Abstract
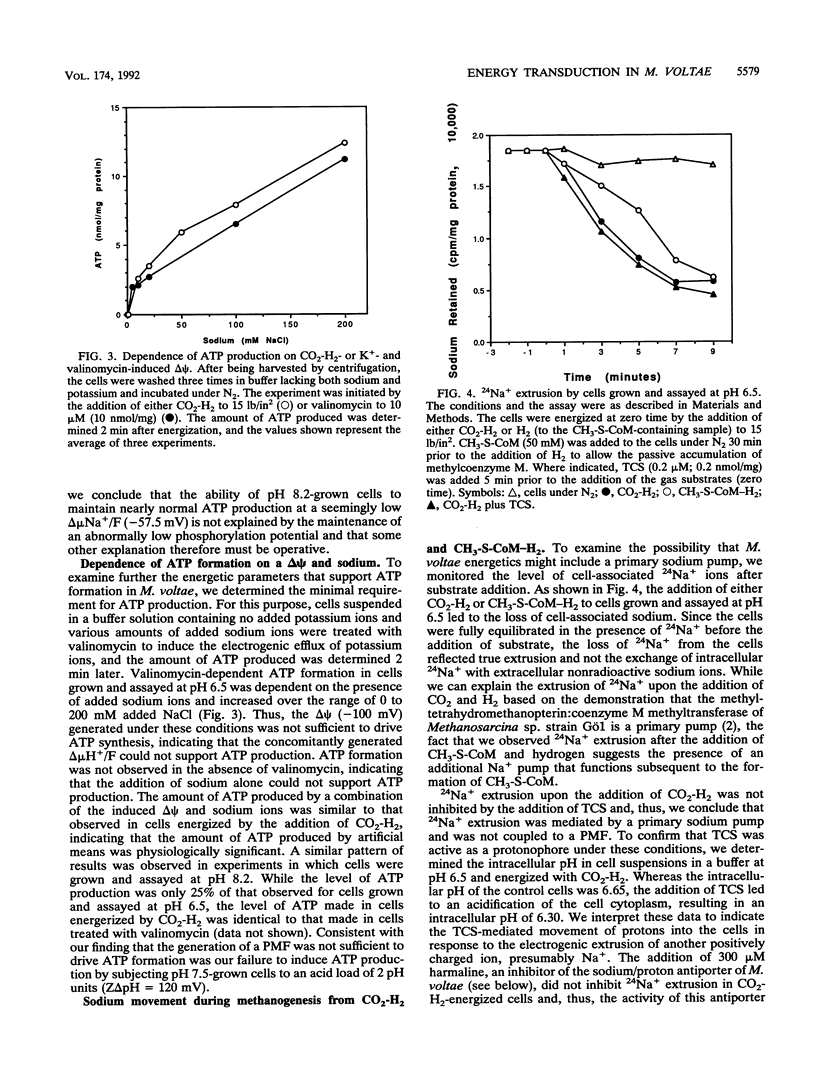
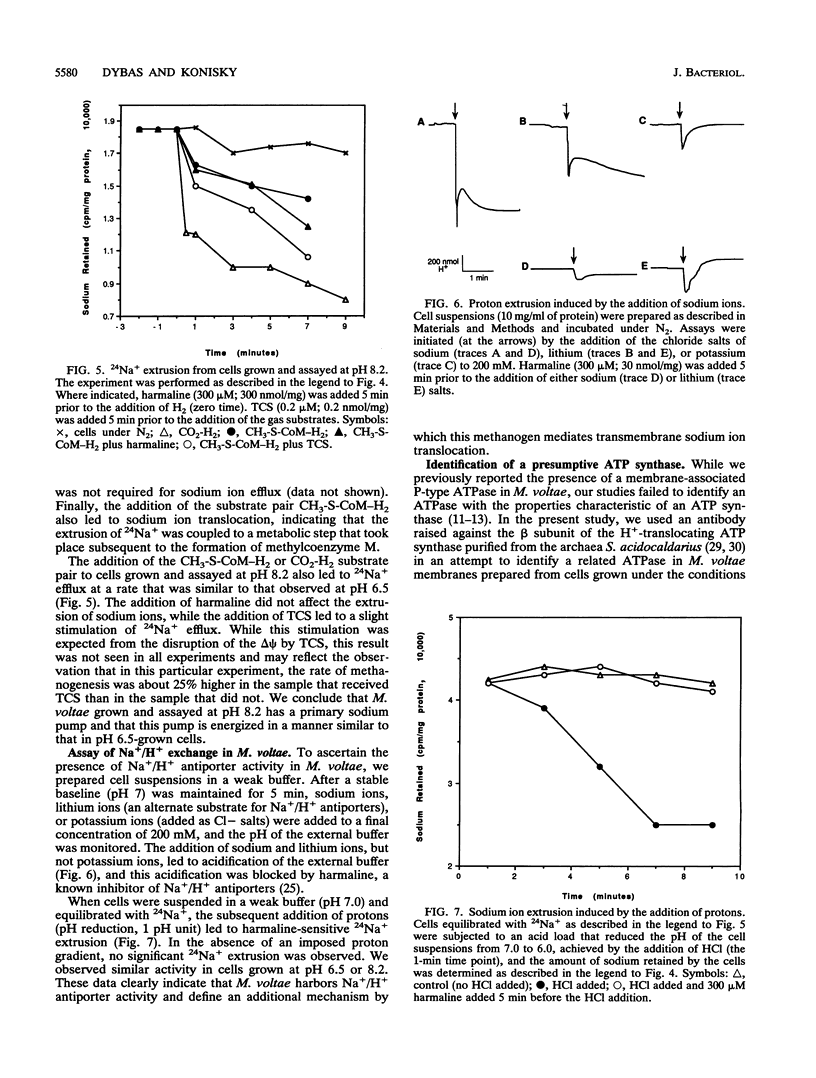
We provide experimental support for the proposal that ATP production in Methanococcus voltae, a methanogenic member of the archaea, is based on an energetic system in which sodium ions, not protons, are the coupling ions. We show that when grown at a pH of 6.0, 7.1, or 8.2, M. voltae cells maintain a membrane potential of approximately -150 mV. The cells maintain a transmembrane pH gradient (pH(in) - pH(out)) of -0.1, -0.2, and -0.2, respectively, values not favorable to the inward movement of protons. The cells maintain a transmembrane sodium concentration gradient (sodium(out)/sodium(in)) of 1.2, 3.4, and 11.6, respectively. While the protonophore 3,3',4',5-tetrachlorosalicylanilide inhibits ATP formation in cells grown at pH 6.5, neither ATP formation nor growth is inhibited in cells grown in medium at pH 8.2. We show that when grown at pH 8.2, cells synthesize ATP in the absence of a favorably oriented proton motive force. Whether grown at pH 6.5 or pH 8.2, M. voltae extrudes Na+ via a primary pump whose activity does not depend on a proton motive force. The addition of protons to the cells leads to a harmaline-sensitive efflux of Na+ and vice versa, indicating the presence of Na+/H+ antiporter activity and, thus, a second mechanism for the translocation of Na+ across the cell membrane. M. voltae contains a membrane component that is immunologically related to the H(+)-translocating ATP synthase of the archaeabacterium Sulfolobus acidocaldarius. Since we demonstrated that ATP production can be driven by an artificially imposed membrane potential only in the presence of sodium ions, we propose that ATP production in M. voltae is mediated by an Na+-translocating ATP synthase whose function is coupled to a sodium motive force that is generated through a primary Na+ pump.
Full text
PDF
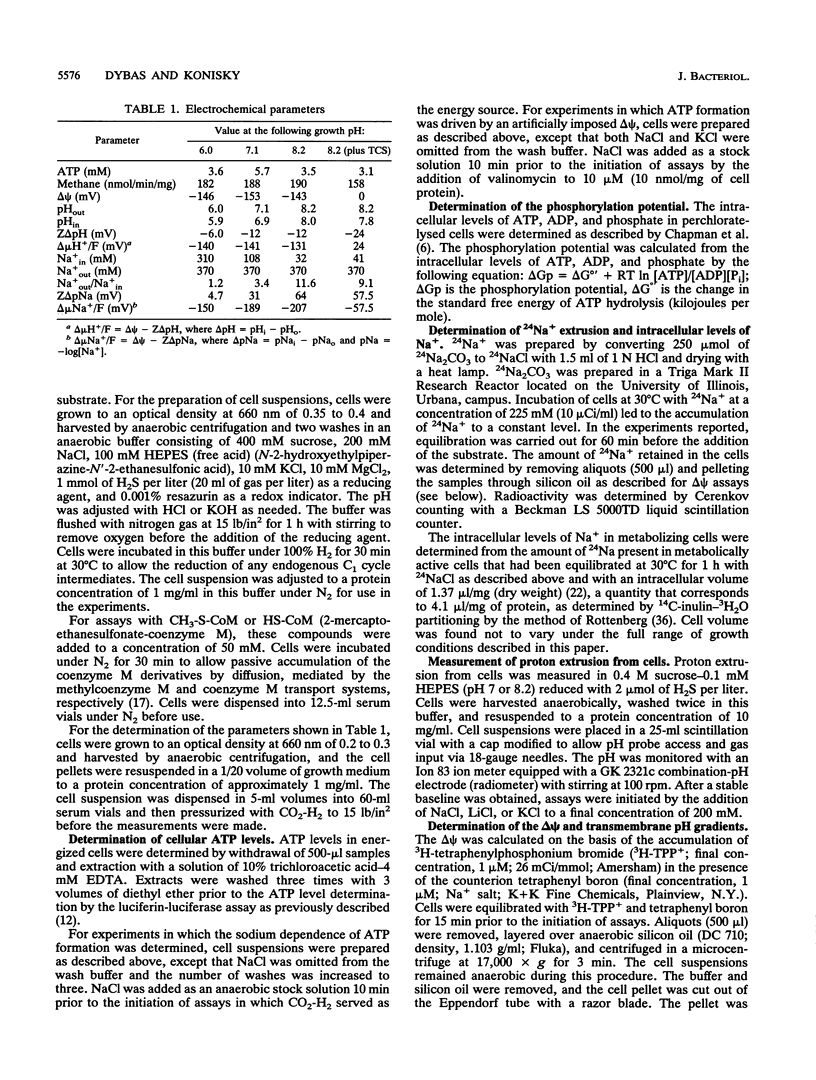
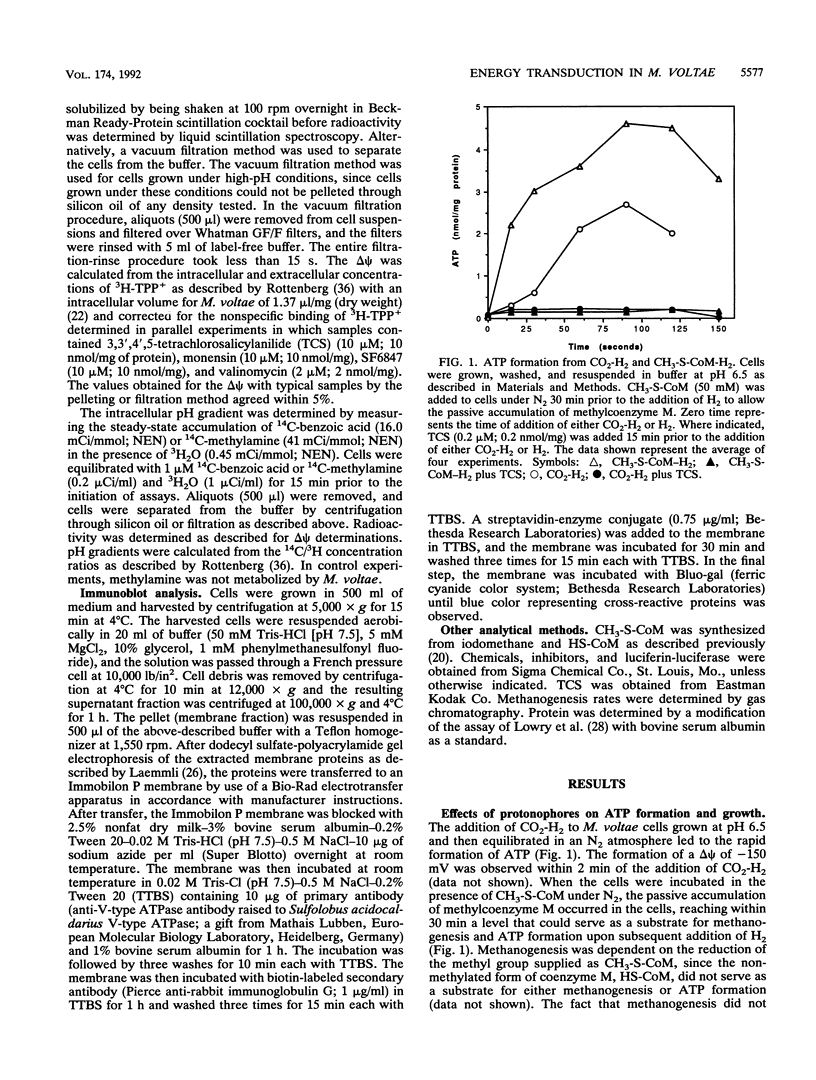
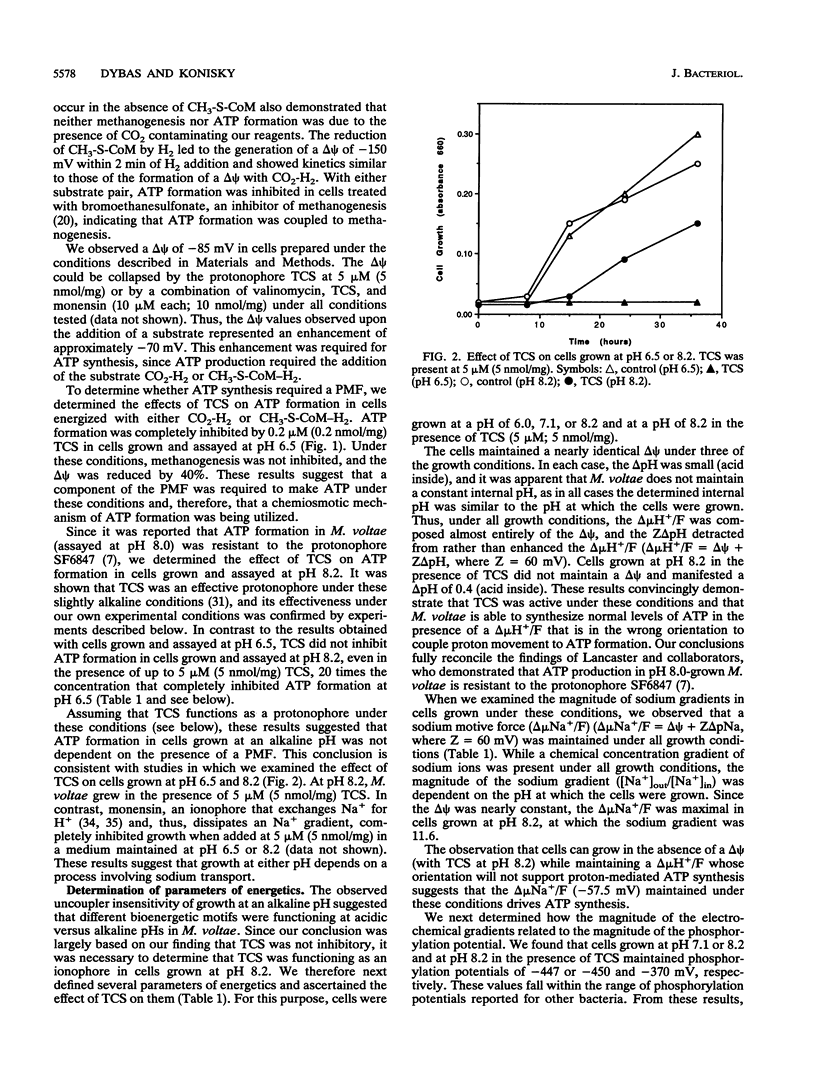



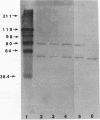
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avetisyan A. V., Dibrov P. A., Semeykina A. L., Skulachev V. P., Sokolov M. V. Adaptation of Bacillus FTU and Escherichia coli to alkaline conditions: the Na(+)-motive respiration. Biochim Biophys Acta. 1991 Dec 3;1098(1):95–104. [PubMed] [Google Scholar]
- Blaut M., Gottschalk G. Coupling of ATP synthesis and methane formation from methanol and molecular hydrogen in Methanosarcina barkeri. Eur J Biochem. 1984 May 15;141(1):217–222. doi: 10.1111/j.1432-1033.1984.tb08178.x. [DOI] [PubMed] [Google Scholar]
- Chapman A. G., Fall L., Atkinson D. E. Adenylate energy charge in Escherichia coli during growth and starvation. J Bacteriol. 1971 Dec;108(3):1072–1086. doi: 10.1128/jb.108.3.1072-1086.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crider B. P., Carper S. W., Lancaster J. R. Electron transfer-driven ATP synthesis in Methanococcus voltae is not dependent on a proton electrochemical gradient. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6793–6796. doi: 10.1073/pnas.82.20.6793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deppenmeier U., Blaut M., Mahlmann A., Gottschalk G. Reduced coenzyme F420: heterodisulfide oxidoreductase, a proton- translocating redox system in methanogenic bacteria. Proc Natl Acad Sci U S A. 1990 Dec 1;87(23):9449–9453. doi: 10.1073/pnas.87.23.9449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmavaram R. M., Konisky J. Characterization of a P-type ATPase of the archaebacterium Methanococcus voltae. J Biol Chem. 1989 Aug 25;264(24):14085–14089. [PubMed] [Google Scholar]
- Dharmavaram R. M., Konisky J. Identification of a vanadate-sensitive, membrane-bound ATPase in the archaebacterium Methanococcus voltae. J Bacteriol. 1987 Sep;169(9):3921–3925. doi: 10.1128/jb.169.9.3921-3925.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmavaram R., Gillevet P., Konisky J. Nucleotide sequence of the gene encoding the vanadate-sensitive membrane-associated ATPase of Methanococcus voltae. J Bacteriol. 1991 Mar;173(6):2131–2133. doi: 10.1128/jb.173.6.2131-2133.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMarco A. A., Bobik T. A., Wolfe R. S. Unusual coenzymes of methanogenesis. Annu Rev Biochem. 1990;59:355–394. doi: 10.1146/annurev.bi.59.070190.002035. [DOI] [PubMed] [Google Scholar]
- Dibrov P. A., Lazarova R. L., Skulachev V. P., Verkhovskaya M. L. The sodium cycle. II. Na+-coupled oxidative phosphorylation in Vibrio alginolyticus cells. Biochim Biophys Acta. 1986 Jul 23;850(3):458–465. doi: 10.1016/0005-2728(86)90114-3. [DOI] [PubMed] [Google Scholar]
- Dimroth P. Sodium ion transport decarboxylases and other aspects of sodium ion cycling in bacteria. Microbiol Rev. 1987 Sep;51(3):320–340. doi: 10.1128/mr.51.3.320-340.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dybas M., Konisky J. Transport of coenzyme M (2-mercaptoethanesulfonic acid) and methylcoenzyme M [(2-methylthio)ethanesulfonic acid] in Methanococcus voltae: identification of specific and general uptake systems. J Bacteriol. 1989 Nov;171(11):5866–5871. doi: 10.1128/jb.171.11.5866-5871.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efiok B. J., Webster D. A. Respiratory-driven Na+ electrical potential in the bacterium Vitreoscilla. Biochemistry. 1990 May 15;29(19):4734–4739. doi: 10.1021/bi00471a030. [DOI] [PubMed] [Google Scholar]
- Gunsalus R. P., Romesser J. A., Wolfe R. S. Preparation of coenzyme M analogues and their activity in the methyl coenzyme M reductase system of Methanobacterium thermoautotrophicum. Biochemistry. 1978 Jun 13;17(12):2374–2377. doi: 10.1021/bi00605a019. [DOI] [PubMed] [Google Scholar]
- Ken-Dror S., Preger R., Avi-Dor Y. Functional characterization of the uncoupler-insensitive Na+ pump of the halotolerant bacterium, Ba1. Arch Biochem Biophys. 1986 Jan;244(1):122–127. doi: 10.1016/0003-9861(86)90100-1. [DOI] [PubMed] [Google Scholar]
- Krulwich T. A. Na+/H+ antiporters. Biochim Biophys Acta. 1983 Dec 30;726(4):245–264. doi: 10.1016/0304-4173(83)90011-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lübben M., Lünsdorf H., Schäfer G. The plasma membrane ATPase of the thermoacidophilic archaebacterium Sulfolobus acidocaldarius. Purification and immunological relationships to F1-ATPases. Eur J Biochem. 1987 Sep 1;167(2):211–219. doi: 10.1111/j.1432-1033.1987.tb13325.x. [DOI] [PubMed] [Google Scholar]
- Lübben M., Schäfer G. Chemiosmotic energy conversion of the archaebacterial thermoacidophile Sulfolobus acidocaldarius: oxidative phosphorylation and the presence of an F0-related N,N'-dicyclohexylcarbodiimide-binding proteolipid. J Bacteriol. 1989 Nov;171(11):6106–6116. doi: 10.1128/jb.171.11.6106-6116.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod R. A., Wisse G. A., Stejskal F. L. Sensitivity of some marine bacteria, a moderate halophile, and Escherichia coli to uncouplers at alkaline pH. J Bacteriol. 1988 Sep;170(9):4330–4337. doi: 10.1128/jb.170.9.4330-4337.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressman B. C. Biological applications of ionophores. Annu Rev Biochem. 1976;45:501–530. doi: 10.1146/annurev.bi.45.070176.002441. [DOI] [PubMed] [Google Scholar]
- Reed P. W. Ionophores. Methods Enzymol. 1979;55:435–454. doi: 10.1016/0076-6879(79)55058-7. [DOI] [PubMed] [Google Scholar]
- Rottenberg H. The measurement of membrane potential and deltapH in cells, organelles, and vesicles. Methods Enzymol. 1979;55:547–569. doi: 10.1016/0076-6879(79)55066-6. [DOI] [PubMed] [Google Scholar]
- Tokuda H., Unemoto T. A respiration-dependent primary sodium extrusion system functioning at alkaline pH in the marine bacterium Vibrio alginolyticus. Biochem Biophys Res Commun. 1981 Sep 16;102(1):265–271. doi: 10.1016/0006-291x(81)91516-3. [DOI] [PubMed] [Google Scholar]
- Tsuchiya T., Shinoda S. Respiration-driven Na+ pump and Na+ circulation in Vibrio parahaemolyticus. J Bacteriol. 1985 May;162(2):794–798. doi: 10.1128/jb.162.2.794-798.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udagawa T., Unemoto T., Tokuda H. Generation of Na+ electrochemical potential by the Na+-motive NADH oxidase and Na+/H+ antiport system of a moderately halophilic Vibrio costicola. J Biol Chem. 1986 Feb 25;261(6):2616–2622. [PubMed] [Google Scholar]
- Whitman W. B., Ankwanda E., Wolfe R. S. Nutrition and carbon metabolism of Methanococcus voltae. J Bacteriol. 1982 Mar;149(3):852–863. doi: 10.1128/jb.149.3.852-863.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]