Abstract
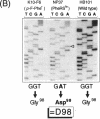
The pheS5 mutation responsible for the thermosensitive phenylalanyl-tRNA synthetase of the classical Escherichia coli NP37 was cloned by a recombination event and identified by DNA sequence analysis. The mutation was subsequently verified by direct sequencing of amplified NP37 DNA generated by an asymmetric polymerase chain reaction. The resulting amino acid exchange, Gly-98 to Asp-98 in the phenylalanyl-tRNA synthetase alpha subunit, might cause subunit disaggregation due to electrostatic repulsion.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 8. Microbiol Rev. 1990 Jun;54(2):130–197. doi: 10.1128/mr.54.2.130-197.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg-Olivier S. A., Tarlinton D., Brown K. D. Defective regulation of the phenylalanine biosynthetic operon in mutants of the phenylalanyl-tRNA synthetase operon. J Bacteriol. 1987 May;169(5):1949–1953. doi: 10.1128/jb.169.5.1949-1953.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brakhage A. A., Putzer H., Shazand K., Röschenthaler R. J., Grunberg-Manago M. Bacillus subtilis phenylalanyl-tRNA synthetase genes: cloning and expression in Escherichia coli and B. subtilis. J Bacteriol. 1989 Feb;171(2):1228–1232. doi: 10.1128/jb.171.2.1228-1232.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böck A., Neidhardt F. C. Genetic mapping of phenylalanyl-sRNA synthetase in Escherichia coli. Science. 1967 Jul 7;157(3784):78–79. doi: 10.1126/science.157.3784.78. [DOI] [PubMed] [Google Scholar]
- Böck A. Relation between subunit structure and temperature-sensitivity of mutant phenylalanyl RNA synthetases of Escherichia coli. Eur J Biochem. 1968 Apr;4(3):395–400. doi: 10.1111/j.1432-1033.1968.tb00225.x. [DOI] [PubMed] [Google Scholar]
- Caillet J., Plumbridge J. A., Springer M. Evidence that pheV, a gene for tRNAPhe of E. coli is transcribed from tandem promoters. Nucleic Acids Res. 1985 May 24;13(10):3699–3710. doi: 10.1093/nar/13.10.3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caillet J., Plumbridge J. A., Springer M., Vacher J., Delamarche C., Buckingham R. H., Grunberg-Manago M. Identification of clones carrying an E. coli tRNAPhe gene by suppression of phenylalanyl-tRNA synthetase thermosensitive mutants. Nucleic Acids Res. 1983 Feb 11;11(3):727–736. doi: 10.1093/nar/11.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatton B., Winsor B., Boulanger Y., Fasiolo F. Cloning and characterization of the yeast methionyl-tRNA synthetase mutation mes1. J Biol Chem. 1987 Nov 5;262(31):15094–15097. [PubMed] [Google Scholar]
- Comer M. M., Böck A. Genes for the alpha and beta subunits of the phenylalanyl-transfer ribonucleic acid synthetase of Escherichia coli. J Bacteriol. 1976 Aug;127(2):923–933. doi: 10.1128/jb.127.2.923-933.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delamarche C., Vacher J., Buckingham R. H. Mutants affecting tRNA(Phe) from Escherichia coli. Studies of the suppression of thermosensitive phenylalanyl-tRNA synthetase. Eur J Biochem. 1987 Oct 15;168(2):365–369. doi: 10.1111/j.1432-1033.1987.tb13428.x. [DOI] [PubMed] [Google Scholar]
- EIDLIC L., NEIDHARDT F. C. PROTEIN AND NUCLEIC ACID SYNTHESIS IN TWO MUTANTS OF ESCHERICHIA COLI WITH TEMPERATURE-SENSITIVE AMINOACYL RIBONUCLEIC ACID SYNTHETASES. J Bacteriol. 1965 Mar;89:706–711. doi: 10.1128/jb.89.3.706-711.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elseviers D., Gallagher P., Hoffman A., Weinberg B., Schwartz I. Molecular cloning and regulation of expression of the genes for initiation factor 3 and two aminoacyl-tRNA synthetases. J Bacteriol. 1982 Oct;152(1):357–362. doi: 10.1128/jb.152.1.357-362.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayat G., Mayaux J. F., Sacerdot C., Fromant M., Springer M., Grunberg-Manago M., Blanquet S. Escherichia coli phenylalanyl-tRNA synthetase operon region. Evidence for an attenuation mechanism. Identification of the gene for the ribosomal protein L20. J Mol Biol. 1983 Dec 15;171(3):239–261. doi: 10.1016/0022-2836(83)90092-x. [DOI] [PubMed] [Google Scholar]
- Goodman R., Schwartz I. Kinetic analysis of an E.coli phenylalanine-tRNA synthetase mutant. Nucleic Acids Res. 1988 Aug 11;16(15):7477–7486. doi: 10.1093/nar/16.15.7477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grüll J. M., Hennecke H., Fröhler J., Thomale J., Nass G., Böck A. Escherichia coli mutants overproducing phenylalanyl- and threonyl-tRNA synthetase. J Bacteriol. 1979 Jan;137(1):480–489. doi: 10.1128/jb.137.1.480-489.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyllensten U. B., Erlich H. A. Generation of single-stranded DNA by the polymerase chain reaction and its application to direct sequencing of the HLA-DQA locus. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7652–7656. doi: 10.1073/pnas.85.20.7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennecke H. Use of mutant enzymes to demonstrate the presence of two active sites on phenylalanyl-tRNA synthetase from Eschericia coli. FEBS Lett. 1976 Dec 15;72(1):182–186. doi: 10.1016/0014-5793(76)80840-x. [DOI] [PubMed] [Google Scholar]
- Hountondji C., Schmitter J. M., Beauvallet C., Blanquet S. Affinity labeling of Escherichia coli phenylalanyl-tRNA synthetase at the binding site for tRNAPhe. Biochemistry. 1987 Aug 25;26(17):5433–5439. doi: 10.1021/bi00391a033. [DOI] [PubMed] [Google Scholar]
- Jasin M., Regan L., Schimmel P. Dispensable pieces of an aminoacyl tRNA synthetase which activate the catalytic site. Cell. 1984 Apr;36(4):1089–1095. doi: 10.1016/0092-8674(84)90059-x. [DOI] [PubMed] [Google Scholar]
- Jasin M., Regan L., Schimmel P. Two mutations in the dispensable part of alanine tRNA synthetase which affect the catalytic activity. J Biol Chem. 1985 Feb 25;260(4):2226–2230. [PubMed] [Google Scholar]
- Jasin M., Schimmel P. Deletion of an essential gene in Escherichia coli by site-specific recombination with linear DNA fragments. J Bacteriol. 1984 Aug;159(2):783–786. doi: 10.1128/jb.159.2.783-786.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. H., McMillan A. J., Fersht A. R., Winter G. Reversible dissociation of dimeric tyrosyl-tRNA synthetase by mutagenesis at the subunit interface. Biochemistry. 1985 Oct 8;24(21):5852–5857. doi: 10.1021/bi00342a024. [DOI] [PubMed] [Google Scholar]
- Kast P., Hennecke H. Amino acid substrate specificity of Escherichia coli phenylalanyl-tRNA synthetase altered by distinct mutations. J Mol Biol. 1991 Nov 5;222(1):99–124. doi: 10.1016/0022-2836(91)90740-w. [DOI] [PubMed] [Google Scholar]
- Kast P., Wehrli C., Hennecke H. Impaired affinity for phenylalanine in Escherichia coli phenylalanyl-tRNA synthetase mutant caused by Gly-to-Asp exchange in motif 2 of class II tRNA synthetases. FEBS Lett. 1991 Nov 18;293(1-2):160–163. doi: 10.1016/0014-5793(91)81176-9. [DOI] [PubMed] [Google Scholar]
- Khodyreva S. N., Moor N. A., Ankilova V. N., Lavrik O. I. Phenylalanyl-tRNA synthetase from E. coli MRE-600: analysis of the active site distribution on the enzyme subunits by affinity labelling. Biochim Biophys Acta. 1985 Aug 8;830(2):206–212. doi: 10.1016/0167-4838(85)90029-9. [DOI] [PubMed] [Google Scholar]
- Mechulam Y., Blanquet S., Fayat G. Dual level control of the Escherichia coli pheST-himA operon expression. tRNA(Phe)-dependent attenuation and transcriptional operator-repressor control by himA and the SOS network. J Mol Biol. 1987 Oct 5;197(3):453–470. doi: 10.1016/0022-2836(87)90558-4. [DOI] [PubMed] [Google Scholar]
- Neidhardt F. C. Roles of amino acid activating enzymes in cellular physiology. Bacteriol Rev. 1966 Dec;30(4):701–719. doi: 10.1128/br.30.4.701-719.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittard J., Praszkier J., Certoma A., Eggertsson G., Gowrishankar J., Narasaiah G., Whipp M. J. Evidence that there are only two tRNA(Phe) genes in Escherichia coli. J Bacteriol. 1990 Oct;172(10):6077–6083. doi: 10.1128/jb.172.10.6077-6083.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plumbridge J. A., Springer M. Escherichia coli phenylalanyl-tRNA synthetase operon: characterization of mutations isolated on multicopy plasmids. J Bacteriol. 1982 Nov;152(2):650–660. doi: 10.1128/jb.152.2.650-660.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plumbridge J. A., Springer M., Graffe M., Goursot R., Grunberg-Manago M. Physical localisation and cloning of the structural gene for E. coli initiation factor IF3 from a group of genes concerned with translation. Gene. 1980 Oct;11(1-2):33–42. doi: 10.1016/0378-1119(80)90084-0. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Schimmel P. Aminoacyl tRNA synthetases: general scheme of structure-function relationships in the polypeptides and recognition of transfer RNAs. Annu Rev Biochem. 1987;56:125–158. doi: 10.1146/annurev.bi.56.070187.001013. [DOI] [PubMed] [Google Scholar]
- Schwartz I., Klotsky R. A., Elseviers D., Gallagher P. J., Krauskopf M., Siddiqui M. A., Wong J. F., Roe B. A. Molecular cloning and sequencing of pheU, a gene for Escherichia coli tRNAPhe. Nucleic Acids Res. 1983 Jul 11;11(13):4379–4389. doi: 10.1093/nar/11.13.4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyamala V., Ames G. F. Amplification of bacterial genomic DNA by the polymerase chain reaction and direct sequencing after asymmetric amplification: application to the study of periplasmic permeases. J Bacteriol. 1989 Mar;171(3):1602–1608. doi: 10.1128/jb.171.3.1602-1608.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer M., Graffe M., Grunberg-Manago M. Genetic organization of the E. coli chromosome around the structural gene for initiation factor IF3 (infC). Mol Gen Genet. 1979 Feb 1;169(3):337–343. doi: 10.1007/BF00382279. [DOI] [PubMed] [Google Scholar]
- Springer M., Mayaux J. F., Fayat G., Plumbridge J. A., Graffe M., Blanquet S., Grunberg-Manago M. Attenuation control of the Escherichia coli phenylalanyl-tRNA synthetase operon. J Mol Biol. 1985 Feb 20;181(4):467–478. doi: 10.1016/0022-2836(85)90420-6. [DOI] [PubMed] [Google Scholar]
- Springer M., Trudel M., Graffe M., Plumbridge J., Fayat G., Mayaux J. F., Sacerdot C., Blanquet S., Grunberg-Manago M. Escherichia coli phenylalanyl-tRNA synthetase operon is controlled by attenuation in vivo. J Mol Biol. 1983 Dec 15;171(3):263–279. doi: 10.1016/0022-2836(83)90093-1. [DOI] [PubMed] [Google Scholar]
- Vacher J., Springer M., Buckingham R. H. Functional mutants of phenylalanine transfer RNA from Escherichia coli. EMBO J. 1985 Feb;4(2):509–513. doi: 10.1002/j.1460-2075.1985.tb03657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R. K., Roe B. A. Presence of the hypermodified nucleotide N6-(delta 2-isopentenyl)-2-methylthioadenosine prevents codon misreading by Escherichia coli phenylalanyl-transfer RNA. Proc Natl Acad Sci U S A. 1989 Jan;86(2):409–413. doi: 10.1073/pnas.86.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrischnik L. A., Higuchi R. G., Stoneking M., Erlich H. A., Arnheim N., Wilson A. C. Length mutations in human mitochondrial DNA: direct sequencing of enzymatically amplified DNA. Nucleic Acids Res. 1987 Jan 26;15(2):529–542. doi: 10.1093/nar/15.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]