:313–65. [Google Scholar]
- Otto T, Eichenbaum H. Neuronal activity in the hippocampus during delayed non-match to sample performance in rats: evidence for hippocampal processing in recognition memory. Hippocampus. 1992;2:323–34. doi: 10.1002/hipo.450020310. [DOI] [PubMed] [Google Scholar]
- Pihlajamaki M, Tanila H, Kononen M, Hanninen A, Soininen H, Aronen HJ. Visual presentation of novel objects and new spatial arrangements of objects differentially activates the medial temporal lobe areas in humans. Eur. J. Neurosci. 2004;19:1939–49. doi: 10.1111/j.1460-9568.2004.03282.x. [DOI] [PubMed] [Google Scholar]
- Prince SE, Daselaar SM, Cabeza R. Neural correlates of relational memory: successful encoding and retrieval of semantic and perceptual associations. J. Neurosci. 2005;25:1203–10. doi: 10.1523/JNEUROSCI.2540-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusky GT, Douglas RM, Nelson L, Shabanpoor A, Sutherland RJ. Visual memory task for rats reveals an essential role for hippocampus and perirhinal cortex. Proc. Natl. Acad. Sci. USA. 2004;101:5064–68. doi: 10.1073/pnas.0308528101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C, Yonelinas AP, Cohen MX, Dy CJ, Tom SM, D'Esposito M. Dissociable correlates of recollection and familiarity within the medial temporal lobes. Neuropsychologia. 2003;42:2–13. doi: 10.1016/j.neuropsychologia.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Ratcliff R, McKoon G, Tindall M. Empirical generality of data from recognition memory receiver-operating characteristic functions and implications for the global memory models. J. Exp. Psychol. Learn Mem. Cogn. 1994;204:763–85. doi: 10.1037//0278-7393.20.4.763. [DOI] [PubMed] [Google Scholar]
- Rempel-Clower NL, Zola SM, Squire LR, Amaral DG. Three cases of enduring memory impairment after bilateral damage limited to the hippocampal formation. J. Neurosci. 1996;16(16):5233–55. doi: 10.1523/JNEUROSCI.16-16-05233.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotello CM, Macmillan NA, Van Tassel G. Recall-to-reject in recognition: evidence from ROC curves. J. Mem. Lang. 2000;43:67–88. [Google Scholar]
- Rugg MD, Herron JE, Morcom AM. Electrophysiological studies of retrieval processing. In: Squire LR, Schacter DL, editors. Neuropsychology of Memory. 3rd ed. Guilford Press; New York: 2002. pp. 154–65. [Google Scholar]
- Rugg MD, Yonelinas AP. Human recognition memory: a cognitive neuroscience perspective. Trends Cogn. Sci. 2003;7:313–19. doi: 10.1016/s1364-6613(03)00131-1. [DOI] [PubMed] [Google Scholar]
- Rutishauser U, Mamelak AN, Schuman EM. Single-trial learning of novel stimuli by individual neurons of the human hippocampus-amygdala complex. Neuron. 2006;49:805–13. doi: 10.1016/j.neuron.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Verfaellie M, Pradere D. The neuropsychology of memory Illusions: false recall and recognition in amnesic patients. J. Mem. Lang. 1996;35:319–34. [Google Scholar]
- Sharot T, Delgado MR, Phelps EA. How emotion enhances the feeling of remembering. Nat. Neurosci. 2004;7:1376–80. doi: 10.1038/nn1353. [DOI] [PubMed] [Google Scholar]
- Slotnick SD, Klein SA, Dodson CS, Shimamura AP. An analysis of signal detection and threshold models of source memory. J. Exp. Psychol. Learn Mem. Cogn. 2000;26:1499–17. doi: 10.1037//0278-7393.26.6.1499. [DOI] [PubMed] [Google Scholar]
- Smith ME. Neurophysiological manifestations of recollective experience during recognition memory judgments. J. Cog. Neurosci. 1993;5(1):1–13. doi: 10.1162/jocn.1993.5.1.1. [DOI] [PubMed] [Google Scholar]
- Squire LR. Declarative and nondeclarative memory: multiple brain systems supporting learning and memory. In: Schacter DL, Tulving E, editors. Memory Systems. MIT Press; Cambridge, MA: 1994. pp. 203–31. [DOI] [PubMed] [Google Scholar]
- Squire LR, Zola S. Episodic memory, semantic memory, and amnesia. Hippocampus. 1998;8:205–11. doi: 10.1002/(SICI)1098-1063(1998)8:3<205::AID-HIPO3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Squire LR, Zola-Morgan S, Chen KS. Human amnesia and animal models of amnesia: performance of amnesic patients on tests designed for the monkey. Behav. Neurosci. 1988;102(2):210–21. doi: 10.1037//0735-7044.102.2.210. [DOI] [PubMed] [Google Scholar]
- Stark CE, Okado Y. Making memories without trying: medial temporal lobe activity associated with incidental memory formation during recognition. J. Neurosci. 2003;23:6748–53. doi: 10.1523/JNEUROSCI.23-17-06748.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark CE, Squire LR. Hippocampal damage equally impairs memory for single items and memory for conjunctions. Hippocampus. 2003;13(2):281–92. doi: 10.1002/hipo.10085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steckler T, Drinkenburg WH, Saghal A, Aggleton JP. Recognition memory in rats. II. Neuroanatomical substrates. Prog. Neurobiol. 1998;54:313–32. doi: 10.1016/s0301-0082(97)00061-0. [DOI] [PubMed] [Google Scholar]
- Steele K, Rawlins JN. The effects of hippocampectomy on performance by rats of a running recognition task using long lists of non-spatial items. Behav. Brain Res. 1993;54:1–10. doi: 10.1016/0166-4328(93)90043-p. [DOI] [PubMed] [Google Scholar]
- Suzuki W, Eichenbaum H. The neurophysiology of memory. Ann. N.Y. Acad. Sci. 2000;911:175–91. doi: 10.1111/j.1749-6632.2000.tb06726.x. [DOI] [PubMed] [Google Scholar]
- Tulving E. How many memory systems are there? Am. Psychol. 1985;40:385–98. [Google Scholar]
- Turriziani P, Fadda L, Caltagirone C, Carlesimo GA. Recognition memory for single items and associations in amnesia patients. Neuropsychologia. 2004;42:426–33. doi: 10.1016/j.neuropsychologia.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Uncapher MR, Otten LJ, Rugg MD. Episodic encoding is more than the sum of its parts: an fMRI investigation of multifeatural contextual encoding. Neuron. 2006;52:547–56. doi: 10.1016/j.neuron.2006.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uncapher MR, Rugg MD. Encoding and the durability of episodic memory: a functional magnetic resonance imaging study. J. Neurosci. 2005;25:7260–67. doi: 10.1523/JNEUROSCI.1641-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vann SD, Brown MW, Erichsen JT, Aggleton JP. Fos imaging reveals differential patterns of hippocampal and parahippocampal subfield activation in rats in response to different spatial memory tests. J. Neurosci. 2000;20:2711–18. doi: 10.1523/JNEUROSCI.20-07-02711.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargha-Khadem F, Gadian DG, Watkins KE, Connelly A, Van Paesschen W, Mishkin M. Differential effects of early hippocampal pathology on episodic and semantic memory. Science. 1997;277(53234):376–80. doi: 10.1126/science.277.5324.376. [DOI] [PubMed] [Google Scholar]
- Verfaellie M, Treadwell JR. The status of recognition memory in amnesia. Neuropsychology. 1993;7:5–13. [Google Scholar]
- Wais PE, Wixted JT, Hopkins RO, Squire LR. The hippocampus supports both the recollection and the familiarity components of recognition memory. Neuron. 2006;49:459–66. doi: 10.1016/j.neuron.2005.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan H, Aggleton JP, Brown MW. Different contributions of the hippocampus and perirhinal cortex to recognition memory. J. Neurosci. 1999;19:1142–48. doi: 10.1523/JNEUROSCI.19-03-01142.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis S, Klaver P, Reul J, Elger CE, Fernandez G. Neural correlates of successful declarative memory formation and retrieval: the anatomical overlap. Cortex. 2004a;40:200–2. doi: 10.1016/s0010-9452(08)70950-x. [DOI] [PubMed] [Google Scholar]
- Weis S, Specht K, Klaver P, Tendolkar I, Willmes K, et al. Process dissociation between contextual retrieval and item recognition. Neuroreport. 2004b;15:2729–33. [PubMed] [Google Scholar]
- Wheeler ME, Buckner RL. Functional-anatomic correlates of remembering and knowing. Neuroimage. 2004;21:1337–49. doi: 10.1016/j.neuroimage.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Wickelgren WA. Chunking and consolidation: a theoretical synthesis of semantic networks, configuring in conditioning, S-R versus cognitive learning, normal forgetting, the amnesic syndrome, and the hippocampal arousal system. Psychol. Rev. 1979;86(1):44–60. [PubMed] [Google Scholar]
- Winters BD, Bussey TJ. Transient inactivation of perirhinal cortex disrupts encoding, retrieval, and consolidation of object recognition memory. J. Neurosci. 2005;25:52–61. doi: 10.1523/JNEUROSCI.3827-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters BD, Forwood SE, Cowell RA, Saksida LM, Bussey TJ. Double dissociation between the effects of peri-postrhinal cortex and hippocampal lesions on tests of object recognition and spatial memory: heterogeneity of function within the temporal lobe. J. Neurosci. 2004;24(26):5901–8. doi: 10.1523/JNEUROSCI.1346-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth S, Yanike M, Frank LM, Smith AC, Brown EN, Suzuki WA. Single neurons in the monkey hippocampus and learning of new associations. Science. 2003;300:1578–81. doi: 10.1126/science.1084324. [DOI] [PubMed] [Google Scholar]
- Witter MP, Groenewegen HJ, Lopes da Silva FH, Lohman AH. Functional organization of the extrinsic and intrinsic circuitry of the parahippocampal region. Prog. Neurobiol. 1989;33:161–253. doi: 10.1016/0301-0082(89)90009-9. [DOI] [PubMed] [Google Scholar]
- Wood E, Dudchenko PA, Eichenbaum H. The global record of memory in hippocampal neuronal activity. Nature. 1999;397:613–16. doi: 10.1038/17605. [DOI] [PubMed] [Google Scholar]
- Wood E, Dudchenko P, Robitsek JR, Eichenbaum H. Hippocampal neurons encode information about different types of memory episodes occurring in the same location. Neuron. 2000;27:623–33. doi: 10.1016/s0896-6273(00)00071-4. [DOI] [PubMed] [Google Scholar]
- Woodruff CC, Hayama HR, Rugg MD. Electrophysiological dissociation of the neural correlates of recollection and familiarity. Brain Res. 2006;1100(1):125–35. doi: 10.1016/j.brainres.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Woodruff CC, Johnson JD, Uncapher MR, Rugg MD. Content-specificity of the neural correlates of recollection. Neuropsychologia. 2005;43:1022–32. doi: 10.1016/j.neuropsychologia.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. Receiver-operating characteristics in recognition memory: evidence for a dual-process model. J. Exp. Psychol. Learn Mem. Cogn. 1994;20(6):1341–54. doi: 10.1037//0278-7393.20.6.1341. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. The contribution of recollection and familiarity to recognition and source-memory judgments: a formal dual-process model and an analysis of receiver operating c haracteristics. J. Exp. Psychol. Learn Mem. Cogn. 1999;25:1415–34. doi: 10.1037//0278-7393.25.6.1415. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. Components of episodic memory: the contribution of recollection and familiarity. Philos. Transcr. R. Soc. London Biol. Sci. 2001;356:1363–74. doi: 10.1098/rstb.2001.0939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP. The nature of recollection and familiarity: a review of 30 years of research. J. Mem. Lang. 2002;46:441–517. [Google Scholar]
- Yonelinas AP, Dobbins I, Szymanski MD, Dhaliwal HS, King L. Signal-detection, threshold, and dual-process models of recognition memory: ROCs and conscious recollection. Conscious Cogn. 1996;5:418–41. doi: 10.1006/ccog.1996.0026. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Jacoby LL. Dissociations of processes in recognition memory: effects of interference and of response speed. Can. J. Exp. Psychol. 1994;484:516–34. doi: 10.1037/1196-1961.48.4.516. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Jacoby LL. The relation between remembering and knowing as bases for recognition: effects of size congruency. J. Mem. Lang. 1995;34(5):622–43. [Google Scholar]
- Yonelinas AP, Kroll NEA, Dobbins I, Lazzara M, Knight RT. Recollection and familiarity deficits in amnesia: convergence of remember-know, process dissociation, and receiver operating characteristic data. Neuropsychology. 1998;12:323–39. doi: 10.1037//0894-4105.12.3.323. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Kroll NEA, Dobbins IG, Soltani M. Recognition memory of faces: when familiarity supports associative recognition judgments. Psychon. Bull. Rev. 1999;6(4):654–61. doi: 10.3758/bf03212975. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Otten LJ, Shaw KN, Rugg MD. Separating the brain regions involved in recollection and familiarity in recognition memory. J. Neurosci. 2005;25(11):3002–8. doi: 10.1523/JNEUROSCI.5295-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP, Quamme JR, Widaman KF, Kroll NEA, Sauve MJ, Knight RT. Mild hypoxia disrupts recollection, not familiarity. Cogn. Affect. Behav. Neurosci. 2004;4:393–400. doi: 10.3758/cabn.4.3.393. [DOI] [PubMed] [Google Scholar]
- Yovel G, Paller KA. The neural basis of the butcher-on-the-bus phenomenon: when a face seems familiar but is not remembered. Neuroimage. 2004;21:789–800. doi: 10.1016/j.neuroimage.2003.09.034. [DOI] [PubMed] [Google Scholar]
- Zola SM, Squire LR, Teng E, Stefanacci L, Buffalo EA, Clark RE. Impaired recognition memory in monkeys after damage limited to the hippocampal region. J. Neurosci. 2000;20:451–63. doi: 10.1523/JNEUROSCI.20-01-00451.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR, Amaral DG. Human amnesia and the medial temporal lobe region: enduring memory impairment following a bilateral lesion limited to field CA1 of the hippocampus. J. Neurosci. 1986;6:2950–67. doi: 10.1523/JNEUROSCI.06-10-02950.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR, Amaral DC, Suzuki WA. Lesions of perirhinal and parahippocampal cortex that spare the amygdala and hippocampal formation produce sever memory impairment. J. Neurosci. 1989;9(12):4355–70. doi: 10.1523/JNEUROSCI.09-12-04355.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]