Abstract
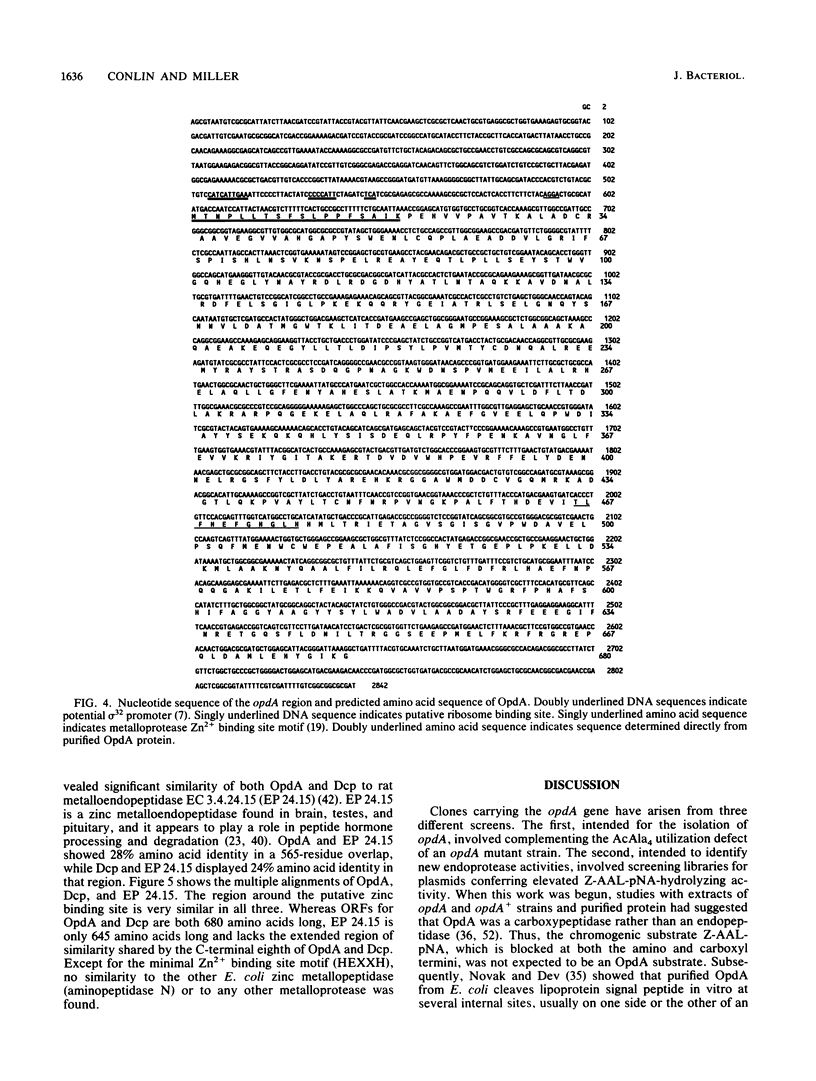
The opdA gene (formerly called optA) of Salmonella typhimurium encodes a metallopeptidase, oligopeptidase A (OpdA), first recognized by its ability to cleave and allow utilization of N-acetyl-L-Ala4 (E. R. Vimr, L. Green, and C. G. Miller, J. Bacteriol. 153:1259-1265, 1983). Derivatives of pBR328 carrying the opdA gene were isolated and shown to express oligopeptidase activity at levels approximately 100-fold higher than that of the wild type. These plasmids complemented all of the phenotypes associated with opdA mutations (failure to use N-acetyl-L-Ala4, defective phage P22 development, and diminished endopeptidase activity). The opdA region of one of these plasmids (pCM127) was defined by insertions of Tn1000 (gamma delta), and these insertions were used as priming sites to determine the nucleotide sequence of a 2,843-bp segment of the insert DNA. This region contained an open reading frame coding for a 680-amino-acid protein, the N terminus of which agreed with that determined for purified OpdA. This open reading frame contained both a sequence motif typical of Zn2+ metalloproteases and a putative sigma 32 promoter. However, no induction was detected upon temperature shift by using a beta-galactosidase operon fusion. The predicted OpdA sequence showed similarity to dipeptidyl carboxypeptidase, the product of the S. typhimurium gene dcp, and to rat metallopeptidase EC 3.4.24.15., which is involved in peptide hormone processing.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Affholter J. A., Fried V. A., Roth R. A. Human insulin-degrading enzyme shares structural and functional homologies with E. coli protease III. Science. 1988 Dec 9;242(4884):1415–1418. doi: 10.1126/science.3059494. [DOI] [PubMed] [Google Scholar]
- Carter T. H., Miller C. G. Aspartate-specific peptidases in Salmonella typhimurium: mutants deficient in peptidase E. J Bacteriol. 1984 Aug;159(2):453–459. doi: 10.1128/jb.159.2.453-459.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castilho B. A., Olfson P., Casadaban M. J. Plasmid insertion mutagenesis and lac gene fusion with mini-mu bacteriophage transposons. J Bacteriol. 1984 May;158(2):488–495. doi: 10.1128/jb.158.2.488-495.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Chung C. H., Goldberg A. L. Purification and characterization of protease So, a cytoplasmic serine protease in Escherichia coli. J Bacteriol. 1983 Apr;154(1):231–238. doi: 10.1128/jb.154.1.231-238.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowing D. W., Bardwell J. C., Craig E. A., Woolford C., Hendrix R. W., Gross C. A. Consensus sequence for Escherichia coli heat shock gene promoters. Proc Natl Acad Sci U S A. 1985 May;82(9):2679–2683. doi: 10.1073/pnas.82.9.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott T., Roth J. R. Characterization of Tn10d-Cam: a transposition-defective Tn10 specifying chloramphenicol resistance. Mol Gen Genet. 1988 Aug;213(2-3):332–338. doi: 10.1007/BF00339599. [DOI] [PubMed] [Google Scholar]
- Goff S. A., Casson L. P., Goldberg A. L. Heat shock regulatory gene htpR influences rates of protein degradation and expression of the lon gene in Escherichia coli. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6647–6651. doi: 10.1073/pnas.81.21.6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutnick D., Calvo J. M., Klopotowski T., Ames B. N. Compounds which serve as the sole source of carbon or nitrogen for Salmonella typhimurium LT-2. J Bacteriol. 1969 Oct;100(1):215–219. doi: 10.1128/jb.100.1.215-219.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer M. S. Uses of the transposon gamma delta in the analysis of cloned genes. Methods Enzymol. 1983;101:362–369. doi: 10.1016/0076-6879(83)01027-7. [DOI] [PubMed] [Google Scholar]
- Hamilton S., Miller C. G. Cloning and nucleotide sequence of the Salmonella typhimurium dcp gene encoding dipeptidyl carboxypeptidase. J Bacteriol. 1992 Mar;174(5):1626–1630. doi: 10.1128/jb.174.5.1626-1630.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayman S., Gatmaitan J. S., Patterson E. K. The relationship of extrinsic and intrinsic metal ions to the specificity of a dipeptidase from Escherichia coli B. Biochemistry. 1974 Oct 22;13(22):4486–4494. doi: 10.1021/bi00719a003. [DOI] [PubMed] [Google Scholar]
- Heiman C., Miller C. G. Salmonella typhimurium mutants lacking protease II. J Bacteriol. 1978 Aug;135(2):588–594. doi: 10.1128/jb.135.2.588-594.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hmiel S. P., Snavely M. D., Florer J. B., Maguire M. E., Miller C. G. Magnesium transport in Salmonella typhimurium: genetic characterization and cloning of three magnesium transport loci. J Bacteriol. 1989 Sep;171(9):4742–4751. doi: 10.1128/jb.171.9.4742-4751.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hmiel S. P., Snavely M. D., Miller C. G., Maguire M. E. Magnesium transport in Salmonella typhimurium: characterization of magnesium influx and cloning of a transport gene. J Bacteriol. 1986 Dec;168(3):1444–1450. doi: 10.1128/jb.168.3.1444-1450.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichihara S., Suzuki T., Suzuki M., Mizushima S. Molecular cloning and sequencing of the sppA gene and characterization of the encoded protease IV, a signal peptide peptidase, of Escherichia coli. J Biol Chem. 1986 Jul 15;261(20):9405–9411. [PubMed] [Google Scholar]
- Jongeneel C. V., Bouvier J., Bairoch A. A unique signature identifies a family of zinc-dependent metallopeptidases. FEBS Lett. 1989 Jan 2;242(2):211–214. doi: 10.1016/0014-5793(89)80471-5. [DOI] [PubMed] [Google Scholar]
- Kroh H. E., Simon L. D. The ClpP component of Clp protease is the sigma 32-dependent heat shock protein F21.5. J Bacteriol. 1990 Oct;172(10):6026–6034. doi: 10.1128/jb.172.10.6026-6034.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukral A. M., Strauch K. L., Maurer R. A., Miller C. G. Genetic analysis in Salmonella typhimurium with a small collection of randomly spaced insertions of transposon Tn10 delta 16 delta 17. J Bacteriol. 1987 May;169(5):1787–1793. doi: 10.1128/jb.169.5.1787-1793.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lasdun A., Reznik S., Molineaux C. J., Orlowski M. Inhibition of endopeptidase 24.15 slows the in vivo degradation of luteinizing hormone-releasing hormone. J Pharmacol Exp Ther. 1989 Nov;251(2):439–447. [PubMed] [Google Scholar]
- Lee H. J., LaRue J. N., Wilson I. B. A simple spectrophotometric assay for amino acyl arylamidases (naphthylamidases, aminopeptidases). Anal Biochem. 1971 Jun;41(2):397–401. doi: 10.1016/0003-2697(71)90157-6. [DOI] [PubMed] [Google Scholar]
- Lesley S. A., Jovanovich S. B., Tse-Dinh Y. C., Burgess R. R. Identification of a heat shock promoter in the topA gene of Escherichia coli. J Bacteriol. 1990 Dec;172(12):6871–6874. doi: 10.1128/jb.172.12.6871-6874.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Whalen W., Das A., Berg C. M. Rapid sequencing of cloned DNA using a transposon for bidirectional priming: sequence of the Escherichia coli K-12 avtA gene. Nucleic Acids Res. 1987 Nov 25;15(22):9461–9469. doi: 10.1093/nar/15.22.9461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. G., Green L. Degradation of abnormal proteins in peptidase-deficient mutants of Salmonella typhimurium. J Bacteriol. 1981 Sep;147(3):925–930. doi: 10.1128/jb.147.3.925-930.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. G., Mackinnon K. Peptidase mutants of Salmonella typhimurium. J Bacteriol. 1974 Oct;120(1):355–363. doi: 10.1128/jb.120.1.355-363.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. G., Schwartz G. Peptidase-deficient mutants of Escherichia coli. J Bacteriol. 1978 Aug;135(2):603–611. doi: 10.1128/jb.135.2.603-611.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. G., Strauch K. L., Kukral A. M., Miller J. L., Wingfield P. T., Mazzei G. J., Werlen R. C., Graber P., Movva N. R. N-terminal methionine-specific peptidase in Salmonella typhimurium. Proc Natl Acad Sci U S A. 1987 May;84(9):2718–2722. doi: 10.1073/pnas.84.9.2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahigashi K., Inokuchi H. Nucleotide sequence between the fadB gene and the rrnA operon from Escherichia coli. Nucleic Acids Res. 1990 Nov 11;18(21):6439–6439. doi: 10.1093/nar/18.21.6439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak P., Dev I. K. Degradation of a signal peptide by protease IV and oligopeptidase A. J Bacteriol. 1988 Nov;170(11):5067–5075. doi: 10.1128/jb.170.11.5067-5075.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak P., Ray P. H., Dev I. K. Localization and purification of two enzymes from Escherichia coli capable of hydrolyzing a signal peptide. J Biol Chem. 1986 Jan 5;261(1):420–427. [PubMed] [Google Scholar]
- Ohlsson B. G., Weström B. R., Karlsson B. W. Enzymoblotting: a method for localizing proteinases and their zymogens using para-nitroanilide substrates after agarose gel electrophoresis and transfer to nitrocellulose. Anal Biochem. 1986 Feb 1;152(2):239–244. doi: 10.1016/0003-2697(86)90404-5. [DOI] [PubMed] [Google Scholar]
- Olsen J., Cowell G. M., Kønigshøfer E., Danielsen E. M., Møller J., Laustsen L., Hansen O. C., Welinder K. G., Engberg J., Hunziker W. Complete amino acid sequence of human intestinal aminopeptidase N as deduced from cloned cDNA. FEBS Lett. 1988 Oct 10;238(2):307–314. doi: 10.1016/0014-5793(88)80502-7. [DOI] [PubMed] [Google Scholar]
- Orlowski M., Michaud C., Molineaux C. J. Substrate-related potent inhibitors of brain metalloendopeptidase. Biochemistry. 1988 Jan 26;27(2):597–602. doi: 10.1021/bi00402a015. [DOI] [PubMed] [Google Scholar]
- Orlowski M., Reznik S., Ayala J., Pierotti A. R. Endopeptidase 24.15 from rat testes. Isolation of the enzyme and its specificity toward synthetic and natural peptides, including enkephalin-containing peptides. Biochem J. 1989 Aug 1;261(3):951–958. doi: 10.1042/bj2610951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierotti A., Dong K. W., Glucksman M. J., Orlowski M., Roberts J. L. Molecular cloning and primary structure of rat testes metalloendopeptidase EC 3.4.24.15. Biochemistry. 1990 Nov 13;29(45):10323–10329. doi: 10.1021/bi00497a006. [DOI] [PubMed] [Google Scholar]
- Sancar A., Hack A. M., Rupp W. D. Simple method for identification of plasmid-coded proteins. J Bacteriol. 1979 Jan;137(1):692–693. doi: 10.1128/jb.137.1.692-693.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson K. E., Roth J. R. Linkage map of Salmonella typhimurium, edition VII. Microbiol Rev. 1988 Dec;52(4):485–532. doi: 10.1128/mr.52.4.485-532.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snavely M. D., Florer J. B., Miller C. G., Maguire M. E. Magnesium transport in Salmonella typhimurium: expression of cloned genes for three distinct Mg2+ transport systems. J Bacteriol. 1989 Sep;171(9):4752–4760. doi: 10.1128/jb.171.9.4752-4760.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stirling C. J., Colloms S. D., Collins J. F., Szatmari G., Sherratt D. J. xerB, an Escherichia coli gene required for plasmid ColE1 site-specific recombination, is identical to pepA, encoding aminopeptidase A, a protein with substantial similarity to bovine lens leucine aminopeptidase. EMBO J. 1989 May;8(5):1623–1627. doi: 10.1002/j.1460-2075.1989.tb03547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Vimr E. R., Green L., Miller C. G. Oligopeptidase-deficient mutants of Salmonella typhimurium. J Bacteriol. 1983 Mar;153(3):1259–1265. doi: 10.1128/jb.153.3.1259-1265.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vimr E. R., Miller C. G. Dipeptidyl carboxypeptidase-deficient mutants of Salmonella typhimurium. J Bacteriol. 1983 Mar;153(3):1252–1258. doi: 10.1128/jb.153.3.1252-1258.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada K., Wada Y., Doi H., Ishibashi F., Gojobori T., Ikemura T. Codon usage tabulated from the GenBank genetic sequence data. Nucleic Acids Res. 1991 Apr 25;19 (Suppl):1981–1986. doi: 10.1093/nar/19.suppl.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way J. C., Davis M. A., Morisato D., Roberts D. E., Kleckner N. New Tn10 derivatives for transposon mutagenesis and for construction of lacZ operon fusions by transposition. Gene. 1984 Dec;32(3):369–379. doi: 10.1016/0378-1119(84)90012-x. [DOI] [PubMed] [Google Scholar]
- Whitfield H. J., Levine G. Isolation and characterization of a mutant of Salmonella typhimurium deficient in a major deoxyribonucleic acid polymerase activity. J Bacteriol. 1973 Oct;116(1):54–58. doi: 10.1128/jb.116.1.54-58.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Yano R., Imai M., Yura T. The use of operon fusions in studies of the heat-shock response: effects of altered sigma 32 on heat-shock promoter function in Escherichia coli. Mol Gen Genet. 1987 Apr;207(1):24–28. doi: 10.1007/BF00331486. [DOI] [PubMed] [Google Scholar]
- Yaron A., Mlynar D., Berger A. A dipeptidocarboxypeptidase from E. coli. Biochem Biophys Res Commun. 1972 May 26;47(4):897–902. doi: 10.1016/0006-291x(72)90577-3. [DOI] [PubMed] [Google Scholar]
- Yen C., Green L., Miller C. G. Degradation of intracellular protein in Salmonella typhimurium peptidase mutants. J Mol Biol. 1980 Oct 15;143(1):21–33. doi: 10.1016/0022-2836(80)90122-9. [DOI] [PubMed] [Google Scholar]
- Yen C., Green L., Miller C. G. Peptide accumulation during growth of peptidase deficient mutants. J Mol Biol. 1980 Oct 15;143(1):35–48. doi: 10.1016/0022-2836(80)90123-0. [DOI] [PubMed] [Google Scholar]
- Youderian P., Sugiono P., Brewer K. L., Higgins N. P., Elliott T. Packaging specific segments of the Salmonella chromosome with locked-in Mud-P22 prophages. Genetics. 1988 Apr;118(4):581–592. doi: 10.1093/genetics/118.4.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidenzaig Y., Shaw W. V. Affinity and hydrophobic chromatography of three variants of chloramphenicol acetyltransferases specified by R factors in Escherichia coli. FEBS Lett. 1976 Mar 1;62(3):266–271. doi: 10.1016/0014-5793(76)80072-5. [DOI] [PubMed] [Google Scholar]