Abstract
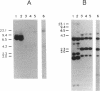
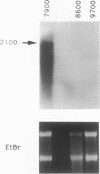
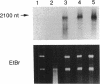
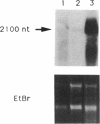
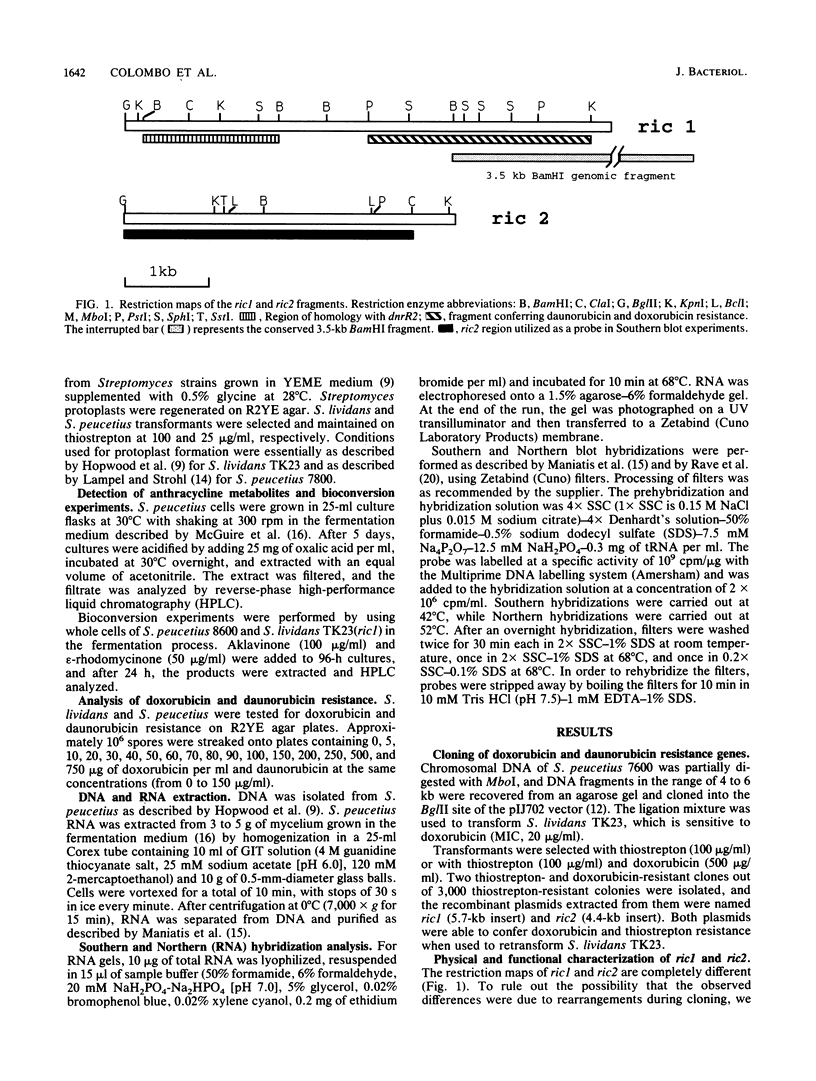
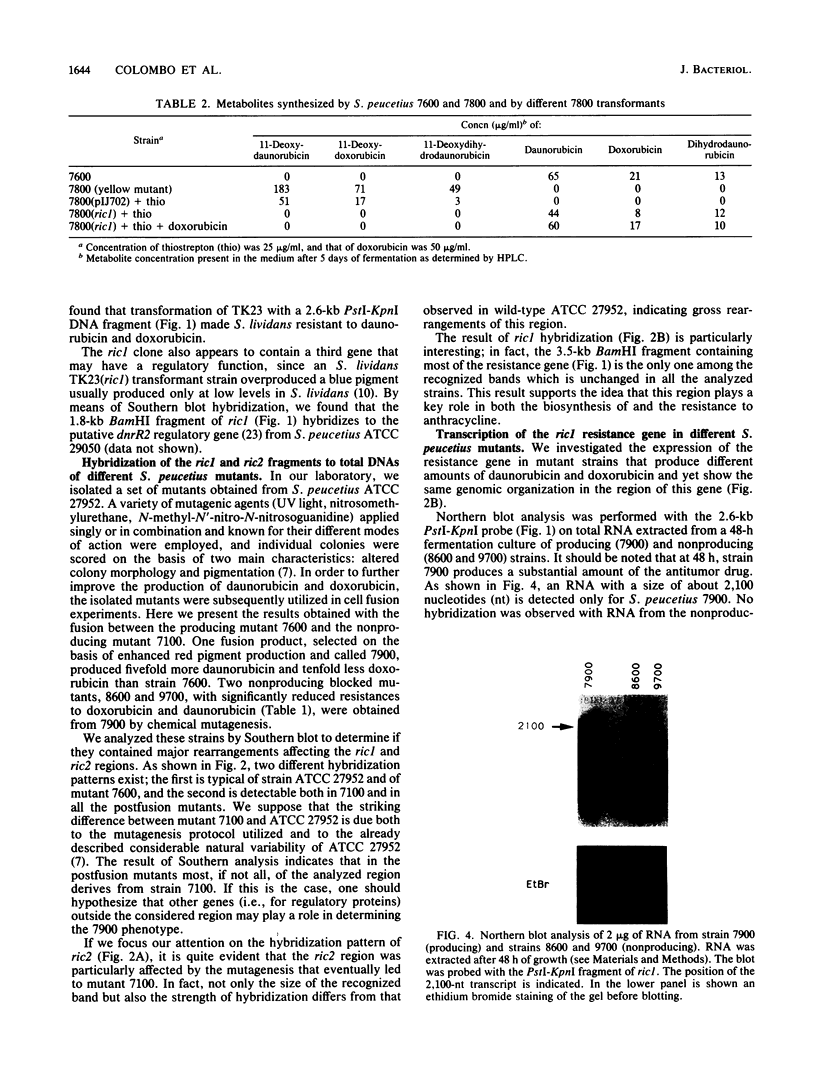
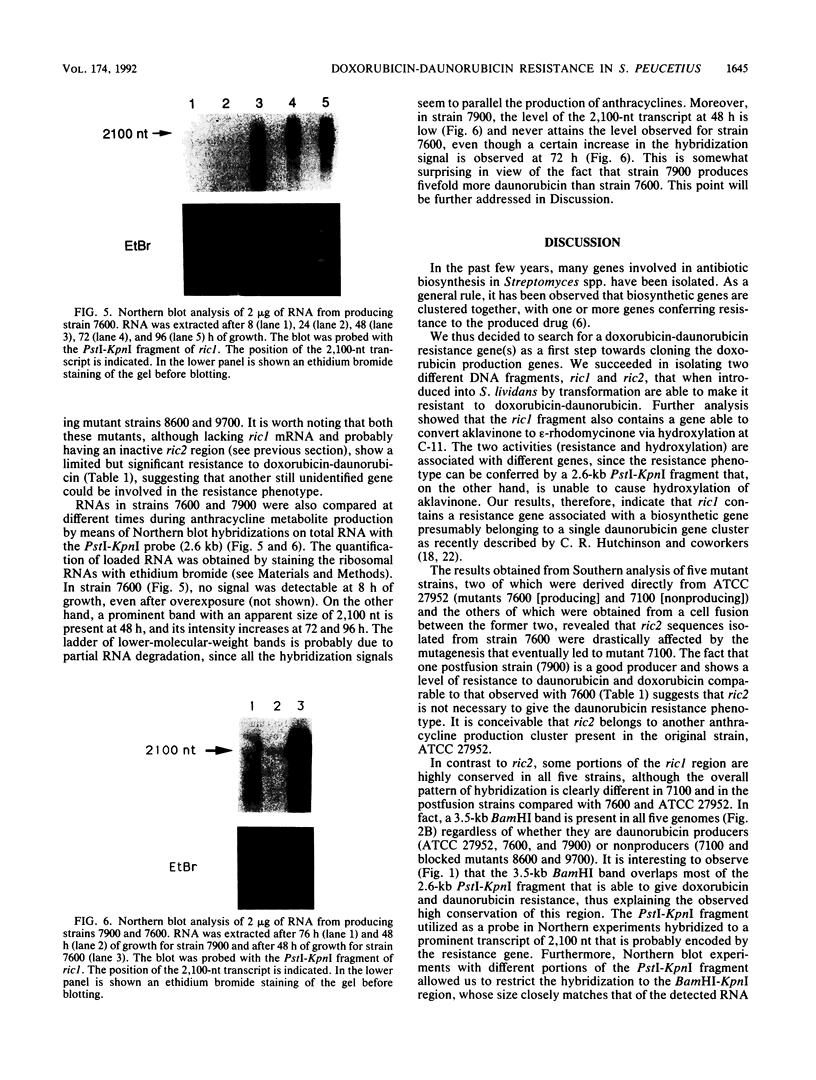
Two DNA fragments, ric1 and ric2, were isolated from the Streptomyces peucetius 7600 mutant, which produces daunorubicin and doxorubicin, on the basis of their abilities to confer doxorubicin and daunorubicin resistance to Streptomyces lividans. These two fragments are unrelated by restriction mapping and do not show any homology by Southern analysis, yet both of them increase the level of resistance 10-fold in transformed S. lividans. Functional analysis revealed that ric1 also contains two genes of daunorubicin biosynthesis: one coding for the aklavinone C-11 hydroxylase and the other corresponding to the putative dnrR2 regulatory gene of wild-type S. peucetius ATCC 29050 (K. J. Stutzman-Engwall, S. L. Otten, and C. R. Hutchinson, J. Bacteriol. 174:144-154, 1992). Northern (RNA) blot experiments, performed with a ric1 fragment containing daunorubicin-doxorubicin resistance gene(s), revealed a transcript of about 2,100 nucleotides that is present only during the phase of anthracycline metabolite production. The amount of this transcript is higher in strain 7600 than in strain 7900, a mutant which produces 5-fold more daunorubicin and 10-fold less doxorubicin than 7600. Furthermore, two 7900-derived blocked mutants, 8600 and 9700, do not express the 2,100-nucleotide transcript in spite of the absence of gross rearrangements in the ric1 region such as occur with the 7900 parental strain.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arcamone F., Cassinelli G., Fantini G., Grein A., Orezzi P., Pol C., Spalla C. Adriamycin, 14-hydroxydaunomycin, a new antitumor antibiotic from S. peucetius var. caesius. Biotechnol Bioeng. 1969 Nov;11(6):1101–1110. doi: 10.1002/bit.260110607. [DOI] [PubMed] [Google Scholar]
- Cassinelli G., Grein A., Masi P., Suarato A., Bernardi L., Arcamone F., Di Marco A., Casazza A. M., Pratesi G., Soranzo C. Preparation and biological evaluation of 4-O-demethyldaunorubicin (carminomycin I) and of its 13-dihydro derivative. J Antibiot (Tokyo) 1978 Mar;31(3):178–184. doi: 10.7164/antibiotics.31.178. [DOI] [PubMed] [Google Scholar]
- Crespi-Perellino N., Grein A., Merli S., Minghetti A., Spalla C. Biosynthetic relationships among daunorubicin, doxorubicin and 13-dihydrodaunorubicin in Streptomyces peucetius. Experientia. 1982 Dec 15;38(12):1455–1456. doi: 10.1007/BF01955767. [DOI] [PubMed] [Google Scholar]
- Hutchinson C. R. The impact of genetic engineering on the commercial production of antibiotics by Streptomyces and related bacteria. Appl Biochem Biotechnol. 1987 Sep-Dec;16:169–190. doi: 10.1007/BF02798365. [DOI] [PubMed] [Google Scholar]
- Katz E., Thompson C. J., Hopwood D. A. Cloning and expression of the tyrosinase gene from Streptomyces antibioticus in Streptomyces lividans. J Gen Microbiol. 1983 Sep;129(9):2703–2714. doi: 10.1099/00221287-129-9-2703. [DOI] [PubMed] [Google Scholar]
- Komiyana T., Matsuzawa Y., Oki T., Inui T., Takahashi Y., Naganawa H., Takeuchi T., Umezawa H. Baumycins, new antitumor antibiotics related to daunomycin. J Antibiot (Tokyo) 1977 Jul;30(7):619–621. doi: 10.7164/antibiotics.30.619. [DOI] [PubMed] [Google Scholar]
- Lampel J. S., Strohl W. R. Transformation and transfection of anthracycline-producing streptomycetes. Appl Environ Microbiol. 1986 Jan;51(1):126–131. doi: 10.1128/aem.51.1.126-131.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oki T., Takatsuki Y., Tobe H., Yoshimoto A., Takeuchi T., Umezawa H. Microbial conversion of daunomycin, carminomycin I and feudomycin A to adriamycin. J Antibiot (Tokyo) 1981 Sep;34(9):1229–1231. doi: 10.7164/antibiotics.34.1229. [DOI] [PubMed] [Google Scholar]
- Otten S. L., Stutzman-Engwall K. J., Hutchinson C. R. Cloning and expression of daunorubicin biosynthesis genes from Streptomyces peucetius and S. peucetius subsp. caesius. J Bacteriol. 1990 Jun;172(6):3427–3434. doi: 10.1128/jb.172.6.3427-3434.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo R., Sciandrello G., Carere A., Bignami M., Velcich A., Sermonti G. Localized mutagenesis in Streptomyces coelicolor A3 (2). Mutat Res. 1976 Sep;36(3):291–302. doi: 10.1016/0027-5107(76)90239-6. [DOI] [PubMed] [Google Scholar]
- Rave N., Crkvenjakov R., Boedtker H. Identification of procollagen mRNAs transferred to diazobenzyloxymethyl paper from formaldehyde agarose gels. Nucleic Acids Res. 1979 Aug 10;6(11):3559–3567. doi: 10.1093/nar/6.11.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutzman-Engwall K. J., Hutchinson C. R. Multigene families for anthracycline antibiotic production in Streptomyces peucetius. Proc Natl Acad Sci U S A. 1989 May;86(9):3135–3139. doi: 10.1073/pnas.86.9.3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutzman-Engwall K. J., Otten S. L., Hutchinson C. R. Regulation of secondary metabolism in Streptomyces spp. and overproduction of daunorubicin in Streptomyces peucetius. J Bacteriol. 1992 Jan;174(1):144–154. doi: 10.1128/jb.174.1.144-154.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto A., Oki T., Takeuchi T., Umezawa H. Microbial conversion of anthracyclinones to daunomycin by blocked mutants of Streptomyces coeruleorubidus. J Antibiot (Tokyo) 1980 Oct;33(10):1158–1166. doi: 10.7164/antibiotics.33.1158. [DOI] [PubMed] [Google Scholar]