Abstract
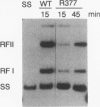
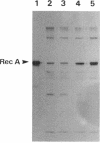
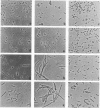
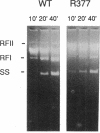
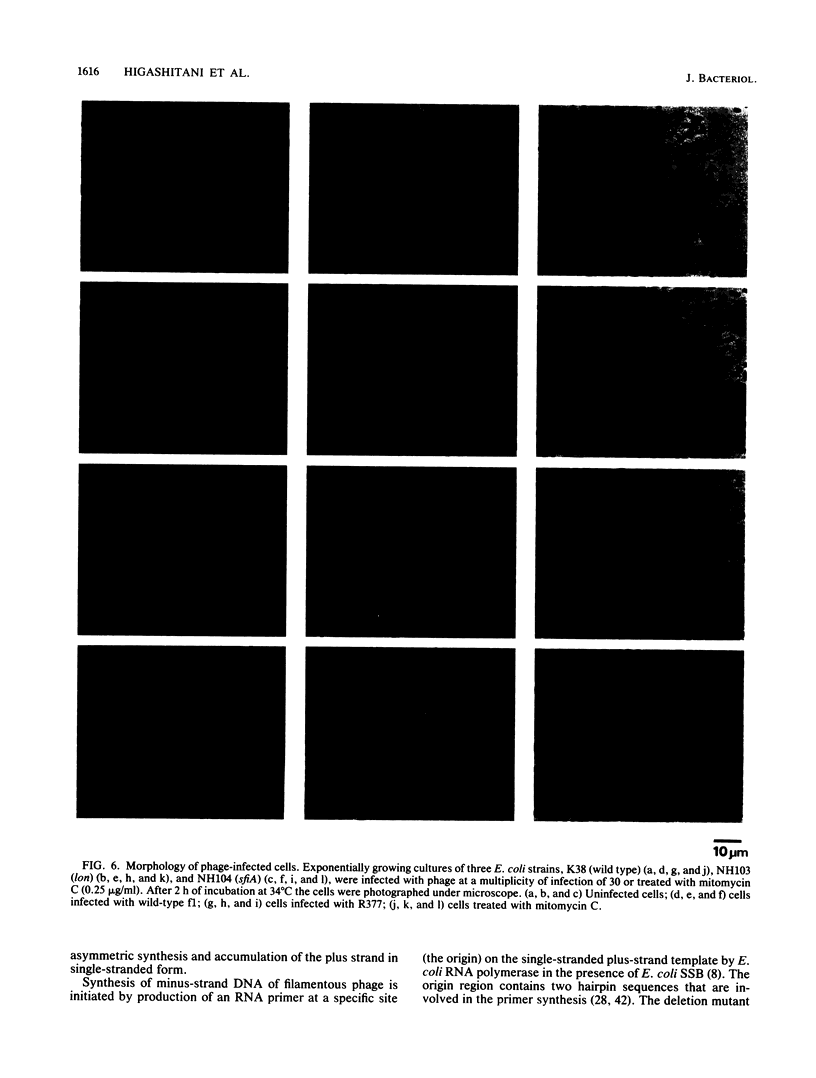
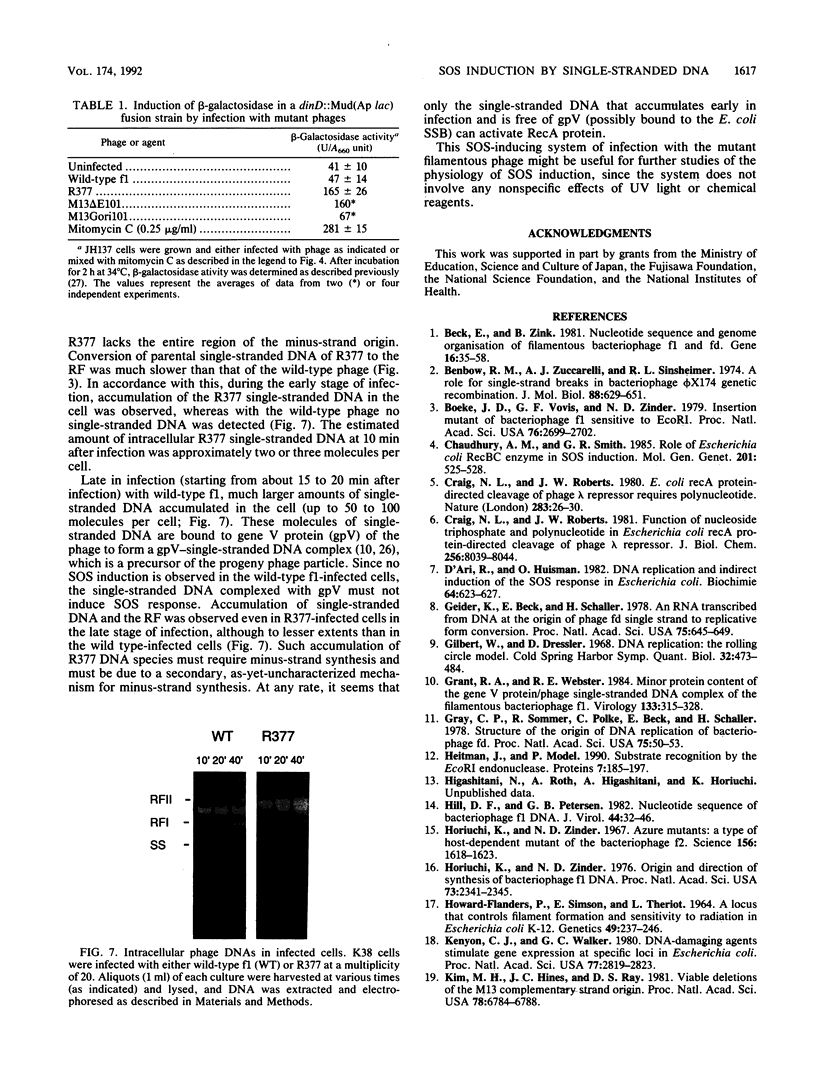
We report that the SOS response is induced in Escherichia coli by infection with mutant filamentous phage that are defective in initiation of the complementary (minus)-strand synthesis. One such mutant, R377, which lacks the entire region of the minus-strand origin, failed to synthesize any detectable amount of primer RNA for minus-strand synthesis. In addition, the rate of conversion of parental single-stranded DNA of the mutant to the double-stranded replicative form in infected cells was extremely slow. Upon infection, R377 induced the SOS response in the cell, whereas the wild-type phage did not. The SOS induction was monitored by (i) induction of beta-galactosidase in a strain carrying a dinD::lacZ fusion and (ii) increased levels of RecA protein. In addition, cells infected with R377 formed filaments. Another deletion mutant of the minus-strand origin, M13 delta E101 (M. H. Kim, J. C. Hines, and D. S. Ray, Proc. Natl. Acad. Sci. USA 78:6784-6788, 1981), also induced the SOS response in E. coli. M13Gori101 (D. S. Ray, J. C. Hines, M. H. Kim, R. Imber, and N. Nomura, Gene 18:231-238, 1982), which is a derivative of M13 delta E101 carrying the primase-dependent minus-strand origin of phage G4, did not induce the SOS response. These observations indicate that single-stranded DNA by itself induces the SOS response in vivo.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck E., Zink B. Nucleotide sequence and genome organisation of filamentous bacteriophages fl and fd. Gene. 1981 Dec;16(1-3):35–58. doi: 10.1016/0378-1119(81)90059-7. [DOI] [PubMed] [Google Scholar]
- Benbow R. M., Zuccarelli A. J., Sinsheimer R. L. A role for single-strand breaks in bacteriophage phi-X174 genetic recombination. J Mol Biol. 1974 Sep 25;88(3):629–651. doi: 10.1016/0022-2836(74)90414-8. [DOI] [PubMed] [Google Scholar]
- Boeke J. D., Vovis G. F., Zinder N. D. Insertion mutant of bacteriophage f1 sensitive to EcoRI. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2699–2702. doi: 10.1073/pnas.76.6.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhury A. M., Smith G. R. Role of Escherichia coli RecBC enzyme in SOS induction. Mol Gen Genet. 1985;201(3):525–528. doi: 10.1007/BF00331350. [DOI] [PubMed] [Google Scholar]
- Craig N. L., Roberts J. W. E. coli recA protein-directed cleavage of phage lambda repressor requires polynucleotide. Nature. 1980 Jan 3;283(5742):26–30. doi: 10.1038/283026a0. [DOI] [PubMed] [Google Scholar]
- Craig N. L., Roberts J. W. Function of nucleoside triphosphate and polynucleotide in Escherichia coli recA protein-directed cleavage of phage lambda repressor. J Biol Chem. 1981 Aug 10;256(15):8039–8044. [PubMed] [Google Scholar]
- D'Ari R., Huisman O. DNA replication and indirect induction of the SOS response in Escherichia coli. Biochimie. 1982 Aug-Sep;64(8-9):623–627. doi: 10.1016/s0300-9084(82)80100-4. [DOI] [PubMed] [Google Scholar]
- Geider K., Beck E., Schaller H. An RNA transcribed from DNA at the origin of phage fd single strand to replicative form conversion. Proc Natl Acad Sci U S A. 1978 Feb;75(2):645–649. doi: 10.1073/pnas.75.2.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert W., Dressler D. DNA replication: the rolling circle model. Cold Spring Harb Symp Quant Biol. 1968;33:473–484. doi: 10.1101/sqb.1968.033.01.055. [DOI] [PubMed] [Google Scholar]
- Grant R. A., Webster R. E. Minor protein content of the gene V protein/phage single-stranded DNA complex of the filamentous bacteriophage f1. Virology. 1984 Mar;133(2):315–328. doi: 10.1016/0042-6822(84)90398-2. [DOI] [PubMed] [Google Scholar]
- Gray C. P., Sommer R., Polke C., Beck E., Schaller H. Structure of the orgin of DNA replication of bacteriophage fd. Proc Natl Acad Sci U S A. 1978 Jan;75(1):50–53. doi: 10.1073/pnas.75.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWARD-FLANDERS P., SIMSON E., THERIOT L. A LOCUS THAT CONTROLS FILAMENT FORMATION AND SENSITIVITY TO RADIATION IN ESCHERICHIA COLI K-12. Genetics. 1964 Feb;49:237–246. doi: 10.1093/genetics/49.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitman J., Model P. Substrate recognition by the EcoRI endonuclease. Proteins. 1990;7(2):185–197. doi: 10.1002/prot.340070207. [DOI] [PubMed] [Google Scholar]
- Hill D. F., Petersen G. B. Nucleotide sequence of bacteriophage f1 DNA. J Virol. 1982 Oct;44(1):32–46. doi: 10.1128/jvi.44.1.32-46.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi K., Zinder N. D. Azure mutants: a type of host-dependent mutant of the bacteriophage f2. Science. 1967 Jun 23;156(3782):1618–1623. doi: 10.1126/science.156.3782.1618. [DOI] [PubMed] [Google Scholar]
- Horiuchi K., Zinder N. D. Origin and direction of synthesis of bacteriophage fl DNA. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2341–2345. doi: 10.1073/pnas.73.7.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon C. J., Walker G. C. DNA-damaging agents stimulate gene expression at specific loci in Escherichia coli. Proc Natl Acad Sci U S A. 1980 May;77(5):2819–2823. doi: 10.1073/pnas.77.5.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M. H., Hines J. C., Ray D. S. Viable deletions of the M13 complementary strand origin. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6784–6788. doi: 10.1073/pnas.78.11.6784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lu C., Echols H. RecA protein and SOS. Correlation of mutagenesis phenotype with binding of mutant RecA proteins to duplex DNA and LexA cleavage. J Mol Biol. 1987 Aug 5;196(3):497–504. doi: 10.1016/0022-2836(87)90027-1. [DOI] [PubMed] [Google Scholar]
- Marvin D. A., Hohn B. Filamentous bacterial viruses. Bacteriol Rev. 1969 Jun;33(2):172–209. doi: 10.1128/br.33.2.172-209.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur B. J., Model P. Regulation of coliphage f1 single-stranded DNA synthesis by a DNA-binding protein. J Mol Biol. 1973 Aug 5;78(2):285–300. doi: 10.1016/0022-2836(73)90117-4. [DOI] [PubMed] [Google Scholar]
- Mazur B. J., Zinder N. D. The role of gene V protein in f1 single-strand synthesis. Virology. 1975 Dec;68(2):490–502. doi: 10.1016/0042-6822(75)90289-5. [DOI] [PubMed] [Google Scholar]
- Moses P. B., Horiuchi K. Effects of transposition and deletion upon coat protein gene expression in bacteriophage f1. Virology. 1982 Jun;119(2):231–244. doi: 10.1016/0042-6822(82)90084-8. [DOI] [PubMed] [Google Scholar]
- Nomura N., Ray D. S. Expression of a DNA strand initiation sequence of ColE1 plasmid in a single-stranded DNA phage. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6566–6570. doi: 10.1073/pnas.77.11.6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray D. S., Hines J. C., Kim M. H., Imber R., Nomura N. M13 vectors for selective cloning of sequences specifying initiation of DNA synthesis on single-stranded templates. Gene. 1982 Jun;18(3):231–238. doi: 10.1016/0378-1119(82)90160-3. [DOI] [PubMed] [Google Scholar]
- Roberts J. W., Roberts C. W. Proteolytic cleavage of bacteriophage lambda repressor in induction. Proc Natl Acad Sci U S A. 1975 Jan;72(1):147–151. doi: 10.1073/pnas.72.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salles B., Defais M. Signal of induction of recA protein in E. coli. Mutat Res. 1984 Feb;131(2):53–59. doi: 10.1016/0167-8817(84)90011-7. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller H., Uhlmann A., Geider K. A DNA fragment from the origin of single-strand to double-strand DNA replication of bacteriophage fd. Proc Natl Acad Sci U S A. 1976 Jan;73(1):49–53. doi: 10.1073/pnas.73.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. L., Oishi M. Early events and mechanisms in the induction of bacterial SOS functions: analysis of the phage repressor inactivation process in vivo. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1657–1661. doi: 10.1073/pnas.75.4.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner W., Brutlag D., Schekman R., Kornberg A. RNA synthesis initiates in vitro conversion of M13 DNA to its replicative form. Proc Natl Acad Sci U S A. 1972 Apr;69(4):965–969. doi: 10.1073/pnas.69.4.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinder N. D., Boeke J. D. The filamentous phage (Ff) as vectors for recombinant DNA--a review. Gene. 1982 Jul-Aug;19(1):1–10. doi: 10.1016/0378-1119(82)90183-4. [DOI] [PubMed] [Google Scholar]
- Zinder N. D., Horiuchi K. Multiregulatory element of filamentous bacteriophages. Microbiol Rev. 1985 Jun;49(2):101–106. doi: 10.1128/mr.49.2.101-106.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]