Abstract
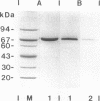
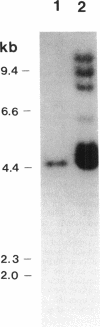
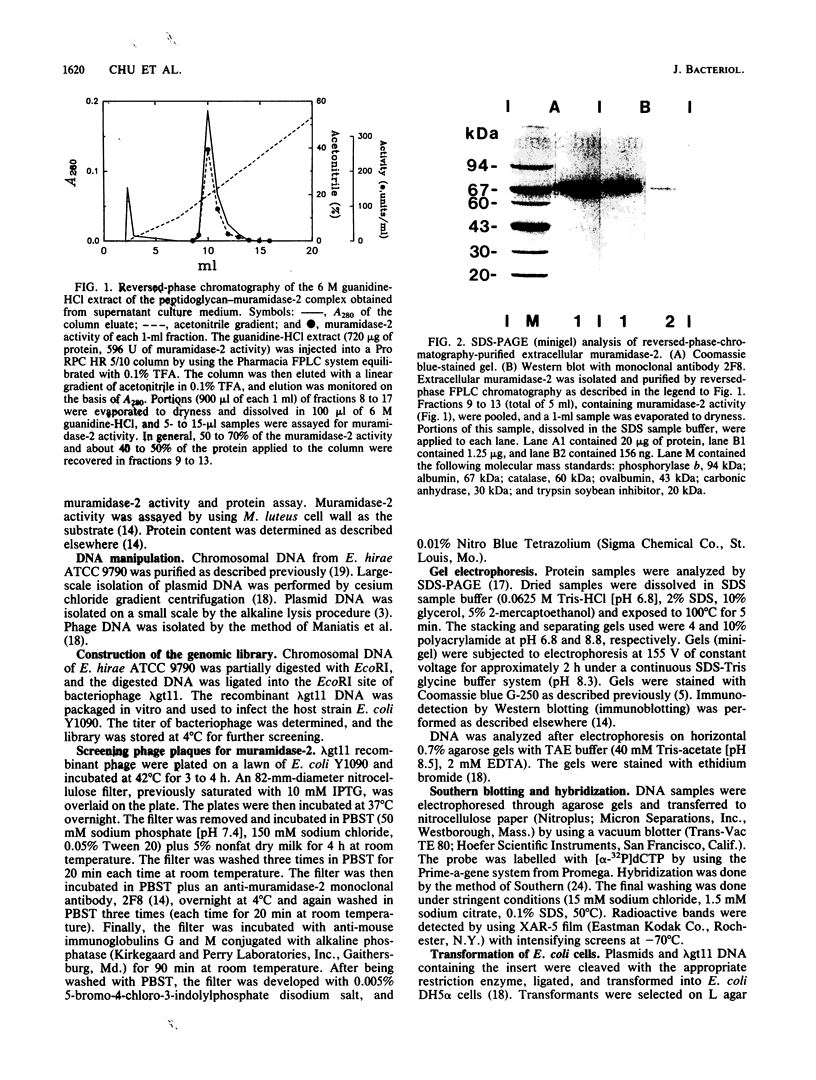
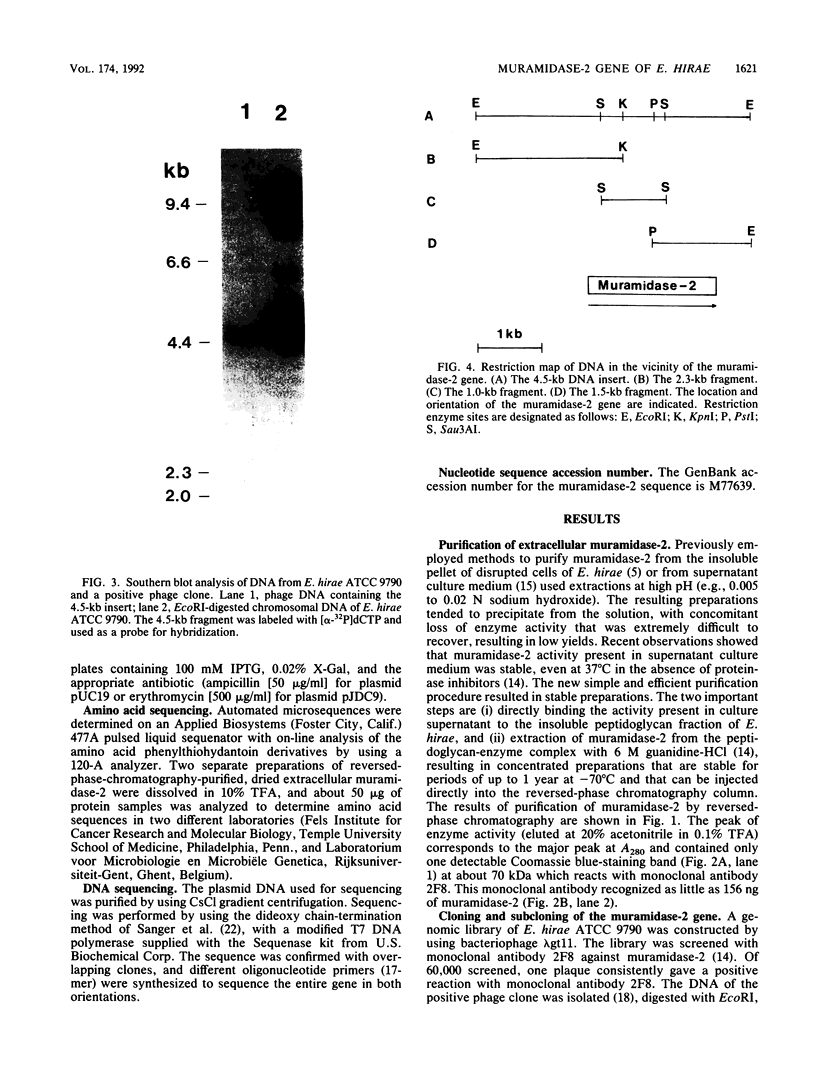
Extracellular muramidase-2 of Enterococcus hirae ATCC 9790 was purified to homogeneity by substrate binding, guanidine-HCl extraction, and reversed-phase chromatography. A monoclonal antibody, 2F8, which specifically recognizes muramidase-2, was used to screen a genomic library of E. hirae ATCC 9790 DNA in bacteriophage lambda gt11. A positive phage clone containing a 4.5-kb DNA insert was isolated and analyzed. The EcoRI-digested 4.5-kb fragment was cut into 2.3-, 1.0-, and 1.5-kb pieces by using restriction enzymes KpnI, Sau3AI, and PstI, and each fragment was subcloned into plasmid pJDC9 or pUC19. The nucleotide sequence of each subclone was determined. The sequence data indicated an open reading frame encoding a polypeptide of 666 amino acid residues, with a calculated molecular mass of 70,678 Da. The first 24 N-terminal amino acids of purified extracellular muramidase-2 were in very good agreement with the deduced amino acid sequence after a 49-amino-acid putative signal sequence. Analysis of the deduced amino acid sequence showed the presence at the C-terminal region of the protein of six highly homologous repeat units separated by nonhomologous intervening sequences that are highly enriched in serine and threonine. The overall sequence showed a high degree of homology with a recently cloned Streptococcus faecalis autolysin.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banas J. A., Russell R. R., Ferretti J. J. Sequence analysis of the gene for the glucan-binding protein of Streptococcus mutans Ingbritt. Infect Immun. 1990 Mar;58(3):667–673. doi: 10.1128/iai.58.3.667-673.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béliveau C., Potvin C., Trudel J., Asselin A., Bellemare G. Cloning, sequencing, and expression in Escherichia coli of a Streptococcus faecalis autolysin. J Bacteriol. 1991 Sep;173(18):5619–5623. doi: 10.1128/jb.173.18.5619-5623.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornett J. B., Johnson C. A., Shockman G. D. Release of autolytic enzyme from Streptococcus, faecium cell walls by treatment with dilute alkali. J Bacteriol. 1979 Jun;138(3):699–704. doi: 10.1128/jb.138.3.699-704.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolinger D. L., Daneo-Moore L., Shockman G. D. The second peptidoglycan hydrolase of Streptococcus faecium ATCC 9790 covalently binds penicillin. J Bacteriol. 1989 Aug;171(8):4355–4361. doi: 10.1128/jb.171.8.4355-4361.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolinger D. L., Schramm V. L., Shockman G. D. Covalent modification of the beta-1,4-N-acetylmuramoylhydrolase of Streptococcus faecium with 5-mercaptouridine monophosphate. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6667–6671. doi: 10.1073/pnas.85.18.6667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahnestock S. R., Alexander P., Nagle J., Filpula D. Gene for an immunoglobulin-binding protein from a group G streptococcus. J Bacteriol. 1986 Sep;167(3):870–880. doi: 10.1128/jb.167.3.870-880.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García E., García J. L., García P., Arrarás A., Sánchez-Puelles J. M., López R. Molecular evolution of lytic enzymes of Streptococcus pneumoniae and its bacteriophages. Proc Natl Acad Sci U S A. 1988 Feb;85(3):914–918. doi: 10.1073/pnas.85.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley C. B., Reynolds R. P. Analysis of E. coli promoter sequences. Nucleic Acids Res. 1987 Mar 11;15(5):2343–2361. doi: 10.1093/nar/15.5.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich P., Rosenstein R., Böhmer M., Sonner P., Götz F. The molecular organization of the lysostaphin gene and its sequences repeated in tandem. Mol Gen Genet. 1987 Oct;209(3):563–569. doi: 10.1007/BF00331163. [DOI] [PubMed] [Google Scholar]
- Hollingshead S. K., Fischetti V. A., Scott J. R. Complete nucleotide sequence of type 6 M protein of the group A Streptococcus. Repetitive structure and membrane anchor. J Biol Chem. 1986 Feb 5;261(4):1677–1686. [PubMed] [Google Scholar]
- Kawamura T., Shockman G. D. Purification and some properties of the endogenous, autolytic N-acetylmuramoylhydrolase of Streptococcus faecium, a bacterial glycoenzyme. J Biol Chem. 1983 Aug 10;258(15):9514–9521. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Procino J. K., Marri L., Shockman G. D., Daneo-Moore L. Tn916 insertional inactivation of multiple genes on the chromosome of Streptococcus mutans GS-5. Infect Immun. 1988 Nov;56(11):2866–2870. doi: 10.1128/iai.56.11.2866-2870.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recsei P. A., Gruss A. D., Novick R. P. Cloning, sequence, and expression of the lysostaphin gene from Staphylococcus simulans. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1127–1131. doi: 10.1073/pnas.84.5.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Uhlén M., Guss B., Nilsson B., Gatenbeck S., Philipson L., Lindberg M. Complete sequence of the staphylococcal gene encoding protein A. A gene evolved through multiple duplications. J Biol Chem. 1984 Feb 10;259(3):1695–1702. [PubMed] [Google Scholar]
- Vlasuk G. P., Inouye S., Ito H., Itakura K., Inouye M. Effects of the complete removal of basic amino acid residues from the signal peptide on secretion of lipoprotein in Escherichia coli. J Biol Chem. 1983 Jun 10;258(11):7141–7148. [PubMed] [Google Scholar]
- Wandersman C. Secretion, processing and activation of bacterial extracellular proteases. Mol Microbiol. 1989 Dec;3(12):1825–1831. doi: 10.1111/j.1365-2958.1989.tb00169.x. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Patterns of amino acids near signal-sequence cleavage sites. Eur J Biochem. 1983 Jun 1;133(1):17–21. doi: 10.1111/j.1432-1033.1983.tb07424.x. [DOI] [PubMed] [Google Scholar]