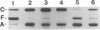Abstract
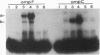
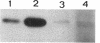
OmpR is a DNA-binding protein that regulates transcription of ompF and ompC. The activity of OmpR is controlled by the inner membrane osmosensor, EnvZ. In order to study the signaling process between EnvZ and OmpR, we analyzed two different envZ strains: the envZ473 strain, in which OmpC is constitutively produced and OmpF is fully repressed, and the envZ3 strain, in which the production of OmpC is greatly reduced and OmpF is not fully repressed by high-osmolarity growth conditions. Using direct sequencing of DNA derived from the polymerase chain reaction amplification method, we identified the mutation in the envZ473 strain as a Val-241-to-Gly substitution and the mutation in the envZ3 as an Ala-219-to-Val substitution. The relative DNA-binding affinity of OmpR derived from the envZ473 strain was dramatically increased for the upstream sequence of both ompF and ompC. In contrast, OmpR derived from the envZ3 strain was not converted to the high-affinity form. The intracellular levels of OmpR-phosphate, as analyzed by the in vivo phosphorylation approach, significantly increased in the envZ473 strain, while in the envZ3 strain the levels were considerably reduced, relative to those found in the parent strain. The intracellular level of OmpR protein in the envZ473 strain was also found to be markedly elevated relative to that of the parent strain. These results are discussed in relation to the role of phosphorylation and relative DNA-binding affinity of OmpR in the expression of ompF and ompC.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiba H., Nakasai F., Mizushima S., Mizuno T. Evidence for the physiological importance of the phosphotransfer between the two regulatory components, EnvZ and OmpR, in osmoregulation in Escherichia coli. J Biol Chem. 1989 Aug 25;264(24):14090–14094. [PubMed] [Google Scholar]
- Aiba H., Nakasai F., Mizushima S., Mizuno T. Phosphorylation of a bacterial activator protein, OmpR, by a protein kinase, EnvZ, results in stimulation of its DNA-binding ability. J Biochem. 1989 Jul;106(1):5–7. doi: 10.1093/oxfordjournals.jbchem.a122817. [DOI] [PubMed] [Google Scholar]
- Brissette R. E., Tsung K. L., Inouye M. Suppression of a mutation in OmpR at the putative phosphorylation center by a mutant EnvZ protein in Escherichia coli. J Bacteriol. 1991 Jan;173(2):601–608. doi: 10.1128/jb.173.2.601-608.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comeau D. E., Ikenaka K., Tsung K. L., Inouye M. Primary characterization of the protein products of the Escherichia coli ompB locus: structure and regulation of synthesis of the OmpR and EnvZ proteins. J Bacteriol. 1985 Nov;164(2):578–584. doi: 10.1128/jb.164.2.578-584.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forst S. A., Delgado J., Inouye M. DNA-binding properties of the transcription activator (OmpR) for the upstream sequences of ompF in Escherichia coli are altered by envZ mutations and medium osmolarity. J Bacteriol. 1989 Jun;171(6):2949–2955. doi: 10.1128/jb.171.6.2949-2955.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forst S., Comeau D., Norioka S., Inouye M. Localization and membrane topology of EnvZ, a protein involved in osmoregulation of OmpF and OmpC in Escherichia coli. J Biol Chem. 1987 Dec 5;262(34):16433–16438. [PubMed] [Google Scholar]
- Forst S., Delgado J., Inouye M. Phosphorylation of OmpR by the osmosensor EnvZ modulates expression of the ompF and ompC genes in Escherichia coli. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6052–6056. doi: 10.1073/pnas.86.16.6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forst S., Delgado J., Ramakrishnan G., Inouye M. Regulation of ompC and ompF expression in Escherichia coli in the absence of envZ. J Bacteriol. 1988 Nov;170(11):5080–5085. doi: 10.1128/jb.170.11.5080-5085.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forst S., Delgado J., Rampersaud A., Inouye M. In vivo phosphorylation of OmpR, the transcription activator of the ompF and ompC genes in Escherichia coli. J Bacteriol. 1990 Jun;172(6):3473–3477. doi: 10.1128/jb.172.6.3473-3477.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forst S., Inouye M. Environmentally regulated gene expression for membrane proteins in Escherichia coli. Annu Rev Cell Biol. 1988;4:21–42. doi: 10.1146/annurev.cb.04.110188.000321. [DOI] [PubMed] [Google Scholar]
- Garrett S., Taylor R. K., Silhavy T. J. Isolation and characterization of chain-terminating nonsense mutations in a porin regulator gene, envZ. J Bacteriol. 1983 Oct;156(1):62–69. doi: 10.1128/jb.156.1.62-69.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall M. N., Silhavy T. J. The ompB locus and the regulation of the major outer membrane porin proteins of Escherichia coli K12. J Mol Biol. 1981 Feb 15;146(1):23–43. doi: 10.1016/0022-2836(81)90364-8. [DOI] [PubMed] [Google Scholar]
- Hall M. N., Silhavy T. J. Transcriptional regulation of Escherichia coli K-12 major outer membrane protein 1b. J Bacteriol. 1979 Nov;140(2):342–350. doi: 10.1128/jb.140.2.342-350.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igo M. M., Ninfa A. J., Silhavy T. J. A bacterial environmental sensor that functions as a protein kinase and stimulates transcriptional activation. Genes Dev. 1989 May;3(5):598–605. doi: 10.1101/gad.3.5.598. [DOI] [PubMed] [Google Scholar]
- Igo M. M., Ninfa A. J., Stock J. B., Silhavy T. J. Phosphorylation and dephosphorylation of a bacterial transcriptional activator by a transmembrane receptor. Genes Dev. 1989 Nov;3(11):1725–1734. doi: 10.1101/gad.3.11.1725. [DOI] [PubMed] [Google Scholar]
- Kawaji H., Mizuno T., Mizushima S. Influence of molecular size and osmolarity of sugars and dextrans on the synthesis of outer membrane proteins O-8 and O-9 of Escherichia coli K-12. J Bacteriol. 1979 Dec;140(3):843–847. doi: 10.1128/jb.140.3.843-847.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama S., Mizuno T., Mizushima S. Interaction between two regulatory proteins in osmoregulatory expression of ompF and ompC genes in Escherichia coli: a novel ompR mutation suppresses pleiotropic defects caused by an envZ mutation. J Bacteriol. 1986 Dec;168(3):1309–1314. doi: 10.1128/jb.168.3.1309-1314.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno T., Kato M., Jo Y. L., Mizushima S. Interaction of OmpR, a positive regulator, with the osmoregulated ompC and ompF genes of Escherichia coli. Studies with wild-type and mutant OmpR proteins. J Biol Chem. 1988 Jan 15;263(2):1008–1012. [PubMed] [Google Scholar]
- Mizuno T., Wurtzel E. T., Inouye M. Osmoregulation of gene expression. II. DNA sequence of the envZ gene of the ompB operon of Escherichia coli and characterization of its gene product. J Biol Chem. 1982 Nov 25;257(22):13692–13698. [PubMed] [Google Scholar]
- Ninfa A. J., Bennett R. L. Identification of the site of autophosphorylation of the bacterial protein kinase/phosphatase NRII. J Biol Chem. 1991 Apr 15;266(11):6888–6893. [PubMed] [Google Scholar]
- Norioka S., Ramakrishnan G., Ikenaka K., Inouye M. Interaction of a transcriptional activator, OmpR, with reciprocally osmoregulated genes, ompF and ompC, of Escherichia coli. J Biol Chem. 1986 Dec 25;261(36):17113–17119. [PubMed] [Google Scholar]
- Rampersaud A., Norioka S., Inouye M. Characterization of OmpR binding sequences in the upstream region of the ompF promoter essential for transcriptional activation. J Biol Chem. 1989 Nov 5;264(31):18693–18700. [PubMed] [Google Scholar]
- Sarma V., Reeves P. Genetic locus (ompB) affecting a major outer-membrane protein in Escherichia coli K-12. J Bacteriol. 1977 Oct;132(1):23–27. doi: 10.1128/jb.132.1.23-27.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slauch J. M., Garrett S., Jackson D. E., Silhavy T. J. EnvZ functions through OmpR to control porin gene expression in Escherichia coli K-12. J Bacteriol. 1988 Jan;170(1):439–441. doi: 10.1128/jb.170.1.439-441.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slauch J. M., Silhavy T. J. Genetic analysis of the switch that controls porin gene expression in Escherichia coli K-12. J Mol Biol. 1989 Nov 20;210(2):281–292. doi: 10.1016/0022-2836(89)90330-6. [DOI] [PubMed] [Google Scholar]
- Slauch J. M., Silhavy T. J. cis-acting ompF mutations that result in OmpR-dependent constitutive expression. J Bacteriol. 1991 Jul;173(13):4039–4048. doi: 10.1128/jb.173.13.4039-4048.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock J. B., Ninfa A. J., Stock A. M. Protein phosphorylation and regulation of adaptive responses in bacteria. Microbiol Rev. 1989 Dec;53(4):450–490. doi: 10.1128/mr.53.4.450-490.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R. K., Hall M. N., Silhavy T. J. Isolation and characterization of mutations altering expression of the major outer membrane porin proteins using the local anaesthetic procaine. J Mol Biol. 1983 May 25;166(3):273–282. doi: 10.1016/s0022-2836(83)80085-0. [DOI] [PubMed] [Google Scholar]
- Tsui P., Huang L., Freundlich M. Integration host factor binds specifically to multiple sites in the ompB promoter of Escherichia coli and inhibits transcription. J Bacteriol. 1991 Sep;173(18):5800–5807. doi: 10.1128/jb.173.18.5800-5807.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsung K., Brissette R. E., Inouye M. Identification of the DNA-binding domain of the OmpR protein required for transcriptional activation of the ompF and ompC genes of Escherichia coli by in vivo DNA footprinting. J Biol Chem. 1989 Jun 15;264(17):10104–10109. [PubMed] [Google Scholar]
- Verhoef C., Lugtenberg B., van Boxtel R., de Graaff P., Verheij H. Genetics and biochemistry of the peptidoglycan-associated proteins b and c of Escherichia coli K12. Mol Gen Genet. 1979 Jan 31;169(2):137–146. doi: 10.1007/BF00271664. [DOI] [PubMed] [Google Scholar]
- Wurtzel E. T., Chou M. Y., Inouye M. Osmoregulation of gene expression. I. DNA sequence of the ompR gene of the ompB operon of Escherichia coli and characterization of its gene product. J Biol Chem. 1982 Nov 25;257(22):13685–13691. [PubMed] [Google Scholar]