Abstract
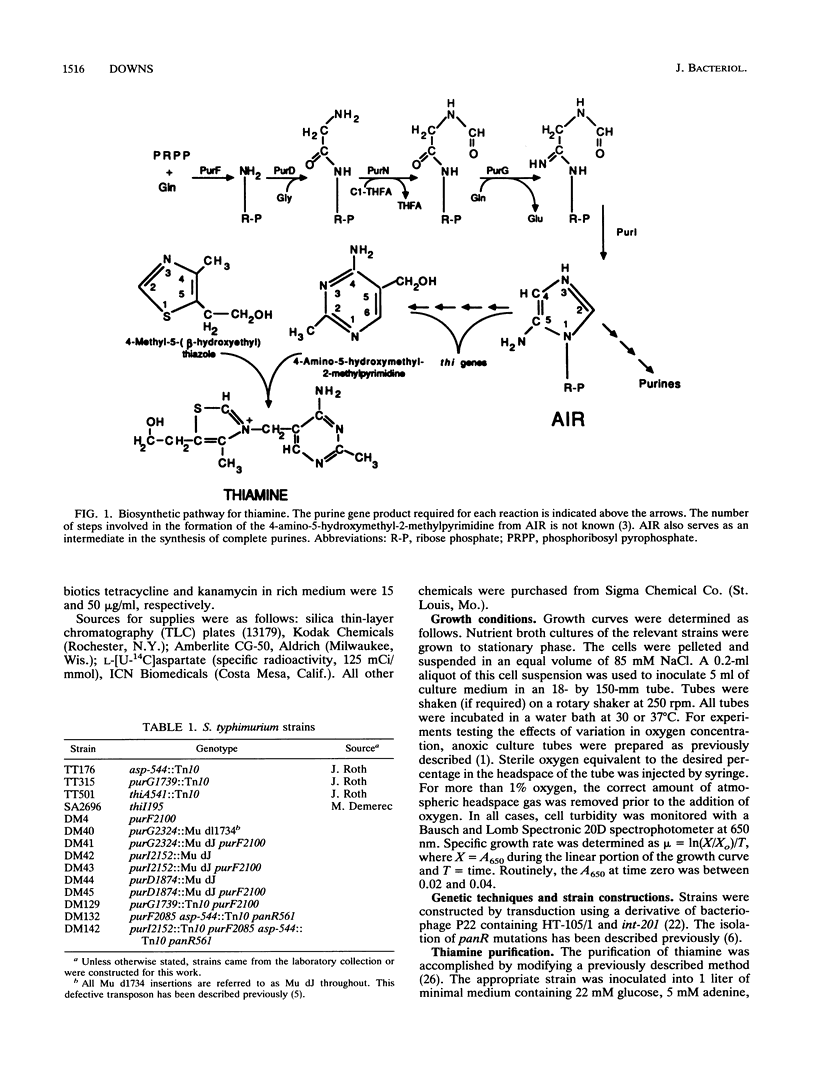
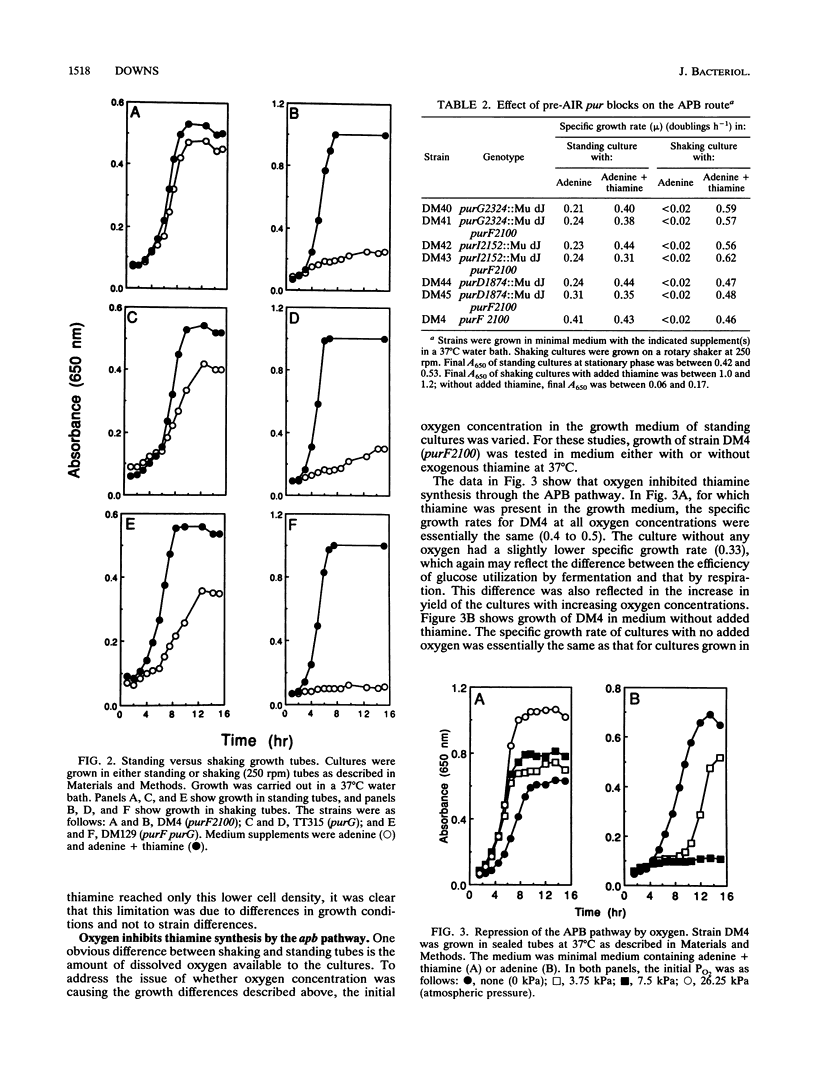
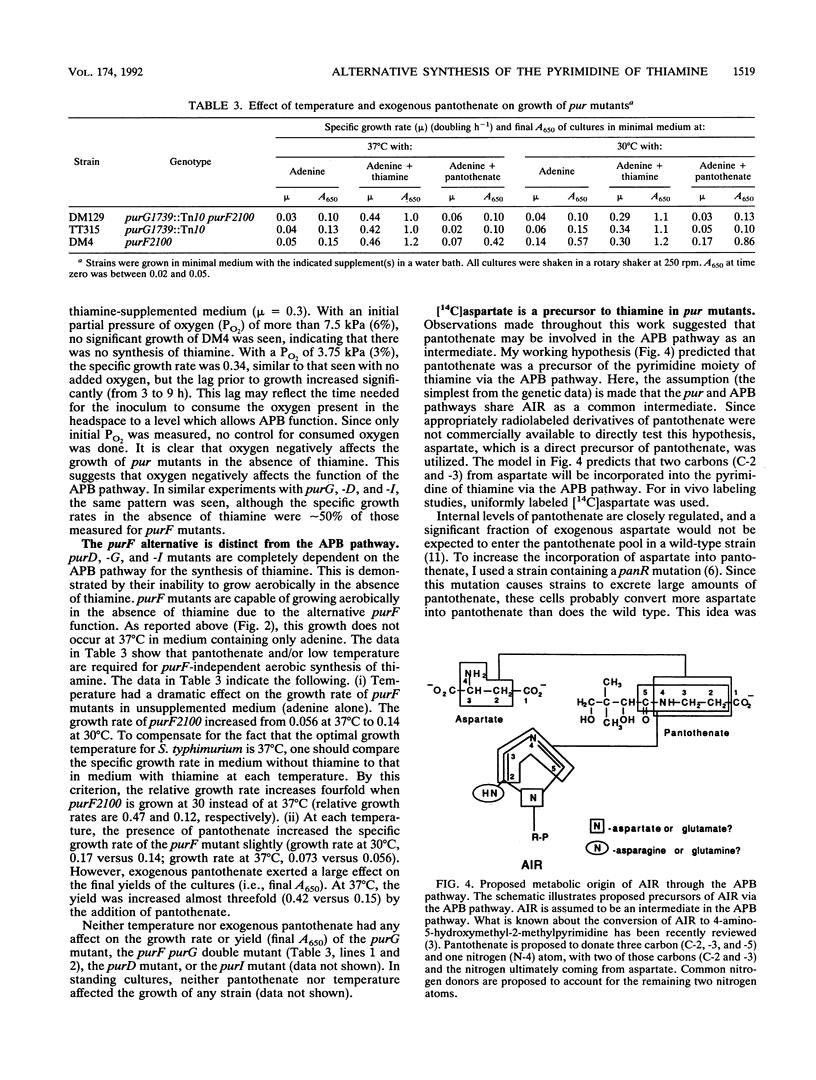
The synthesis of the pyrimidine moiety of thiamine (vitamin B1) shares five reactions with the de novo purine biosynthetic pathway. Aminoimidazole ribotide (AIR) is the last common intermediate before the two pathways diverge. Evidence for the existence of a new pathway to the pyrimidine which bypasses the de novo purine biosynthetic pathway is reported here. This pathway is only expressed under anaerobic growth conditions and is denoted alternative pyrimidine biosynthesis or APB. Labeling studies are consistent with pantothenate being a precursor to the pyrimidine moiety of thiamine that is synthesized by the APB pathway. The APB pathway is independent of the alternative purF function which was proposed previously (D. M. Downs and J. R. Roth, J. Bacteriol. 173:6597-6604, 1991). The alternative purF function is shown here to be affected by temperature and exogenous pantothenate. Although the evidence suggests that the APB pathway is separate from the alternative purF function, the relationship between this function and the APB pathway is not yet clear.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROOKE M. S., MAGASANIK B. The metabolism of purines in Aerobacter aerogenes: a study of purineless mutants. J Bacteriol. 1954 Dec;68(6):727–733. doi: 10.1128/jb.68.6.727-733.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch W. E., Wolfe R. S. New approach to the cultivation of methanogenic bacteria: 2-mercaptoethanesulfonic acid (HS-CoM)-dependent growth of Methanobacterium ruminantium in a pressureized atmosphere. Appl Environ Microbiol. 1976 Dec;32(6):781–791. doi: 10.1128/aem.32.6.781-791.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARLSON G. L., BROWN G. M. The natural occurrence, enzymatic formation, and biochemical significance of a hydroxyethyl derivative of thiamine pyrophosphate. J Biol Chem. 1961 Jul;236:2099–2108. [PubMed] [Google Scholar]
- Castilho B. A., Olfson P., Casadaban M. J. Plasmid insertion mutagenesis and lac gene fusion with mini-mu bacteriophage transposons. J Bacteriol. 1984 May;158(2):488–495. doi: 10.1128/jb.158.2.488-495.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs D. M., Roth J. R. Synthesis of thiamine in Salmonella typhimurium independent of the purF function. J Bacteriol. 1991 Oct;173(20):6597–6604. doi: 10.1128/jb.173.20.6597-6604.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estramareix B., Gaudry D., Thérisod M. Biosynthèse du thiazole de la thiamine chez Escherichia coli. Biochimie. 1977;59(10):857–859. doi: 10.1016/s0300-9084(77)80219-8. [DOI] [PubMed] [Google Scholar]
- Estramareix B., Therisod M. La tyrosine, facteur de la biosynthèse du thiazole de la thiamine chez Escherichia coli. Biochim Biophys Acta. 1972 Jul 19;273(2):275–282. [PubMed] [Google Scholar]
- GOLDSTEIN G. A., BROWN G. M. THE BIOSYNTHESIS OF THIAMINE. V. STUDIES CONCERNING PRECURSORS OF THE PYRIMIDINE MOIETY. Arch Biochem Biophys. 1963 Dec;103:449–452. doi: 10.1016/0003-9861(63)90436-3. [DOI] [PubMed] [Google Scholar]
- Jackowski S., Rock C. O. Regulation of coenzyme A biosynthesis. J Bacteriol. 1981 Dec;148(3):926–932. doi: 10.1128/jb.148.3.926-932.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki T. Determination of thiamin and its phosphate esters by high-performance liquid chromatography. Methods Enzymol. 1986;122:15–20. doi: 10.1016/0076-6879(86)22141-2. [DOI] [PubMed] [Google Scholar]
- Kilstrup M., Meng L. M., Neuhard J., Nygaard P. Genetic evidence for a repressor of synthesis of cytosine deaminase and purine biosynthesis enzymes in Escherichia coli. J Bacteriol. 1989 Apr;171(4):2124–2127. doi: 10.1128/jb.171.4.2124-2127.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell P. C., Tucker R. G. Biosynthesis of the pyrimidine moiety of thiamine. A new route of pyrimidine biosynthesis involving purine intermediates. Biochem J. 1968 Jan;106(1):279–287. doi: 10.1042/bj1060279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell P. C., Tucker R. G. New pyrimidine pathway involved in the biosynthesis of the pyrimidine of thiamine. Nature. 1967 Sep 23;215(5108):1384–1385. doi: 10.1038/2151384a0. [DOI] [PubMed] [Google Scholar]
- Newell P. C., Tucker R. G. Precursors of the pyrimidine moiety of thiamine. Biochem J. 1968 Jan;106(1):271–277. doi: 10.1042/bj1060271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfes R. J., Zalkin H. Escherichia coli gene purR encoding a repressor protein for purine nucleotide synthesis. Cloning, nucleotide sequence, and interaction with the purF operator. J Biol Chem. 1988 Dec 25;263(36):19653–19661. [PubMed] [Google Scholar]
- Rolfes R. J., Zalkin H. Purification of the Escherichia coli purine regulon repressor and identification of corepressors. J Bacteriol. 1990 Oct;172(10):5637–5642. doi: 10.1128/jb.172.10.5637-5642.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson K. E., Roth J. R. Linkage map of Salmonella typhimurium, edition VII. Microbiol Rev. 1988 Dec;52(4):485–532. doi: 10.1128/mr.52.4.485-532.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmieger H. Phage P22-mutants with increased or decreased transduction abilities. Mol Gen Genet. 1972;119(1):75–88. doi: 10.1007/BF00270447. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- White R. H., Rudolph F. B. Biosynthesis of the pyrimidine moiety of thiamin in Escherichia coli: incorporation of stable isotope-labeled glycines. Biochemistry. 1979 Jun 12;18(12):2632–2636. doi: 10.1021/bi00579a031. [DOI] [PubMed] [Google Scholar]
- White R. H., Rudolph F. B. The origin of the nitrogen atom in the thiazole ring of thiamine in Escherichia coli. Biochim Biophys Acta. 1978 Aug 17;542(2):340–347. doi: 10.1016/0304-4165(78)90029-6. [DOI] [PubMed] [Google Scholar]
- White R. H. Stable isotope studies on the biosynthesis of the thiazole moiety of thiamin in Escherichia coli. Biochemistry. 1978 Sep 5;17(18):3833–3840. doi: 10.1021/bi00611a024. [DOI] [PubMed] [Google Scholar]
- Yamada K., Kumaoka H. Precursor of carbon atom five and hydroxymethyl carbon atom of the pyrimidine moiety of thiamin in Escherichia coli. J Nutr Sci Vitaminol (Tokyo) 1983 Aug;29(4):389–398. doi: 10.3177/jnsv.29.389. [DOI] [PubMed] [Google Scholar]


