Abstract
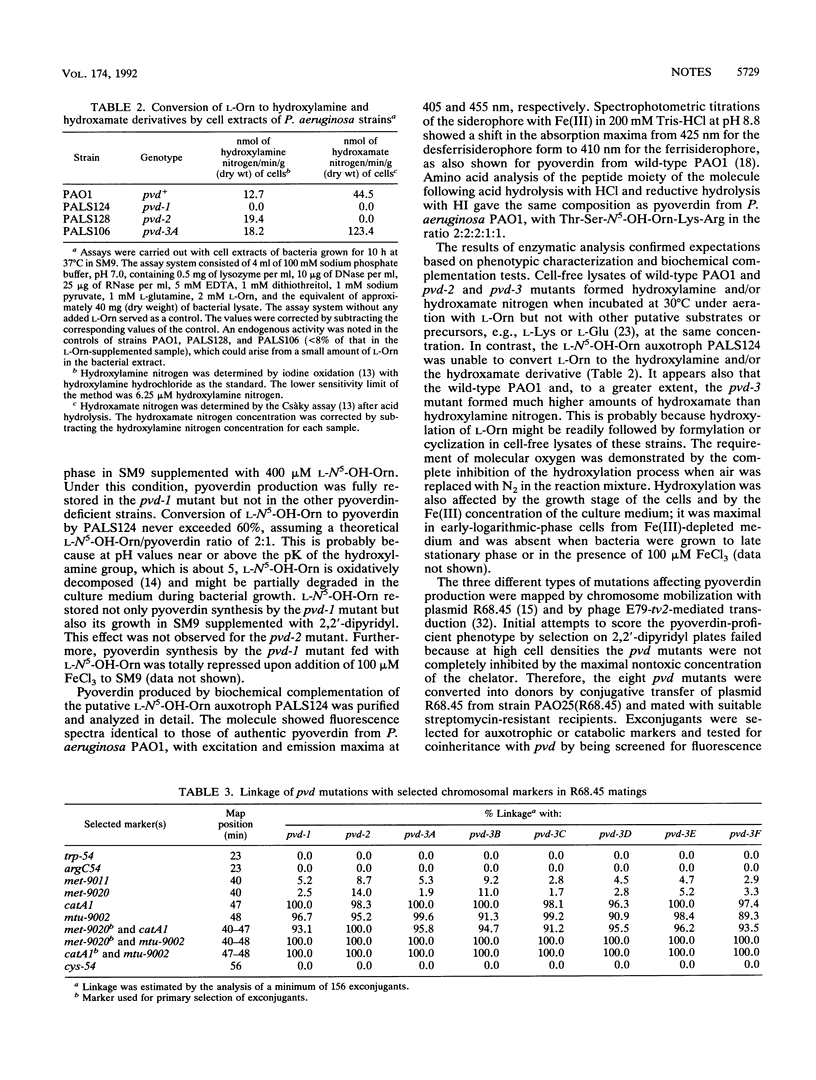
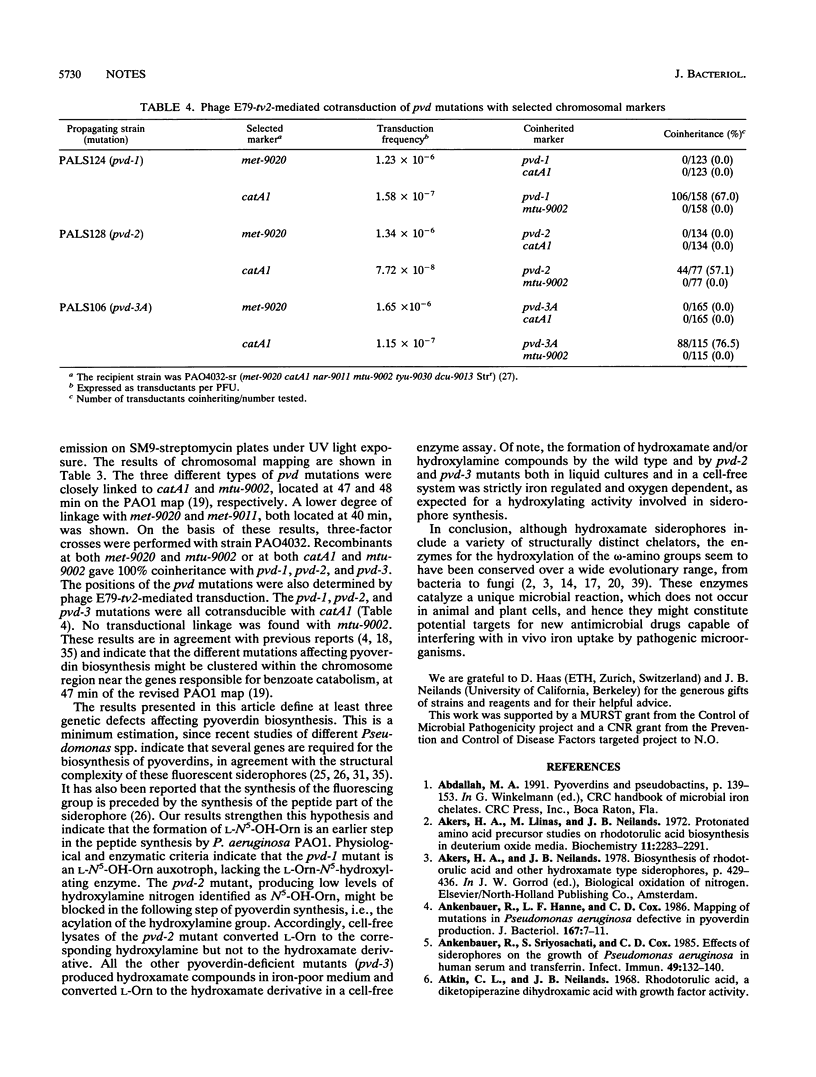
We have isolated and characterized by chemical and enzymatic analyses three distinct types of pyoverdin-defective (pvd) mutants of Pseudomonas aeruginosa PAO1. The pvd-1 mutant is an L-N5-hydroxyornithine (L-N5-OH-Orn) auxotroph unable to hydroxylate L-ornithine (L-Orn) in a cell-free system and requiring L-N5-OH-Orn for pyoverdin production. The other two types of mutants appear to be blocked in further steps of the biosynthetic pathway leading to pyoverdin, namely, the acylation of L-N5-OH-Orn (pvd-2) and chromophore synthesis (pvd-3). The different pvd mutations were all found to be located in the catA1 region at 47 min of the genetic map of P. aeruginosa PAO1.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akers H. A., Llinás M., Neilands J. B. Protonated amino acid precursor studies on rhodotorulic acid biosynthesis in deuterium oxide media. Biochemistry. 1972 Jun 6;11(12):2283–2291. doi: 10.1021/bi00762a012. [DOI] [PubMed] [Google Scholar]
- Ankenbauer R., Hanne L. F., Cox C. D. Mapping of mutations in Pseudomonas aeruginosa defective in pyoverdin production. J Bacteriol. 1986 Jul;167(1):7–11. doi: 10.1128/jb.167.1.7-11.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankenbauer R., Sriyosachati S., Cox C. D. Effects of siderophores on the growth of Pseudomonas aeruginosa in human serum and transferrin. Infect Immun. 1985 Jul;49(1):132–140. doi: 10.1128/iai.49.1.132-140.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkin C. L., Neilands J. B. Rhodotorulic acid, a diketopiperazine dihydroxamic acid with growth-factor activity. I. Isolation and characterization. Biochemistry. 1968 Oct;7(10):3734–3739. doi: 10.1021/bi00850a054. [DOI] [PubMed] [Google Scholar]
- Callahan L. T., 3rd Pseudomonas aeruginosa exotoxin: purification by preparative polyacrylamide gel electrophoresis and the development of a highly specific antitoxin serum. Infect Immun. 1976 Jul;14(1):55–61. doi: 10.1128/iai.14.1.55-61.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonna B., Nicoletti M., Visca P., Casalino M., Valenti P., Maimone F. Composite IS1 elements encoding hydroxamate-mediated iron uptake in FIme plasmids from epidemic Salmonella spp. J Bacteriol. 1985 Apr;162(1):307–316. doi: 10.1128/jb.162.1.307-316.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelis P., Hohnadel D., Meyer J. M. Evidence for different pyoverdine-mediated iron uptake systems among Pseudomonas aeruginosa strains. Infect Immun. 1989 Nov;57(11):3491–3497. doi: 10.1128/iai.57.11.3491-3497.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox C. D., Graham R. Isolation of an iron-binding compound from Pseudomonas aeruginosa. J Bacteriol. 1979 Jan;137(1):357–364. doi: 10.1128/jb.137.1.357-364.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox C. D., Rinehart K. L., Jr, Moore M. L., Cook J. C., Jr Pyochelin: novel structure of an iron-chelating growth promoter for Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4256–4260. doi: 10.1073/pnas.78.7.4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery T. F. Initial steps in the biosynthesis of ferrichrome. Incorporation of delta-N-hydroxyornithine and delta-N-acetyl-delta-N-hydroxyornithine. Biochemistry. 1966 Nov;5(11):3694–3701. doi: 10.1021/bi00875a045. [DOI] [PubMed] [Google Scholar]
- Gross R., Engelbrecht F., Braun V. Genetic and biochemical characterization of the aerobactin synthesis operon on pColV. Mol Gen Genet. 1984;196(1):74–80. doi: 10.1007/BF00334095. [DOI] [PubMed] [Google Scholar]
- Guterman S. K. Colicin B: mode of action and inhibition by enterochelin. J Bacteriol. 1973 Jun;114(3):1217–1224. doi: 10.1128/jb.114.3.1217-1224.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas D., Holloway B. W. R factor variants with enhanced sex factor activity in Pseudomonas aeruginosa. Mol Gen Genet. 1976 Mar 30;144(3):243–251. doi: 10.1007/BF00341722. [DOI] [PubMed] [Google Scholar]
- Heinrichs D. E., Young L., Poole K. Pyochelin-mediated iron transport in Pseudomonas aeruginosa: involvement of a high-molecular-mass outer membrane protein. Infect Immun. 1991 Oct;59(10):3680–3684. doi: 10.1128/iai.59.10.3680-3684.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero M., de Lorenzo V., Neilands J. B. Nucleotide sequence of the iucD gene of the pColV-K30 aerobactin operon and topology of its product studied with phoA and lacZ gene fusions. J Bacteriol. 1988 Jan;170(1):56–64. doi: 10.1128/jb.170.1.56-64.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac J. H., Holloway B. W. Control of arginine biosynthesis in Pseudomonas aeruginosa. J Gen Microbiol. 1972 Dec;73(3):427–438. doi: 10.1099/00221287-73-3-427. [DOI] [PubMed] [Google Scholar]
- LIN E. C., LERNER S. A., JORGENSEN S. E. A method for isolating constitutive mutants for carbohydrate-catabolizing enzymes. Biochim Biophys Acta. 1962 Jul 2;60:422–424. doi: 10.1016/0006-3002(62)90423-7. [DOI] [PubMed] [Google Scholar]
- Marugg J. D., van Spanje M., Hoekstra W. P., Schippers B., Weisbeek P. J. Isolation and analysis of genes involved in siderophore biosynthesis in plant-growth-stimulating Pseudomonas putida WCS358. J Bacteriol. 1985 Nov;164(2):563–570. doi: 10.1128/jb.164.2.563-570.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto H., Nakazawa T., Ohta S., Terawaki Y. Chromosomal locations of catA, pobA, dcu and chu genes in Pseudomonas aeruginosa. Genet Res. 1981 Dec;38(3):251–266. doi: 10.1017/s0016672300020590. [DOI] [PubMed] [Google Scholar]
- Meyer J. M., Hohnadel D., Khan A., Cornelis P. Pyoverdin-facilitated iron uptake in Pseudomonas aeruginosa: immunological characterization of the ferripyoverdin receptor. Mol Microbiol. 1990 Aug;4(8):1401–1405. doi: 10.1111/j.1365-2958.1990.tb00719.x. [DOI] [PubMed] [Google Scholar]
- Moore A. T., Nayudu M., Holloway B. W. Genetic mapping in Methylophilus methylotrophus AS1. J Gen Microbiol. 1983 Mar;129(3):785–799. doi: 10.1099/00221287-129-3-785. [DOI] [PubMed] [Google Scholar]
- Moores J. C., Magazin M., Ditta G. S., Leong J. Cloning of genes involved in the biosynthesis of pseudobactin, a high-affinity iron transport agent of a plant growth-promoting Pseudomonas strain. J Bacteriol. 1984 Jan;157(1):53–58. doi: 10.1128/jb.157.1.53-58.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan A. F. Transduction of Pseudomonas aeruginosa with a mutant of bacteriophage E79. J Bacteriol. 1979 Jul;139(1):137–140. doi: 10.1128/jb.139.1.137-140.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray G. J., Clark G. E., Parniak M. A., Viswanatha T. Effect of metabolites on epsilon-N-hydroxylysine formation in cell-free extracts of Aerobacter aerogenes 62-1. Can J Biochem. 1977 Jun;55(6):625–629. doi: 10.1139/o77-090. [DOI] [PubMed] [Google Scholar]
- Poole K., Neshat S., Heinrichs D. Pyoverdine-mediated iron transport in Pseudomonas aeruginosa: involvement of a high-molecular-mass outer membrane protein. FEMS Microbiol Lett. 1991 Feb;62(1):1–5. [PubMed] [Google Scholar]
- Rombel I. T., Lamont I. L. DNA homology between siderophore genes from fluorescent pseudomonads. J Gen Microbiol. 1992 Jan;138(1):181–187. doi: 10.1099/00221287-138-1-181. [DOI] [PubMed] [Google Scholar]
- Royle P. L., Matsumoto H., Holloway B. W. Genetic circularity of the Pseudomonas aeruginosa PAO chromosome. J Bacteriol. 1981 Jan;145(1):145–155. doi: 10.1128/jb.145.1.145-155.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwyn B., Neilands J. B. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. 1987 Jan;160(1):47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- Sokol P. A., Woods D. E. Demonstration of an iron-siderophore-binding protein in the outer membrane of Pseudomonas aeruginosa. Infect Immun. 1983 May;40(2):665–669. doi: 10.1128/iai.40.2.665-669.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Budde A. D., Leong S. A. Analysis of ferrichrome biosynthesis in the phytopathogenic fungus Ustilago maydis: cloning of an ornithine-N5-oxygenase gene. J Bacteriol. 1989 May;171(5):2811–2818. doi: 10.1128/jb.171.5.2811-2818.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J. M., Holloway B. W. Suppressor mutations in Pseudomonas aeruginosa. J Bacteriol. 1976 Mar;125(3):780–786. doi: 10.1128/jb.125.3.780-786.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]


