Abstract
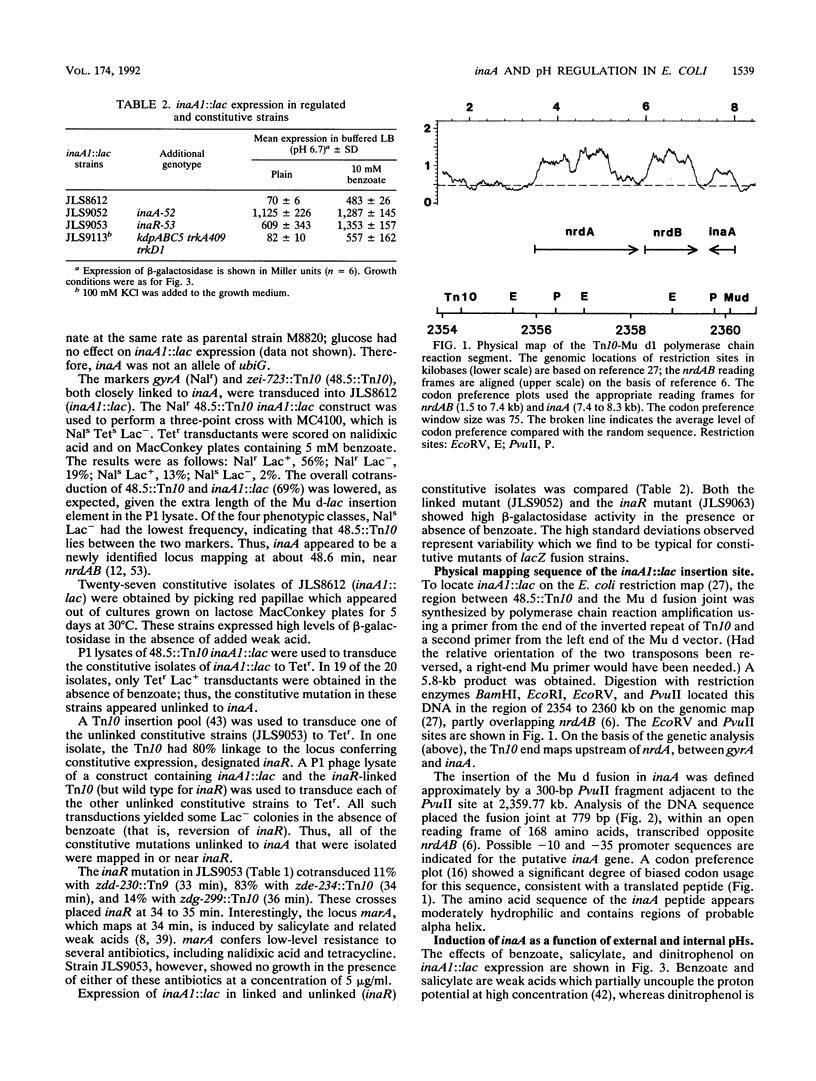
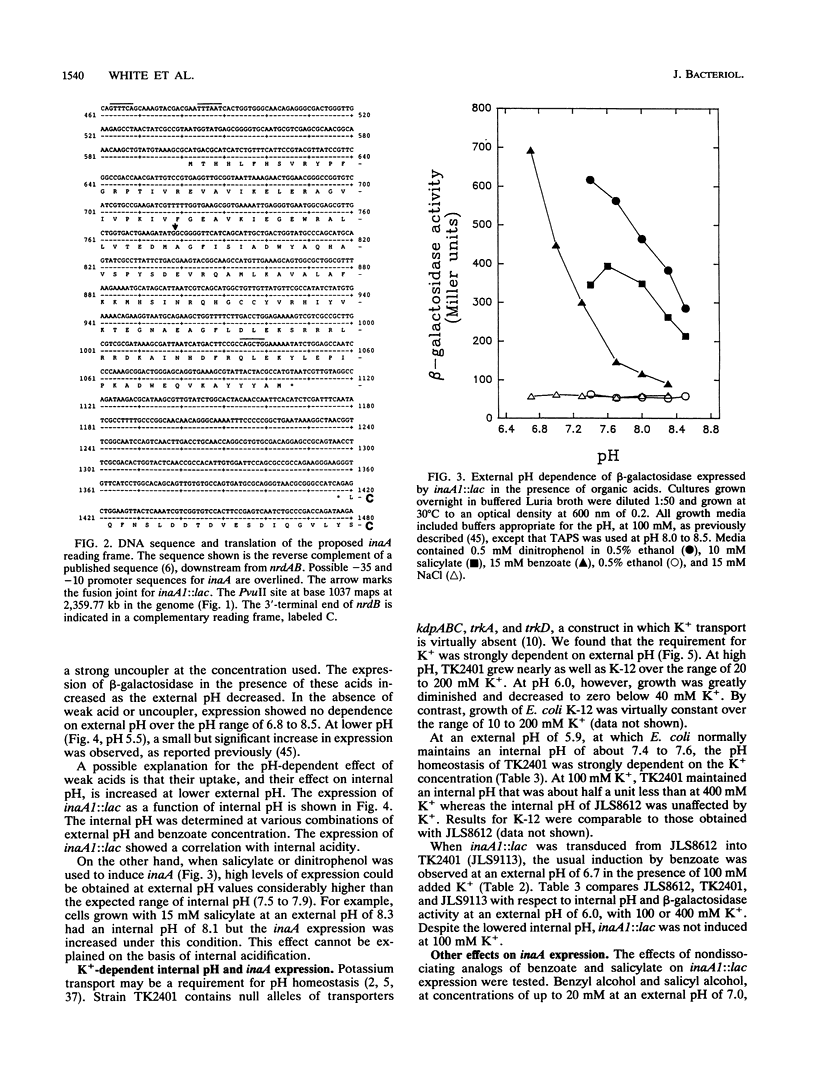
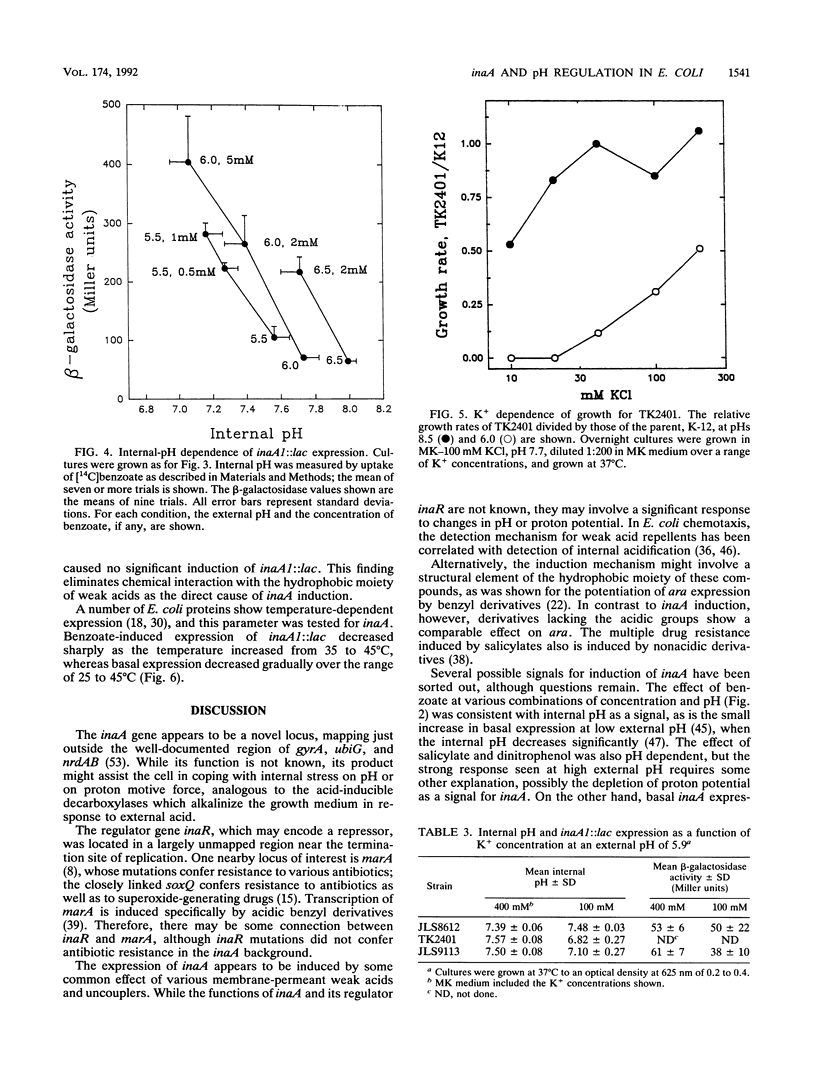
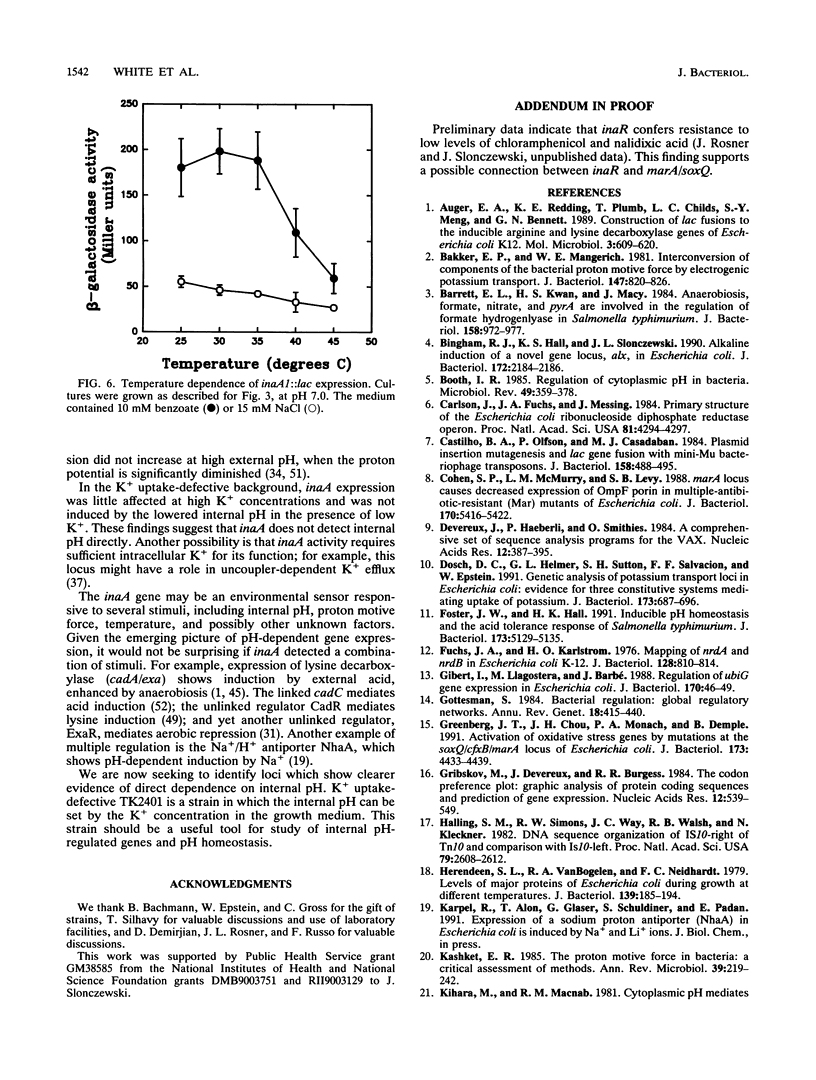
The weak-acid-inducible locus inaA in Escherichia coli was mapped to 48.6 min by P1 cotransduction of inaA Mud lac fusions and linked Tn10 insertions. The inaA1::lac fusion tested negative for phenotypes characteristic of mutations in the nearby locus ubiG. Sequence analysis of a fragment amplified by polymerase chain reaction located the inaA1::lac fusion joint within an open reading frame 311 nucleotides downstream of nrdB, transcribed in the opposite direction, encoding a 168-amino-acid polypeptide. Constitutive mutant strains identified on lactose MacConkey revealed a novel regulatory locus unlinked to inaA, which mapped at 34 min (designated inaR). Expression of inaA1::lac increased slightly with external acidification; the presence of benzoate, a membrane-permeant weak acid, greatly increased the acid effect. The expression at various combinations of benzoate and external pH correlated with the decrease in intracellular pH. The uncouplers salicylate and dinitrophenol also caused acid-dependent induction of inaA, but substantial induction was seen at external pH values higher than the internal pH; this effect cannot be caused by internal acidification. Nondissociating analogs of benzoate and salicylate, benzyl alcohol and salicyl alcohol, did not induce inaA. Expression of inaA was inversely related to growth temperature over the range of 30 to 45 degrees C. The inaA1::lac fusion was transferred to a strain defective for K+ uptake (kdpABC trkA trkD) in which pH homeostasis was shown to depend on the external K+ concentration. In this construct, inaA1::lac retained pH-dependent induction by benzoate but was not induced at low K+ concentrations. Induction of inaA appears to involve several factors in addition to internal pH. inaR may be related to the nearby locus marA/soxQ, which is inducible by acidic benzyl derivatives.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auger E. A., Redding K. E., Plumb T., Childs L. C., Meng S. Y., Bennett G. N. Construction of lac fusions to the inducible arginine- and lysine decarboxylase genes of Escherichia coli K12. Mol Microbiol. 1989 May;3(5):609–620. doi: 10.1111/j.1365-2958.1989.tb00208.x. [DOI] [PubMed] [Google Scholar]
- Bakker E. P., Mangerich W. E. Interconversion of components of the bacterial proton motive force by electrogenic potassium transport. J Bacteriol. 1981 Sep;147(3):820–826. doi: 10.1128/jb.147.3.820-826.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett E. L., Kwan H. S., Macy J. Anaerobiosis, formate, nitrate, and pyrA are involved in the regulation of formate hydrogenlyase in Salmonella typhimurium. J Bacteriol. 1984 Jun;158(3):972–977. doi: 10.1128/jb.158.3.972-977.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham R. J., Hall K. S., Slonczewski J. L. Alkaline induction of a novel gene locus, alx, in Escherichia coli. J Bacteriol. 1990 Apr;172(4):2184–2186. doi: 10.1128/jb.172.4.2184-2186.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth I. R. Regulation of cytoplasmic pH in bacteria. Microbiol Rev. 1985 Dec;49(4):359–378. doi: 10.1128/mr.49.4.359-378.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson J., Fuchs J. A., Messing J. Primary structure of the Escherichia coli ribonucleoside diphosphate reductase operon. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4294–4297. doi: 10.1073/pnas.81.14.4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castilho B. A., Olfson P., Casadaban M. J. Plasmid insertion mutagenesis and lac gene fusion with mini-mu bacteriophage transposons. J Bacteriol. 1984 May;158(2):488–495. doi: 10.1128/jb.158.2.488-495.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. P., McMurry L. M., Levy S. B. marA locus causes decreased expression of OmpF porin in multiple-antibiotic-resistant (Mar) mutants of Escherichia coli. J Bacteriol. 1988 Dec;170(12):5416–5422. doi: 10.1128/jb.170.12.5416-5422.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosch D. C., Helmer G. L., Sutton S. H., Salvacion F. F., Epstein W. Genetic analysis of potassium transport loci in Escherichia coli: evidence for three constitutive systems mediating uptake potassium. J Bacteriol. 1991 Jan;173(2):687–696. doi: 10.1128/jb.173.2.687-696.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J. W., Hall H. K. Inducible pH homeostasis and the acid tolerance response of Salmonella typhimurium. J Bacteriol. 1991 Aug;173(16):5129–5135. doi: 10.1128/jb.173.16.5129-5135.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs J. A., Karlström H. O. Mapping of nrdA and nrdB in Escherichia coli K-12. J Bacteriol. 1976 Dec;128(3):810–814. doi: 10.1128/jb.128.3.810-814.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman S. Bacterial regulation: global regulatory networks. Annu Rev Genet. 1984;18:415–441. doi: 10.1146/annurev.ge.18.120184.002215. [DOI] [PubMed] [Google Scholar]
- Greenberg J. T., Chou J. H., Monach P. A., Demple B. Activation of oxidative stress genes by mutations at the soxQ/cfxB/marA locus of Escherichia coli. J Bacteriol. 1991 Jul;173(14):4433–4439. doi: 10.1128/jb.173.14.4433-4439.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribskov M., Devereux J., Burgess R. R. The codon preference plot: graphic analysis of protein coding sequences and prediction of gene expression. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):539–549. doi: 10.1093/nar/12.1part2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halling S. M., Simons R. W., Way J. C., Walsh R. B., Kleckner N. DNA sequence organization of IS10-right of Tn10 and comparison with IS10-left. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2608–2612. doi: 10.1073/pnas.79.8.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herendeen S. L., VanBogelen R. A., Neidhardt F. C. Levels of major proteins of Escherichia coli during growth at different temperatures. J Bacteriol. 1979 Jul;139(1):185–194. doi: 10.1128/jb.139.1.185-194.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashket E. R. The proton motive force in bacteria: a critical assessment of methods. Annu Rev Microbiol. 1985;39:219–242. doi: 10.1146/annurev.mi.39.100185.001251. [DOI] [PubMed] [Google Scholar]
- Kihara M., Macnab R. M. Cytoplasmic pH mediates pH taxis and weak-acid repellent taxis of bacteria. J Bacteriol. 1981 Mar;145(3):1209–1221. doi: 10.1128/jb.145.3.1209-1221.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline E. L., West R. W., Jr, Ink B. S., Kline P. M., Rodriguez R. L. Benzyl derivative facilitation of transcription in Escherichia coli at the ara and lac operon promoters: metabolite gene regulation (MGR). Mol Gen Genet. 1984;193(2):340–348. doi: 10.1007/BF00330691. [DOI] [PubMed] [Google Scholar]
- Kobayashi H. A proton-translocating ATPase regulates pH of the bacterial cytoplasm. J Biol Chem. 1985 Jan 10;260(1):72–76. [PubMed] [Google Scholar]
- Kobayashi H., Suzuki T., Kinoshita N., Unemoto T. Amplification of the Streptococcus faecalis proton-translocating ATPase by a decrease in cytoplasmic pH. J Bacteriol. 1984 Jun;158(3):1157–1160. doi: 10.1128/jb.158.3.1157-1160.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. S. Alternative dideoxy sequencing of double-stranded DNA by cyclic reactions using Taq polymerase. DNA Cell Biol. 1991 Jan-Feb;10(1):67–73. doi: 10.1089/dna.1991.10.67. [DOI] [PubMed] [Google Scholar]
- Mat-Jan F., Williams C. R., Clark D. P. Anaerobic growth defects resulting from gene fusions affecting succinyl-CoA synthetase in Escherichia coli K12. Mol Gen Genet. 1989 Jan;215(2):276–280. doi: 10.1007/BF00339728. [DOI] [PubMed] [Google Scholar]
- Médigue C., Bouché J. P., Hénaut A., Danchin A. Mapping of sequenced genes (700 kbp) in the restriction map of the Escherichia coli chromosome. Mol Microbiol. 1990 Feb;4(2):169–187. doi: 10.1111/j.1365-2958.1990.tb00585.x. [DOI] [PubMed] [Google Scholar]
- Padan E., Schuldiner S. Intracellular pH and membrane potential as regulators in the prokaryotic cell. J Membr Biol. 1987;95(3):189–198. doi: 10.1007/BF01869481. [DOI] [PubMed] [Google Scholar]
- Padan E., Zilberstein D., Rottenberg H. The proton electrochemical gradient in Escherichia coli cells. Eur J Biochem. 1976 Apr 1;63(2):533–541. doi: 10.1111/j.1432-1033.1976.tb10257.x. [DOI] [PubMed] [Google Scholar]
- Pan J. W., Macnab R. M. Steady-state measurements of Escherichia coli sodium and proton potentials at alkaline pH support the hypothesis of electrogenic antiport. J Biol Chem. 1990 Jun 5;265(16):9247–9250. [PubMed] [Google Scholar]
- Repaske D. R., Adler J. Change in intracellular pH of Escherichia coli mediates the chemotactic response to certain attractants and repellents. J Bacteriol. 1981 Mar;145(3):1196–1208. doi: 10.1128/jb.145.3.1196-1208.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoads D. B., Waters F. B., Epstein W. Cation transport in Escherichia coli. VIII. Potassium transport mutants. J Gen Physiol. 1976 Mar;67(3):325–341. doi: 10.1085/jgp.67.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner J. L., Chai T. J., Foulds J. Regulation of ompF porin expression by salicylate in Escherichia coli. J Bacteriol. 1991 Sep;173(18):5631–5638. doi: 10.1128/jb.173.18.5631-5638.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner J. L. Nonheritable resistance to chloramphenicol and other antibiotics induced by salicylates and other chemotactic repellents in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8771–8774. doi: 10.1073/pnas.82.24.8771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmond C. V., Kroll R. G., Booth I. R. The effect of food preservatives on pH homeostasis in Escherichia coli. J Gen Microbiol. 1984 Nov;130(11):2845–2850. doi: 10.1099/00221287-130-11-2845. [DOI] [PubMed] [Google Scholar]
- Singer M., Baker T. A., Schnitzler G., Deischel S. M., Goel M., Dove W., Jaacks K. J., Grossman A. D., Erickson J. W., Gross C. A. A collection of strains containing genetically linked alternating antibiotic resistance elements for genetic mapping of Escherichia coli. Microbiol Rev. 1989 Mar;53(1):1–24. doi: 10.1128/mr.53.1.1-24.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slonczewski J. L., Gonzalez T. N., Bartholomew F. M., Holt N. J. Mu d-directed lacZ fusions regulated by low pH in Escherichia coli. J Bacteriol. 1987 Jul;169(7):3001–3006. doi: 10.1128/jb.169.7.3001-3006.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slonczewski J. L., Macnab R. M., Alger J. R., Castle A. M. Effects of pH and repellent tactic stimuli on protein methylation levels in Escherichia coli. J Bacteriol. 1982 Oct;152(1):384–399. doi: 10.1128/jb.152.1.384-399.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slonczewski J. L., Rosen B. P., Alger J. R., Macnab R. M. pH homeostasis in Escherichia coli: measurement by 31P nuclear magnetic resonance of methylphosphonate and phosphate. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6271–6275. doi: 10.1073/pnas.78.10.6271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock J. B., Stock A. M., Mottonen J. M. Signal transduction in bacteria. Nature. 1990 Mar 29;344(6265):395–400. doi: 10.1038/344395a0. [DOI] [PubMed] [Google Scholar]
- Tabor H., Hafner E. W., Tabor C. W. Construction of an Escherichia coli strain unable to synthesize putrescine, spermidine, or cadaverine: characterization of two genes controlling lysine decarboxylase. J Bacteriol. 1980 Dec;144(3):952–956. doi: 10.1128/jb.144.3.952-956.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson N., Dunyak D. S., Rosey E. L., Slonczewski J. L., Olson E. R. Identification of elements involved in transcriptional regulation of the Escherichia coli cad operon by external pH. J Bacteriol. 1992 Jan;174(2):530–540. doi: 10.1128/jb.174.2.530-540.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb G., Rohatgi K., Courtright J. B. Location of gyrA on the physical map of the Escherichia coli chromosome. J Bacteriol. 1990 Dec;172(12):6617–6617. doi: 10.1128/jb.172.12.6617.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberstein D., Agmon V., Schuldiner S., Padan E. Escherichia coli intracellular pH, membrane potential, and cell growth. J Bacteriol. 1984 Apr;158(1):246–252. doi: 10.1128/jb.158.1.246-252.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberstein D., Agmon V., Schuldiner S., Padan E. The sodium/proton antiporter is part of the pH homeostasis mechanism in Escherichia coli. J Biol Chem. 1982 Apr 10;257(7):3687–3691. [PubMed] [Google Scholar]



