Abstract
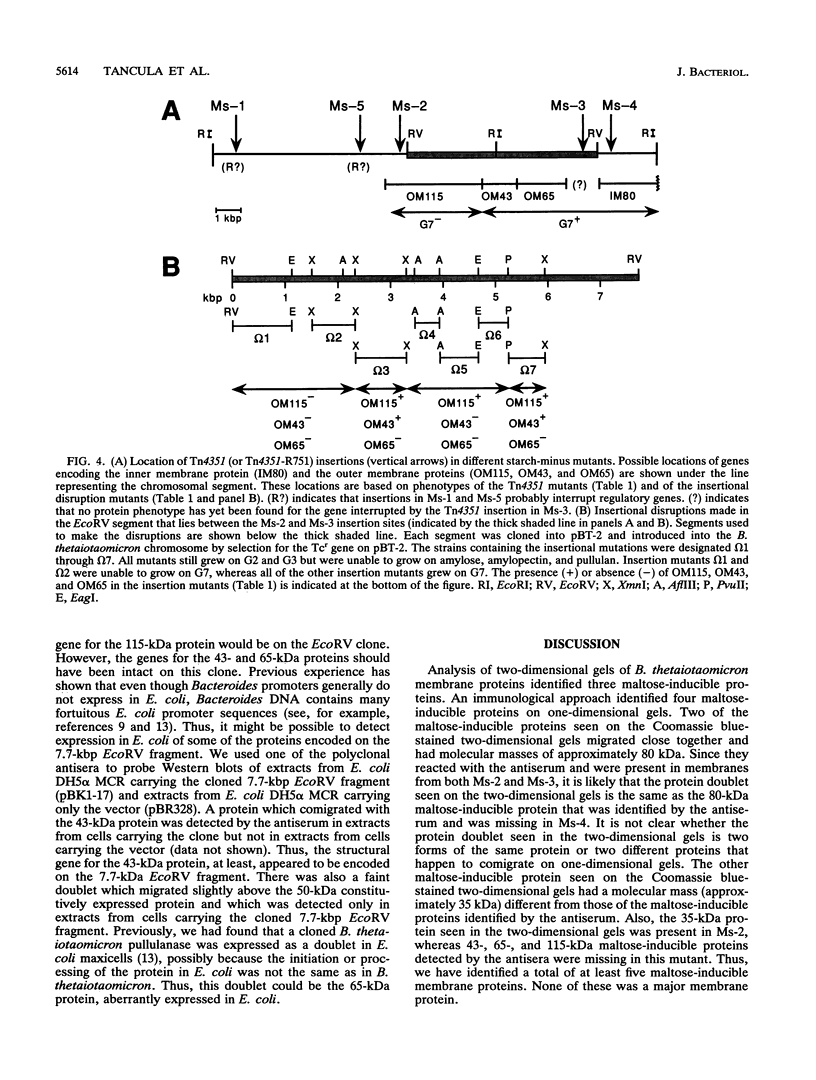
Previous studies of starch utilization by the gram-negative anaerobe Bacteroides thetaiotaomicron have demonstrated that the starch-degrading enzymes are cell associated rather than extracellular, indicating that the first step in starch utilization is binding of the polysaccharide to the bacterial surface. Five transposon-generated mutants of B. thetaiotaomicron which were defective in starch binding (Ms-1 through Ms-5) had been isolated, but initial attempts to identify membrane proteins missing in these mutants were not successful. We report here the use of an immunological approach to identify four maltose-inducible membrane proteins, which were missing in one or more of the starch-binding mutants of B. thetaiotaomicron. Three of the maltose-inducible proteins were outer membrane proteins (115, 65, and 43 kDa), and one was a cytoplasmic membrane protein (80 kDa). The genes encoding these proteins were shown to be clustered in an 8.5-kbp segment of the B. thetaiotaomicron chromosome. Two other loci defined by transposon insertions, which appeared to contain regulatory genes, were located within 7 kbp of the cluster of membrane protein genes. The 115-kDa outer membrane protein was essential for utilization of maltoheptaose (G7), whereas loss of the other proteins affected growth on starch but not on G7. Not all of the proteins missing in the mutants were maltose regulated. We also detected two constitutively produced proteins (32 and 50 kDa) that were less prominent in all of the mutants than in the wild type. Both of these were outer membrane proteins.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson K. L., Salyers A. A. Biochemical evidence that starch breakdown by Bacteroides thetaiotaomicron involves outer membrane starch-binding sites and periplasmic starch-degrading enzymes. J Bacteriol. 1989 Jun;171(6):3192–3198. doi: 10.1128/jb.171.6.3192-3198.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson K. L., Salyers A. A. Genetic evidence that outer membrane binding of starch is required for starch utilization by Bacteroides thetaiotaomicron. J Bacteriol. 1989 Jun;171(6):3199–3204. doi: 10.1128/jb.171.6.3199-3204.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gherardini F. C., Salyers A. A. Purification and characterization of a cell-associated, soluble mannanase from Bacteroides ovatus. J Bacteriol. 1987 May;169(5):2038–2043. doi: 10.1128/jb.169.5.2038-2043.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King S. W., Morrow K. J., Jr Monoclonal antibodies produced against antigenic determinants present in complex mixtures of proteins. Biotechniques. 1988 Oct;6(9):856–861. [PubMed] [Google Scholar]
- Kotarski S. F., Linz J., Braun D. M., Salyers A. A. Analysis of outer membrane proteins which are associated with growth of Bacteroides thetaiotaomicron on chondroitin sulfate. J Bacteriol. 1985 Sep;163(3):1080–1086. doi: 10.1128/jb.163.3.1080-1086.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotarski S. F., Salyers A. A. Isolation and characterization of outer membranes of Bacteroides thetaiotaomicron grown on different carbohydrates. J Bacteriol. 1984 Apr;158(1):102–109. doi: 10.1128/jb.158.1.102-109.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolich M. P., Shoemaker N. B., Salyers A. A. A Bacteroides tetracycline resistance gene represents a new class of ribosome protection tetracycline resistance. Antimicrob Agents Chemother. 1992 May;36(5):1005–1012. doi: 10.1128/aac.36.5.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salyers A. A., Vercellotti J. R., West S. E., Wilkins T. D. Fermentation of mucin and plant polysaccharides by strains of Bacteroides from the human colon. Appl Environ Microbiol. 1977 Feb;33(2):319–322. doi: 10.1128/aem.33.2.319-322.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker N. B., Barber R. D., Salyers A. A. Cloning and characterization of a Bacteroides conjugal tetracycline-erythromycin resistance element by using a shuttle cosmid vector. J Bacteriol. 1989 Mar;171(3):1294–1302. doi: 10.1128/jb.171.3.1294-1302.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker N. B., Getty C., Gardner J. F., Salyers A. A. Tn4351 transposes in Bacteroides spp. and mediates the integration of plasmid R751 into the Bacteroides chromosome. J Bacteriol. 1986 Mar;165(3):929–936. doi: 10.1128/jb.165.3.929-936.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. A., Salyers A. A. Cell-associated pullulanase from Bacteroides thetaiotaomicron: cloning, characterization, and insertional mutagenesis to determine role in pullulan utilization. J Bacteriol. 1989 Apr;171(4):2116–2123. doi: 10.1128/jb.171.4.2116-2123.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. A., Salyers A. A. Characterization of a neopullulanase and an alpha-glucosidase from Bacteroides thetaiotaomicron 95-1. J Bacteriol. 1991 May;173(9):2962–2968. doi: 10.1128/jb.173.9.2962-2968.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speer B. S., Salyers A. A. Novel aerobic tetracycline resistance gene that chemically modifies tetracycline. J Bacteriol. 1989 Jan;171(1):148–153. doi: 10.1128/jb.171.1.148-153.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens A. M., Shoemaker N. B., Salyers A. A. The region of a Bacteroides conjugal chromosomal tetracycline resistance element which is responsible for production of plasmidlike forms from unlinked chromosomal DNA might also be involved in transfer of the element. J Bacteriol. 1990 Aug;172(8):4271–4279. doi: 10.1128/jb.172.8.4271-4279.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine P. J., Salyers A. A. Analysis of proteins associated with growth of Bacteroides ovatus on the branched galactomannan guar gum. Appl Environ Microbiol. 1992 May;58(5):1534–1540. doi: 10.1128/aem.58.5.1534-1540.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]