Abstract
Quantitative trait loci (QTL) analysis was conducted in bread wheat for 14 important traits utilizing data from four different mapping populations involving different approaches of QTL analysis. Analysis for grain protein content (GPC) suggested that the major part of genetic variation for this trait is due to environmental interactions. In contrast, pre-harvest sprouting tolerance (PHST) was controlled mainly by main effect QTL (M-QTL) with very little genetic variation due to environmental interactions; a major QTL for PHST was detected on chromosome arm 3AL. For grain weight, one QTL each was detected on chromosome arms 1AS, 2BS and 7AS. QTL for 4 growth related traits taken together detected by different methods ranged from 37 to 40; nine QTL that were detected by single-locus as well as two-locus analyses were all M-QTL. Similarly, single-locus and two-locus QTL analyses for seven yield and yield contributing traits in two populations respectively allowed detection of 25 and 50 QTL by composite interval mapping (CIM), 16 and 25 QTL by multiple-trait composite interval mapping (MCIM) and 38 and 37 QTL by two-locus analyses. These studies should prove useful in QTL cloning and wheat improvement through marker aided selection.
Keywords: Quantitative trait loci (QTL) analysis, Grain quality traits, Grain protein content, Pre-harvest sprouting tolerance (PHST), Grain weight, Mapping populations, Bread wheat
INTRODUCTION
In bread wheat (Triticum aestivum L.), grain quality, growth and yield are important traits. A genetic dissection of these traits through quantitative trait loci (QTL) analysis has been an important area of research. However, in majority of QTL studies on bread wheat involving different traits, QTL that have main effects were detected, leaving out many QTL that do not have any main effect but interact among themselves and with the environment. It is now known that interactions among loci (epistasis) or between genes/QTL and environment make a substantial contribution to variation in complex traits. It has also been recognized that the power of QTL discovery can be substantially improved by making provision for the detection and estimations of these interactions among loci (epistasis) and between genes/QTL and evironment. Therefore, statistical methods are being regularly developed and improved for the study of these interactions. In our study involving four mapping populations, QTL analysis for the above traits was conducted using single-locus and two-locus analyses. Results of the above study, which are largely published, are summarized in this communication.
MATERIALS AND METHODS
Mapping populations and their evaluation
The four different mapping populations (PI to PIV) used in the present study were each evaluated in 4~6 different environments comprising locations and years (Table 1).
Table 1.
Details of four mapping populations of bread wheat and their evaluation
| No. | Cross | RIL population designation | Number of environments |
| 1 | PH132 (high GPC)×WL711 (low GPC) | PI | 5 |
| 2 | W7984 (synthetic wheat)×Opata85 (cultivar) | PII | 4 |
| 3 | SPR8198 (PHS tolerant)×HD2329 (PHS susceptible) | PIII | 6 |
| 4 | Rye selection 111 (high GW)×Chinese spring (low GW) | PIV | 6 |
GPC: Grain protein content; PHS: Pre-harvest sprouting; GW: Grain weight; RIL: Recombinant inbreed lines
Molecular maps
Whole genome maps were used for conducting QTL analysis in PI and PII populations (Prasad et al., 2003; Kulwal et al., 2004; 2005a; Kumar et al., 2007) and partial maps were used in PIII (chromosome 3A) and PIV (chromosomes 1A, 2B and 7A) populations.
QTL analysis
The main effect QTL (M-QTL) was identified by single-locus QTL analysis using QTL Cartographer. A LOD (logarithm of odds) score of 2.5 was used for suggesting the presence of a putative QTL. Threshold LOD scores, calculated using 1 000 permutations, were used for declaring definitive QTL. Two-locus analysis that identifies main effect QTL (M-QTL), epistatic QTL (E-QTL), QTL×environment (QE) and QTL×QTL×environment (QQE) interactions was conducted using QTLMapper/QTLNetwork Version 2.0 (Table 2). The relative contribution of a genetic component was calculated as the proportion of the phenotypic variance explained (PVE) by that component.
Table 2.
Details of traits studied and methods/software used for QTL analysis in four mapping populations
| Mapping population | Trait studied | Method employed | Software used | References |
| PI | GPC, growth traits, yield traits | Single-locus (SMA, SIM, CIM, MCIM), two-locus | QTL Cartographer, QTLMapper, QTLNetwork | Wang et al., 1999; 2004; Prasad et al., 2003; Yang et al., 2005; Kumar et al., 2007 |
| PII | GPC, PHST, yield traits | Single-locus (CIM, MCIM), two-locus | QTL Cartographer, QTLMapper, QTLNetwork | Wang et al., 1999; 2004; Kulwal et al., 2004; 2005a; Yang et al., 2005; Kumar et al., 2007 |
| PIII | PHST | Single-locus (CIM) | QTL Cartographer | Wang et al., 2004; Kulwal et al., 2005b |
| PIV | GW | Single-locus (SMA, CIM) | QTL Cartographer | Wang et al., 2004; Kumar et al., 2006 |
GPC: Grain protein content; PHST: Pre-harvest sprouting tolerance; GW: Grain weight; SMA: Single marker analysis, SIM: Simple interval mapping, CIM: Composite interval mapping, MCIM: Multiple-trait composite interval mapping
RESULTS AND DISCUSSION
QTL analysis for grain protein content (GPC)
1. Single-locus QTL analysis
The results of single-locus QTL analysis involving estimation of M-QTL for GPC in PI and PII were reported earlier (Prasad et al., 2003; Kulwal et al., 2005a). In PI a total of 10 QTL were resolved following composite interval mapping (CIM) (Table 3); of these, only 7 QTL were detected at a LOD score equal to or above the threshold values. The PVE by individual QTL ranged from 2.95% to 32.44%. In PII, 7 M-QTL were resolved through CIM, which included 3 definitive and 4 suggestive QTL (Table 3). Only one definitive QTL (QGpc.ccsu-2D.7) was detected in more than one environment. The PVE by individual QTL varied from 8.38% to 16.58% (Kulwal et al., 2005a).
Table 3.
A summary of the results of single-locus QTL analysis following composite interval mapping (CIM) in four mapping populations of bread wheat
| Mapping population | Traits | Number of QTL identified | Chromosome |
| PH132×WL711 (PI) | GPC | 10 | 2A, 2B, 2D, 3D, 4A, 6B, 7A |
| Yield traits | |||
| TPP | 3 | 3A, 7A, 7B | |
| BY | 2 | 2B, 4A | |
| GY | 8 | 1D, 2D, 3D, 4A, 4D, 7A | |
| HI | 3 | 2B, 3A, 4A | |
| SL | 2 | 2B, 2D | |
| SPS | 3 | 2B, 4A, 6A | |
| GPS | 4 | 2A, 4B, 7A | |
| Growth traits | |||
| DH | 12 | 1D, 2A, 2D, 4A, 5B, 7A, 7D | |
| DM | 13 | 2B, 2D, 5A, 5B, 5D, 6B, 7A, 7B, 7D | |
| EGH | 11 | 2A, 2B, 3B, 4D, 5B, 5D, 7A, 7B | |
| PH |
4 |
2A, 2B |
|
| W7984×Opata85 (PII=ITMIpop) | GPC | 7 | 1D, 2D, 2A, 5A, 3A, 7D |
| PHST | 5 | 2B, 2D, 3B, 3D, 3B | |
| Yield traits | |||
| TPP | 8 | 1A, 1B, 3B, 3D, 4A, 6D, 7A | |
| BY | 6 | 1A, 2A, 2B, 3B, 6D, 7A | |
| GY | 6 | 1A, 2A, 2D, 4B, 6D | |
| HI | 5 | 2D, 3B, 4B, 6A | |
| SL | 9 | 1A, 1B, 1D, 2D, 4A, 5A, 5D | |
| SPS | 6 | 2D, 4A, 4D, 5A, 6A | |
| GPS |
10 |
1A, 1B, 2B, 2D, 3B, 3D, 7A |
|
| SPR8198×HD2329 (PIII) |
PHST |
1 |
3A |
| RS111×CS (PIV) | GW | 3 | 1A, 2B, 7A |
RS: Rye selection; CS: Chinese spring; GPC: Grain protein content; PHST: Pre-harvest sprouting tolerance; GW: Grain weight; TPP: Tiller per plant; BY: Biological yield; GY: Grain yield; HI: Harvest index; SL: Spike length; SPS: Spike lets per spike; GPS: Grains per spike; DH: Days to heading; DM: Days to maturity; EGH: Early growth habit; PH: Plant height
2. Two-locus QTL analysis
Using two-locus analysis, 26 QTL for GPC were detected in both populations taken together (14 QTL in PI and 12 QTL in PII). These QTL included M-QTL, E-QTL, QE and QQE interactions. However, none of the individual QTL was detected in both populations.
M-QTL: In each of the two mapping populations, 5 M-QTL for GPC were detected, which accounted for a mere 7.22% (PII) to 7.24% (PI) of the phenotypic variation. The desirable alleles for high GPC were distributed in both the parents of the two populations studied.
QQ, QE and QQE interactions: In PI, 2 digenic QQ epistatic interactions involved 4 E-QTL, and in PII, 3 digenic QQ epistatic interactions involved 6 E-QTL (Table 4). The epistatic interactions accounted for as low as 2.68% (PI) and 6.04% (PII) of the phenotypic variation. QE interactions together accounted for a substantial proportion (24.24% in PI and 21.19% in PII) of the phenotypic variation in both populations. Substantial QQE interactions (26.80% PVE) were also noticed in PII, although in PI these interactions were rather minimal (1.67% PVE). Taken together, the results indicated that a substantial proportion (25.91% in PI and 47.99% in PII) of the phenotypic variation is contributed by QE and QQE interactions.
Table 4.
A summary of the results of two-locus analysis showing QTL involved in epistasis, and epistasis by environmental interactions in two mapping populations of bread wheat
| Mapping population | Trait | 1st QTL | 2nd QTL | Significant epistatic and epistatic×environment interactions |
| PH132×WL711 (PI) | GPC | QGpc.ccsu-1A.1 | QGpc.ccsu-2B.2 | aae2 |
| QGpc.ccsu-2B.1 | QGpc.ccsu-4A.3 | aa, aae3 | ||
| QGpc.ccsu-2D.4 | QGpc.ccsu-7A.3 | aae4 | ||
| QGpc.ccsu-5B.1 | QGpc.ccsu-7A.2 | aa, aae4 | ||
| Yield traits | ||||
| TPP | QTp.ccsu-3A.2 | QTp.ccsu-6A.2 | aae4, aae6 | |
| GY | QGy.ccsu-2D.4 | QGy.ccsu-4D.1 | aa, aae4 | |
| QGy.ccsu-3B.4 | QGy.ccsu-4A.2 | aa, aae2, aae3, aae4 | ||
| SL | QSl.ccsu-1A.5 | QSl.ccsu-5B.2 | aa | |
| QSl.ccsu-1B.4 | QSl.ccsu-7B.3 | aa | ||
| QSl.ccsu-1B.5 | QSl.ccsu-3B.2 | aa | ||
| QSl.ccsu-1B.5 | QSl.ccsu-7B.2 | aa | ||
| QSl.ccsu-2A.5 | QSl.ccsu-5B.3 | aa | ||
| QSl.ccsu-2D.6 | QSl.ccsu-4A.4 | aa | ||
| QSl.ccsu-2D.7 | QSl.ccsu-4A.5 | aa | ||
| QSl.ccsu-4B.1 | QSl.ccsu-7B.6 | aa | ||
| SPS | QSps.ccsu-2A.2 | QSps.ccsu-7A.3 | aa | |
| QSps.ccsu-3A.1 | QSps.ccsu-7A.2 | aa | ||
| GPS | QGps.ccsu-4B.5 | QGps.ccsu-7A.4 | aa | |
| Growth traits | ||||
| DH | QDh.ccsu-1A.1 | QDh.ccsu-2D.8 | aa, aae4 | |
| QDh.ccsu-2B.1 | QDh.ccsu-3D.3 | aa | ||
| QDh.ccsu-2B.1 | QDh.ccsu-3D.2 | aa, aae4 | ||
| QDh.ccsu-3B.1 | QDh.ccsu-6A.1 | aa | ||
| DM | QDm.ccsu-1B.1 | QDm.ccsu-7A.2 | aa, aae4 | |
| QDm.ccsu-1B.2 | QDm.ccsu-4B.1 | aa, aae1, aae2, aae4 | ||
| QDm.ccsu-2A.3 | QDm.ccsu-6A.2 | aa | ||
| QDm.ccsu-2B.1 | QDm.ccsu-7A.2 | aa | ||
| EGH | QEgh.ccsu-2A.5 | QEgh.ccsu-3D.1 | aae1, aae4, aae5, aae6 | |
| QEgh.ccsu-2B.1 | QEgh.ccsu-3A.2 | aa | ||
| QEgh.ccsu-6A.2 | QEgh.ccsu-6D.1 | aa | ||
| QEgh.ccsu-6A.1 | QEgh.ccsu-6D.2 | aa | ||
| PH | QPh.ccsu-1D.2 | QPh.ccsu-6A.1 | aa | |
| QPh.ccsu-1D.2 | QPh.ccsu-6A.2 | aa | ||
| QPh.ccsu-2D.3 | QPh.ccsu-5A.2 | aa, aae2 | ||
|
QPh.ccsu-4B.1 |
QPh.ccsu-4D.1 |
aa |
||
| W7984×Opata85 (PII=ITMIpop) | GPC | QGpc.ccsu-1A.2 | QGpc.ccsu-2A.5 | aa |
| QGpc.ccsu-2A.3 | QGpc.ccsu-2D.5 | aa, aae1, aae2, aae3, aae4 | ||
| QGpc.ccsu-5D.1 | QGpc.ccsu-7A.4 | aa | ||
| QGpc.ccsu-1B.1 | QGpc.ccsu-1D.1 | aae4 | ||
| QGpc.ccsu-2A.4 | QGpc.ccsu-3B.1 | aae1, aae2, aae3, aae4 | ||
| PHST | QPhs.ccsu-2B.1 | QPhs.ccsu-3B.1 | aa | |
| QPhs.ccsu-3B.3 | QPhs.ccsu-3B.4 | aa | ||
| QPhs.ccsu-3B.5 | QPhs.ccsu-3D.1 | aa | ||
| QPhs.ccsu-3D.2 | QPhs.ccsu-5B.1 | aa | ||
| QPhs.ccsu-2B.2 | QPhs.ccsu-6A.1 | aae1, aae3, aae4 | ||
| QPhs.ccsu-3B.1 | QPhs.ccsu-7B.1 | aae3 | ||
| Yield traits | ||||
| TPP | QTp.ccsu-1D.2 | QTp.ccsu-3A.2 | aa | |
| BY | QBy.ccsu-1A.2 | QBy.ccsu-2B.1 | aa, aae1, aae3 | |
| QBy.ccsu-4B.2 | QBy.ccsu-7A.6 | aa | ||
| GY | QGy.ccsu-1A.3 | QGy.ccsu-5B.1 | aa, aae1, aae4 | |
| QGy.ccsu-1A.3 | QGy.ccsu-3A.1 | aa | ||
| HI | QHi.ccsu-3A.3 | QHi.ccsu-5B.1 | aa, aae1 | |
| QHi.ccsu-4A.3 | QHi.ccsu-5A.2 | aae4 | ||
| SL | QSl.ccsu-6A.3 | QSl.ccsu-6A.4 | aa | |
| SPS | QSps.ccsu-3B.2 | QSps.ccsu-5D.1 | aa | |
| GPS | QGps.ccsu-4B.5 | QGps.ccsu-7A.4 | aa |
GPC: Grain protein content; PHST: Pre-harvest sprouting tolerance; TPP: Tiller per plant; BY: Biological yield; GY: Grain yield; HI: Harvest index; SL: Spike length; SPS: Spike lets per spike; GPS: Grains per spike; DH: Days to heading; DM: Days to maturity; EGH: Early growth habit; PH: Plant height
The above results suggest that often more than one mapping population derived from genetically diverse parents should be used to identify as many QTL as possible for the complete genetic dissection of the trait. It is also inferred that, although, improvement in GPC is possible without the concurrent loss in grain yield, the available QTL in hexaploid wheat, at best, may lead to only marginal improvement of GPC through marker assisted selection (MAS), since no more than a quarter (PI) to one eighth (PII) of the total variation is fixable.
QTL analysis for pre-harvest sprouting tolerance (PHST)
1. Single-locus QTL analysis
QTL analysis for PHST was carried out using two mapping populations (PII and PIII). Using PII, as many as 5 QTL were detected (Table 3), of which 3 QTL each were identified in more than one environment, but none of them could be detected in all the four environments. Two of the above 5 QTL were definitive. The PVE by individual QTL ranged from 8.12% to 17.39%.
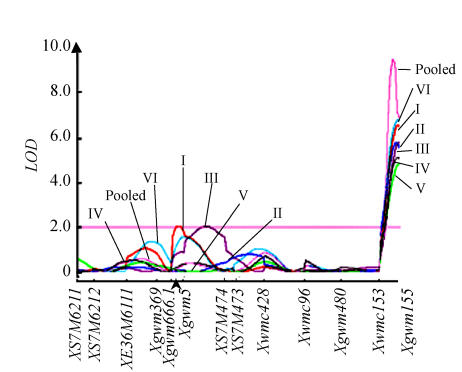
In another study in PIII, a major QTL for PHST on chromosome arm 3AL (Fig.1), explaining 24.68% to 35.21% variation in individual environments and 78.03% variation in pooled environments, was detected (Kulwal et al., 2005b). Positive QTL effect suggested that an allele of the above QTL for PHST is available in the PHS tolerant parental genotype SPR8198. The marker allele associated with this superior QTL in SPR8198 is currently being exploited by us in MAS for the transfer of this QTL allele into some elite Indian cultivars to obtain white-grained PHS tolerant genotypes.
Fig. 1.
A QTL Cartographer plot for chromosome 3A obtained following composite interval mapping (CIM) for pre-harvest sprouting tolerance (PHST) in population PIII for six different environments (I~VI) and the pooled data
Marker designations are given at the bottom of the horizontal line; arrow indicates centromere [modified from Kulwal et al.(2005b)]
2. Two-locus QTL analysis
M-QTL and E-QTL: Out of a total of 14 QTL detected in PII using QTLMapper, 8 were M-QTL, which together accounted for 47.95% of the total phenotypic variation (Table 2).
QQ, QE and QQE: Eight E-QTL (including 5 of the above M-QTL) were involved in 4 digenic epistatic interactions (QQ) and accounted for 28.73% of the PVE (Table 4). None of these 4 epistatic combinations exhibited QQE interaction. Four QTL were involved in two interactions and accounted for 3.24% of the phenotypic variation. Two QTL that were detected in more than one environment at LOD scores above the threshold values were located on chromosome arms 3BL and 3DL in the vicinity of the dormancy gene TaVp1. Another QTL was found to be located on chromosome 3B, perhaps in close proximity of R gene for red grain color. None of the 8 M-QTL showed significant QE interaction with any of the 4 environments. In contrast, 1 QTL, located on chromosome 5D (QPhs.ccsu-5D.1) had neither any main effect nor any epistatic effect, but was involved in QE interaction.
From the above results, it is apparent that besides M-QTL, there are other QTL that do not have any main effect but are involved in interactions with other QTL and/or with the environment. These QTL do contribute to the total variance of a trait. Hence ignoring these QTL will cause bias in QTL analysis. Also for a trait like PHST, selection would be effective as the amount of variation explained by M-QTL is more than three quarters.
QTL analysis for yield contributing traits
1. Single-locus QTL analysis
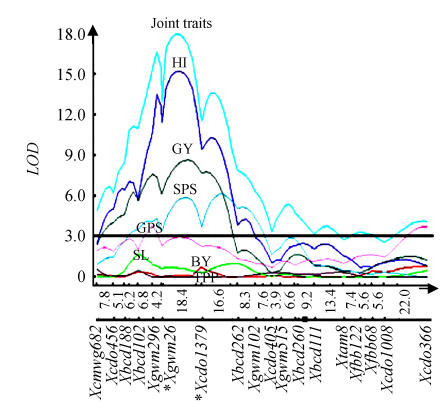
For seven yield and yield contributing traits following single-locus analysis, 25 QTL in PI and 50 QTL in PII were detected at LOD scores above the threshold values (Table 3). In PI, a QTL for spike lets per spike (SPS), which was consistent in four of the seven environments, was coincident with one QTL each for two other traits [biological yield (BY) and harvest index (HI)]; it is possible that these 3 QTL for 3 traits represent one pleiotropic QTL. Another QTL for spike length (SL) (on chromosome arm 2BL) was consistent over all the environments. Similarly in PII, 6 sets of QTL were detected, each set having more than one coincident QTL; 4 of these 6 sets of QTL represent each a pleiotropic QTL as determined through MCIM and joint MCIM (Fig.2). Also in PII, 6 QTL were consistent, which included one QTL each for tiller per plant (TPP), grain yield (GY), harvest index (HI), spike lets per spike (SPS), spike length (SL) and grain per spike (GPS); some of these consistent QTL also figured among the sets of coincident QTL suggesting that they are definitive and reliable QTL.
Fig. 2.
QTL Cartographer plot for chromosome arm 2DS obtained using MCIM and joint MCIM involving seven yield traits in PII. The plot shows a pleiotropic QTL on chromosome arm 2DS
Marker designations are given below and the genetic distances (cM) are given above the horizontal line. Solid black rectangle indicates centromere; asterisks (*) indicate flanking markers of the QTL. Trait abbreviations are on peaks of curves (HI: Harvest index; GY: Grain yield; SPS: Spike lets per spike; GPS: Grains per spike; SL: Spike length; BY: Biological yield; TPP: Tiller per plant) [modified from Kumar et al.(2007)]
As many as 16 QTL in PI and 25 QTL in PII were detected in MCIM; all these QTL were also included among the 86 QTL in PI and 92 QTL in PII that were detected in joint MCIM. The LOD scores for an individual QTL ranged from 3.0 to 17.9 in two populations. Relatively fewer QTL were detected in PI than in PII. This may be partly due to a lower density of marker loci on PI framework map (173) than in PII (521) used in the present study and partly due to more divergent parents used for developing PII population.
2. Two-locus QTL analysis
M-QTL and E-QTL: For 7 yield traits, a total of 38 QTL in PI and 37 QTL in PII were detected using two-locus analysis. Of these QTL, 11 QTL in PI and 18 QTL in PII were M-QTL; the remaining QTL were mainly E-QTL except 3 QTL in PI (1 QTL involved in QE and 2 QTL involved in QQE interactions) and 2 QTL in PII (involved in QQE interactions).
QQ, QE and QQE interactions: Four M-QTL in PI and 10 M-QTL in PII also exhibited QE interactions (Table 4). In PI, however, there was an additional QTL [for GY (grain yield)], which had no main effect, but was involved in QE interactions. The other QTL involved in QQ and QQE interactions were either E-QTL or QTL with no main or epistatic effects. There were 14 QQ interactions involving 27 QTL in PI and 10 QQ interactions involving 19 QTL in PII. In PI, for the traits BY and HI, no QQ interactions were detected, but in PII QQ interactions were observed for all the traits. QQE interactions (6 QTL) were also observed and included 3 QQE interactions (involving 6 QTL) in PI; out of 6 QTL, 4 were E-QTL (for GY) and the remaining 2 QTL (for TPP) were only involved in QQE interactions. Similarly, in PII 4 QQE interactions (8 QTL) were detected; out of 8 QTL, 6 were E-QTL involving 3 traits (BY, GY and HI) and remaining 2 QTL (for HI) were involved in QQE interactions only. None of the QQ for SL, SPS and GPS in PI and for TPP, SL, SPS and GPS in PII exhibited interaction with the environment (Table 4).
In the present study, 4 homoeologous groups (1, 4, 5 and 6) were also found to carry QTL for the same trait on seemingly similar positions on 2 of the 3 homoeologous chromosomes. As many as 10 QTL belong to this class in PII. No such homoeo-QTL were detected in PI. This shows that perhaps the presence of triplicate loci is a characteristic of bread wheat, although all the 3 QTL may not be detected in the same populations.
QTL analysis for grain weight (GW)
In PIV, single-locus QTL analysis following CIM identified 3 QTL for GW (Table 3). Two of 3 QTL (QGw.ccsu-2B.1 and QGw.ccsu-7A.1) were definitive and were detected in at least 4 different environments and also in the pooled environment. The individual QTL explained 9.06% to 19.85% phenotypic variation (Kumar et al., 2006).
QTL analysis for growth traits
1. Single-locus QTL analysis
For the four growth related traits [DH (days to heading), DM (days to maturity), EGH (early growth habit) and PH (plant height)], single-locus QTL analysis detected 40 QTL through CIM and 37 QTL through MCIM/joint MCIM analyses, which were not very different (Table 3). Five pleiotropic QTL detected for the three correlated traits (DH, DM and EGH) also figured both in MCIM and joint MCIM analyses; four of these pleiotropic QTL were coincident with QTL detected by CIM. However, one additional set of QTL (for DH and DM on chromosome arm 2BL) detected by CIM in adjacent intervals also figured among pleiotropic QTL. This places higher level of confidence in MCIM than in CIM for detecting pleiotropic QTL.
2. Two-locus QTL analysis
Two-locus analysis detected 38 QTL for the 4 growth related traits. Nine of these QTL were also detected by single-locus analysis, of which 8 (except QDh.ccsu-4B.1 on chromosome arm 4BS) were M-QTL (detected by two-locus analysis), which were detected either in the same or adjacent regions (CIM/joint MCIM). It is also interesting to note that the QTL detected by CIM and M-QTL that were also detected by two-locus analysis were not involved in epistatic (QQ or QQE) interactions except for a solitary common QTL (QEgh.ccsu-2B.1) for EGH on chromosome arm 2BL. Thus, the M-QTL accounting for additive component of variation, which is fixable, may be used in plant breeding. As many as 15 epistatic QQ interactions were detected in the present study; only 6 of these QQ exhibited significant interactions with environments (Table 4).
CONCLUSION
Based on the above results and those of our recent earlier studies, it is concluded that a network of M-QTL and interacting QTL (involved in QQ, QE and QQE interactions) control most of the important agronomic traits in common wheat (Kulwal et al., 2005a; Kumar et al., 2007). However, the relative importance of epistasis and environment interactions may vary for different traits. This information needs to be collected using several mapping populations to prove useful in designing breeding strategies for improvement of individual traits.
Acknowledgments
Thanks are due to Prof. Jun Zhu, Zhejiang University, Hangzhou, China for conducting two-locus QTL analysis and to Govind Ballabh Pant University of Agriculture and Technology, Pantnagar and Punjab Agriculture University, Ludhiana, India for their help in conducting field trials.
Footnotes
Project supported by the National Agricultural Technology Project of Indian Council of Agricultural Research, Department of Biotechnology of Government of India, Council of Scientific and Industrial Research of India and Indian National Science Academy
References
- 1.Kulwal PL, Singh R, Balyan HS, Gupta PK. Genetic basis of pre-harvest sprouting tolerance using single-locus and two-locus QTL analyses in bread wheat. Funct Integr Genomics. 2004;4(2):94–101. doi: 10.1007/s10142-004-0105-2. [DOI] [PubMed] [Google Scholar]
- 2.Kulwal PL, Kumar N, Kumar A, Gupta RK, Balyan HS, Gupta PK. Gene networks in hexaploid wheat: interacting quantitative trait loci for grain protein content. Funct Integr Genomics. 2005;5(4):254–259. doi: 10.1007/s10142-005-0136-3. [DOI] [PubMed] [Google Scholar]
- 3.Kulwal PL, Kumar N, Gaur A, Khurana P, Khurana JP, Tyagi AK, Balyan HS, Gupta PK. Mapping of a major QTL for pre-harvest sprouting tolerance on chromosome 3A in bread wheat. Theor Appl Genet. 2005;111(6):1052–1059. doi: 10.1007/s00122-005-0021-4. [DOI] [PubMed] [Google Scholar]
- 4.Kumar N, Kulwal PL, Gaur A, Tyagi AK, Khurana JP, Khurana P, Balyan HS, Gupta PK. QTL analysis for grain weight in common wheat. Euphytica. 2006;151(2):135–144. doi: 10.1007/s10681-006-9133-4. [DOI] [Google Scholar]
- 5.Kumar N, Kulwal PL, Balyan HS, Gupta PK. QTL mapping for yield and yield contributing traits in two mapping populations of bread wheat. Mol Breeding. 2007;19(2):163–177. doi: 10.1007/s11032-006-9056-8. [DOI] [Google Scholar]
- 6.Prasad M, Kumar N, Kulwal PL, Röder M, Balyan HS, Dhaliwal HS, Gupta PK. QTL analysis for grain protein content using SSR markers and validation studies using NILs in bread wheat. Theor Appl Genet. 2003;106(4):659–667. doi: 10.1007/s00122-002-1114-y. [DOI] [PubMed] [Google Scholar]
- 7.Wang DL, Zhu J, Li ZK, et al. A Computer Software for Mapping Quantitative Trait Loci: QTL with Main Effects, Epistatic Effects and QTL-Environment Interactions. User Manual for QTL Map Version 1.0. Hangzhou, China: Zhejiang University Press; 1999. [Google Scholar]
- 8.Wang S, Basten CJ, Zeng Z. Window QTL Cartographer. V. 2.0 Program in Statistical Genetics. North Carolina: North Carolina State University; 2004. [Google Scholar]
- 9.Yang J, Hu CC, Ye XZ, et al. QTLNetwork 2.0. Institute of Bioinformatics. Hangzhou, China: Zhejiang University; 2005. [Google Scholar]